Abstract
In recent years, there has been a dramatic increase in the use of poly(dimethylsiloxane) (PDMS) devices for cell-based studies. Commonly, the negative tone photoresist, SU8, is used to pattern features onto silicon wafers to create masters (SU8-Si) for PDMS replica molding. However, the complexity in the fabrication process, low feature reproducibility (master-to-master variability), silane toxicity, and short life span of these masters have been deterrents for using SU8-Si masters for the production of cell culture based PDMS microfluidic devices. While other techniques have demonstrated the ability to generate multiple devices from a single master, they often do not match the high feature resolution (∼0.1 μm) and low surface roughness that soft lithography masters offer. In this work, we developed a method to fabricate epoxy-based masters that allows for the replication of features with high fidelity directly from SU8-Si masters via their PDMS replicas. By this method, we show that we could obtain many epoxy based masters with equivalent features to a single SU8-Si master with a low feature variance of 1.54%. Favorable feature transfer resolutions were also obtained by using an appropriate Tg epoxy based system to ensure minimal shrinkage of features ranging in size from ∼100 μm to <10 μm in height. We further show that surface coating epoxy masters with Cr/Au lead to effective demolding and yield PDMS chambers that are suitable for long-term culturing of sensitive primary hippocampal neurons. Finally, we incorporated pillars within the Au-epoxy masters to eliminate the process of punching media reservoirs and thereby reducing substantial artefacts and wastage.
I. INTRODUCTION
Poly(dimethylsiloxane) (PDMS) microdevices have been increasing in popularity for in vitro cell based studies such as biochemical analyses, single molecule transport, and drug based studies. PDMS is a favorable material for cell culture devices due its gas permeability, biocompatibility, and optical transparency, which makes high quality imaging possible.1–5 Standard lithography processes such as photoresist processing and etching are used to generate masters for PDMS casting. Negative tone photoresist SU8 spun and UV cured onto silicon wafers is commonly used for soft lithography.
Standard lithography processes have been shown to produce high grade masters characterized by high feature resolution (∼0.1 μm) dimensions and low surface roughness.6,7 However, there are some drawbacks regarding these masters. For example: (i) silicon based masters have a short life expectancy due to their fragility; (ii) fabrication of masters using standard lithography is a low throughput process involving complex and numerous processing steps per master making it impractical for large-scale production; (iii) variation in conditions with time that affect fabrication protocols, such as changes in humidity and SU8 composition (i.e., solvent evaporation) contribute to batch-to-batch master variability; and (iv) fabrication processes require the use of clean room facility equipment making it expensive and inaccessible to most labs.8–12
Alternative fabrication techniques that do not require lithography are able to produce durable and sturdy metal masters with less complicated fabrication steps. High precision micromilling, as an example, is a microfabrication technique used to generate sturdy metal masters for hot embossing and injection molding of polymer microfluidic devices. It can generate a variety of microstructures on different materials as well as create multilevel structures. Masters generated from this process can be used to produce several identical polymer devices via hot embossing and have very long life expectancies. This is suitable for rapid prototyping of microdevices and mass production of finished product. However, this technique has some limitations as it cannot match the fine feature resolution (∼0.1 μm) that standard lithography masters offer. Large surface roughness and the inability of the milling process to produce sharp inner corners are also some of the drawbacks of micromilled masters.13
Replica molding can produce masters with the same grade of quality that standard lithography masters have to offer. It has been used widely for manufacturing compact discs and microtools because of its rapid replication fidelity for high resolution features.14 This technique is capable of duplicating complex relief structures from PDMS molds that were cast against silicon based masters in a single step. The advantage of this technique is that it generates multiple masters from a single soft lithography master. An example is the use of PDMS as a master mold for casting PDMS microdevices using a double casting method. The challenge with this is the difficulty with the demolding step as result of PDMS to PDMS adhesion. This destroys features upon release and would require complex derivatization and potentially toxic surface treatments on the master to facilitate effective release of the PDMS replica.15,16 A more rigid master template created via replica molding was demonstrated by Desai et al. through the use of room temperature curable polyurethanes.17 They obtained low shrinkage of ∼4% in feature resolution during feature transfer from PDMS substrate to the cured plastic master. However, a challenge with low temperature curable plastic masters is the low feature preservation during repeated use of the master for multiple PDMS castings. This comes as a result of shrinkage of the plastic master when using higher PDMS curing temperatures above the glass transition temperature (Tg) of the plastic master.
Our motivation behind this work was to produce biocompatible masters for cell culture based PDMS devices suitable for mass production. Our model system was to produce masters for previously described neuron culture devices.18,19 These chambers consist of feature sizes ranging from 4 μm to 140 μm, and therefore it was critical to ensure that the replication method could achieve these identical dimensions. We describe a replica molding process used to produce thermally curable epoxy masters and evaluate feature transfer resolution and precision. We also evaluate the throughput of this process by assessing batch-to-batch variability which we hypothesize would be improved over the variability of SU8-Si masters. Further, we tested the suitability of Cr/Au surface coating on the epoxy masters as an alternative to silane coating. Our findings indicate that Cr/Au coating is biocompatible with PDMS culture devices for sensitive cultures such as primary neurons as well as effective for demolding PDMS devices from epoxy masters. Finally, we demonstrate the addition of pillars within these masters. This enables PDMS-based devices to be formed without punching media reservoirs, thus eliminating debris and greatly facilitating production.
II. MATERIALS AND METHODS
A. Materials list
Epotek® epoxy (Epoxy Technologies, Billerica, MA, part number 301–2); EasyCast® epoxy from Environmental Tech Inc. (Fields Landing, California); Sylgard® 184 Silicone elastomer Kit from Dow Corning (Midland, MI); Trichloro-octylsilane from Sigma Aldrich (St. Louis, MO); propidium Iodide from Molecular probes (Grand Island, NY); CellTracker™ Green CMFDA Dye from Molecular probes (Grand Island, NY); primary Chicken antipeptide β-Tubulin III from Aves Labs (Tigard, OR); and DAPI from Sigma Aldrich (St. Louis, MO).
B. Epoxy master production
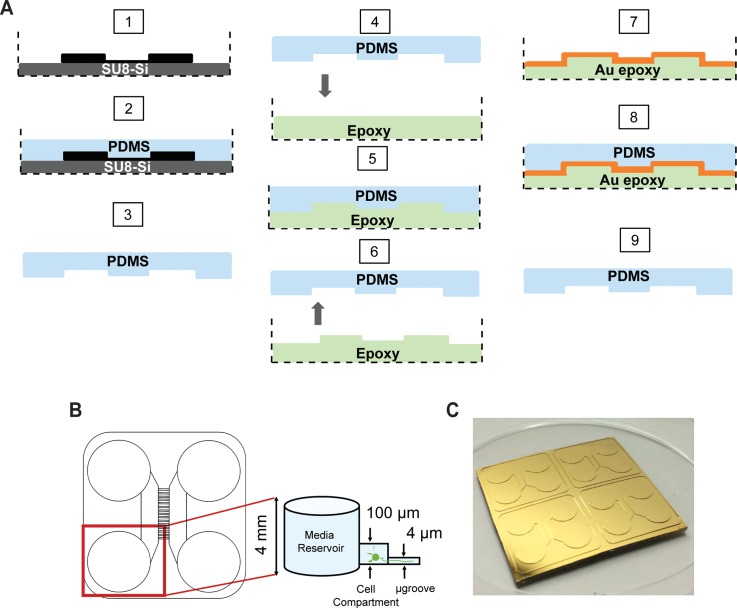
Epoxy masters as seen in Figure 1(a) were generated from PDMS devices that were cast from SU8-Si masters using replica molding.7 In step 1, an SU8-Si master was placed in a standard 100 mm × 15 mm petri dish as a holding container. In step 2, 20 g of premixed PDMS polymer was slowly poured over the SU8-Si master and the petri dish was then placed in a convection oven at 65 °C for a 12 h cure. Next in step 3, the PDMS was demolded from the SU8-Si master after the petri dish was cooled down to room temperature. Steps 4–7 demonstrate the making of the epoxy mold. In a separate petri dish 10 g of premixed and degassed epoxy was poured in a similar petri dish used in step 1 (step 4). The PDMS cast obtained from step 3 was gently placed over the epoxy with the micro features in contact with the epoxy (step 5). A thorough degassing was applied to remove any trapped bubbles between the uncured epoxy and PDMS cast. Shortly after, the PDMS over the epoxy was left to cure according to the epoxy curing schedule as recommended from the manufacturers. In step 6, the PDMS was demolded from the cured epoxy. Following this, the epoxy mold was subjected to a chromium and gold surface sputter deposition (step 7). Steps 8 and 9 demonstrate the use of an epoxy mold for soft lithography. Briefly, 20 g of premixed degassed PDMS polymer was poured over the Au-epoxy master and placed in a convection oven at 65 °C for cure. After a complete cure and cool down of the Au-epoxy master to room temperature, a PDMS cast was demolded.
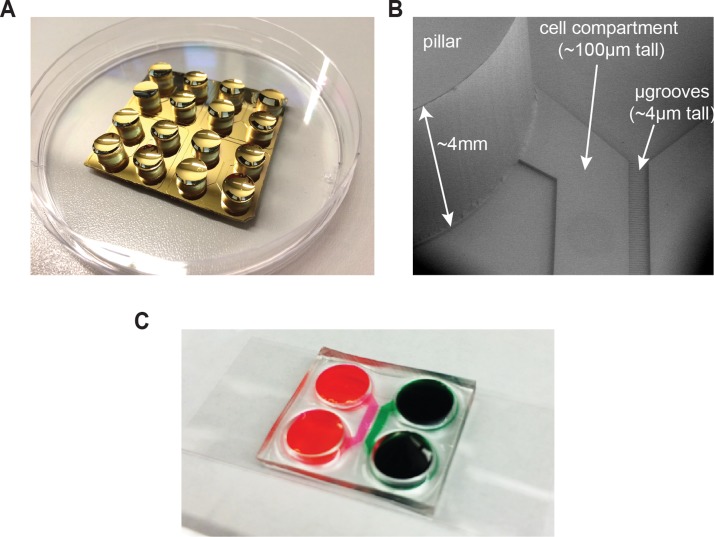
FIG. 1.
Au-epoxy master production. (a) Schematic of the replica molding process starting from the SU8-Si master (step 1) to the Au-epoxy master (step 7) to the generation of a PDMS device (step 9). A PDMS replica of the SU8-Si master (steps 1–3) is used to mold the epoxy contained within a suitable container, such as a petri dish (dashed lines) (steps 4–6). The surface of the epoxy is then coated with Cr/Au to facilitate future PDMS demolding (steps 7–9). (b) Diagram of a PDMS microfluidic culture device for neurons which contains varying feature heights: ∼4 mm wells for loading solution into the channels; ∼100 μm high cell compartments which house the neurons; and ∼4 μm tall microgrooves which allow growth of axons and dendrites, but not cell bodies. We used this microfluidic device configuration as a model for the remainder of this study. (c) Photograph of a Au-epoxy master contained in a standard 100 mm × 50 mm petri dish for PDMS casting.
C. Epoxy master surface coatings
1. Silane coating
The surface of the epoxy mold was initially exposed to plasma oxidation at 50 W for 30 s and then coated with a self-assembled monolayer of trichloro-octylsilane in a vacuum chamber for 12 h at room temperature. Masters were rinsed in 70% (v/v) EtOH to dissolve any physisorbed silane molecules.
2. Gold coating
Gold metal sputter deposition on the epoxy masters was done using the PVD 75 magnetron sputtering system (DC), operating at a substrate temperature of 25 °C with O2 gas at a chamber pressure of 0.05 mTorr. First, Cr was coated at a deposition rate of 2 Å/s and thickness of 0.05 kÅ, followed by Au at a deposition rate of 2 Å/s and thickness of 1.0 kÅ.
D. SU8-Si master fabrication
SU8 on Si master fabrication has been reported elsewhere.18–20 Masks to generate the SU8-Si masters were drafted in AutoCAD (Autodesk Inc.) and chrome masks were generated (Photo Sciences, Inc.). Masters were fabricated in the Chapel Hill Analytical & Nanofabrication Laboratory (CHANL) at UNC as described previously.21 SU8–2005 (Microchem) was spun onto a silicon wafer to generate the 4 μm layer. SU8–2050 was spun on at a thickness of 120 μm. PDMS was molded onto a SU8-Si master, as described previously. Devices were sterilized in 70% EtOH and placed onto poly-D-lysine coated glass coverslip substrates as described previously.22 We used 500–550 kDa poly-d-lysine (BD Biosciences) and incubated the glass for >6 h at 37 °C.
E. SEM imaging
Electron micrographs of the micro features in masters were obtained using FEI Quanta 200 Field emission gun scanning electron microscope at low vacuum mode (∼0.5 Torr). Conditions during imaging included: (i) an applied accelerating voltage of 10 kV; (ii) a magnification of 200× for low magnification images and 800× for high magnification images; and (iii) a beam spot size of 3.0. A tilt angle of 15° was used for further elucidation of compartment wall features and microgroove features. SEM images of the epoxy master were taken with uncoated samples, and thus appear to have bright regions due to the surface charging effects of SEM the nonconductive epoxy surface.
F. Profilometry
Height measurements of the micro features on both SU8-Si masters (n = 2) and Au-epoxy master replicates (n = 2) were performed with the KLA Tencor P-6 stylus profiler system. Linear scans were performed with a 2 μm tip radius a speed of 20 mm·s−1 at a sampling rate of 20 Hz with an applied force of 2 g. One SU8-Si master consisted of 4 multicompartment device feature reliefs centrally placed in a 2 × 2 arrangement. Each device relief on a master consisted of an array of approximately 150 parallel microgrooves flanked by 2 compartments. Profilometry scans were made across at least 4 to 6 microgrooves from each device relief. Sampling locations were distributed equally on all 4 device reliefs per master. An average reading was obtained from each scan and a minimum of 4 scans per master was performed. These same locations were used for all the Au-epoxy master replicates from the SU8-Si masters. Compartment height measurements were also taken from all device reliefs with a minimum of 6 readings per master.
G. Optical measurements
For optical analysis of the master micro features, a Nikon Eclipse LV150 optical upright microscope attached to a CCD camera was used to capture images using bright field mode with 10× (NA 0.3), 20× (NA 0.45), and 50× (NA 0.80) objective lenses. NIS-Elements imaging software was used to process and analyze microgroove widths from the obtained images. The same sampling procedure for the width measurements was performed as explained under Sec. II F. We obtained 12 width readings from each sample.
H. Cell culture
Primary embryonic rat hippocampal neuron cells were used as model cells for this study. All experiments were performed in compliance with relevant laws and institutional guidelines; all procedures were approved by the UNC Institutional Animal Care and Use Committee. Hippocampal cells were prepared from embryonic day 17–18 Sprague Dawley embryos of either sex as described previously.21,22 We used 5–10 μl of cell suspension per well at a density of 12 million cells/ml.
I. Viability assay and cell imaging
CellTracker Green CMFDA Dye (1 μM: Invitrogen) was used as a live cell dye and Propidium iodide (1: 3000; Invitrogen) to label dead cells. Staining was performed according to Invitrogen protocols.21
J. Microscopy and image analysis
Images were acquired using a spinning disk confocal imaging system (Yokogawa CSU-X1) configured for an Olympus IX81 zero-drift microscope (Andor Revolution XD system). Excitation was provided by 50 mW 488 nm, 50 mW 561 nm, and 100 mW 640 nm lasers. Andor iQ software was used to acquire images. Imaging conditions for the live cells were taken with a 20× Olympus dry objective (NA 0.75) using 488 nm excitation with exposure of 200 ms at a laser intensity of 20%; for the dead cells, we used 561 nm excitation with an exposure of 300 ms at a laser intensity set to 25%. Images were processed using NIH ImageJ. To count the live cells and dead cells, we converted the images to 8 bit depth, and applied appropriate thresholds for both the CellTracker green dye images and propidium iodide dye images. We used the ‘analyze particle’ command to count cells using the regions >2 μm2 for both channels. To calculate the percent viability, we divided the number of live cells by the total number of cells (live plus dead) and normalized the ratio to the mean of the control sample.
III. RESULTS AND DISCUSSION
A. Design and production of Au-epoxy master
A critical aspect when selecting epoxy resins for generating masters for PDMS casting is the thermal stability of the resin. Epoxy resins should be structurally stable and have minimal volume shrinkage at the range curing temperatures required for PDMS casting. PDMS can be cured at various temperatures with the appropriate curing times. A significant thermal property of epoxy resins is the Tg. This is the temperature at which cured epoxy changes from a glassy state to rubbery state and as a consequence dictates the molecular stability of the epoxy resin.23 Among the factors that influence the Tg of an epoxy are the crosslinking density and the curing temperature.24 In our case, we applied a PDMS curing temperature of 65 °C for 12 h for our in house conventional SU8-Si masters which were placed in polystyrene petri dishes with a Tg of 80 °C as holding containers. This curing temperature was carefully selected to be below the Tg of the petri dish in order to prevent the petri dish from warping at temperatures higher than its Tg.
We evaluated two commercially available epoxies with different Tg's and curing schedules in order to generate durable and stiff masters for PDMS casting. The first epoxy we tested was EasyCast epoxy, a room temperature epoxy with a Tg of 53 °C and the second epoxy had a higher curing temperature and Tg of 80 °C, referred to as Epotek epoxy. In addition, epoxy physical properties as such low viscosity and longer pot life were considered in order to allow time for efficient degassing of the epoxy before hardening.
B. Feature replication resolution and fidelity; replication precision; and Au-epoxy durability
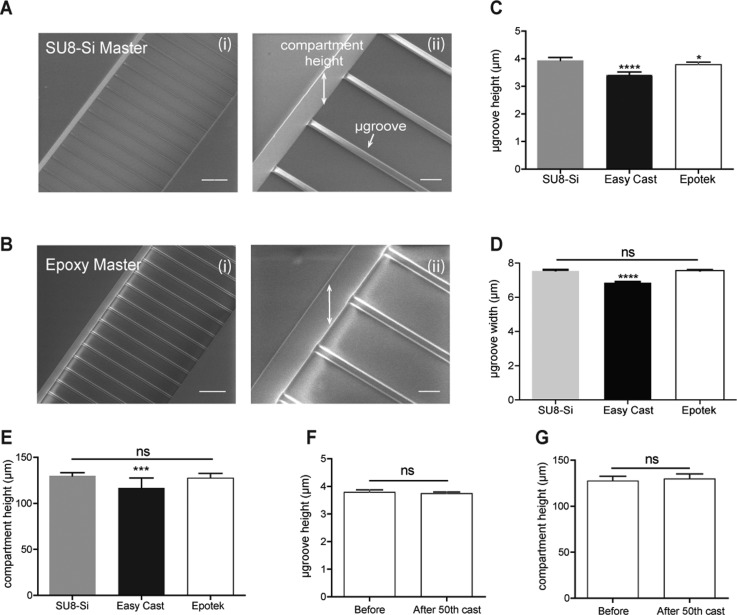
Feature transfer precision from the SU8-Si master to the epoxy master was determined by comparing the feature height and width dimensions of both masters. Stylus profilometry measurements were used to determine the microgroove height dimensions (range of 3–5 μm) and compartment height dimensions (range of 130–140 μm). Microgroove width dimensions were determined by using an optical microscope (range of 7–8 μm). Our data as seen in Figures 2(c), 2(d), and 2(e) indicate that there was significant feature dimension shrinkage of 13.2% in microgroove height, 9.1% in microgroove width, and 7.3% in cell compartment height for the low Tg EasyCast epoxy master. However, for the higher Tg Epotek epoxy master, a low shrinkage was observed of 3.8% in microgroove height (Fig. 2(c)), and insignificant shrinkages of 0.5% in microgroove width (Fig. 2(d)) and 1.1% in cell compartment height (Fig. 2(e)).
FIG. 2.
Feature replication precision from SU8-Si master to epoxy master and epoxy master durability. (a-i) SEM image of parallel SU8 microgrooves flanked by 2 SU8 compartments on a representative SU8-Si master. (a-ii) Close-up SEM image of the microgrooves and compartment side wall on the SU8-Si master. (b-i) SEM image of replicated parallel microgrooves on a representative epoxy master. (b-ii) Close-up SEM image of the microgrooves and compartment side wall features formed into the epoxy master. (c) Graph of the microgroove height measurements of the SU8-Si master 3.938 ± 0.11 μm (n = 8), low Tg Easy Cast Au-epoxy master 3.417 ± 0.11 μm (n = 12), and high Tg Epotek Au epoxy masters 3.785 ± 0.08 μm (n = 8). (d) Graph of microgroove width measurements of the SU8-Si masters 7.567 ± 0.21 μm (n = 24), low Tg Easy Cast Au-epoxy masters 6.883 ± 0.14 μm (n = 24), and high Tg Epotek Au-epoxy masters 7.533 ± 0.22 μm (n = 24). (e) Graph of compartment height measurements of the SU8-Si masters 129.9 ± 3.4 μm (n = 14), low Tg Easy Cast Au-epoxy masters 120.3 ± 7.39 μm (n = 14), and high Tg Epotek Au-epoxy masters 127.7 ± 4.85 μm (n = 10). (f) Graph of microgroove height measurements of an Epotek master before the first PDMS cast 3.785 ± 0.08 μm (n = 8) and after 50 PDMS castings 3.748 ± 0.02 μm (n = 12); p = 0.2274. (g) Graph of compartment height measurements of an Epotek master before the first PDMS cast 127.7 ± 4.85 μm (n = 10) and after 50 PDMS castings 130.5 ± 5.077 μm (n = 10); p = 0.2332. Both microgroove height measurements and compartment height measurements were obtained from a scanning profilometer, while microgroove width measurements were obtained using an optical microscope. For (c)–(e), two masters were measured for each condition. SEM images for the epoxy master were taken with uncoated samples, and thus appear to have bright regions due to the surface charging effects of SEM. Scale bars, 100 μm for low magnification SEM images and 20 μm for high magnification SEM images.
To evaluate the stability and durability of the Epotek epoxy master, we measured the microgroove and compartment heights for a Au-epotek master before its first PDMS casting, compared to the same master after the 50th PDMS casting. Results shown in Figures 2(e) and 2(f) indicate no significant changes in microgroove height (p = 0.2274) and compartment height (p = 0.2331) as determined by Unpaired Student's t-test. However, for the EasyCast master we found significant changes in the compartment height dimensions and observed the appearance of cracks on the gold surface coating of Au-epoxy cast masters with continued use, suggesting considerable thermal changes in the EasyCast epoxy during the curing process of PDMS culture chambers. Studies have shown that at temperatures below the Tg of an epoxy, the rate of thermal expansion and modulus is lower, as we observed with the minimal changes of the dimensions in Epotek epoxy master (Tg = 80 °C) during PDMS curing temperature of 65 °C. While at temperatures above the Tg of the epoxy will result in higher rates of thermal expansion (changes in molecular structure) and epoxy modulus, as we observed in the case of the EasyCast epoxy (Tg = 54 C).24,25 Keeping this in mind, we selected Epotek epoxy to generate rigid masters with minimal changes in feature dimension due to its suitable physical properties for our application.
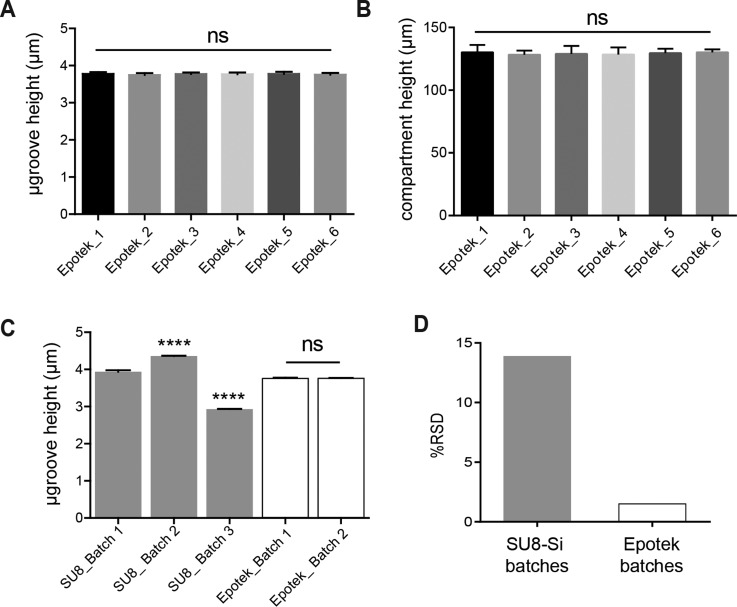
One of the challenging aspects of fabricating SU8-Si masters is the low batch-to-batch reproducibility which hinders large-scale production. The high variability observed in feature dimensions from one SU8-Si master to another is mainly due to the complexity of the fabrication process. In particular, for our application, the challenge lies in the spin casting steps of the SU8 photoresist onto the silicon wafer. Solvent evaporation from the SU8 photoresist results in viscosity changes with time. This affects the batch-to-batch reproducibility of SU8-Si masters. Although there exist master microfabrication prototyping methods such as hot embossing which utilizes a single master mold to generate multiple polymer replicates with high reproducibility, it is still difficult to obtain a master with feature dimensions that are less than 10 μm.13 In our neuron culture device, 4 μm tall straight microgrooves allow the extension of axons and dendrites, but prevents cell bodies from entering. Without strict adherence to these dimensions, we would observe cell body migration across the barrier for taller and wider channels. The attractive aspect of generating masters through replica molding such as performed here is that we could expect to generate multiple identical master molds with high batch-to-batch reproducibility from a single SU8-Si master. In Figure 3, we measured the degree of variability in the microgroove height and the compartment height within one batch of 6 Epotek epoxy masters generated from a single SU8-Si master via replica molding. The resulting variance in the microgroove heights was 1.89%, while the variance for the compartment height was 3.30%. Next, we analyzed the variability of microgroove feature dimensions from 3 different batches of SU8-Si masters produced via lithography and 2 different batches Epotek masters produced from a single master (Figures 3(c) and 3(d)). As expected, our results show a higher variation in microgroove dimensions for the SU8-Si batches at 13.9% compared to 1.54% for Epotek batches (Fig. 3(d)).
FIG. 3.
Batch-to-batch feature reproducibility of Au-epoxy masters compared with SU8-Si masters. (a) Graph of microgroove heights for a batch of 6 epoxy masters, mean height of 3.67 ± 0.071 μm (RSD 1.89%; n = 48 from the 6 masters). (b) Graph of compartment heights for the same batch of 6 masters; mean height of 127.5 ± 4.3 μm (RSD 3.3%; n = 24 from 6 masters). (c) Graph showing microgroove height variability from 3 different batches of SU8-Si masters and 2 different batches of Epotek masters produced from a single SU8-Si master. Mean heights of SU8-Si batch 1 (2 masters) was 3.786 ± 0.067 μm (RSD 1.74%; n = 24); SU8-Si batch 2 (1 master) was 4.358 ± 0.04 μm (RSD 0.87%; n = 12); SU8-Si batch 3 (1 master) was 2.932 ± 0.03 μm (RSD 0.89%; n = 12); Epotek batch 1 (2 masters) was 3.764 ± 0.06 μm (RSD 1.72%; n = 18); and Epotek batch 2 (12 masters) was 3.768 ± 0.06 μm (RSD 1.52%; n = 120). (d) Overall microgroove feature variation between SU8-Si batches (RSD 13.91%; n = 48) and Epotek batches (RSD 1.54%; n = 138).
C. Biocompatibility
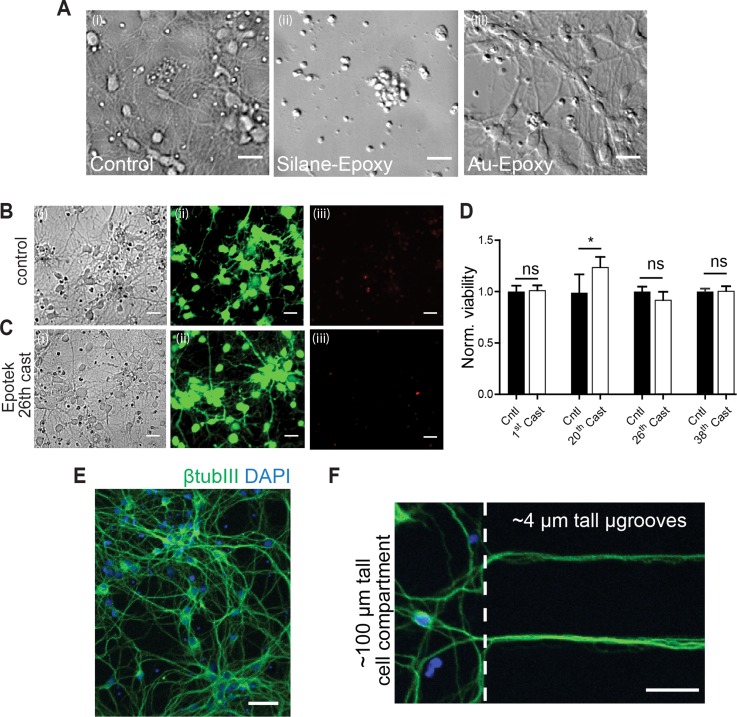
Epoxy surface passivation was necessary for PDMS demolding and without it, we could not demold the PDMS casts due to epoxy to PDMS adhesion. In order to ensure effective demolding as well as biocompatibility of the PDMS culture devices from epoxy masters, we initially coated the epoxy surface with a silane monolayer. Silane surface coating of the epoxy mold proved to be adequate for demolding. However, we observed poor viability with the neurons cultured in the resulting PDMS devices (Fig. 4(a-ii)). This was repeatedly observed for the first 3 PDMS casts from the silane coated epoxy masters despite the application of PDMS sterilization techniques. Due to the inherent porous structure of PDMS polymer,26 organic elements such as silane can be absorbed and leached into culture media causing toxicity when culturing cells.27 As an alternative viable option, we coated epoxy masters with the biocompatible surface metal, gold, using chromium as an adhesion promoter. Cr/Au metal deposition has been used as an effective surface coating for polymers.28,29 We observed effortless demolding of the PDMS casts from these molds, indicative of effective adhesion of the Au on the epoxy surface and passivation of reactive moieties on the surface.
FIG. 4.
Biocompatibility of PDMS devices cast from Au-epoxy masters. (a) Representative DIC micrographs of rat hippocampal neurons (6 DIV) within a cell compartment of PDMS devices cast from: (i) SU8-Si masters; (ii) epoxy masters coated with silane, and (iii) Au-epoxy masters. (b) and (c) Representative images of live neurons cultured within PDMS devices from SU8-Si masters (control) and the 26th cast of Epotek masters. Micrographs show (i) DIC, (ii) staining with the live cell marker CellTracker Green, and (iii) dead cell labelling using propidium iodide. Scale bars, 25 μm. (d) Quantification of the number of live cells relative to the total number of cells (live plus dead) for PDMS devices from the 1st, 20th, 26th, and 38th casts of Au-epoxy masters and normalized to controls from PDMS chambers molded from SU8-Si Masters for each cast tested. Two devices were used per condition and three frames per device. (e) and (f) Merged images of rat hippocampal neurons (6 DIV) within a PDMS device cast from Au-epoxy masters immunolabeled for β-tubulin III (green) and counterstained for DAPI (blue). Scale bar, 75 μm and 40 μm, respectively. (f) Neurons cultured with Au-epoxy-derived PDMS chambers extend axons into 4 μm tall microgrooves.
The cell viability in PDMS devices derived from Au-epoxy masters was evaluated using primary hippocampal neuron cultures. Viability was assessed using live/dead stains as described previously.21 PDMS chambers were treated with a live stain marker [Figs. 4(b-ii) and 4(c-ii)] and a dead cell marker propidium iodide [Figs. 4(b-iii) and 4(c-iii)]. Neurons grown on SU8-Si derived PDMS chambers were used as a reference control (Fig. 4(b)) to gauge viability. In addition, an evaluation of the viability of subsequent PDMS casts from Au-epoxy masters was done. Our data shown in Figures 4(b) and 4(c) indicate that the percent viability in the Au-epoxy-derived PDMS chambers were comparable or better to those of the controls for the 1st cast (p = 0.71), 20th cast (p = 0.014), 26th cast (p = 0.056), and 37th cast (p = 0.93). Axonal growth and extension across the 450 μm microgroove barrier were normal and similar compared to controls. Further, Figures 4(e) and 4(f) show expression of the neuron-specific marker β-tubulin III in neurons and axons within the compartments and microgrooves by 6 DIV. Together, our viability data confirm the biocompatibility and durability of the Au-epotek masters and the feature dimension stability due to the observed favorable axonal growth within the PDMS chambers.
D. Au-epoxy master with pillars
An advantage of replica molding over photolithography is the potential to create taller structures such as pillars. Incorporating pillars within a master allows us to obtain culture media reservoirs in the PDMS devices without punching. To fabricate the pillars, we produced an epoxy replica from a PDMS device that had precise punched holes in the media reservoirs. The pillars bore a diameter of 8 mm and 4–6 mm in height (Figure 5). Cr/Au coating on the pillars facilitated the removal of the PDMS casts without any difficulty. By adding the pillars to the masters: (i) we eliminated the need for punching the PDMS devices which reduced the amount debris in the microfeatures introduced by punching (this is critical for quality control); (ii) we also reduced wastage produced as a result poorly punched devices; and (iii) reduced labor time.
FIG. 5.
Tall pillar structures in Au-epoxy masters. (a) Photograph of a Au-epoxy master with pillars within a 100 mm petri dish. (b) SEM image of a section of the pillar and relief features on the Au-epoxy master. (c) PDMS device molded from a Au-epoxy master with pillars and assembled onto coverglass. Food coloring was used to highlight the cell compartments.
IV. CONCLUSIONS
In this study, we demonstrate a simple replication process for the rapid production of highly reproducible epoxy resin masters for culture based applications with a batch-to-batch variation of only 1.54%. Epotek epoxy resin with a Tg of 80 °C was thermally stable and durable giving >50 PDMS castings from a single master. Au coating on the epoxy masters enabled effective demolding of the PDMS chambers without feature destruction and ensured the replication of biocompatible PDMS chambers for sensitive cultures such as primary neurons. Further, pillars were incorporated within the masters to eliminate mechanical punching of media reservoirs.
ACKNOWLEDGMENTS
We thank Bob Geil and Wallace Ambrose for assistance and Tharkika Nagendren for supplying primary neuron cultures. We acknowledge the UNC CHANL facility for use of their clean room and microscopy equipment. This work was supported by the National Institutes of Health Grant No. R41MH097377 (A.M.T.). A.M.T. is an Alfred P. Sloan Research Fellow.
There is potential competing interest. A.M.T. is an inventor of the microfluidic chambers to compartmentalize neurons (US 7419822 B2) and has financial interest in Xona Microfluidics, LLC.
References
- 1. Meyvantsson I. and Beebe D. J., “ Cell culture models in microfluidic systems,” Annu. Rev. Anal. Chem. 1, 423–449 (2008). 10.1146/annurev.anchem.1.031207.113042 [DOI] [PubMed] [Google Scholar]
- 2. McDonald J. C. and Whitesides G. M., “ Poly(dimethylsiloxane) as a material for fabricating microfluidic devices,” Acc. Chem. Res. 35(7), 491–499 (2002). 10.1021/ar010110q [DOI] [PubMed] [Google Scholar]
- 3. Merkel T. C., Bondar V. I., Nagai K. et al. , “ Gas sorption, diffusion, and permeation in poly(dimethylsiloxane),” J. Polym. Sci., Part B: Polym. Phys. 38(3), 415–434 (2000). [DOI] [Google Scholar]
- 4. Mata A., Fleischman A. J., and Roy S., “ Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems,” Biomed. Microdevices 7(4), 281–293 (2005). 10.1007/s10544-005-6070-2 [DOI] [PubMed] [Google Scholar]
- 5. Taylor A. M., Menon S., and Gupton S. L., “ Passive microfluidic chamber for long-term imaging of axon guidance in response to soluble gradients,” Lab Chip 15, 2781–2789 (2015) 10.1039/C5LC00503E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Duffy D. C., McDonald J. C., Schueller O. J. A. et al. , “ Rapid prototyping of microfluidic systems in poly(dimethylsiloxane),” Anal. Chem. 70(23), 4974–4984 (1998). 10.1021/ac980656z [DOI] [PubMed] [Google Scholar]
- 7. Xia Y. and Whitesides G. M., “ Soft lithography,” Angew. Chem. Int. Ed. 37, 550–575 (1998). [DOI] [PubMed] [Google Scholar]
- 8. Briones M. P. P., Honda T., Yamaguchi Y. et al. , “ A practical method for rapid microchannel fabrication in polydimethylsiloxane by replica molding without using silicon photoresist,” J. Chem. Eng. Jpn. 39(10), 1108–1114 (2006). 10.1252/jcej.39.1108 [DOI] [Google Scholar]
- 9. Esch M. B., Kapur S., Irizarry G. et al. , “ Influence of master fabrication techniques on the characteristics of embossed microfluidic channels,” Lab Chip 3(2), 121–127 (2003). 10.1039/b300730h [DOI] [PubMed] [Google Scholar]
- 10. Bubendorfer A., Liu X., and Ellis A. V., “ Microfabrication of PDMS microchannels using SU-8/PMMA moldings and their sealing to polystyrene substrates,” Smart Mater. Struct. 16(2), 367–371 (2007). 10.1088/0964-1726/16/2/015 [DOI] [Google Scholar]
- 11. Larsson M. P., Syms R. R. A., and Wojcik A. G., “ Improved adhesion in hybrid Si-polymer MEMS via micromechanical interlocking,” J. Micromech. Microeng. 15(11), 2074–2082 (2005). 10.1088/0960-1317/15/11/012 [DOI] [Google Scholar]
- 12. Khoo H. S., Liu K. K., and Tseng F. G., “ Mechanical strength and interfacial failure analysis of cantilevered SU-8 microposts,” J. Micromech. Microeng. 13(6), 822–831 (2003). 10.1088/0960-1317/13/6/305 [DOI] [Google Scholar]
- 13. Hupert M., Guy W. J., Llopis S. et al. , “ Evaluation of micromilled metal mold masters for the replication of microchip electrophoresis devices,” Microfluid. Nanofluid. 3(1), 1–11 (2007). 10.1007/s10404-006-0091-x [DOI] [Google Scholar]
- 14. Xia Y. N., McClelland J. J., Gupta R. et al. , “ Replica molding using polymeric materials: A practical step toward nanomanufacturing,” Adv. Mater. 9(2), 147–149 (1997). 10.1002/adma.19970090211 [DOI] [Google Scholar]
- 15. Park S., Kim K., Manohara H. M. et al. , “ Massive replication of polymeric high-aspect-ratio microstructures using PDMS casting,” Proc. SPIE 4334, 271–279 (2001). 10.1117/12.436611 [DOI] [Google Scholar]
- 16. Gitlin L., Schulze P., and Belder D., “ Rapid replication of master structures by double casting with PDMS,” Lab Chip 9(20), 3000–3002 (2009). 10.1039/b904684d [DOI] [PubMed] [Google Scholar]
- 17. Desai S. P., Freeman D. M., and Voldman J., “ Plastic masters—rigid templates for soft lithography,” Lab Chip 9(11), 1631–1637 (2009). 10.1039/b822081f [DOI] [PubMed] [Google Scholar]
- 18. Taylor A. M., Blurton-Jones M., Rhee S. W. et al. , “ A microfluidic culture platform for CNS axonal injury, regeneration and transport,” Nat. Methods 2(8), 599–605 (2005). 10.1038/nmeth777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Taylor A. M., Rhee S. W., Tu C. H. et al. , “ Microfluidic multicompartment device for neuroscience research,” Langmuir 19(5), 1551–1556 (2003). 10.1021/la026417v [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park J. W., Vahidi B., Taylor A. M. et al. , “ Microfluidic culture platform for neuroscience research,” Nat. Protoc. 1(4), 2128–2136 (2006). 10.1038/nprot.2006.316 [DOI] [PubMed] [Google Scholar]
- 21. Hallfors N., Khan A., Dickey M. D. et al. , “ Integration of pre-aligned liquid metal electrodes for neural stimulation within a user-friendly microfluidic platform,” Lab Chip 13(4), 522–526 (2013). 10.1039/C2LC40954B [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taylor A. M., Wu J., Tai H. C. et al. , “ Axonal translation of beta-catenin regulates synaptic vesicle dynamics,” J. Neurosci. 33(13), 5584–5589 (2013). 10.1523/JNEUROSCI.2944-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hutchinson J. M., “ Determination of the glass transition temperature,” J. Therm. Anal. Calorim. 98(3), 579–589 (2009). 10.1007/s10973-009-0268-0 [DOI] [Google Scholar]
- 24. Carbas R. J. C., Marques E. A. S., da Silva L. F. M. et al. , “ Effect of cure temperature on the glass transition temperature and mechanical properties of epoxy adhesives,” J. Adhes. 90(1), 104–119 (2013). 10.1080/00218464.2013.779559 [DOI] [Google Scholar]
- 25. Odegard G. M. and Bandyopadhyay A., “ Physical aging of epoxy polymers and their composites,” J. Polym. Sci., Part B: Polym. Phys. 49(24), 1695–1716 (2011). 10.1002/polb.22384 [DOI] [Google Scholar]
- 26. Khorasani M. T., Mirzadeh H., and Kermani Z., “ Wettability of porous polydimethylsiloxane surface: Morphology study,” Appl. Surf. Sci. 242(3–4), 339–345 (2005). 10.1016/j.apsusc.2004.08.035 [DOI] [Google Scholar]
- 27. Bayliss S. C., Buckberry L. D., Harris P. J. et al. , “ Nature of the silicon-animal cell interface,” J. Porous Mater. 7(1–3), 191–195 (2000). 10.1023/A:1009686704506 [DOI] [Google Scholar]
- 28. Nordstrom M., Johansson A., Nogueron E. S. et al. , “ Investigation of the bond strength between the photo-sensitive polymer SU-8 and gold,” Microelectron. Eng. 78–79, 152–157 (2005). 10.1016/j.mee.2004.12.021 [DOI] [Google Scholar]
- 29. Moazzez B., O'Brien S. M., and Merschrod E. F. S., “ Improved adhesion of gold thin films evaporated on polymer resin: Applications for sensing surfaces and MEMS,” Sensors 13(6), 7021–7032 (2013). 10.3390/s130607021 [DOI] [PMC free article] [PubMed] [Google Scholar]