Abstract
Introduction
HIV-1 near full-length genome (HIV-NFLG) sequencing from plasma is an attractive multidimensional tool to apply in large-scale population-based molecular epidemiological studies. It also enables genotypic resistance testing (GRT) for all drug target sites allowing effective intervention strategies for control and prevention in high-risk population groups. Thus, the main objective of this study was to develop a simplified subtype-independent, cost- and labour-efficient HIV-NFLG protocol that can be used in clinical management as well as in molecular epidemiological studies.
Methods
Plasma samples (n=30) were obtained from HIV-1B (n=10), HIV-1C (n=10), CRF01_AE (n=5) and CRF01_AG (n=5) infected individuals with minimum viral load >1120 copies/ml. The amplification was performed with two large amplicons of 5.5 kb and 3.7 kb, sequenced with 17 primers to obtain HIV-NFLG. GRT was validated against ViroSeq™ HIV-1 Genotyping System.
Results
After excluding four plasma samples with low-quality RNA, a total of 26 samples were attempted. Among them, NFLG was obtained from 24 (92%) samples with the lowest viral load being 3000 copies/ml. High (>99%) concordance was observed between HIV-NFLG and ViroSeq™ when determining the drug resistance mutations (DRMs). The N384I connection mutation was additionally detected by NFLG in two samples.
Conclusions
Our high efficiency subtype-independent HIV-NFLG is a simple and promising approach to be used in large-scale molecular epidemiological studies. It will facilitate the understanding of the HIV-1 pandemic population dynamics and outline effective intervention strategies. Furthermore, it can potentially be applicable in clinical management of drug resistance by evaluating DRMs against all available antiretrovirals in a single assay.
Keywords: HIV-1 NFLG sequencing, subtype independent, molecular epidemiology
Introduction
The increasing HIV diversity and evolution of circulating and unique recombinants forms (CRFs and URFs) pose a major threat to accurate identification of the circulating HIV strains in an epidemic. This has an impact on development, implementation and maintenance of effective preventive and treatment intervention strategies including vaccine development [1,2]. Due to high genetic heterogeneity, methods for universal amplification and sequencing of diverse HIV-1 subtypes remain inadequate [3]. Regularly, a smaller gene portion is used to characterize the subtype, which limits accurate identification of the HIV-1 strains, especially for the recombinant forms. Our earlier study identified a significantly higher prevalence of recombinant forms when two or more genes were used for subtyping [4]. Moreover, using a smaller portion of the genome limits the identification of epidemiological signatures and recombination hot spots [5], adaptations in a host population and immune recognition at whole genome level [6,7].
In current clinical practice of the genotypic resistance testing (GRT), most of the commercial or in-house assays provide information about the HIV-1 drug resistance mutations (DRMs) restricted to full-length protease (PR) (1–99) and partial reverse transcriptase (RT) (1–335). However, a growing body of scientific evidence suggests the role of connection domain mutations, for example, N348I [8], in conferring resistance to reverse transcriptase inhibitors (RTIs). Furthermore, distal non-drug target HIV-1 Gag mutations have been described to confer strong resistance to protease inhibitor (PI) [9,10].
Several studies have attempted to develop protocols for HIV-1 near full-length genome (HIV-NFLG) sequencing. However, most of the studies were restricted to sequencing of single subtypes [2,11] or identification of CRFs [12,13]. Moreover, genetic material was mainly derived from cultured cells or as proviral DNA from blood mononuclear cells [14–16]. Data directly from plasma samples are scarce, albeit they are routinely used in clinical drug resistance testing [2,17] and reflect the most recent viral population in the host [18].
Among the HIV-1 groups, the M-group dominates in global infections. Among the M-group subtypes, HIV-1 subtype C (HIV-1C); HIV-1A (A1 and A2); HIV-1B; CRF02_AG and CRF01_AE account for nearly 84% of the global infections [19]. In our recent study from the Swedish InfCare cohort based on the pol gene, we identified a highly diverse HIV-1 epidemic dominated by HIV-1B (47%), HIV-1C (18%) and CRF01_AE (12%) [20].
The aim of the present study was to develop a simple, cost and labour-efficient protocol for HIV-NFLG sequencing for diverse HIV-1 subtypes. This protocol could be used routinely in large-scale population-based molecular epidemiological studies. Additionally, this protocol can also be implemented for extended drug resistance genotyping with full-length Gag for predictors of PI-DRMs, full-length PR and RT, Integrase (IN) for Integrase Inhibitor (INI) as well as genotypic co-receptor tropism testing for co-receptor antagonists. Here, we amplified, sequenced and assembled HIV-1B, HIV-1C, CRF01_AE and CRF02_AG NFLG. Therefore, this protocol might potentially serve as a single tool for both epidemiological and clinical studies, independent of HIV-1 subtypes.
Methods
Ethical consideration
Ethical permissions were obtained from the Regional Ethics Committee Stockholm (Dnr: 2006/1367-31/4). The patient information was anonymized and de-linked prior to analysis. Single peripheral blood samples were obtained during the routine viral load testing and GRT using ViroSeq™ HIV-1 Genotyping System (Celera Diagnostics, Alameda, CA, USA).
Patients material and RNA extraction
The patients were followed-up at the Infectious Disease Clinic at Karolinska University Hospital, Stockholm, Sweden, as part of a large cohort, InfCare HIV [20]. Based on pol gene subtyping, a total of 30 samples from four different HIV-1 subtypes (HIV-1B (n=10), HIV-1C (n=10), 01_AE (n=5) and 02_AG (n=5)) were attempted for HIV-NFLG sequencing. Viral RNA was extracted using QIAamp viral RNA extraction kit (Qiagen, Hilden, Germany) from 140 µl of plasma. RNA was quantified and purity checked using NanoDrop (Thermo Scientific, DE, USA) and stored at −80°C until used. Among the 30 samples, four samples gave A260/A280 and 260/A230 ratio below one and were therefore excluded from the study.
Primer and cDNA synthesis
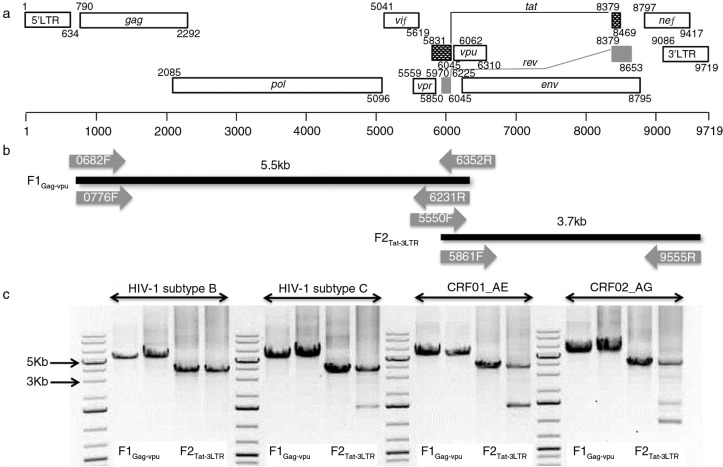
The near full-length genome from the samples was amplified in two fragments using nested and semi-nested polymerase chain reactions (PCR) with seven sets of primers numbered as HXB2 [GenBank:K03455] co-ordinates (Figure 1a and Table 1). First-strand cDNA synthesis was performed using the SuperScript® III RT enzyme (Invitrogen, Life Technologies, MA, USA). For fragment 1 Gag-Vpu (herein F1Gag-Vpu), cDNA was synthesized with the primer 6352R (10 pmol) and for fragment 2, Tat-3LTR (herein F2Tat-3LTR), an oligo (dT)18 primer (50 pmol) (Thermo Scientific) was used. A first master mix of 5 µl extracted viral RNA was combined with 1 µl of 10 mM dNTP mix, 1 µl of 6352R or oligo(dT)18 primer and 5 µl of PCR grade water. The reaction mix was heated to 65°C for five minutes and immediately placed on ice for two minutes. A second master mix consisting of 4 µl of 5× First-Strand Buffer (250 mM Tris-HCl, 375 mM KCl, 15 mM MgCl2), 1 µl 0.1 M DTT, 1 µl of RiboLock RNase inhibitor (40 U/µl; Thermo Scientific) and 2 µl of SuperScript-III RT (200 U/µl) was subsequently added to the first mastermix. The final 20 µl reaction mix was then incubated at 25°C for five minutes followed by 55°C for one hour and finally 70°C for 10 minutes to terminate the reaction.
Figure 1.
Amplification strategy of HIV-1 NFLG. (a) HIV-1 gene map in HXB2 [GenBank:K03455] co-ordinates Adapted from Los Alamos Database (www.hiv.lanl.gov). (b) The locations of all primers used for cDNA synthesis, nested and semi-nested PCR are shown. The 8.8 kb genome is amplified into two fragments: fragment 1, 5.5 kb (gag-vpu) and fragment 2, 3.7 kb (vif-3′-LTR) with 400 bp overlap. (c) Representative amplification of all four subtypes is presented.
Table 1.
Primers used in this study
| Primer_ID | Seq (5′→3′) | HXB2 position | Ref. |
|---|---|---|---|
| Amplification primers | |||
| F1Gag-Vpu | |||
| 0682F | TCTCTCGACGCAGGACTCGGCTTGCTG | 0682→0708 | [4,21] |
| 0776F | CTAGAAGGAGAGAGAGATGGGTGCGAG | 0776→0800 | [4] |
| 6352R | GGTACCCCATAATAGACTGTRACCCACAA | 6352→6324 | [17] |
| 6231R | CTCTCATTGCCACTGTCTTCTGCTC | 6231→6207 | [17] |
| F2Vif-3LTR | |||
| 5550F | AGARGAYAGATGGAACAAGCCCCAG | 5550→5574 | [17] |
| 5861F | TGGAAGCATCCRGGAAGTCAGCCT | 5861→5884 | [17] |
| 9555R | TCTACCTAGAGAGACCCAGTACA | 9555→9533 | Present study |
| Sequencing primers | |||
| F1Gag-Vpu | |||
| 0776F | CTAGAAGGAGAGAGAGATGGGTGCGAG | 0776→0800 | [4] |
| 1231F | TCACCTAGAACTTTRAATGCATGGG | 1231→1255 | Present study |
| 1817F | TAGAAGAAATGATGACAG | 1817→1834 | [22] |
| 2586F | AAGCCAGGAATGGATGGCCCA | 2586→2606 | [23] |
| 2713R | GGATTTTCAGGCCCAATTTTTG | 2713→2692 | Present study |
| 3885R | CTGCTCCATCTACATAGAA | 3885→3867 | [24] |
| 4350R | CACAGCTAGCTACTATTTCTTTTGC | 4350→4326 | [24] |
| 4900F | GGGTTTATTACAGGGACAGCAGAG | 4900→4923 | [25] |
| 5066R | ATCATCACCTGCCATCTGTTTTCCAT | 5066→5041 | [26] |
| 6231R | CTCTCATTGCCACTGTCTTCTGCTC | 6231→6207 | [17] |
| F2Vif-3LTR | |||
| 5861F | TGGAAGCATCCRGGAAGTCAGCCT | 5861→5884 | [17] |
| 6559F | GGGATCAAAGCCTAAAGCCATGTGTAA | 6559→6585 | [22] |
| 7002F | TTRTTAAATGGTAGTATAGC | 7002→7021 | Present study |
| 7373R | GAAAAATTCTCCTCYACAATTAAA | 7373→7350 | Present study |
| 7761F | GTGGGAATAGGAGCTGTGTTCCTTGGG | 7761→7787 | [22] |
| 8445R | CTCTCTCTCCACCTTCTTCTTC | 8445→8424 | [22] |
| 9555R | TCTACCTAGAGAGACCCAGTACA | 9555→9533 | Present study |
Source of the primers are given. The primer IDs are changed from the original for the ease of identification. The numbering is as per HXB2 (K03455).
NFLG PCR and sequencing
All PCR reactions were performed with high fidelity KAPA HiFi HotStart ReadyMix (2×) (KAPA Biosystem, MA, USA) with 15 pmol of each primer in 50 µl total volume. For F1Gag-Vpu, the first round PCR was performed with 0682F and 6352R primers and was followed by the second round nested with 0776F and 6231R primers, which yielded an amplicon of approximately 5.5 kb (Figure 1b). The condition for both PCRs was as follows: initial denaturation at 95°C for five minutes followed by 30 cycles of 98°C for 20 sec, 65°C for 15 sec and 72°C for three minutes and final extension at 72°C for five minutes. The F2Tat-3LTR fragment was amplified in a semi-nested manner with the first and second round forward primers 5550F and 5831F, respectively, with a common reverse primer 9555R, yielding a 3.7 kb amplicon (Figure 1b). The condition for both the PCRs was as follows: initial denaturation at 95°C for five minutes followed by 30 cycles of 98°C for 20 sec, 65°C for 15 sec and 72°C for two minutes and final extension at 72°C for five minutes. The PCR amplicon was gel purified using QIAamp Gel Extraction Kit (Qiagen). Sequencing was performed with a set of 17 primers (Table 1). Representative PCR amplification of all the four subtypes was presented in Figure 1c.
Sequence assembly, visualization and quality control
The sequencing primers were designed in such a way that there will be 100 bases overlapping with 800 bp sequencing read (Table 1). The sequencing was performed with the Applied Biosystems® 3730xl DNA Analyzer (Life Technologies, CA, USA). The sequences were auto-clipped with a quality score ≥10. CAP3 Sequence Assembly Program with default parameter was used to assemble the final sequence from all available contigs [27]. Due to high genetic variability, only the major peak was considered in the consensus sequence. Single-base frame shifts due to sequencing errors were curated manually after observation of the chromatogram or alignment with the subtype-specific consensus sequences in ClustalX2 [28]. A multiple sequence alignment against the HXB2 reference genome was generated and analyzed with an in-house Perl script (Supplementary file 1) that recognizes the nucleotide changes from the HXB2 sequence [GenBank:K03455] and creates a corresponding number code as per HXB2 co-ordinates. The resulting matrix was plotted using the TraMineR package [29] in R version 3.1.2 [30]. Sequence quality control was performed using Quality Control Tool available in Los Alamos database (www.hiv.lanl.gov/) and submitted to GenBank under accession nos. KP411822 to KP411845.
Subtyping, drug resistance and co-receptor tropism
HIV-1 subtyping was performed with COMET-HIV, which uses adaptive context-based modelling for ultrafast HIV-1 subtype identification [31]. A maximum likelihood phylogenetic tree with reference sequences was generated in MEGA6 software [32] using a General Time Reversible (GTR) model with inverse gamma distribution. The reference sequences were downloaded from Los Alamos database (www.hiv.lanl.gov). GRT was performed with whole pol gene that provides DRM profile of PR, RT and IN. The results were compared with the ViroSeq™ HIV-1 Genotyping System (Life Technologies), which provide DRM profile of full PR and first 335 amino acids of RT. Co-receptor tropism analysis was performed using Geno2pheno[co-receptor] with 10% false-positive rate [33].
Results
The patients (n=26) demographic and clinical characteristics along with the amplification proficiency are given in Table 2.
Table 2.
Demographic and clinical parameters and amplification of HIV-1 fragment of the study samples
| PID | Age | Sex | Viral loada | CD4a | Therapy | Country of transmission | Route of transmissiona | F1Gag-Vpu | F2vif-3LTR |
|---|---|---|---|---|---|---|---|---|---|
| SE600001 | 51 | Male | 1,905,461 | 4 | Naïve | Sweden | IVDU | + | + |
| SE600012 | 39 | Male | 707,946 | 190 | Naïve | Sweden | MSM | + | + |
| SE600023 | 41 | Male | 194,984 | 150 | Naïve | Sweden | Heterosexual | + | + |
| SE600034 | 52 | Male | 3,467,369 | 220 | Naïve | Sweden | MSM | + | + |
| SE600035 | 44 | Female | 309,030 | 260 | Naïve | Sweden | IVDU | + | + |
| SE600046 | 50 | Male | 6,606,934 | 9 | Naïve | NA | MSM | + | + |
| SE600057 | 45 | Male | 50,400 | 180 | Naïve | Sweden | Heterosexual | + | + |
| SE600063 | 45 | Female | 7600 | 316 | Naïve | Sweden | Heterosexual | + | + |
| SE600068 | 40 | Female | 1300 | 421 | Naïve | Sweden | NA | − | + |
| SE600099 | 27 | Female | 1120 | 510 | Naïve | Sweden | Heterosexual | + | − |
| SE600108 | 35 | Male | 150,000 | 270 | Naïve | Sweden | IVDU | + | + |
| SE600119 | 39 | Male | 158,000 | 184 | Naïve | Ethiopia | Heterosexual | + | + |
| SE600210 | 42 | Male | 338,844 | 133 | Naïve | Ethiopia | Heterosexual | + | + |
| SE600311 | 58 | Male | 3990 | 110 | Experience | Ethiopia | Heterosexual | + | + |
| SE600412 | 58 | Male | 18,400 | 300 | Naïve | Eritrea | Heterosexual | + | + |
| SE600213 | 25 | Female | 110,000 | 169 | Naïve | Ethiopia | Heterosexual | + | + |
| SE600314 | 40 | Female | 219,000 | 400 | Naïve | Zimbabwe | Heterosexual | + | + |
| SE600415 | 30 | Male | 575,000 | 125 | Naïve | Eritrea | Heterosexual | + | + |
| SE600122 | 38 | Female | 6580 | 460 | Naïve | Eretria | Heterosexual | + | + |
| SE600516 | 37 | Female | 1,000,000 | 304 | Naïve | Sweden | Heterosexual | + | + |
| SE601017 | 34 | Female | 2,300,000 | 16 | Naïve | Thailand | Heterosexual | + | + |
| SE601018 | 35 | Female | 260,000 | 350 | Naïve | Thailand | Heterosexual | + | + |
| SE601021 | 40 | Female | 40,800 | 249 | Naïve | Thailand | NA | + | + |
| SE602019 | 41 | Female | 129,000 | 400 | Naïve | Senegal | Blood transfusion | + | + |
| SE602020 | 24 | Female | 162,000 | 90 | Experience | Cameroon | Heterosexual | + | + |
| SE602024 | 35 | Female | 3060 | 360 | Naïve | Cameroon | Heterosexual | + | + |
Viral load in copies/ml, CD4 in cells/mm3. IVDU: intravenous drug user; MSM: men who have sex with men; NA: not available.
Among the 26 samples with viral loads ranging from 1120 to 6,606,934 copies/ml, both PCR fragments were amplified and sequenced from 24 samples (efficiency 92%). In two samples with viral loads below 2000 copies/ml (1300 and 1120 copies/ml respectively), either one of the fragments was not amplified. Amplification was successful in all remaining samples with the lowest viral load being 3060 copies/ml.
For F1Gag-Vpu, 10 primers were used which gave complete coverage from the HXB2 positions 790 to 6200. The F2Tat-3LTR amplicon is considerably shorter (~3.7 kb) but still required seven sequencing primers. This was due to the high intra-individual HIV-1 genetic diversity and the presence of poly-A stretches in the region as poly-A stretches can abruptly terminate the sequencing read.
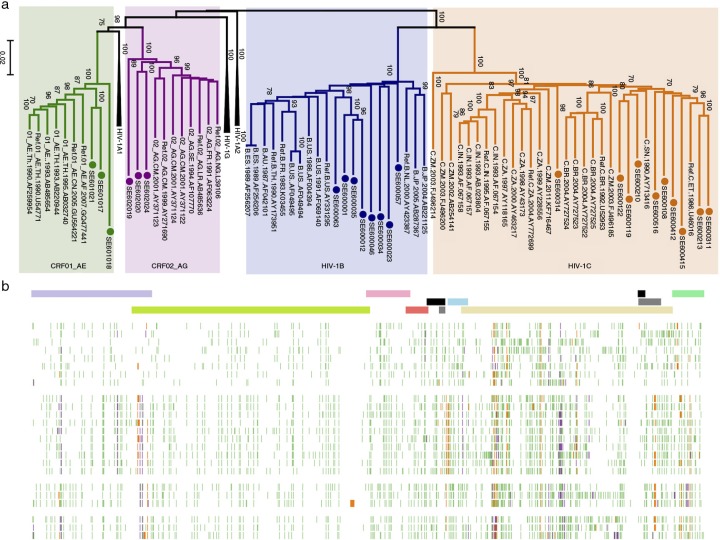
The phylogenetic analysis identified the same subtype as identified by the pol region with 10 as HIV-1C, 8 as HIV-1B and 3 each as 01_AE and 02_AG (Figure 2a). The sequence variability of the 24 samples compared to HXB2 sequence is presented in Figure 2b. This indicates higher sequence variability in the env region and the subtype-specific signatures over the genome specifically in the Gag-p6 region.
Figure 2.
Phylogenetic and variability analysis of sequenced Swedish HIV-1 strains. (a) Maximum likelihood phylogenetic tree with reference HIV-1 sequences downloaded from Los Alamos Database. Four subtypes are indicated: HIV-1B (dark blue), HIV-1C (orange), CRF01_AG (green) and CRF02_AG (purple). The Swedish strains are indicated with filled circle with a respective colour. (b) Genetic diversity of HIV-1 subtypes: all the 24 HIV-1 genomes were aligned with reference to HXB2 [GenBank:K03455]. For each sequence, every nucleotide differing from the reference HXB2 strain (mutation) is shown as a green line, an insertion is shown in orange, and a deletion is shown in purple. The top panel shows the open-reading frame of HIV-1 genes: gag (violet), pol (lemon green), vif (pink), vpr (light red), tat exon 1 and 2 (black), rev exon 1 and 2 (grey), vpu (cyan), env (light yellow) and nef (green).
DRM analysis based on the ViroSeq™ HIV-1 Genotyping System and the current HIV-NFLG assay is presented in Table 3. It should be noted that the HIV-NFLG and the ViroSeq™ showed >99% concordance at 71 DRM positions (PR: 33 positions and RT: 38, excluding N348I, which is not detected by ViroSeq™) in 24 samples (total codon analysis 1704 and three mismatch). In two samples, ViroSeq™ identified PI mutation L10IL (SE600314) and RTI mutations Y318YF (SE602020) in contradiction to the current assay. On the contrary, the V11I mutation was detected by NFLG in one sample (SE600057) but not by ViroSeq™. In two samples, NFLG identified additional N348I mutations due to an extended genomic coverage. Moreover, the current assay potentially can identify the INI-DRMs. The co-receptor tropism identified 18 CCR5-tropic viruses and six as CXCR4-tropic virus (Table 3).
Table 3.
Comparative drug resistance analysis of current protocol and ViroSeq™ genotypic resistance testing
| ViroSeq genotypic system | Whole genome sequencing | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||
| PID | PI major | PI minor | NRTI-DRM | NNRTI-DRM | PI major | PI minor | NRTI-DRM | NNRTI-DRM | INT-DRM-major | INT-DRM-accessory | Co-receptor |
| SE600001 | None | None | None | None | None | None | None | None | None | None | CCR5 |
| SE600012 | None | None | None | None | None | None | None | None | None | None | CXCR4 |
| SE600023 | None | None | T215S | None | None | None | T215S | None | None | None | CCR5 |
| SE600034 | None | None | None | V90I | None | None | None | V90I | None | None | CCR5 |
| SE600035 | None | None | None | None | None | None | None | None | None | None | CCR5 |
| SE600046 | None | None | None | None | None | None | None | None | None | None | CXCR4 |
| SE600057 | None | None | None | None | None | V11I | None | None | None | E157Q | CCR5 |
| SE600063 | None | None | None | None | None | None | None | None | None | None | CXCR4 |
| SE600108 | None | None | None | None | None | None | None | N348I | None | None | CCR5 |
| SE600119 | None | None | None | None | None | None | None | None | None | None | CCR5 |
| SE600210 | None | None | None | None | None | None | None | None | None | None | CCR5 |
| SE600311 | None | None | None | V90I | None | None | None | V90I | None | None | CCR5 |
| SE600412 | None | None | None | None | None | None | None | None | None | None | CCR5 |
| SE600213 | None | None | None | None | None | None | None | None | None | None | CCR5 |
| SE600314 | None | L10IL | None | E138A | None | None | None | E138A | None | None | CCR5 |
| SE600415 | None | None | None | None | None | None | None | N348I | None | None | CCR5 |
| SE600122 | None | V11I | None | None | None | V11I | None | None | None | None | CCR5 |
| SE600516 | None | None | None | None | None | None | None | None | None | None | CCR5 |
| SE601017 | None | None | None | None | None | None | None | None | None | L74I | CXCR4 |
| SE601018 | None | None | None | E138A | None | None | None | E138A | None | None | CCR5 |
| SE601021 | None | None | None | None | None | None | None | None | None | None | CXCR4 |
| SE602019 | None | L10I, K20I | None | None | None | L10I, K20I | None | None | None | None | CCR5 |
| SE602020 | None | K20I | M184V | V106A, F227L, Y318FY | None | K20I | M184V | V106A, F227L | None | None | CXCR4 |
| SE602024 | None | K20I | None | None | None | K20I | None | None | None | None | CCR5 |
The mismatches are marked in bold.
Discussion
In the present study, we have developed a simple, labour and time-efficient HIV-NFLG sequencing protocol that can be used for large-scale molecular epidemiological studies. This assay can identify the phylogenetic transmission cluster in a local HIV-1 epidemic. Therefore, it has a potential role in the prevention of HIV-1 transmission in the high-risk groups from a public health perspective. Moreover, the assay extends the genotypic GRT to cover all HIV-1 drug target sites (PR inhibitors, RTIs, IN and fusion inhibitors). It also includes genotypic co-receptor tropism analysis and genetic analysis of the non-drug target sites that potentially affect the drug efficiency as well as resistance (e.g. Gag).
Molecular epidemiological studies often use smaller gene fragments that can potentially underestimate the event of recombination. Our earlier study showed significant increase in recombinants when two or more genes from different parts of the HIV-1 genome (e.g. gag, pol and env) were used [4]. Therefore, the use of NFLG is ideal to be used as a tool for HIV-1 subtyping [34]. It also enables an understanding of the dynamics of the HIV pandemic at a population level [3]. Earlier studies that developed full-length genome are either expensive, time consuming or hampered by low throughput that is often restricted to one HIV-1 subtype [2]. A HIV-NFLG sequencing protocol from plasma viral RNA has been developed using two or three fragments to cover 9 kb genome [17]. However, the method was limited by its efficiency in samples having a viral load <10,000 copies/ml and a subtype-independent applicability was not mentioned. If a high-quality RNA sample is provided, NFLG sequences from patients with viral loads as low as 3000 copies/ml can be obtained, which is a great advantage of the presented assay compared to the earlier method [17].
Most importantly, the current method can detect four major subtypes and recombinant forms (HIV-1B, HIV-1C, 01_AE and 02_AG), which are responsible for >80% of global infections when combined [19]. The method can be potentially applied to all major pure and recombinant HIV-1 strains as the primers applied here had >95% sequence identity to them. Recently, a universal amplification protocol has been developed that amplifies HIV-1 genome in four PCR fragments followed by sequencing in a next generation sequencing (NGS) platform [3]. However, the method is more labour-intensive and the application of the method is limited in low- and middle-income countries (LMICs) due to limited availability of NGS and corresponding experts to run the assays. The calculated costs for NGS strongly depend on the number of samples processed and increase if only a few numbers of samples have to be analyzed. In our current NFLG method, the cost per sample is between $130 and $140 and even one sample can be run without any change in effective cost. The overall time taken for the method is five days.
The current method can also be applied for genotypic resistance testing as it showed high concordance in determining the DRM compared to gold standard ViroSeq™ System. Additionally, it can determine clinically important N348I that confers resistance to RTI [8]. Recently, a single assay for HIV-1 GRT and co-receptor tropism assay, based on deep sequencing, has been developed [35]. Though the assay is high throughput, the application of the method is limited in LMICs, due to limited availability of NGS in those settings. However, with this current method, in a single assay it is now possible to determine PI, RTI and INI resistance mutations and co-receptor tropism in LMICs also. Finally, Gag has shown to act synergistically to confer resistance to PIs [9,10]. The insertions in Gag-p6 have recently shown to be associated with PI-therapy failure in Indian HIV-1C viruses [36]. The current method is also helpful in determining the full Gag including p6 region.
Our study has certain technical aspects that merit comments. First, the quality of RNA is important for successful amplification. Therefore, highly lipemic or lysed samples should not be used. Second, the sequencing of the Env region may require more primers [22]. This is due to higher occurrence of poly-A stretches in the env variable regions (V1 to V5; more specifically in the V4 region), which can suddenly stop the sequencing reaction. Third, in two positions mixed populations were detected by ViroSeq™ but not by NFLG. This kind of results was also noted in earlier studies [37,38] and might be due to the variant calling. The method is less efficient in samples with viral load <3000 copies/ml. Furthermore, extensive mutations in the primer binding sites can result in failure of amplification as observed in most HIV-diagnostic assays. However, a major merit of this assay is its subtype independency. In the current study, the samples were from patients who got HIV infected in different countries – Sweden, Zimbabwe, Ethiopia, Thailand, Senegal, Cameroon and Eritrea – with four major subtypes. Another important factor is the use of this method in clinical management of drug resistance. The input plasma sample volume is only 140 µl. Thus, no additional blood sample is required and the assay can be merged with routine viral load and CD4 testing.
Conclusions
In conclusion, we demonstrated a subtype-independent HIV-NFLG sequencing method that is a simple, cost and labour-efficient and promising approach. The method can be used in large-scale population-based molecular epidemiological studies that can identify the HIV-1 recombinant forms more accurately as well as population dynamics of HIV-1 spread. It can also be used in extended genotypic resistance testing that can evaluate DRMs in PR, RT and IN genes. Genotypic co-receptor tropism for evaluation of co-receptor antagonist usage can also be done in one single assay. Therefore, the application of this method in clinical care can improve patient management strategies through the accurate identification the strains. A better understanding of the population dynamics of HIV-1 within pandemics could enable the development of an effective intervention for control and prevention.
Supplementary Material
Acknowledgements
Authors thank Prof. Anders Sönnerborg, Karolinska Institutet for technical discussions and Amanda Häggblom, Karolinska Institutet for extracting patient's data from the database.
Competing interests
None to declare.
Authors' contributions
UN designed the study and wrote the first draft of the manuscript. SG and UN performed the laboratory and bioinformatics analysis. PN contributed with the clinical data and clinical input in the study and critical revision of the manuscript. All authors have read and approved the final version of the manuscript.
Funding sources
This study is partly funded by Swedish Research Council and Karolinska Institutet Research Foundation Grants (2014fobi41250) to UN. SG acknowledges the support received from Erasmus+ Mobility for Traineeships.
References
- 1.Brenner B, Wainberg MA, Roger M. Phylogenetic inferences on HIV-1 transmission: implications for the design of prevention and treatment interventions. AIDS. 2013;27:1045–57. doi: 10.1097/QAD.0b013e32835cffd9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rousseau CM, Birditt BA, McKay AR, Stoddard JN, Lee TC, McLaughlin S, et al. Large-scale amplification, cloning and sequencing of near full-length HIV-1 subtype C genomes. J Virol Methods. 2006;136:118–25. doi: 10.1016/j.jviromet.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Gall A, Ferns B, Morris C, Watson S, Cotten M, Robinson M, et al. Universal amplification, next-generation sequencing, and assembly of HIV-1 genomes. J Clin Microbiol. 2012;50:3838–44. doi: 10.1128/JCM.01516-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neogi U, Bontell I, Shet A, De Costa A, Gupta S, Diwan V, et al. Molecular epidemiology of HIV-1 subtypes in India: origin and evolutionary history of the predominant subtype C. PLoS One. 2012;7:e39819. doi: 10.1371/journal.pone.0039819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smyth RP, Schlub TE, Grimm AJ, Waugh C, Ellenberg P, Chopra A, et al. Identifying recombination hot spots in the HIV-1 genome. J Virol. 2014;88:2891–902. doi: 10.1128/JVI.03014-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cotton LA, Kuang XT, Le AQ, Carlson JM, Chan B, Chopera DR, et al. Genotypic and functional impact of HIV-1 adaptation to its host population during the North American epidemic. PLoS Genet. 2014;10:e1004295. doi: 10.1371/journal.pgen.1004295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henn MR, Boutwell CL, Charlebois P, Lennon NJ, Power KA, Macalalad AR, et al. Whole genome deep sequencing of HIV-1 reveals the impact of early minor variants upon immune recognition during acute infection. PLoS Pathog. 2012;8:e1002529. doi: 10.1371/journal.ppat.1002529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yap SH, Sheen CW, Fahey J, Zanin M, Tyssen D, Lima VD, et al. N348I in the connection domain of HIV-1 reverse transcriptase confers zidovudine and nevirapine resistance. PLoS Med. 2007;4:e335. doi: 10.1371/journal.pmed.0040335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fun A, Wensing AM, Verheyen J, Nijhuis M. Human immunodeficiency virus gag and protease: partners in resistance. Retrovirology. 2012;9:63. doi: 10.1186/1742-4690-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dam E, Quercia R, Glass B, Descamps D, Launay O, Duval X, et al. Gag mutations strongly contribute to HIV-1 resistance to protease inhibitors in highly drug-experienced patients besides compensating for fitness loss. PLoS Pathog. 2009;5:e1000345. doi: 10.1371/journal.ppat.1000345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Riva C, Romano L, Saladini F, Lai A, Carr JK, Francisci D, et al. Identification of a possible ancestor of the subtype A1 HIV type 1 variant circulating in the former Soviet Union. AIDS Res Hum Retroviruses. 2008;24:1319–25. doi: 10.1089/aid.2008.0119. [DOI] [PubMed] [Google Scholar]
- 12.Ng OT, Eyzaguirre LM, Carr JK, Chew KK, Lin L, Chua A, et al. Identification of new CRF51_01B in Singapore using full genome analysis of three HIV type 1 isolates. AIDS Res Hum Retroviruses. 2012;28:527–30. doi: 10.1089/aid.2011.0177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao J, Tang S, Ragupathy V, Carr JK, Wolfe ND, Awazi B, et al. Identification and genetic characterization of a novel CRF22_01A1 recombinant form of HIV type 1 in Cameroon. AIDS Res Hum Retroviruses. 2010;26:1033–45. doi: 10.1089/aid.2009.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratner L, Haseltine W, Patarca R, Livak KJ, Starcich B, Josephs SF, et al. Complete nucleotide sequence of the AIDS virus, HTLV-III. Nature. 1985;313:277–84. doi: 10.1038/313277a0. [DOI] [PubMed] [Google Scholar]
- 15.Salminen MO, Koch C, Sanders-Buell E, Ehrenberg PK, Michael NL, Carr JK, et al. Recovery of virtually full-length HIV-1 provirus of diverse subtypes from primary virus cultures using the polymerase chain reaction. Virology. 1995;213:80–6. doi: 10.1006/viro.1995.1548. [DOI] [PubMed] [Google Scholar]
- 16.Lole KS, Bollinger RC, Paranjape RS, Gadkari D, Kulkarni SS, Novak NG, et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J Virol. 1999;73:152–60. doi: 10.1128/jvi.73.1.152-160.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nadai Y, Eyzaguirre LM, Constantine NT, Sill AM, Cleghorn F, Blattner WA, et al. Protocol for nearly full-length sequencing of HIV-1 RNA from plasma. PLoS One. 2008;3:e1420. doi: 10.1371/journal.pone.0001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neogi U, Shet A, Sahoo PN, Bontell I, Ekstrand ML, Banerjea AC, et al. Human APOBEC3G-mediated hypermutation is associated with antiretroviral therapy failure in HIV-1 subtype C-infected individuals. J Int AIDS Soc. 2013;16:18472. doi: 10.7448/IAS.16.1.18472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hemelaar J, Gouws E, Ghys PD, Osmanov S, WHO-UNAIDS Network for HIV Isolation and Characterisation Global trends in molecular epidemiology of HIV-1 during 2000–2007. AIDS. 2011;25:679–89. doi: 10.1097/QAD.0b013e328342ff93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neogi U, Haggblom A, Santacatterina M, Bratt G, Gisslen M, Albert J, et al. Temporal trends in the Swedish HIV-1 epidemic: increase in non-B subtypes and recombinant forms over three decades. PLoS One. 2014;9:e99390. doi: 10.1371/journal.pone.0099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sengupta S, Khetawat D, Jana S, Sarkar K, Bhattacharya SK, Chakrabarti S. Polymorphism of HIV-1 gag (p17) gene from female sex workers in Calcutta, India. Arch Virol. 2005;150:2117–24. doi: 10.1007/s00705-005-0562-5. [DOI] [PubMed] [Google Scholar]
- 22.Sanders-Buell E, Salminen MO, McCutchan FE. Sequencing primers for HIV-1, p. III-15-III-21. In The human retroviruses and AIDS compendium. 1995. Available from: http://www.hiv.lanl.gov/content/sequence/HIV/COMPENDIUM/1995/PART-III/3.pdf.
- 23.Kully C, Yerly S, Erb P, Kind C, Krautheim A, Perrin L, et al. Codon 215 mutations in human immunodeficiency virus-infected pregnant women. Swiss Collaborative ‘HIV and pregnancy’ study. J Infect Dis. 1999;179:705–8. doi: 10.1086/314615. [DOI] [PubMed] [Google Scholar]
- 24.Brehm JH, Koontz DL, Wallis CL, Shutt KA, Sanne I, Wood R, et al. Frequent emergence of N348I in HIV-1 subtype C reverse transcriptase with failure of initial therapy reduces susceptibility to reverse-transcriptase inhibitors. Clin Infect Dis. 2012;55:737–45. doi: 10.1093/cid/cis501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bibollet-Ruche F, Loussert-Ajaka I, Simon F, Mboup S, Ngole EM, Saman E, et al. Genetic characterization of accessory genes from human immunodeficiency virus type 1 group O strains. AIDS Res Hum Retroviruses. 1998;14:951–61. doi: 10.1089/aid.1998.14.951. [DOI] [PubMed] [Google Scholar]
- 26.Ritchie A, Cai F, Smith N, Chen S, Song H, Brackenridge S, et al. Recombination-mediated escape from primary CD8+ T cells in acute HIV-1 infection. Retrovirology. 2014;11:69. doi: 10.1186/s12977-014-0069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang X, Madan A. CAP3: a DNA sequence assembly program. Genome Res. 1999;9:868–77. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, et al. Clustal W and clustal X version 2.0. Bioinformatics. 2007;23:2947–8. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- 29.Gabadinho A, Ritschard G, Mueller NS, Studer M. Analyzing and visualizing state sequences in R with TraMineR. J Stat Software. 2011;40:1–37. [Google Scholar]
- 30.R Development Core Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 31.Struck D, Lawyer G, Ternes AM, Schmit JC, Bercoff DP. COMET: adaptive context-based modelling for ultrafast HIV-1 subtype identification. Nucleic Acids Res. 2015;42:e144. doi: 10.1093/nar/gku739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–9. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta S, Neogi U, Srinivasa H, Banerjea AC, Shet A. HIV-1 coreceptor tropism in India: increasing proportion of X4-tropism in subtype C strains over two decades. J Acquir Immune Defic Syndr. 2014;65:397–404. doi: 10.1097/QAI.0000000000000046. [DOI] [PubMed] [Google Scholar]
- 34.Wilkinson E, Holzmayer V, Jacobs G, de Oliveira T, Brennan C, Hackett J, Jr., et al. Sequencing and phylogenetic analysis of near full-length HIV-1 subtypes A, B, G and unique recombinant AC and AD viral strains identified in South Africa. AIDS Res Hum Retroviruses. 2015;31:412–20. doi: 10.1089/aid.2014.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gibson RM, Meyer AM, Winner D, Archer J, Feyertag F, Ruiz-Mateos E, et al. Sensitive deep-sequencing-based HIV-1 genotyping assay to simultaneously determine susceptibility to protease, reverse transcriptase, integrase, and maturation inhibitors, as well as HIV-1 coreceptor tropism. Antimicrob Agents Chemother. 2014;58:2167–85. doi: 10.1128/AAC.02710-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neogi U, Rao SD, Bontell I, Verheyen J, Rao VR, Gore SC, et al. Novel tetra-peptide insertion in Gag-p6 ALIX-binding motif in HIV-1 subtype C associated with protease inhibitor failure in Indian patients. AIDS. 2014;28:2319–22. doi: 10.1097/QAD.0000000000000419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandit A, Mackay WG, Steel C, van Loon AM, Schuurman R. HIV-1 drug resistance genotyping quality assessment: results of the ENVA7 Genotyping Proficiency Programme. J Clin Virol. 2008;43:401–6. doi: 10.1016/j.jcv.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 38.Chaturbhuj DN, Nirmalkar AP, Paranjape RS, Tripathy SP. Evaluation of a cost effective in-house method for HIV-1 drug resistance genotyping using plasma samples. PLoS One. 2014;9:e87441. doi: 10.1371/journal.pone.0087441. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.