Abstract
Perforations, leaks and fistula involving gastrointestinal (GI) tract are increasing encountered in clinical practice. There is a changing paradigm for their management with surgical approach being replaced by conservative approach including endoscopic therapy. Clips (through the scope and over the scope) and covered stent are front runners for endotherapy for GI leaks and fistula. Over the scope clips introduced recently, can treat larger defects compared to through the scope clips. Covered stents are suited for larger defects and those associated with luminal narrowing. However cervical esophagus, gastro-esophageal junction, stomach and right colonic lesions may be better for clip therapy rather than stenting. Recent developments in this field include use of endovac therapy which consists of a sponge with suction device, biodegradable stent, use of fibrin glue and some endo-suturing device. Conservative therapy with no surgical or endoscopic intervention, may be suitable for a small subset of patients. An algorithm based on location, size of defect, associated stricture, infection and available expertise needs to be developed to reduce the mortality and morbidity of this difficult clinical problem.
Keywords: Fistula, Leak, Perforation, Post operative, Endoscopy, Endoscopic, Surgery, Stent, Suture, Endoclip, Clip
Core tip: Gastrointestinal (GI) leaks and fistula are increasingly recognized in our day to day practice. While these patients were earlier managed by surgical interventions, more and more such patients are now considered for endoscopic therapy. Endotherapy for GI leaks include endoclips (through the scope and over the scope), covered stents, fibrin glue, suture devices and more recently introduced endoscopic vacuum therapy using bioactive sponge. Since the experience with these modalities is limited, there are hardly any clear guidelines to treat these difficult patients. This review article deals with endotherapy of GI leaks and fistula and presents an updated experience as well some guidance to select appropriate modality.
INTRODUCTION
Gastrointestinal (GI) leaks and fistula constitute disruption of GI wall. GI leaks and fistula can be either spontaneous due to GI pathology or may be iatrogenic. There seems to be increase in prevalence of GI leaks and this seems primarily due to increasing complexity of GI surgery and endoscopic interventions. There is a changing paradigm in management strategy of GI leaks and fistula. While majority of these complicated patients were managed by surgery 15-20 years back, non-operative treatment including endoscopy presently constitute the primary modality of therapy[1]. There is evidence to suggest that this changing paradigm in form of endoscopic therapy is associated with improved outcome and shortened length of hospital stay[1]. This review deals with endoscopic techniques and their present status in the management of GI leaks and fistula.
Management of pancreatic and bile ductal leaks is however, not discussed.
DEFINITION AND ETIOLOGY
Perforation, fistula and leaks are terms, which are often used interchangeably. However in strict terms, they are somewhat different. Perforation refers to acute full thickness defect in GI tract. Leaks are defined as disruption of surgical anastomosis resulting in a fluid collection[2]. The term fistula usually means an abnormal communication between two epithelialized surfaces[2]. Table 1 enumerates the causes of GI leaks and fistula[3-17], while Table 2 distinguishes the underlying etiology for leaks and fistula. Table 3 details the endoscopic procedures associated with increased risk of perforation[18].
Table 1.
Etiology of gastrointestinal leaks and fistula
| Diagnostic endoscopy including endoscopic ultrasound |
| Dilation: bougie, balloon, achalasia |
| Polypectomy/EMR/ESD |
| Foreign body |
| Endoscopic variceal therapy including ligation |
| POEM |
| Anastomotic dehiscence |
| Boerhaave’s syndrome |
| Diverticulitis |
| Laser |
| PEG |
| Endoscopic sphincterotomy |
| Biliary stent migration |
| Ampullectomy |
| Appendicular abscess |
| Empyema |
EMR: Endoscopic mucosal resection; ESD: Endoscopic submucosal dissection; POEM: Peroral endoscopic myotomy; PEG: Percutaneous endoscopic gastrotomy.
Table 2.
Causes of leaks and fistula
| Leaks | Fistula |
| Iatrogenic (60%) | Malignant (50%) |
| Endoscopy | Benign |
| EVL | Stents |
| Dilatation | Tuberculosis |
| ESD/EMR | Crohn’s |
| POEM | Iatrogenic |
| Spontaneous | Trauma |
| Boerhaave’s | Surgical |
| Foreign body | AIDS |
| Surgical | |
| Trauma |
EVL: Endoscopic variceal ligation; EMR: Endoscopic mucosal resection; ESD: Endoscopic submucosal dissection; POEM: Peroral endoscopic myotomy; AIDS: Acquired immune deficiency syndrome.
Table 3.
Endoscopic procedures in different parts of gastrointestinal tract associated with increased risk of iatrogenic perforation
| Esophagus and stomach | |
| Dilatation | ESD |
| EMR | Foreign body removal |
| POEM | EVL |
| Small bowel | |
| Altered anatomy | DBE in altered anatomy |
| Dilatation in Crohn’s | ESD |
| Dilatation of GJ stricture after gastric bypass | |
| Colon | |
| EMR | Balloon dilatation |
| ESD | Old age, co-morbidity |
| Inexperience | Inflammatory colonic disease |
ESD: Endoscopic submucosal dissection; EMR: Endoscopic mucosal resection; POEM: Peroral endoscopic myotomy; EVL: Endoscopic variceal ligation; DBE: Double-balloon; GJ: Gastrojejunostomy.
TOOLS AND TECHNIQUES
The two options for managing GI leaks and fistula include surgery and endotherapy. The choice between two is decided by size of disruption, location and accessibility of lesion, presence of contamination, time of diagnosis and availability of expertise. Whatever be the choiced option for repairing the disruption, the management needs to include bowel rest, institution of appropriate antibiotics, drainage of associated collection, pneumoperitoneum, pneumothorax and maintenance of nutrition. Proton pump inhibitors are instituted, if leaks are located in upper GI tract. As highlighted in a recently published Position Statement of European Society of Gastrointestinal Endoscopy, it is important to have a systematic approach for diagnosis and treatment of GI perforations[18]. Endoscopist must record details of findings, attending physician must evaluate the clinical profile, necessary investigations which may include a CT scan and a blood picture should be carried out, a decision should then be taken whether to perform endotherapy or surgery and finally post endotherapy monitoring must be done to evaluate success or failure of the endotherapy[18]. Table 4 lists the endoscopic modalities, which can be used for closure of GI leaks and fistula. Of these, endoclips and covered stents are the two modalities, which are most commonly used and have most consistent results.
Table 4.
Modalities for endotherapy of leaks/fistula
| Closure |
| Endoclips |
| Suture |
| Sealant: Fibrin, cyanoacrylate |
| Diversion |
| Covered stents |
Endoclips
Endoclips, which are more frequently used for arresting GI hemorrhage can also be used for closing the GI wall disruptions and work like surgical sutures or staples[3,4,19]. First report of endoclipping for closure of GI perforation came from Germany[20]. This report discussed successful endotherapy of a perforation after endoscopic removal of gastric leiomyoma[20]. Endoclips can either be through the scope (TTS) clips, where clip applicator with loaded clip is introduced through the biopsy channel of the endoscope or recently available over the scope (OTS) clips, which are mounted over the scope tip like variceal band ligator device and released by a similar technique. TTS clips (Figure 1) are available in various designs and sizes: Quick clip (Olympus, America Inc., Center Valley PA, United States), Resolution clip (Boston Scientific Inc., Natick, United States) and Instinct clip (Cook Medical Inc.; Bloomington, IN, United States). Some of these are rotatable and re–openable, making them convenient to appropriately align the disrupted tissue. Figure 2 shows an esophageal tear treated by TTS clips.
Figure 1.
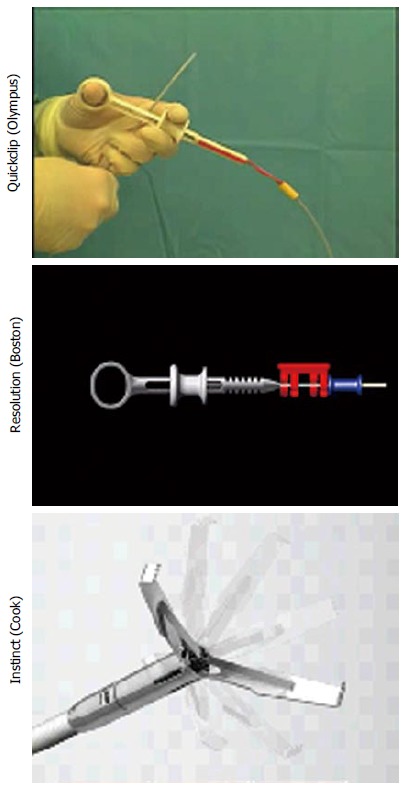
Through the scope clips.
Figure 2.
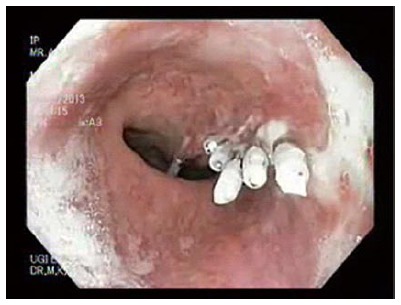
Endo clips (Through the scope) used to close an esophageal defect due to Boerhaave’s syndrome.
OTS clips (Figure 3) from Ovesco Endoscopy GmbH (Tuebingen, Germany) are nitinol, super elastic, biocompatible clips with teeth designed in the shape of a bear trap and can produce a full thickness closure. OTS clips are available in various shapes and sizes and selection of a particular size depends upon the size of the defect. For larger defect, one can use accessories like anchor and twin grasper, which can pull the defective mucosa into the OTS cylinder or reduce the gap of the defect respectively (Figure 3). One should carefully avoid capturing twin grasper or anchor while releasing the clip. Figure 4 illustrates the use of OTS clips in a patient with two defects in gastric wall located diagonally opposite one another following a Whipple’s surgery. Two OTS clips were placed with the help of anchor and twin grasper through a double channel endoscope. Follow-up CT scan confirmed the complete closure of defects.
Figure 3.
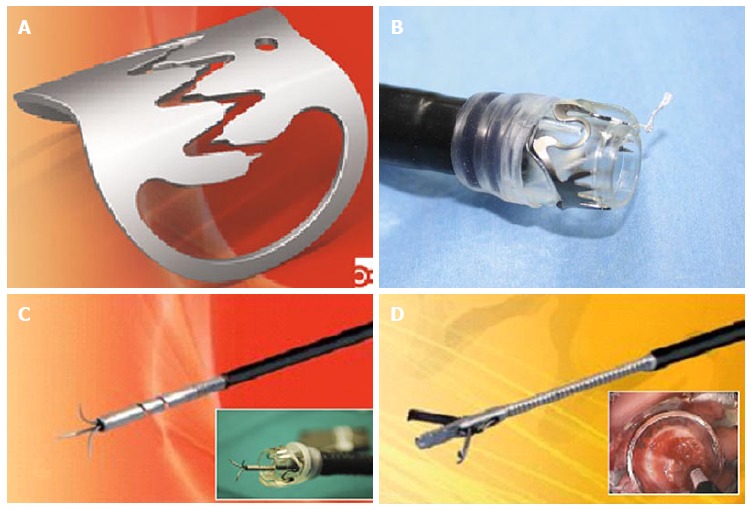
Over the scope clips (ovesco) (A) clip, (B) clip mounted on the endoscope, (C) anchor, (D) twin grasper.
Figure 4.
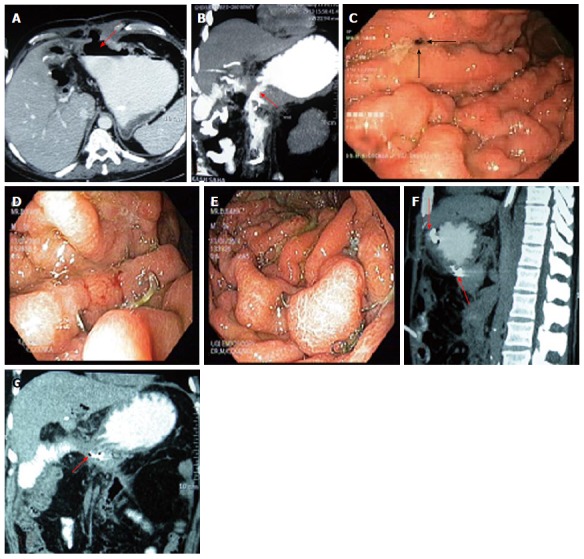
Dual gastric leak following Whipple’s Surgery treated by over the scope clips. A and B: Anterior and posterior defects (arrows); C: Endoscopic view showing leak on anterior gastric wall (arrows); D and E: The OTS clips placed on anterior and posterior defects respectively; F and G: Follow-up CT scan showing the clips (arrows) with demonstration of closure of leaks. OTS: Over the scope.
In general, it is believed that OTS clips cover a larger defect and one OTS clip can be compared with results obtained with 5 TTS clips. In large defects, such as after Endoscopic Submucosal Dissection (ESD), multiple TTS clips can be used to fix an endoloop at the margin of the defect and then pulling the loop and closing it can obliterate the defect. There are case reports of OTS clips applied under laparoscopic control in order to achieve greater success[21].
Both TTS and OTS clips have been used to close fistula and leaks located in esophagus, stomach as well as colon[20,22-30]. While most of these studies involve small number of patients, large series have reported results of clips to close leaks following ESD and endoscopic mucosal resection (EMR)[23,31]. Minami et al[23], in a series of 117 patients with gastric leak following EMR, demonstrated a success rate of 98.3% with TTS clips. Interestingly, they found a similar recovery rate for patients with perforation treated by clips and non perforated patients[23]. Of the 39 patients with perforations following ESD reported by Jeon et al[32] managed by endoclip, there was no failure. Overall success rate is higher for esophagus and stomach and somewhat moderate for colonic leaks. Voermans et al[22] reported their experience of OTS clips in 36 patients with iatrogenic perforations (esophageal: 5, gastric: 6, duodenal: 12, colonic: 13). Overall success rate of OTS clips was 89% with only one patient having endotherapy related complications. A large muti-center retrospective study by Chavez et al[33] involved 188 patients with GI leaks and fistula treated with OTS clips. 27 patients were lost during follow-up. Of the remaining patients, OTS was used as primary treatment in 97 patients and as rescue therapy in 64 patients. The success rate was 75% in first group and 47% in second group. Overall success rate was 64% (103 out of 161 patients). The result was better for perforation (95%) and leaks (80%), compared to fistula (45%).
In general clips are preferred over stents, if the leak is located in proximal esophagus or in distal most esophagus as well as for stomach and right colon[34]. While TTS clips are effective for leaks smaller than 10 mm[35], OTS clips are preferred if defect is larger than 20-30 mm. Prior ablation at edges of defect to make it raw, may help in clip placement[36]. While closing a large leak, it may be worthwhile to attempt to include adjacent omental patch within the clip, akin to surgical practice[37]. Because of possibility of leakage of air during the procedure, it may be a good idea to use CO2 insufflation during endotherapy of leaks and fistula[18]. In order to get best results, it is important to apply endoclips early after detection of leaks and perforations[35]. There is no reported risk of peritoneal dissemination or tumor recurrence after endoclips used for perforations following ESD or EMR performed for early cancers[38,39].
Luminal stenting
A large variety of stents are available to close luminal defects (Figure 5). These stents are covered (at least partially), so as to seal the defect and avoid contamination of the disrupted area. Mostly these stents are self - expanding metallic stent except for a single design of plastic stent (Polyflex, Boston). Fully covered stents, because of their ease at removability, are generally preferred particularly in the setting of benign disease. Figure 6 shows a patient with leak following gastrojejunostomy done for distal duodenal obstruction. One of the major issues with use of covered stent for closing of the GI defects is the risk of migration in absence of any obstructive pathology. This can be reduced by using large sized stents (Mega stents by Niti or Danis stent by Ella Figure 7), modified stents designs with extra covering in the shaft of stent (Figure 8) or by anchoring the stent by using endoclips or externalised threads (Figure 9)[40].
Figure 5.
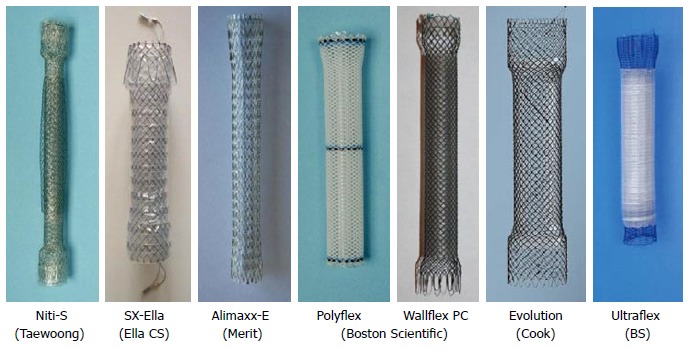
Stents for gastrointestinal leaks/fistula.
Figure 6.

Leak after duodeno-jejunostomy managed by luminal stenting. A: Contrast introduced through the surgical drain site shows the leak (arrow); B: Stent being deployed; C: Fully deployed covered stent; D: Contrast through the surgical drain shows the closure of the leak.
Figure 7.
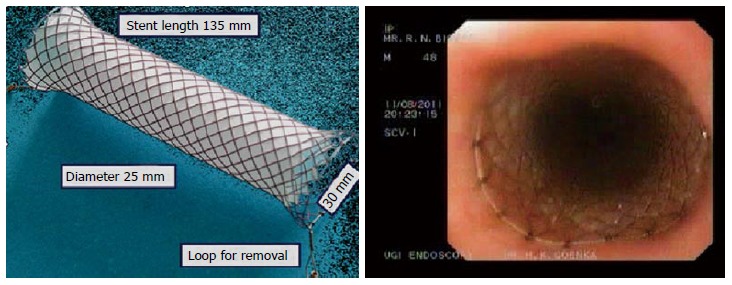
Danis stent.
Figure 8.

Modified stent design to prevent stent migration.
Figure 9.
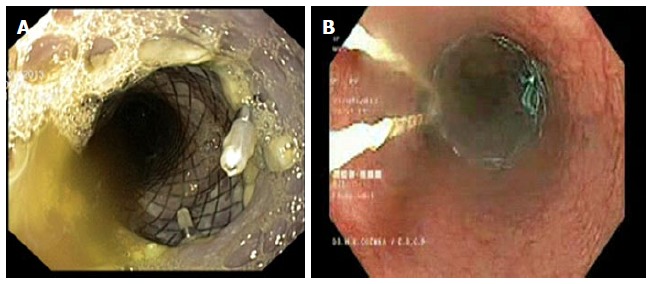
Anchoring of stent using (A) clip and (B) externalized thread.
Stents have been used mostly in esophagus, duodenum and colon. Van Boeckel et al[41] reported the results of 25 studies with luminal stent for iatrogenic esophageal leaks. In the cumulative data involving 267 patients, they reported a clinical success of 85% for closure of leak with no difference between plastic stent, fully covered or partially covered metal stents (84%, 85% and 86% respectively P = 0.097). Overall complication rate was 34%. Migration rate was somewhat higher for plastic stents compared to fully covered and partially covered stents (31% vs 26% vs 12% respectively). There was however, no difference in other complications such as tumor in-growth or over-growth. Freeman et al[42] recently reported that factors associated with failure of leak closure with stent placement include leak at cervical esophagus and esophagogastric junction, injury greater than 6 cm and additional distal leak. Tables 5 and 6 gives details of results of case series with endotherapy in esophageal and gastric iatrogenic perforation respectively[22-24,39,43-56]. As shown most of the series with gastric perforation have used clips, while both clips and stents have been used for esophageal perforation.
Table 5.
Result of endotherapy for iatrogenic esophageal perforation1
| Ref. | Type of treatment | Patients (n) | Technical success (%) | Complications (%) |
| 2Freeman et al[43] | SEPS | 19 | 100 | 24 |
| Vallböhmer et al[44] | SEMS | 12 | 100 | 8 |
| 2van Heel et al[45] | SEMS/ SEPS | 31 | 100 | 33 |
| Schimdt et al[46] | SEMS ± clip (1) | 22 | 100 | NA |
| Swinnen et al[47] | SEMS | 23 | 100 | NA |
| D’Cunha et al[48] | SEMS/ SEPS | 15 | 95 | 13 |
| Biancari et al[49], | Stents ± clip (1) | 12 | 100 | 25 |
| Schweigert et al[50] | SEMS/ SEPS | 13 | 100 | 85 |
| 2Heits et al[51] | Vaccum therapy | 10 | 100 | 20 |
| Biancari et al[52] | SEMS/clip | 67 | 100 | 34 |
1Only studies with 10 or more patients have been included;
Study design was prospective, rest were all retrospective. NA: Not available; OTSC: Over-the-scope clip; SEMS: Self-expandable metal stent; SEPS: Self expandable plastic stent.
Table 6.
Result of endotherapy for iatrogenic gastric perforation
| Ref. | n | Additional procedures | Success rate (%) |
| TTS | |||
| Tsunada et al[53] | 7 | Omental patch (1 case) | 100 |
| Fujishiro et al[39] | 11 | - | 100 |
| Minami et al[23] | 121 | > 1 cm: omental patch | 98.3 |
| Shi et al[54] | 20 | Endoloop | 100 |
| Zhong et al[55] | 14 | Endoloop | 100 |
| OTS | |||
| Kirschniak et al[24] | 72 | - | 100 |
| 1Voermans et al[22] | 6 | - | 100 |
| Nishiyama et al[56] | 7 | - | 86 |
Only studies with 5 or more patients are included.
All studies were retrospective except Voermans et al[22];
13 OTS clips were placed in 7 patients.
Bariatic surgery is not uncommonly complicated by leaks and fistula. In a retrospective study, over a period of 6 years involving 1499 bariatric surgery, Spyropoulos et al[57] reported a 2% incidence of luminal leak. Leaks were noted in sleeve itself, at staple line or at anastomosis site (gastrojejunostomy or enteroenteral). Of the 30 patients with leak, stents were used in 9, while surgery was performed in 3 patients and conservative approach was followed in 18 patients. Another recent study by EI Mourad et al[58] reported success of stent to close leaks following bariatric surgery in 41 out of 47 patients. Mega stents with a diameter of 30 mm are best suited for these indications.
Suturing and sealants
While suturing and use of sealants have been used to close GI leaks and fistula, results are mixed and experience is limited. Some of the suturing devices include EndoCinch suturing device (C.R. Bard, Inc, Boston, Mass, United States) Sefestitch (Safestitch, Medical Inc, Miami, Florida), Medical Power System (Power Medical Interventions, Longtrome, Pennsylvania), ESD Flexible Endoscopic Suturing devices by Wilson- Cook Medical (Winston- Salem, North Carolina) and Eagle Claw (Olympus Corporation, Tokyo, Japan)[59]. All these devices are either being still investigated or have not stood the test of time. Apollo Overstitch system (Figure 10) introduced recently, has been shown to have encouraging results[60,61]. This device is front-loaded onto a double - channel endoscope and allows continuous or interrupted stitches to be made with a cinching device. The merits of this approved device include the ability to reload the device inside the body eliminating the need to remove it between stitches as well as predictability of tissue needle penetration due to it being not suction based. Moreover, the device allows one endoscopic channel to be free to allow passage of grasping forcep for better tissue apposition[61].
Figure 10.
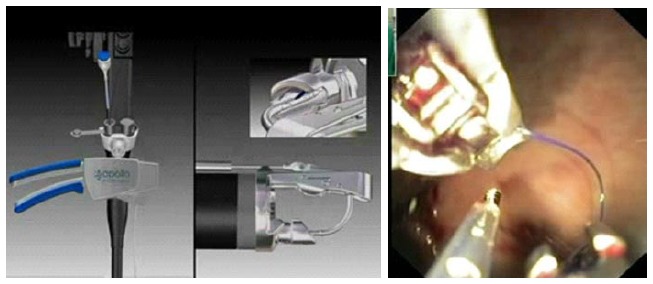
Apollo overstitch device.
Sealants which have been used to obliterate GI leaks and fistula include Cyanoacryate and Fibrin glue[62,63]. In a study by Rábago et al[62], 15 patients with post operative GI fistula were treated with Fibrin glue (combination of thrombin with fibrinogen). Complete sealing was obtained in 86.6% with a mean 2.5 sessions (range: 1 to 5) and a mean healing time of 16 d (range 5-40 d). Cyanoacrylate has been successfully used to close an esophagojejunal anastomotic leak after failed conservative therapy[63].
LIMITATIONS AND COMPLICATIONS
While endotherapy is exciting and results are encouraging, it has limitations in situations such as large perforation, difficult endoscopic position, fibrosis at the edge of the defect, evidence of abscess or fecal contamination etc[64]. Additional procedures or surgical alternatives should be considered in these circumstances. It is important to identify patients with failed clip closure as surgery should be promptly instituted in these patients in order to avoid sepsis and its consequences[65]. Monitoring should therefore be done by clinical profile and repeated blood counts. While endotherapy is safe if performed judiciously, complications such as perforation and bleeding are known. In particular, one must be careful while introducing endoscopes loaded with OTS clips, since the bigger insertion diameter can lead to iatrogenic perforations[22].
RECENT DEVELOPMENTS
Some of the recent techniques used to close GI leaks and fistula include Endovac therapy, Plugs and grafts, Biodegradable stents and Cardiac septal occluder. Endoscopic vacuum assisted closure sponge or Endovac therapy has been used in setting of leaks associated with infections (Figure 11)[66-69]. Ahrens et al[68] reported 5 patients with post esophageal surgery anastomotic leaks treated by endovac therapy. Polyurethane sponges with a drainage tube fixed to it allowing continuous suction was positioned endoscopically in the wound cavity and sponge was changed at regular interval. All 5 patients had closure of leak after a median of 9 sponge changes, median duration of drainage being 28 d. Two patients did require bougie dilatation for esophageal stenosis and one of them had fatal outcome due to aortoanastomotic fistula after dilatation. Loske et al[67] reported success in 13 out of 14 patients with esophageal leak treated by Endovac therapy with sponge being placed in the esophageal lumen (intraluminal method) or in the extraluminal wound cavity (intracavitary method). Similar technique has also been used for colonic anastomotic leaks with success in 28 out of 29 patients.
Figure 11.
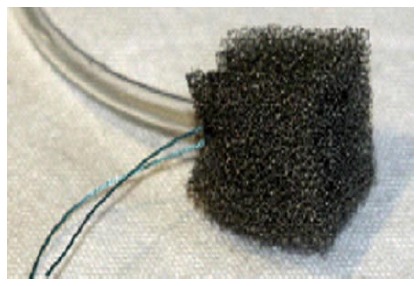
Endoscopic vacuum-assisted closure sponge (Endovac Therapy).
Plugs and grafts used include Vicryl plug and Surgisis. Surgisis soft tissue graft (Cook Biotech Inc, West Latayette, Ind) is an acellular bioactive prosthetic biomatrix produced from sheep intestinal submucosa[70]. In contrast to synthetic prosthetic material which has inherent risk of foreign body reaction, sepsis and secondary fistula formation, surgisis has been shown to be safe in contaminated tissues. It has been used successfully to treat complicated infected fistula after surgical resections including bariatric surgeries[71-73]. Vicryl mesh in combination with fibrin glue (covering the mesh as well as injected into the submucosa at the edge of the defect) used by Böhm et al[74] has also shown success in 13 out of 15 patients with leaks or fistula in upper GI tract following surgery for cancer. One to four sessions were used for this purpose.
Biodegradable stents have been used in a small series of 5 patients with esophageal leaks[75]. Four out of these 5 patients responded, inspite of 3 stents migrating during follow up. Cardiac septal occluder (Amplatzer Occluder, AGA Medical Corp, Plymouth, MN) used for cardiac septal defects have been used successfully by Repici et al[76] to close esophago - tracheal fistula. More data is however, required with these modalities before they are included in routine clinical practice.
CONSERVATIVE TREATMENT
While majority of patient with GI wall disruptions are candidates for either surgery or endotherapy, a small selected group of patients with iatrogenic perforation can be managed by conservative approach[18]. This subset includes stable patients who have perforations in cervical esophagus or a small number of patients with gastric or duodenal perforation, which are diagnosed late (> 12 h), are asymptomatic, have no signs of peritonitis and do not show free fluid or contrast extravasation at CT scan[18]. Somatostatin and its analogue octreotide, which decrease intestinal secretions, have also been used to improve the results of this conservative approach both in adults as well as children[77-79]. However, their role has been primarily considered for post-operative dehiscence, particularly after pancreatic surgery[78,79].
In conclusion, leaks and fistula involving GI tract are increasingly encountered in our routine practice. In a small select group of patients, there is a scope for conservative treatment of perforation and leaks. However, majority of patients are treated by surgery or endoscopic therapy. Techniques such as Endoclips (TTS and OTS) and covered metal stents have made endotherapy a preferred method to treat GI leaks and fistula. In general small leaks (< 10 mm) can be managed by traditional TTS clips, larger leaks require covered stents or OTS clips. Leaks and fistula associated with luminal strictures should be managed by luminal stenting. Recent developments with use of Endovac, Plugs and Graft and Biodegradable stents are encouraging. In particular, use of Endovac in the setting of sepsis seems promising. In view of multiple endoscopic modalities available with us, an algorithm based on location, size and associated features need to be developed to use these techniques judiciously.
Footnotes
Conflict-of-interest: We hereby declare that that we have no conflict of interest in relation to this review article.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: November 1, 2014
First decision: February 7, 2015
Article in press: April 7, 2015
P- Reviewer: Alsolaiman M, Gurkan A, Luo HS S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
References
- 1.Carrott PW, Low DE. Advances in the management of esophageal perforation. Thorac Surg Clin. 2011;21:541–555. doi: 10.1016/j.thorsurg.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Kumar N, Thompson CC. Endoscopic therapy for postoperative leaks and fistulae. Gastrointest Endosc Clin N Am. 2013;23:123–136. doi: 10.1016/j.giec.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Raju GS. Endoscopic clip closure of gastrointestinal perforations, fistulae, and leaks. Dig Endosc. 2014;26 Suppl 1:95–104. doi: 10.1111/den.12191. [DOI] [PubMed] [Google Scholar]
- 4.Raymer GS, Sadana A, Campbell DB, Rowe WA. Endoscopic clip application as an adjunct to closure of mature esophageal perforation with fistulae. Clin Gastroenterol Hepatol. 2003;1:44–50. doi: 10.1053/jcgh.2003.50007. [DOI] [PubMed] [Google Scholar]
- 5.Baron TH, Gostout CJ, Herman L. Hemoclip repair of a sphincterotomy-induced duodenal perforation. Gastrointest Endosc. 2000;52:566–568. [PubMed] [Google Scholar]
- 6.Yoshikane H, Hidano H, Sakakibara A, Ayakawa T, Mori S, Kawashima H, Goto H, Niwa Y. Endoscopic repair by clipping of iatrogenic colonic perforation. Gastrointest Endosc. 1997;46:464–466. doi: 10.1016/s0016-5107(97)70045-2. [DOI] [PubMed] [Google Scholar]
- 7.Kim HS, Lee DK, Jeong YS, Kim KH, Baik SK, Kwon SO, Cho MY. Successful endoscopic management of a perforated gastric dysplastic lesion after endoscopic mucosal resection. Gastrointest Endosc. 2000;51:613–615. doi: 10.1016/s0016-5107(00)70305-1. [DOI] [PubMed] [Google Scholar]
- 8.Rosés LL, Ramirez AG, Seco AL, Blanco ES, Alonso DI, Avila S, Lopez BU. Clip closure of a duodenal perforation secondary to a biliary stent. Gastrointest Endosc. 2000;51:487–489. doi: 10.1016/s0016-5107(00)70454-8. [DOI] [PubMed] [Google Scholar]
- 9.Cipolletta L, Bianco MA, Rotondano G, Marmo R, Piscopo R, Meucci C. Endoscopic clipping of perforation following pneumatic dilation of esophagojejunal anastomotic strictures. Endoscopy. 2000;32:720–722. doi: 10.1055/s-2000-7032. [DOI] [PubMed] [Google Scholar]
- 10.Seibert DG. Use of an endoscopic clipping device to repair a duodenal perforation. Endoscopy. 2003;35:189. doi: 10.1055/s-2003-37004. [DOI] [PubMed] [Google Scholar]
- 11.Abe N, Sugiyama M, Hashimoto Y, Itoh N, Nakaura H, Izumisato Y, Matsuoka H, Masaki T, Nakashima M, Mori T, et al. Endoscopic nasomediastinal drainage followed by clip application for treatment of delayed esophageal perforation with mediastinitis. Gastrointest Endosc. 2001;54:646–648. doi: 10.1067/mge.2001.117155. [DOI] [PubMed] [Google Scholar]
- 12.Shimamoto C, Hirata I, Umegaki E, Katsu K. Closure of an esophageal perforation due to fish bone ingestion by endoscopic clip application. Gastrointest Endosc. 2000;51:736–739. doi: 10.1067/mge.2000.105729. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson BC, Briggs DR, Carr-Locke DL. Endoscopic closure of a colovesical fistula. Gastrointest Endosc. 2001;54:248–250. doi: 10.1067/mge.2001.116886. [DOI] [PubMed] [Google Scholar]
- 14.van Bodegraven AA, Kuipers EJ, Bonenkamp HJ, Meuwissen SG. Esophagopleural fistula treated endoscopically with argon beam electrocoagulation and clips. Gastrointest Endosc. 1999;50:407–409. doi: 10.1053/ge.1999.v50.97234. [DOI] [PubMed] [Google Scholar]
- 15.Lee SO, Jeong YJ. Colonoscopic clipping of fecal fistula that occurred as a postoperative complication in patients with perforated appendicitis: two case reports. Gastrointest Endosc. 2001;54:245–247. doi: 10.1067/mge.2001.114411. [DOI] [PubMed] [Google Scholar]
- 16.Rodella L, Laterza E, De Manzoni G, Kind R, Lombardo F, Catalano F, Ricci F, Cordiano C. Endoscopic clipping of anastomotic leakages in esophagogastric surgery. Endoscopy. 1998;30:453–456. doi: 10.1055/s-2007-1001307. [DOI] [PubMed] [Google Scholar]
- 17.Mizobuchi S, Kuge K, Maeda H, Matsumoto Y, Yamamoto M, Sasaguri S. Endoscopic clip application for closure of an esophagomediastinal-tracheal fistula after surgery for esophageal cancer. Gastrointest Endosc. 2003;57:962–965. doi: 10.1016/s0016-5107(03)70054-6. [DOI] [PubMed] [Google Scholar]
- 18.Paspatis GA, Dumonceau JM, Barthet M, Meisner S, Repici A, Saunders BP, Vezakis A, Gonzalez JM, Turino SY, Tsiamoulos ZP, et al. Diagnosis and management of iatrogenic endoscopic perforations: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy. 2014;46:693–711. doi: 10.1055/s-0034-1377531. [DOI] [PubMed] [Google Scholar]
- 19.Shimizu Y, Kato M, Yamamoto J, Nakagawa S, Komatsu Y, Tsukagoshi H, Fujita M, Hosokawa M, Asaka M. Endoscopic clip application for closure of esophageal perforations caused by EMR. Gastrointest Endosc. 2004;60:636–639. doi: 10.1016/s0016-5107(04)01960-1. [DOI] [PubMed] [Google Scholar]
- 20.Binmoeller KF, Grimm H, Soehendra N. Endoscopic closure of a perforation using metallic clips after snare excision of a gastric leiomyoma. Gastrointest Endosc. 1993;39:172–174. doi: 10.1016/s0016-5107(93)70060-7. [DOI] [PubMed] [Google Scholar]
- 21.Schlag C, Wilhelm D, von Delius S, Feussner H, Meining A. EndoResect study: endoscopic full-thickness resection of gastric subepithelial tumors. Endoscopy. 2013;45:4–11. doi: 10.1055/s-0032-1325760. [DOI] [PubMed] [Google Scholar]
- 22.Voermans RP, Le Moine O, von Renteln D, Ponchon T, Giovannini M, Bruno M, Weusten B, Seewald S, Costamagna G, Deprez P, et al. Efficacy of endoscopic closure of acute perforations of the gastrointestinal tract. Clin Gastroenterol Hepatol. 2012;10:603–608. doi: 10.1016/j.cgh.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Minami S, Gotoda T, Ono H, Oda I, Hamanaka H. Complete endoscopic closure of gastric perforation induced by endoscopic resection of early gastric cancer using endoclips can prevent surgery (with video) Gastrointest Endosc. 2006;63:596–601. doi: 10.1016/j.gie.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Kirschniak A, Subotova N, Zieker D, Königsrainer A, Kratt T. The Over-The-Scope Clip (OTSC) for the treatment of gastrointestinal bleeding, perforations, and fistulas. Surg Endosc. 2011;25:2901–2905. doi: 10.1007/s00464-011-1640-2. [DOI] [PubMed] [Google Scholar]
- 25.Zolotarevsky E, Kwon Y, Bains M, Schattner M. Esophagobronchial fistula closure using a novel endoscopic over-the-scope-clip. Ann Thorac Surg. 2012;94:e69–e70. doi: 10.1016/j.athoracsur.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Markar SR, Koehler R, Low DE, Ross A. Novel multimodality endoscopic closure of postoperative esophageal fistula. Int J Surg Case Rep. 2012;3:577–579. doi: 10.1016/j.ijscr.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manta R, Manno M, Bertani H, Barbera C, Pigò F, Mirante V, Longinotti E, Bassotti G, Conigliaro R. Endoscopic treatment of gastrointestinal fistulas using an over-the-scope clip (OTSC) device: case series from a tertiary referral center. Endoscopy. 2011;43:545–548. doi: 10.1055/s-0030-1256196. [DOI] [PubMed] [Google Scholar]
- 28.von Renteln D, Denzer UW, Schachschal G, Anders M, Groth S, Rösch T. Endoscopic closure of GI fistulae by using an over-the-scope clip (with videos) Gastrointest Endosc. 2010;72:1289–1296. doi: 10.1016/j.gie.2010.07.033. [DOI] [PubMed] [Google Scholar]
- 29.Jacobsen GR, Coker AM, Acosta G, Talamini MA, Savides TJ, Horgan S. Initial experience with an innovative endoscopic clipping system. Surg Technol Int. 2012;22:39–43. [PubMed] [Google Scholar]
- 30.Raju GS, Saito Y, Matsuda T, Kaltenbach T, Soetikno R. Endoscopic management of colonoscopic perforations (with videos) Gastrointest Endosc. 2011;74:1380–1388. doi: 10.1016/j.gie.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Yokoi C, Gotoda T, Hamanaka H, Oda I. Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc. 2006;64:212–218. doi: 10.1016/j.gie.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 32.Jeon SW, Jung MK, Kim SK, Cho KB, Park KS, Park CK, Kwon JG, Jung JT, Kim EY, Kim TN, et al. Clinical outcomes for perforations during endoscopic submucosal dissection in patients with gastric lesions. Surg Endosc. 2010;24:911–916. doi: 10.1007/s00464-009-0693-y. [DOI] [PubMed] [Google Scholar]
- 33.Chavez YH, Kratt T, Law JK, Arezzo A, Sharaiha RZ, Poley JW, Kahaleh M, Thompson CC, Ryan MB, Choksi N, et al. A Large International Multicenter Experience With an Over-the- Scope Clipping Device for Endoscopic Management of Gastrointestinal Perforations, Fistulae, and Leaks in 188 Patients. Gastroint Endos. 2013;77:AB148. doi: 10.1016/j.gie.2014.03.049. [DOI] [PubMed] [Google Scholar]
- 34.Fischer A, Schrag HJ, Goos M, von Dobschuetz E, Hopt UT. Nonoperative treatment of four esophageal perforations with hemostatic clips. Dis Esophagus. 2007;20:444–448. doi: 10.1111/j.1442-2050.2007.00652.x. [DOI] [PubMed] [Google Scholar]
- 35.Qadeer MA, Dumot JA, Vargo JJ, Lopez AR, Rice TW. Endoscopic clips for closing esophageal perforations: case report and pooled analysis. Gastrointest Endosc. 2007;66:605–611. doi: 10.1016/j.gie.2007.03.1028. [DOI] [PubMed] [Google Scholar]
- 36.Felsher J, Farres H, Chand B, Farver C, Ponsky J. Mucosal apposition in endoscopic suturing. Gastrointest Endosc. 2003;58:867–870. doi: 10.1016/s0016-5107(03)02312-5. [DOI] [PubMed] [Google Scholar]
- 37.Díez-Redondo P, Blanco JI, Lorenzo-Pelayo S, De-la-Serna-Higuera C, Gil-Simón P, Alcaide-Suárez N, Pérez-Miranda M. A novel system for endoscopic closure of iatrogenic colon perforations using the Ovesco® clip and omental patch. Rev Esp Enferm Dig. 2012;104:550–552. doi: 10.4321/s1130-01082012001000009. [DOI] [PubMed] [Google Scholar]
- 38.Ikehara H, Gotoda T, Ono H, Oda I, Saito D. Gastric perforation during endoscopic resection for gastric carcinoma and the risk of peritoneal dissemination. Br J Surg. 2007;94:992–995. doi: 10.1002/bjs.5636. [DOI] [PubMed] [Google Scholar]
- 39.Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Kobayashi K, Hashimoto T, Yamamichi N, Tateishi A, et al. Successful nonsurgical management of perforation complicating endoscopic submucosal dissection of gastrointestinal epithelial neoplasms. Endoscopy. 2006;38:1001–1006. doi: 10.1055/s-2006-944775. [DOI] [PubMed] [Google Scholar]
- 40.Fischer A, Bausch D, Richter-Schrag HJ. Use of a specially designed partially covered self-expandable metal stent (PSEMS) with a 40-mm diameter for the treatment of upper gastrointestinal suture or staple line leaks in 11 cases. Surg Endosc. 2013;27:642–647. doi: 10.1007/s00464-012-2507-x. [DOI] [PubMed] [Google Scholar]
- 41.van Boeckel PG, Sijbring A, Vleggaar FP, Siersema PD. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther. 2011;33:1292–1301. doi: 10.1111/j.1365-2036.2011.04663.x. [DOI] [PubMed] [Google Scholar]
- 42.Freeman RK, Ascioti AJ, Giannini T, Mahidhara RJ. Analysis of unsuccessful esophageal stent placements for esophageal perforation, fistula, or anastomotic leak. Ann Thorac Surg. 2012;94:959–964; discussion 964-965. doi: 10.1016/j.athoracsur.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 43.Freeman RK, Van Woerkom JM, Vyverberg A, Ascioti AJ. Esophageal stent placement for the treatment of spontaneous esophageal perforations. Ann Thorac Surg. 2009;88:194–198. doi: 10.1016/j.athoracsur.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Vallböhmer D, Hölscher AH, Hölscher M, Bludau M, Gutschow C, Stippel D, Bollschweiler E, Schröder W. Options in the management of esophageal perforation: analysis over a 12-year period. Dis Esophagus. 2010;23:185–190. doi: 10.1111/j.1442-2050.2009.01017.x. [DOI] [PubMed] [Google Scholar]
- 45.van Heel NC, Haringsma J, Spaander MC, Bruno MJ, Kuipers EJ. Short-term esophageal stenting in the management of benign perforations. Am J Gastroenterol. 2010;105:1515–1520. doi: 10.1038/ajg.2010.104. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt SC, Strauch S, Rösch T, Veltzke-Schlieker W, Jonas S, Pratschke J, Weidemann H, Neuhaus P, Schumacher G. Management of esophageal perforations. Surg Endosc. 2010;24:2809–2813. doi: 10.1007/s00464-010-1054-6. [DOI] [PubMed] [Google Scholar]
- 47.Swinnen J, Eisendrath P, Rigaux J, Kahegeshe L, Lemmers A, Le Moine O, Devière J. Self-expandable metal stents for the treatment of benign upper GI leaks and perforations. Gastrointest Endosc. 2011;73:890–899. doi: 10.1016/j.gie.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 48.D’Cunha J, Rueth NM, Groth SS, Maddaus MA, Andrade RS. Esophageal stents for anastomotic leaks and perforations. J Thorac Cardiovasc Surg. 2011;142:39–46.e1. doi: 10.1016/j.jtcvs.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 49.Biancari F, Gudbjartsson T, Mennander A, Hypén L, Salminen P, Kuttila K, Viktorzon M, Böckelman C, Tarantino E, Tiffet O, et al. Treatment of esophageal perforation in octogenarians: a multicenter study. Dis Esophagus. 2014;27:715–718. doi: 10.1111/dote.12148. [DOI] [PubMed] [Google Scholar]
- 50.Schweigert M, Beattie R, Solymosi N, Booth K, Dubecz A, Muir A, Moskorz K, Stadlhuber RJ, Ofner D, McGuigan J, et al. Endoscopic stent insertion versus primary operative management for spontaneous rupture of the esophagus (Boerhaave syndrome): an international study comparing the outcome. Am Surg. 2013;79:634–640. doi: 10.1177/000313481307900627. [DOI] [PubMed] [Google Scholar]
- 51.Heits N, Stapel L, Reichert B, Schafmayer C, Schniewind B, Becker T, Hampe J, Egberts JH. Endoscopic endoluminal vacuum therapy in esophageal perforation. Ann Thorac Surg. 2014;97:1029–1035. doi: 10.1016/j.athoracsur.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 52.Biancari F, Saarnio J, Mennander A, Hypén L, Salminen P, Kuttila K, Victorzon M, Böckelman C, Tarantino E, Tiffet O, et al. Outcome of patients with esophageal perforations: a multicenter study. World J Surg. 2014;38:902–909. doi: 10.1007/s00268-013-2312-2. [DOI] [PubMed] [Google Scholar]
- 53.Tsunada S, Ogata S, Ohyama T, Ootani H, Oda K, Kikkawa A, Ootani A, Sakata H, Iwakiri R, Fujimoto K. Endoscopic closure of perforations caused by EMR in the stomach by application of metallic clips. Gastrointest Endosc. 2003;57:948–951. doi: 10.1016/s0016-5107(03)70051-0. [DOI] [PubMed] [Google Scholar]
- 54.Shi Q, Chen T, Zhong YS, Zhou PH, Ren Z, Xu MD, Yao LQ. Complete closure of large gastric defects after endoscopic full-thickness resection, using endoloop and metallic clip interrupted suture. Endoscopy. 2013;45:329–334. doi: 10.1055/s-0032-1326214. [DOI] [PubMed] [Google Scholar]
- 55.Zhong YS, Shi Q, Yao LQ, Zhou PH, Xu MD, Ma LL, Chen T. Complete closure of gastric wall defect after endoscopic full-thick resection with metal clips and endoloop snare. Zhonghua Weichang Waike Zazhi. 2012;15:280–284. [PubMed] [Google Scholar]
- 56.Nishiyama N, Mori H, Kobara H, Rafiq K, Fujihara S, Kobayashi M, Oryu M, Masaki T. Efficacy and safety of over-the-scope clip: including complications after endoscopic submucosal dissection. World J Gastroenterol. 2013;19:2752–2760. doi: 10.3748/wjg.v19.i18.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Spyropoulos C, Argentou MI, Petsas T, Thomopoulos K, Kehagias I, Kalfarentzos F. Management of gastrointestinal leaks after surgery for clinically severe obesity. Surg Obes Relat Dis. 2012;8:609–615. doi: 10.1016/j.soard.2011.04.222. [DOI] [PubMed] [Google Scholar]
- 58.El Mourad H, Himpens J, Verhofstadt J. Stent treatment for fistula after obesity surgery: results in 47 consecutive patients. Surg Endosc. 2013;27:808–816. doi: 10.1007/s00464-012-2517-8. [DOI] [PubMed] [Google Scholar]
- 59.Hu B, Chung SC, Sun LC, Kawashima K, Yamamoto T, Cotton PB, Gostout CJ, Hawes RH, Kalloo AN, Kantsevoy SV, et al. Transoral obesity surgery: endoluminal gastroplasty with an endoscopic suture device. Endoscopy. 2005;37:411–414. doi: 10.1055/s-2005-861196. [DOI] [PubMed] [Google Scholar]
- 60.Bonin EA, Wong Kee Song LM, Gostout ZS, Bingener J, Gostout CJ. Closure of a persistent esophagopleural fistula assisted by a novel endoscopic suturing system. Endoscopy. 2012;44 Suppl 2 UCTN:E8–E9. doi: 10.1055/s-0031-1291494. [DOI] [PubMed] [Google Scholar]
- 61.Kantsevoy SV, Thuluvath PJ. Successful closure of a chronic refractory gastrocutaneous fistula with a new endoscopic suturing device (with video) Gastrointest Endosc. 2012;75:688–690. doi: 10.1016/j.gie.2011.04.031. [DOI] [PubMed] [Google Scholar]
- 62.Rábago LR, Ventosa N, Castro JL, Marco J, Herrera N, Gea F. Endoscopic treatment of postoperative fistulas resistant to conservative management using biological fibrin glue. Endoscopy. 2002;34:632–638. doi: 10.1055/s-2002-33237. [DOI] [PubMed] [Google Scholar]
- 63.Pramateftakis MG, Vrakas G, Kanellos I, Mantzoros I, Angelopoulos S, Eleftheriades E, Lazarides C. Endoscopic application of n-butyl-2-cyanoacrylate on esophagojejunal anastomotic leak: a case report. J Med Case Rep. 2011;5:96. doi: 10.1186/1752-1947-5-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dişibeyaz S, Köksal AŞ, Parlak E, Torun S, Şaşmaz N. Endoscopic closure of gastrointestinal defects with an over-the-scope clip device. A case series and review of the literature. Clin Res Hepatol Gastroenterol. 2012;36:614–621. doi: 10.1016/j.clinre.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 65.Cho SB, Lee WS, Joo YE, Kim HR, Park SW, Park CH, Kim HS, Choi SK, Rew JS. Therapeutic options for iatrogenic colon perforation: feasibility of endoscopic clip closure and predictors of the need for early surgery. Surg Endosc. 2012;26:473–479. doi: 10.1007/s00464-011-1903-y. [DOI] [PubMed] [Google Scholar]
- 66.Loske G, Schorsch T, Müller C. Endoscopic intracavitary vacuum sponge therapy of anastomotic leakage in the proximal colon after right-sided colectomy. Endoscopy. 2010;42 Suppl 2:E171–E172. doi: 10.1055/s-0029-1244177. [DOI] [PubMed] [Google Scholar]
- 67.Loske G, Schorsch T, Müller C. Intraluminal and intracavitary vacuum therapy for esophageal leakage: a new endoscopic minimally invasive approach. Endoscopy. 2011;43:540–544. doi: 10.1055/s-0030-1256345. [DOI] [PubMed] [Google Scholar]
- 68.Ahrens M, Schulte T, Egberts J, Schafmayer C, Hampe J, Fritscher-Ravens A, Broering DC, Schniewind B. Drainage of esophageal leakage using endoscopic vacuum therapy: a prospective pilot study. Endoscopy. 2010;42:693–698. doi: 10.1055/s-0030-1255688. [DOI] [PubMed] [Google Scholar]
- 69.Weidenhagen R, Gruetzner KU, Wiecken T, Spelsberg F, Jauch KW. Endoscopic vacuum-assisted closure of anastomotic leakage following anterior resection of the rectum: a new method. Surg Endosc. 2008;22:1818–1825. doi: 10.1007/s00464-007-9706-x. [DOI] [PubMed] [Google Scholar]
- 70.Tringali A, Daniel FB, Familiari P, Perri V, Mutignani M, Vitelli CE, Costamagna G. Endoscopic treatment of a recalcitrant esophageal fistula with new tools: stents, Surgisis, and nitinol staples (with video) Gastrointest Endosc. 2010;72:647–650. doi: 10.1016/j.gie.2009.11.047. [DOI] [PubMed] [Google Scholar]
- 71.Franklin ME, Gonzalez JJ, Michaelson RP, Glass JL, Chock DA. Preliminary experience with new bioactive prosthetic material for repair of hernias in infected fields. Hernia. 2002;6:171–174. doi: 10.1007/s10029-002-0078-9. [DOI] [PubMed] [Google Scholar]
- 72.Toussaint E, Eisendrath P, Kwan V, Dugardeyn S, Devière J, Le Moine O. Endoscopic treatment of postoperative enterocutaneous fistulas after bariatric surgery with the use of a fistula plug: report of five cases. Endoscopy. 2009;41:560–563. doi: 10.1055/s-0029-1214606. [DOI] [PubMed] [Google Scholar]
- 73.Maluf-Filho F, Hondo F, Halwan B, de Lima MS, Giordano-Nappi JH, Sakai P. Endoscopic treatment of Roux-en-Y gastric bypass-related gastrocutaneous fistulas using a novel biomaterial. Surg Endosc. 2009;23:1541–1545. doi: 10.1007/s00464-009-0440-4. [DOI] [PubMed] [Google Scholar]
- 74.Böhm G, Mossdorf A, Klink C, Klinge U, Jansen M, Schumpelick V, Truong S. Treatment algorithm for postoperative upper gastrointestinal fistulas and leaks using combined vicryl plug and fibrin glue. Endoscopy. 2010;42:599–602. doi: 10.1055/s-0029-1244165. [DOI] [PubMed] [Google Scholar]
- 75.Černá M, Köcher M, Válek V, Aujeský R, Neoral Č, Andrašina T, Pánek J, Mahathmakanthi S. Covered biodegradable stent: new therapeutic option for the management of esophageal perforation or anastomotic leak. Cardiovasc Intervent Radiol. 2011;34:1267–1271. doi: 10.1007/s00270-010-0059-9. [DOI] [PubMed] [Google Scholar]
- 76.Repici A, Presbitero P, Carlino A, Strangio G, Rando G, Pagano N, Romeo F, Rosati R. First human case of esophagus-tracheal fistula closure by using a cardiac septal occluder (with video) Gastrointest Endosc. 2010;71:867–869. doi: 10.1016/j.gie.2009.08.036. [DOI] [PubMed] [Google Scholar]
- 77.Lam JC, Aters S, Tobias JD. Initial experience with octreotide in the pediatric population. Am J Ther. 2001;8:409–415. doi: 10.1097/00045391-200111000-00005. [DOI] [PubMed] [Google Scholar]
- 78.Kingsnorth AN, Berg JD, Gray MR. A novel reconstructive technique for pylorus-preserving pancreaticoduodenectomy: avoidance of early postoperative gastric stasis. Ann R Coll Surg Engl. 1993;75:38–42. [PMC free article] [PubMed] [Google Scholar]
- 79.Gouillat C, Faucheron JL, Balique JG, Gayet B, Saric J, Partensky C, Baulieux J, Chipponi J. Natural history of the pancreatic stump after duodenopancreatectomy of the pancreatic head. Ann Chir. 2002;127:467–476. doi: 10.1016/s0003-3944(02)00804-0. [DOI] [PubMed] [Google Scholar]


