Abstract
AIM: To identify the features of early signet ring cell gastric carcinoma using magnification endoscopy with narrow band imaging (NBI).
METHODS: A retrospective review was conducted of 12 cases of early signet ring cell gastric carcinoma who underwent treatment in a single institution between January 2009 and April 2013. All patients had magnification endoscopy with NBI and indigo carmine contrast to closely examine the mucosal architecture, including the microvasculature and arrangement of gastric pits. Histologic examination of the final endoscopic submucosal dissection or gastrectomy specimen was performed and compared with the endoscopic findings to identify patterns specific to signet ring cell carcinoma.
RESULTS: Twelve patients with early signet ring cell gastric carcinoma were identified; 75% were male, and average age was 61 years. Most of the lesions were stage T1a (83%), while the remainder were T1b (17%). The mean lesion size was 1.4 cm2. On standard endoscopy, all 12 patients had a pale, flat lesion without any evidence of mucosal abnormality such as ulceration, elevation, or depression. On magnification endoscopy with NBI, all of the patients had irregularities in the glands and microvasculature consistent with early gastric cancer. In addition, all 12 patients exhibited the “stretch sign”, an elongation or expansion of the architectural structure. Histologic examination of the resected specimens demonstrated an expanded and edematous mucosal layer infiltrated with tumor cells.
CONCLUSION: The “stretch sign” appears to be specific for signet ring cell carcinoma and may aid in the early diagnosis and treatment of this aggressive pathology.
Keywords: Signet ring cells, Early gastric cancer, Magnification endoscopy, Narrow band imaging, Stretch sign, Endoscopic submucosal dissection
Core tip: With aggressive screening, gastric cancer can be detected in the early stages, leading to the possibility of successful minimally invasive treatments, such as endoscopic submucosal dissection. A rare type of gastric cancer, signet ring cell carcinoma, has aggressive biological features, but patients treated in the early stages may actually fare better than those with adenocarcinoma. Here we present findings specific for signet ring cell carcinoma that can be identified on magnification endoscopy, potentially securing a diagnosis in the early stages of the disease without the need to rely on random biopsies.
INTRODUCTION
Gastric cancer can be detected in the early stages by aggressively screening asymptomatic patients. In Japan, where such rigorous screening is conducted, half of gastric cancers are now diagnosed in the early stages of the disease[1]. Early detection affords the opportunity for less invasive treatment options, such as endoscopic submucosal dissection (ESD). However, signet ring cell carcinoma, an unfavorable subtype of gastric cancer that may require more aggressive treatment, has been reported in up to 29% of gastric cancer patients in the United States[2] and over 10% in Japan[3]. If treated early, signet ring cell carcinoma has a better prognosis than other subtypes; however, advanced signet ring cell carcinoma has a prognosis that is even worse than undifferentiated adenocarcinoma[4]. Early diagnosis of signet ring cell carcinoma is therefore critical to guide optimal treatment, but the typical presentation during conventional endoscopy is a pale, flat lesion that can easily be missed even by experienced endoscopists.
Advanced endoscopy using a magnifying endoscope and narrow band imaging (NBI) technology may play an important role. Previous studies have reported fine mucosal patterns of gastric pits and microvasculature that can be identified and classified using magnification endoscopy with NBI[5-7], and that it is possible to predict the depth of invasion of early gastric carcinomas prior to histologic assessment[8,9]. We postulate that magnification endoscopy and NBI can be further applied to the early detection of signet ring cell carcinoma.
The purpose of this study was to review our experience with magnification endoscopy with NBI in 12 cases of early signet cell gastric carcinoma, and to identify specific endoscopic patterns that may predict the final pathologic diagnosis.
MATERIALS AND METHODS
Patient selection
A retrospective chart review was performed to identify patients who underwent endoscopic and/or surgical intervention for signet ring cell gastric carcinoma in a single institution (Showa University Northern Yokohama Hospital). We identified 12 cases of signet ring cell gastric carcinoma during the study period from January 2009 to April 2013.
Magnification endoscopy
Diagnostic procedures were performed following the ingestion of 5 cc of viscous 2% lidocaine and administration of light intravenous sedation. Magnification endoscopy was performed in a single center utilizing high-resolution magnifying upper endoscopes (Olympus Evis Lucera Spectrum, GIF-H260Z, Tokyo, Japan) with 10.8 mm diameter tips and color charge-coupled-device (CCD) optical lenses with a 140 degree field of view. A distal attachment with 3 mm depth (MB-162, Olympus, Tokyo, Japan) was utilized as described by Yao et al[10], and images were recorded with NBI both before and after administration of 0.3% indigo carmine dye. Endoscopic images were reviewed by expert endoscopists and assessed for irregularity of the gastric pits and/or microvasculature.
Histopathology
Histologic examination of the final specimen following ESD or laparoscopic-assisted partial or total gastrectomy was performed by pathologists specializing in gastrointestinal pathology, and lesions were classified according to the Japanese classification system[11]. Pathologists did not have access to the endoscopic findings.
Biostatistics
The data presented are a qualitative analysis of a single cohort. No statistical tests were performed.
RESULTS
Patient demographics and clinical outcomes
Patient demographic data are summarized in Table 1. Of the 12 patients with signet ring type early gastric cancer, mean age was 61.3 (range 39-87), and 75% were male; the lesions had a maximum dimension of 1.3 cm on average (range 0.5-2.2 cm) with a mean area of 1.4 cm2 (range 0.2-4.2 cm2); 83% of lesions were T1a, and 17% were T1b. ESD was performed in 83% of cases; laparoscopic-assisted distal gastrectomy or laparoscopic-assisted total gastrectomy was performed in the remaining 17% due to pre-operative suspicion of lymph node metastases. There was 100% disease-free survival at a median follow-up of 2.5 years.
Table 1.
Demographic data and tumor characteristics of 12 patients with signet ring cell early gastric carcinoma
| No. | Age | Sex | Location of tumor | Size (mm) | Depth | Operation | F/U (mo) |
| 1 | 64 | F | Lower body/posterior | 10 × 9 | T1a | ESD | 54 |
| 2 | 77 | M | Pyloric/lesser curve | 5 × 4 | T1a | ESD | 45 |
| 3 | 46 | M | Mid body/posterior | 5 × 5 | T1a | LATG | 41 |
| 4 | 87 | F | Mid body/greater curve | 20 × 16 | T1b | ESD | 38 |
| 5 | 39 | M | Lower body/posterior | 12 × 6 | T1a | ESD | 38 |
| 6 | 46 | M | Pyloric/greater curve | 10 × 8 | T1a | ESD | 35 |
| 7 | 60 | M | Pyloric/greater curve | 6 × 6 | T1a | ESD | 26 |
| 8 | 72 | F | Pyloric/greater curve | 22 × 19 | T1a | ESD | 18 |
| 9 | 71 | M | Pyloric/greater curve | 11 × 10 | T1a | ESD | 16 |
| 10 | 48 | M | Pyloric/lesser curve | 20 × 11 | T1a | LADG | 14 |
| 11 | 82 | M | Pyloric/greater curve | 20 × 14 | T1b | ESD | 13 |
| 12 | 44 | M | Pyloric/lesser curve | 10 × 3 | T1a | ESD | 10 |
F/U: Follow-up; ESD: Endoscopic submucosal dissection; LATG: Laparoscopic-assisted total gastrectomy; LADG: Laparoscopic-assisted distal gastrectomy.
Endoscopic findings
On standard white light endoscopy, all 12 patients with signet ring early gastric cancer had pale, flat lesions without gross mucosal abnormality such as ulceration, elevation, or depression. On magnification endoscopy, each of the patients had irregularities in the glands and microvasculature, consistent with early gastric cancer; however, in addition, the architecture appeared to be expanded or elongated, as if it had been “stretched”, within a portion of the lesion for all 12 patients (Figures 1 and 2).
Figure 1.
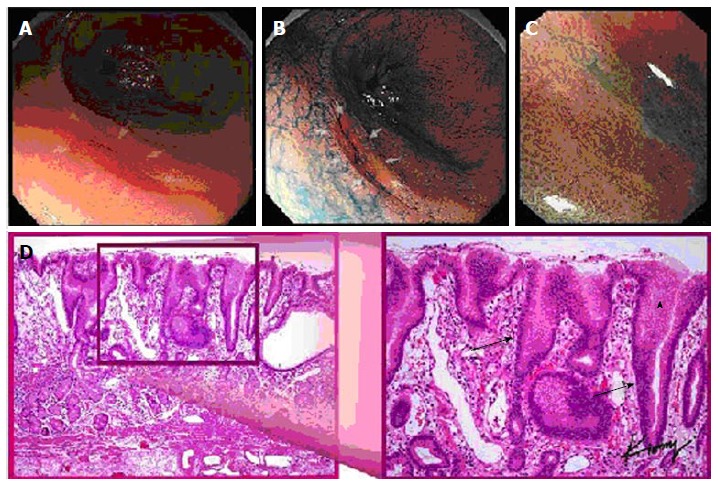
Multiple views of a signet ring cell gastric carcinoma in a single patient: (A) standard white light endoscopy, (B) chromoendoscopy, (C) magnification endoscopy, and (D) histopathology demonstrating elongated gastric glands (arrows) infiltrated with tumor cell (arrowhead).
Figure 2.
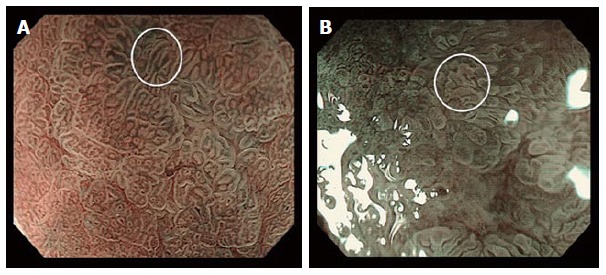
Magnification endoscopy of the stomach: (A) normal polygonal architecture (bottom left, underlying “a”) and a signet ring cell gastric carcinoma demonstrating an elongated or “stretched” gastric gland (white circle); (B) a non-signet ring cell adenocarcinoma demonstrating irregular (non-polygonal) but non-elongated glands (white circle).
Pathologic correlation
On histologic examination, patients with signet ring early gastric cancer demonstrated an expanded and edematous mucosal layer infiltrated with tumor cells (Figure 1).
DISCUSSION
In all 12 cases of signet ring-type early gastric cancer in our institution, we identified the “stretch” sign - elongation of the architecture of the submucosa. Anecdotally, we do not note any architectural elongation in our non-signet ring early gastric cancer patients.
All 12 of our signet ring early gastric cancer patients underwent either endoscopic or surgical resection and are doing well at 2.5 years median follow-up; however; the optimal treatment for this subgroup of patients has not yet been determined. The current Japanese guidelines recommend ESD for non-ulcerated pT1a undifferentiated gastric cancer with tumor size ≤ 2 cm, but while some studies have shown only a 4% rate of lymph node metastases for tumors limited to the mucosa (as compared with 92% for tumors with submucosal spread)[12].
In an animal study, signet ring cells originated from the lamina propria at the level of the gland neck and spread through the mucosal[13]. We postulate that this proliferation of signet ring cells along the lamina propria results in clusters of tumor cells, causing the “stretched” appearance of the gastric pits and microvasculature that we observe on magnification endoscopy (Figures 3 and 4).
Figure 3.
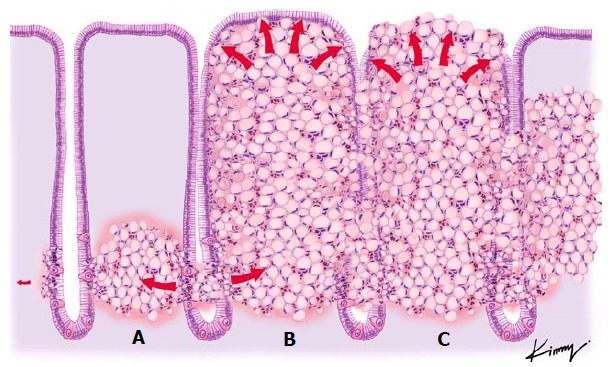
Theoretical view of the pathophysiology of signet ring cell differentiation: (A) tumor cells originating in the neck of the gland and spreading to the submucosal space; (B) an increasing number of tumor cells being packed together, resulting in a barrel shape; and (C) the previously non-exposed tumor becoming exposed through necrosis and formation of an ulcer.
Figure 4.
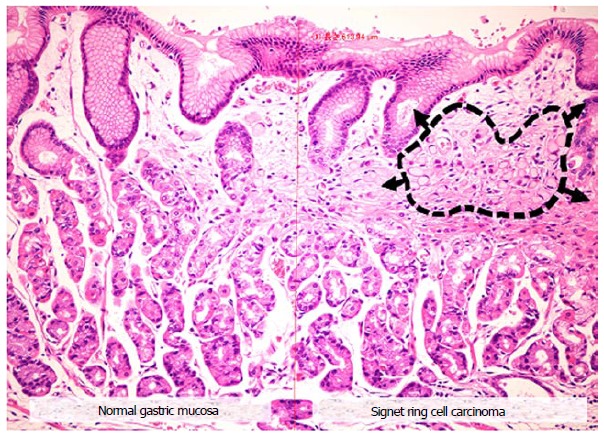
Microscopic view of a signet ring cell gastric carcinoma, demonstrating: (1) normal appearing gastric mucosa (left); and (2) signet ring cells (black dashed circle) causing distortion of the gastric glands (right), consistent with the endoscopic finding of the “stretch sign.”
Our study is limited by its retrospective design and the small numbers associated with the relative rarity of early stage signet ring cell gastric cancer.
Additional studies are needed to further identify unique microendoscopic features of signet ring cell gastric cancer and to more accurately determine the sensitivity and specificity of the “stretch sign”. Given that ESD is still considered an investigational treatment in the presence of signet ring cells due to the more aggressive biology and unfavorable prognosis[11,14], the presence of the “stretch sign” may help to identify patients with signet ring cells and perhaps guide more aggressive treatment, such as wider margins during ESD or earlier progression to formal surgical resection.
In conclusion, we found that signet ring cell carcinoma can be identified by the expansion or “stretching” of the gastric pits and microvasculature. This may allow for the diagnosis of signet ring cell carcinoma in the early stages using magnification endoscopy, reducing the impact of sampling error if random biopsies are taken, and perhaps guiding more aggressive treatment.
ACKNOWLEDGMENTS
The authors would like to thank Chananya Hokieti (“Kimmy”) for her help with the illustrations.
COMMENTS
Background
Gastric cancers are aggressive tumors of the stomach that are often asymptomatic in the early stages. By the time patients develop symptoms, the tumors are often advanced and may be incurable. Aggressive screening regimens have been introduced in countries with a high prevalence of gastric cancer, such as Japan, leading to more gastric cancers being diagnosed in the early stages.
Research frontiers
Signet ring cell carcinoma, a rare subtype of gastric cancer, is unique in its biology and progression. Compared to “standard” adenocarcinoma, patients with early stage signet ring cell carcinomas have a better prognosis; meanwhile, patients with later stage signet ring cell carcinomas have a much worse prognosis than those with adenocarcinoma. Identifying and treating signet ring cell carcinoma in its early stages is therefore critical.
Innovations and breakthroughs
Early gastric cancers can be examined with magnification endoscopes using a narrow band imaging to reveal the architecture of the most superficial layers of the stomach, the mucosa and submucosa. This reveals the shapes of the gastric glands and the organization of the tiny submucosal blood vessels. In this study the authors present 12 patients with signet ring cell carcinoma; all of the patients have unique changes to the architecture of the glands and blood vessels (specifically, “stretching”) that the authors have only seen when signet ring cells are present.
Applications
Use of the “stretch sign” during magnification endoscopy can potentially be used to identify patients who have signet ring cell carcinoma, allowing their prognosis and treatment to be tailored to the more aggressive biology of their cancer.
Terminology
Magnification endoscopes are upper endoscopes with a special tip and image processing equipment that can zoom in to see the organization of groups of cells. Narrow band imaging uses a small range of light (rather than the full “white light” spectrum) to highlight borders between normal and abnormal areas of the stomach. The “stretch sign” is the authors’ term for elongation or “stretching” of the usual architecture of the gastric glands and the tiny submucosal vessels.
Peer-review
It is a concise and easy to read paper which brings a new progress in the field of early gastric cancer diagnosis.
Footnotes
Ethics approval: This study was conducted in accordance with the ethical standards of the Helsinki Declaration of 1964, as revised in 2013. Retrospective review of pathologic specimens is exempt from formal approval according to the policies and procedures of the Institutional Review Board (IRB) of Showa University Northern Yokohama Hospital, Yokohama, Japan.
Informed consent: All study participants, or their legal guardian, provided pre-operative informed written consent for the endoscopic or surgical procedures, as well as the collection and analysis of pathologic specimens.
Conflict-of-interest: Dr. Inoue has received fees for serving as an advisory committee member for Olympus Corporation, Tokyo, Japan. The other authors have no conflicts of interest or financial ties to disclose.
Data sharing: Technical appendix and dataset are available from the corresponding author at haruinoue777@yahoo.co.jp. Consent was not obtained but the presented data are anonymized and the risk of identification is low.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: September 11, 2014
First decision: November 14, 2014
Article in press: April 7, 2015
P- Reviewer: Kashida H, Osian G S- Editor: Song XX L- Editor: A E- Editor: Wu HL
References
- 1.Maruyama K, Kaminishi M, Hayashi K, Isobe Y, Honda I, Katai H, Arai K, Kodera Y, Nashimoto A. Gastric cancer treated in 1991 in Japan: data analysis of nationwide registry. Gastric Cancer. 2006;9:51–66. doi: 10.1007/s10120-006-0370-y. [DOI] [PubMed] [Google Scholar]
- 2.Antonioli DA, Goldman H. Changes in the location and type of gastric adenocarcinoma. Cancer. 1982;50:775–781. doi: 10.1002/1097-0142(19820815)50:4<775::aid-cncr2820500425>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 3.Otsuji E, Yamaguchi T, Sawai K, Takahashi T. Characterization of signet ring cell carcinoma of the stomach. J Surg Oncol. 1998;67:216–220. doi: 10.1002/(sici)1096-9098(199804)67:4<216::aid-jso2>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 4.Kim JP, Kim SC, Yang HK. Prognostic significance of signet ring cell carcinoma of the stomach. Surg Oncol. 1994;3:221–227. doi: 10.1016/0960-7404(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 5.Calès P, Oberti F, Delmotte JS, Baslé M, Casa C, Arnaud JP. Gastric mucosal surface in cirrhosis evaluated by magnifying endoscopy and scanning electronic microscopy. Endoscopy. 2000;32:614–623. doi: 10.1055/s-2000-9019. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez S. Red-flag technologies in gastric neoplasia. Gastrointest Endosc Clin N Am. 2013;23:581–595. doi: 10.1016/j.giec.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Nagahama T, Yao K, Maki S, Yasaka M, Takaki Y, Matsui T, Tanabe H, Iwashita A, Ota A. Usefulness of magnifying endoscopy with narrow-band imaging for determining the horizontal extent of early gastric cancer when there is an unclear margin by chromoendoscopy (with video) Gastrointest Endosc. 2011;74:1259–1267. doi: 10.1016/j.gie.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Yagi K, Saka A, Nozawa Y, Nakamura A, Umezu H. Prediction of submucosal gastric cancer by narrow-band imaging magnifying endoscopy. Dig Liver Dis. 2014;46:187–190. doi: 10.1016/j.dld.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Kikuchi D, Iizuka T, Hoteya S, Yamada A, Furuhata T, Yamashita S, Domon K, Nakamura M, Matsui A, Mitani T, et al. Usefulness of magnifying endoscopy with narrow-band imaging for determining tumor invasion depth in early gastric cancer. Gastroenterol Res Pract. 2013;2013:217695. doi: 10.1155/2013/217695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yao K, Oishi T, Matsui T, Yao T, Iwashita A. Novel magnified endoscopic findings of microvascular architecture in intramucosal gastric cancer. Gastrointest Endosc. 2002;56:279–284. doi: 10.1016/s0016-5107(02)70194-6. [DOI] [PubMed] [Google Scholar]
- 11.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 12.Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, Kato Y. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–225. doi: 10.1007/pl00011720. [DOI] [PubMed] [Google Scholar]
- 13.Sugihara H, Hattori T, Imamura Y, Noriki S, Fukuda M, Katsura K, Tsuchihashi Y, Fujita S. Morphology and modes of cell proliferation in earliest signet-ring-cell carcinomas induced in canine stomachs by N-ethyl-N’-nitro-N-nitrosoguanidine. J Cancer Res Clin Oncol. 1991;117:197–204. doi: 10.1007/BF01625425. [DOI] [PubMed] [Google Scholar]
- 14.Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, Fujisaki J, Sano T, Yamaguchi T. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148–152. doi: 10.1007/s10120-009-0515-x. [DOI] [PubMed] [Google Scholar]


