Abstract
Objective:
To assess the role of social risk factors on adherence to tyrosine kinase inhibitors (TKI) therapy in chronic myeloid leukemia (CML) patients.
Methods:
This is a retrospective study and eligible patients were adults with CML on TKI treatment. Cases of no adherence to treatment were confirmed during pharmacists’ consultation (patient-reported adherence). Baseline characteristics between groups were compared between cases and controls groups. Risk factors identified in bivariate analysis (p<0.2) were included in multivariate model. A qualitative investigation assessed whether such predictors of non-adherence had causal relationship.
Results:
Of 151 patients with CML consulted by pharmacists, 21% had adherence problems. Despite patients with secondary school (p=0.03), most of investigated social risk factors did not differ between groups. However, by using a qualitative approach, patients’ level of education could not explain low adherence rates behavior.
Conclusions:
Social determinants of health, herein investigated, were unlikely to predict adherence to treatment. Regression techniques may lead to untrue statements, so future researches should consider investigating the causes, not only the statistical estimates.
Keywords: Medication Adherence, Risk Factors, Leukemia, Myeloid, Protein Kinase Inhibitors, Qualitative Research, Brazil
INTRODUCTION
Chronic Myeloid Leukemia (CML) accounts for almost 20% of all adult blood malignancies. The introduction of Tyrosine Kinase Inhibitors (TKI) to treat CML have changed its natural disease history.1 In TKI era, adult patients are likely to live long periods, and as much as 85% of the young adults diagnosed with CML survive more than five years.2 Herein, some studies advocate that adherence to TKI drugs are related to improved clinical outcomes, such as complete or major molecular response (defined, respectively, as undetectable BCR-ABL gene transcription products after 2 blood samples or 3 log reduction from baseline levels shown at diagnosis).3,4
Non-adherence to TKI can be considered a public health problem, and achieving adequate drug intake rates should be promoted. CML acute phase5 resembles other preventable acute medical urgencies, such as myocardial infarction in uncontrolled hypertensive patients, or type 2 Diabetes mellitus induced nephropathy.6,7 Blast crisis resulted by incorrect TKI use have certainly led to unnecessary economic and social expenditures. Therefore, clinical pharmacy services can play an important role to improve adherence rates8, but in CML, only few reports have demonstrated such benefit.9
Notwithstanding, it is widely known that adherence to pharmacological treatment should not be solely analyzed as one binary variable, because adherence is also determined by patients’ social and economic status.10 According to World Health Organization (WHO), five dimensions can affect adherence to treatment: Health System Factors, Condition-related Factors, Therapy-Related Factors, Patient-Related Factors and Social/Economic Status.10
Regarding social and economic influence, previous publications suggested that such determinants of health could not be considered independent predictors of non-adherence.10 On the other hand, important studies on CML and adherence – such as the ADAGIO study – suggested the opposite11, so age, sex and employment status could predict adherence to TKI. In this conflicting scenario, one contribution to better understand the role of social and economic status on low adherence rates would certainly improve patient care process and further researches.
Given the importance of TKI adherence to CML patients’ outcomes, and that social factors are poorly investigated in international literature, the present paper aimed to explore the role of social and economic determinants of adherence on TKI therapy.
METHODS
Study Design and Inclusion Criteria
This is a retrospective study conducted in an ambulatory care setting. All adult CML patients were included in this research if: using TKI therapy (imatinib, dasatinib and nilotinib) and were consulted by clinical pharmacists in 2014. Patients were excluded if they were on blast crisis, presence of Philadelphia gene mutations (i.e.: T315I mutation, resistant to all TKI) and those who refused to participate.
Setting
This study was conducted in one hematology reference hospital in Curitiba / Brazil, where 300 patients receive TKI therapy every year
Every Monday, the Ambulatory Pharmaceutical Care service provides adherence consultations and counseling sessions to all CML patients, before physicians’ consultations. Therefore, on Fridays, clinical pharmacists assess patients that will have consultations on the next Monday, by collecting medication history, assessing clinical and laboratorial data, social and allergy histories.
This clinical documentation review aims to identify patients at: (a) risk of non-adherence due to adverse reactions; (b) potential drug interactions; (c) contraindications; (d) need for renal dose adjustments; (e) physicians’ perception of low adherent patients; (f) hematological and fluctuating molecular response and their possible relation with non-adherence; (g) detecting patients that are starting TKI therapy.
All medication-related problems found – including adherence problems – were registered at patients’ clinical documentations (hospital’s formulary and one specific chart for pharmacist-driven CML patients’ consultations and follow up), and discussed with experienced Hematology Medical Doctors (MD). The product of this discussion is a collaborative intervention towards better adherence rates, by providing information on potential drugs to avoid with TKI, adjusting their dose according to daily activities, counseling on what to do if missing one dose, managing adverse drug reactions and changing any drug therapy according to patients’ needs.
Definitions of TKI Non-adherence
Cases of non-adherence to treatment were suspected by clinical pharmacists during chart review (on Fridays) and confirmed at consultation (on Mondays). During consultations, clinical pharmacists confronted patients with their molecular response (BCR/ABL ratios) by asking: “we observed a good tendency in your molecular response (BCR/ABL), and suddenly you started to lose it.” “Did you miss any doses?” “Do you know what were reasons to miss those doses?” Within these simple questions, we considered that our cases of non-adherence were assumed non-adherents and gave directions to individualized counseling. Other drug therapy problems identified on Fridays were also addressed with MD staff.
On the other hand, “controls” (adherents) were patients with optimal BCR/ABL ratio: sustained major or complete molecular response. Patients who did not assume a non-adherence condition, even after confronting them against fluctuating levels of BCR/ABL transcripts, were not considered adequate controls and did not fulfilled inclusion criteria from this study.
More information about not using a validated tool to assess adherence were detailed at discussion session.
Assessed Risk Factors
We defined demographic and social factors according to World Health Organization guideline.10 The studied social and demographic factors were: age, sex, educational level, employment status, presence of supportive care (presence of one caregiver) and distance to treatment center.
Other important social factors that could predict non-adherence were history of alcohol and tobacco use. Patients’ income was not quantified because our Health System (Sistema Único de Saúde) provides TKI therapy for all CML Brazilian citizens. Moreover, some of them have their transport paid by public system, so including “income” as variable could lead to untrue assessments.
Data Analysis: Quantitative and Qualitative
We conducted two analyses: one to determine statistical relation between risk factors and adherence, and a second, to assess if such variables could qualitatively explain an association.
Therefore, we quantitatively explored data by dividing patients in cases (non-adherence) and controls (adherence). All variables were compared between groups by using independent t-test, chi-square and Fisher test, as adequate. Risk factors identified in this bivariate analysis (p<0.2) were included in a multivariable model, whereby we applied a logistic regression to investigate the ability of one variable to predict non-adherence in CML patients.
In the logistic regression analysis, non-adherence was set as dependent variable and all analysis were conducted with a specialized software (SPSS v.20). Statistical significance was determined according to 95% Confidence Interval (95%CI) and by rejecting null hypothesis when p-value was lower than 5%.
Additionally, we conducted a qualitative investigation (subgroup analysis) to assess whether such independent predictors of non-adherence – found with the aforementioned quantitative analysis – were robust and could have causal relations. Thus, risk factors determined by logistic regression (quota sampling determined the subjects of study) were reassessed by using pharmacists’ clinical interview records.12,13 After assessing the documented reasons to not adhere to TKI, we transcribed them into affinity themes. In other words, the reasons for non-adherence were grouped as: incorrect administration, dose omission, etc. Those affinity themes were reanalyzed and grouped into same association characteristics, such as: adverse events that led to non-adherence, forgot to bring to consultations, etc. After analyzing each association of non-adherence, repeated association were excluded, as it is not an object of qualitative analysis. All remaining association of non-adherence was fully described.
Ethics
This study was approved by the Local Bioethics Committee and complies with Helsinki’s Declaration. All included patients signed a consent term and agreed to participate in this study.
RESULTS
In 2014, of 300 patients treating CML with TKI, 151 (about 50%) patients were consulted by pharmacists. The prevalence of TKI adherence problems was 21% (n=32). In general, variables between groups were homogeneous (p>0.05). Most of investigated social and demographic factors did not differ between TKI adherence and non-adherence groups (Table 1). Exceptions were physical activity status, BMI and high school level of education, which were considered critical factors (p<0.2) and were included to multivariable analysis (Table 2).
Table 1.
Baseline Characteristics.
| Variables | TKI Adherence N=119 | TKI non-adherence N=32 | p-value |
|---|---|---|---|
| Gender, male (%) | 72 (60.5) | 17 (53.1) | 0.45 |
| Age, years (sd) | 52 (15.3) | 51 (18.7) | 0.73 |
| Physical Activity | |||
| Active* (%) | 35 (29.4) | 5 (15.6) | 0.17 |
| BMI, kg/m2 (sd) | 26.98 (4.37) | 28.37 (3.7) | 0.10 |
| Geographic Adscription and Access to Health System | |||
| Curitiba (%) | 37 (31.1) | 11 (34.4) | 0.72 |
| Metropolitan Region (%) | 17 (14.3) | 6 (18.7) | 0.53 |
| Paraná (%) | 37 (31.1) | 11 (34.4) | 0.72 |
| Other State (%) | 28 (23.5) | 4 (12.5) | 0.23 |
| Occupation / Autonomy | |||
| Retired (%) | 53 (44.5) | 15 (46.9) | 0.81 |
| Caregiver Dependent (%) | 14 (11.8) | 3 (9.4) | 0.77 |
| Other Life Habits | |||
| Alcohol Use (%) | 13 (10.9) | 3 (9.4) | 1.00 |
| Never Smoked (%) | 95 (79.8) | 25 (78.1) | 0.83 |
| Education Level | |||
| Incomplete Basic School (%) | 45 (37.8) | 14 (43.8) | 0.54 |
| Basic School (%) | 11 (9.2) | 4 (12.5) | 0.59 |
| High School (%) | 42 (35.3) | 5 (15.6) | 0.03 |
| College (%) | 21 (17.6) | 5 (15.6) | 0.79 |
TKI (Tyrosine Kinase Inhibitor), sd (standard deviation)
(physical activity was defined as walking or other prescribed exercise by physical therapists) and BMI (Body Mass Index).
Table 2.
Multivariable Model and Included Variables.
| Variable | p-value | OR | 95% CI |
|---|---|---|---|
| BMI | 0.040 | 1.108 | 1.005 - 1.221 |
| Physical Activity | 0.059 | 2.904 | 0.961 - 8.776 |
| High School | 0.035 | 3.088 | 1.086 - 8.785 |
BMI (Body Mass Index), OR (odd ratio), 95% CI (95% Confidence Interval).
By including three critical variables (p<0.2) to a multivariable model, only BMI and high school were considered risk factors to non-adherence. We observed that every 1 point increase in BMI, there was a 10% increased risk of TKI non-adherence; and patients with complete high school level of education had three times more chance of being non-adherent (Table 2). On the other hand, physical activity could not be interpreted as critical factor to non-adherence to TKI.
Qualitative Analysis
Initially, we explored 34 clinical registries made by pharmacists. Considering risk factors determined by logistic regression, we identified 24 patients that had BMI>25 kg/m2, ten who had high school level of education and eight that had both risk factors (Figure 1).
Figure 1.
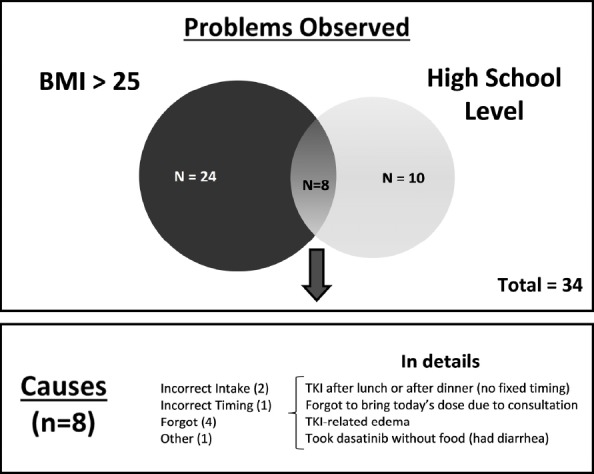
Problems and Causes of No Adherence to TKI Therapy.
In those 34 patients, we grouped the main reasons to not adhere and defined six themes: incorrect drug, incorrect intake, incorrect timing, discontinuation, forgot to take, and other less common causes for non-adherence. By analyzing the reasons to not adhering to TKI therapy in patients with both risk factors, we identified two scenarios: lack of organization (forgot to bring to consultation or forgot to bring to work) and TKI-related adverse events were the most important causes of non-adherence (patient experienced edema or had intense diarrhea because took dasatinib without food) (Figure 1).
DISCUSSION
According to our main findings, every five patients using TKI, one will experience adherence problems, which are unlikely to be associated or predicted by one of the studied social determinants. In other words, qualitative findings of non-adherence could not be explained by quantitative results (risk factors). Therefore, high BMI values and completion of high school could not explain a non-adherence behavior due to lack of organization or an adverse reaction. By counseling patients to modify or prevent some risky situations, such as taking dasatinib with food to avoid diarrhea, promptly improved adherence status.
Furthermore, we found a poor linear correlation between social determinants and adherence to treatment, which was already addressed before.10 On the other hand, some studies on CML patients advocated that there was an association between social risk factors and adherence based only on statistical significance.11,14
Curiously, one review16 identified three studies that assessed demographic or social predictors of adherence. The results are conflicting, so both higher age11 and lower age (<50 years old)14,15 could predict low adherence rates. Other non-sense results include: living alone11, male sex11, secondary school level of education, – or even higher levels (post-graduate studies)11 – could paradoxically be related to low adherence rates. Unfortunately, no further investigations were conducted to assess whether such statistical findings are reliable or clinically important.
Herein, we walked through this scientific gap so this study explored and tried to clarify such inconsistencies. We found that “high school level of education” was an independent risk factor, but there is no dose-response relationship between this variable and adherence to treatment. In other words, neither lower levels of education were associated with lower adherence rates, nor college level education was associated with higher rates of adherence. As fact, education has a role on adherence to treatment17,18, especially on patient’s understanding about importance for TKI adherence and disease progression, misconceptions, concerns about adverse reactions, etc. However, there is no possibility for generalizations and numerical relations have been leading to superficial statements.11
Finally, by conducting a study that reduced adherence as binary outcome would only reproduce previous inconclusive results11,14,15,16, so qualitative methods were essential to illustrate what were the uninvestigated issues that could be related to non-adherence: daily organization and adverse events.
Adherence to Treatment: Focus on Daily Organization and Adverse Events Management
As aforementioned, we identified four situations found in non-adherent patients:
Lack of personal organization made patients forget to bring TKI to medical consultations and may happen in other contexts, such as traveling and sudden routine changes.
In some patients, there were not fixed timing to take imatinib (once daily administration), especially because one forgets to bring to work and, eventually, takes it after lunch or before dinner. Fortunately, this patient did not experience an adverse event by taking it without meal at night, and taking again in the morning. At counseling sessions, we promoted a daily organization to improve intake adequacy and adequate TKI serum levels.
TKI-related adverse reactions influenced adherence to treatment, whereby some were avoidable and needed prompt intervention – for example, when he forget to take imatinib with food and had diarrhea. With these patients, we demonstrated the problem & effect of taking that TKI without meals, which led to an undesired diarrhea. We could change this behavior and possibly prevented future TKI holidays or dose omissions.
Finally, edema is expected to happen with TKI use and treatment is adapted according to patients’ clinical specificities. In one case, edema was solved by changing calcium channel blocker drug (amlopidine) to a thiazide-based antihypertensive therapy.
Some practical assignments learned from the aforementioned examples were already discussed in international literature.17,18,19,21 Herein, to prevent non-adherence behavior, we suggest the following considerations:
There is a need to improve the quality of information exchanged between Health Care Providers (HCP) and patients. There is evidence that elucidation of misconceptions about disease and TKI may promote better adherence to treatment.18 Other studies suggest that counseling based on the following topics could be related to improved outcomes: explaining treatments’ purposes, the importance of adherence, some of the expected adverse reactions and their management, what to do if missing one dose, performing feedback and other issues17,20;
Quality of social support: in case of elderly patients, instead of one passive caregiver, the presence of a dedicated person would certainly help to improve the correct use of medications17;
Counseling according to patients’ own timing is one way to individualize CML care. Patients may be in different stages of live-disease comprehension and some theoretical models suggest that HCP and patients may exchange information respecting their stage of: crisis, hope, adaptation or uncertainty regarding TKI therapy and CML.19
It is important to say that all three TKI may have different administration instructions (nilotinib should be taken without food, while dasatinib and imatinib are commonly prescribed once a day) but similar adverse events profile: leg edema, gastrointestinal complaints, risk of hepatotoxicity, myelotoxicity and dermatologic reactions.21 Despite these characteristics were important to TKI-related diarrhea in patients taking imatinib without food, previous research have not investigated if there were different rates of adherence between drugs.21 Therefore, future studies could investigate the impact of each side effect profile on adherence rates.
The Role of Social Determinants of Health and Adherence to TKI
Statistical assumptions cannot solely predict how social determinants can influence adherence to treatment: deeper discussions are needed, as we observed with this study.
When assessing levels of education, researchers should take into account that patients could have received different quality of education, and social responsibility, clinical understanding and willingness to improve their health are variables that are not a routine or easy to study.11,14 Herein, patients’ own beliefs regarding health literacy and importance to adhere to treatment cannot be simply dichotomized, categorized into scales, or reliably counted.
Social determinants of adherence should be individualized according to every situation. Statistical regression techniques may lead to generalized statements that mask patients’ needs and opportunities for HCP to solve one problem. Notwithstanding, HCP do not assume previous researches on TKI and adherence as applicable to daily clinical practice. Thus, likewise CML HCP, researchers should qualitatively investigate the reasons of non-adherence and convert all efforts to understand how social and economic status have been impacting public health and every patient with CML. We believe the aforementioned arguments and recently published researches11,14 corroborate with the idea that adherence problems and social determinants of health could be elucidated with other investigation approaches.22,23,24
Limitations
This study has several limitations. First of all, this is a retrospective study and clinical registries may not be adequately documented, so essential information could be missed; even if researchers were involved with data collection and patient care. Still on study’s design, we preferred not to use well-known adherence scales, because their accuracy is still undetermined to CML patients. Moreover, Possession Ratio did not apply for our setting [3], so pharmacists’ clinical consultations and patient-reported adherence were used. It is important to say that in our institution, patients may receive it directly from clinical trials sponsors, so there were no dispensation registries in the service that could feasibly ensure patient’s TKI possession. Moreover, we did not apply any specific technology or electronic device that could count the number of TKI taken from recipient. On the other hand, our controls (assumed to adhere) may be non-adherents. Although we tried to attenuate this fact by confronting their answer with BCR/ABL fluctuations, but such approach still is not a validated method. It is important to say that no clinical guidelines promote the use of adherence tools, so its applicability to CML patient care still is unknown.21
Other confounders10,11,24 that affect adherence were poorly investigated, and these could have influenced our findings. We tried to include most of relevant social determinants published before11 to reproduce in a study focused on exploring adherence and social determinants. For example: one of the variables that were not assessed – income – was assumed to be irrelevant in this manuscript due to Health Systems’ characteristics: free access to products and services, including transportation to health care centers. However, it is likely that opportunity costs can impact patients’ lives, so that should be addressed in future studies.
Lastly, as interaction between variables was mostly investigated in international literature only by statistical techniques, we do not know how each social determinant influence adherence. Moreover, there still is only a few researches that explored qualitative methods22,23,24 and some assumptions are still theoretical in our study and other publications19, but we believe that further researches will benefit from our findings and discussion.
CONCLUSIONS
Social and demographic determinants of health, which are presumably not quantifiable, were unlikely to predict adherence to treatment.
Notwithstanding, independent risk factors herein investigated, impacted adherence to TKI therapy. Thus, a qualitative approach22,23,24 would significantly contribute for assessing and addressing interventions to improve adherence in CML patients.
Lastly, regression techniques may lead to untrue statements, so future researches should consider investigating more the associations between risk factors and adherence behaviors, and less about rates of adherence.
Footnotes
CONFLICT OF INTEREST
We declare not having any conflicts of interests and there were no funding sources for this project.
Contributor Information
Lucas M. Okumura, Pharmacy Department, Clinical Hospital, Federal University of Paraná. Curitiba, PR (Brazil). lucasokumura@yahoo.com.br
Valquíria D. Antunes, Pharmacy Department, Clinical Hospital, Federal University of Paraná. Curitiba, PR (Brazil). Antunes.vdc@gmail.com
Karina S. Aguiar, Pharmacy Department, Clinical Hospital, Federal University of Paraná. Curitiba, PR (Brazil). karinasilva.aguiar@gmail.com
Tatiane Farias, Pharmacy Department, Clinical Hospital, Federal University of Paraná. Curitiba, PR (Brazil). tatifernandes_1@hotmail.com.
Vânia M. Andrzejevski, Pharmacy Department, Clinical Hospital, Federal University of Paraná. Curitiba, PR (Brazil). salvivania@gmail.com
Vaneuza M. Funke, Hematopoietic Stem Cell Transplantation Division, Clinical Hospital, Federal University of Paraná. Curitiba, PR (Brazil). vaneuzamf@uol.com.br
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics 2014. CA Cancer J Clin. 2014;64(1):9–29. doi: 10.3322/caac.21208. doi:10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Brunner AM, Campigotto F, Sadrzadeh H, Drapkin BJ, Chen YB, Neuberg DS, Fathi AT. Trends in all-cause mortality among patients with chronic myeloid leukemia: a Surveillance, Epidemiology, and End Results database analysis. Cancer. 2013;119(14):2620262–2620269. doi: 10.1002/cncr.28106. doi:10.1002/cncr.28106. [DOI] [PubMed] [Google Scholar]
- 3.de Almeida MH, Pagnano KB, Vigorito AC, Lorand-Metze I, de Souza CA. Adherence to tyrosine kinase inhibitor therapy for chronic myeloid leukemia: a Brazilian single-center cohort. Acta Haematol. 2013;130(1):16–22. doi: 10.1159/000345722. doi:10.1159/000345722. [DOI] [PubMed] [Google Scholar]
- 4.Hirji I, Gupta S, Goren A, Chirovsky DR, Moadel AB, Olavarria E, Victor TW, Davis CC. Chronic myeloid leukemia (CML): association of treatment satisfaction, negative medication experience and treatment restrictions with health outcomes, from the patient's perspective. Health Qual Life Outcomes. 2013;11:559. doi: 10.1186/1477-7525-11-167. doi:10.1186/1477-7525-11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jabbour E, Kantarjian H, O’Brien S, Rios MB, Abruzzo L, Verstovsek S, Garcia-Manero G, Cortes J. Sudden blastic transformation in patients with chronic myeloid leukemia treated with imatinib mesylate. Blood. 2006;107(2):480–482. doi: 10.1182/blood-2005-05-1816. [DOI] [PubMed] [Google Scholar]
- 6.Kauf TL, Velazquez EJ, Crosslin DR, Weaver WD, Diaz R, Granger CB, McMurray JJ, Rouleau JL, Aylward PE, White HD, Califf RM, Schulman KA. The cost of acute myocardial infarction in the new millennium: evidence from a multinational registry. Am Heart J. 2006;151(1):206–212. doi: 10.1016/j.ahj.2005.02.028. [DOI] [PubMed] [Google Scholar]
- 7.Nichols GA, Vupputuri S, Lau H. Medical care costs associated with progression of diabetic nephropathy. Diabetes Care. 2011;34(11):2374–2378. doi: 10.2337/dc11-0475. doi:10.2337/dc11-0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santoleri F, Sorice P, Lasala R, Rizzo RC, Costantini A. Patient adherence and persistence with Imatinib, Nilotinib, Dasatinib in clinical practice. PLoS One. 2013;8(2):559. doi: 10.1371/journal.pone.0056813. doi:10.1371/journal.pone.0056813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Darkow T, Henk HJ, Thomas SK, Feng W, Baladi JF, Goldberg GA, Hatfield A, Cortes J. Treatment interruptions and non-adherence with imatinib and associated healthcare costs: a retrospective analysis among managed care patients with chronic myelogenous leukaemia. Pharmacoeconomics. 2007;25(6):481–496. doi: 10.2165/00019053-200725060-00004. [DOI] [PubMed] [Google Scholar]
- 10.Sabaté E, editor. Geneva, Switzerland: World Health Organization; 2003. Adherence to Long-Term Therapies: Evidence for Action. [Google Scholar]
- 11.Noens L, van Lierde MA, De Bock R, Verhoef G, Zachée P, Berneman Z, Martiat P, Mineur P, Van Eygen K, MacDonald K, De Geest S, Albrecht T, Abraham I. Prevalence, determinants, and outcomes of non-adherence to imatinib therapy in patients with chronic myeloid leukemia: the ADAGIO study. Blood. 2009;113(22):5401–5411. doi: 10.1182/blood-2008-12-196543. doi:10.1182/blood-2008-12-196543. [DOI] [PubMed] [Google Scholar]
- 12.Nkwi P, Nyamongo I, Ryan G. Washington, DC: UNESCO; 2001. [accessed 3 May 2015]. [internet] Field Research into Social Issues: Methodological Guidelines. Available at: http://www.ccs.neu.edu/course/is4800sp12/resources/qualmethods.pdf . [Google Scholar]
- 13.Pope C, Mays N. London: BMJ Books; 2000. Qualitative Research in Health Care. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marin D, Bazeos A, Mahon FX, Eliasson L, Milojkovic D, Bua M, Apperley JF, Szydlo R, Desai R, Kozlowski K, Paliompeis C, Latham V, Foroni L, Molimard M, Reid A, Rezvani K, de Lavallade H, Guallar C, Goldman J, Khorashad JS. Adherence is the critical factor for achieving molecular responses in patients with chronic myeloid leukemia who achieve complete cytogenetic responses on imatinib. Clin Oncol. 2010;28(14):2381–2388. doi: 10.1200/JCO.2009.26.3087. doi:10.1200/J339.2009.26.3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.StCharles M, Bollu VK, Hornyak E, Coombs J, Blanchette CM, DeAngelo DJ. Predictors of treatment non-adherence in patients treated with imatinib mesylate for chronic myeloid leukemia. Blood (ASH Annual Meeting Abstracts) 2009;114:2209. [Google Scholar]
- 16.Jabbour EJ, Kantarjian H, Eliasson L, Cornelison AM, Marin D. Patient adherence to tyrosine kinase inhibitor therapy in chronic myeloid leukemia. Am J Hematol. 2012;87(7):687–691. doi: 10.1002/ajh.23180. doi:10.1002/ajh.23180. [DOI] [PubMed] [Google Scholar]
- 17.Efficace F, Baccarani M, Rosti G, Cottone F, Castagnetti F, Breccia M, Alimena G, Iurlo A, Rossi AR, Pardini S, Gherlinzoni F, Salvucci M, Tiribelli M, Vignetti M, Mandelli F. Investigating factors associated with adherence behaviour in patients with chronic myeloid leukemia: an observational patient-centered outcome study. Br J Cancer. 2012;107(6):904–909. doi: 10.1038/bjc.2012.348. doi:10.1038/bjc.2012.348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen LC, Chen TC, Huang YB, Chang CS. Disease acceptance and adherence to imatinib in Taiwanese chronic myeloid leukaemia outpatients. Int J Clin Pharm. 2014;36(1):120–127. doi: 10.1007/s11096-013-9867-8. doi:10.1007/s11096-013-9867-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guilhot F, Coombs J, Szczudlo T, Zernovak O, Paolantonio M, Bender C, Macdonald NJ, Shapiro A. The patient journey in chronic myeloid leukemia patients on tyrosine kinase inhibitor therapies: qualitative insights using a global ethnographic approach. Patient. 2013;6(2):81–92. doi: 10.1007/s40271-013-0006-3. doi:10.1007/s40271-013-0006-3. [DOI] [PubMed] [Google Scholar]
- 20.Okumura LM, Rotta I, Correr CJ. Assessment of pharmacist-led patient counseling in randomized controlled trials: a systematic review. Int J Clin Pharm. 2014;36(5):882–891. doi: 10.1007/s11096-014-9982-1. doi:10.1007/s11096-014-9982-1. [DOI] [PubMed] [Google Scholar]
- 21.O’Brien S, Radich JP, Abboud CN, Akhtari M, Altman JK, Berman E, Curtin P, DeAngelo DJ, Deininger M, Devine S, Fathi AT, Gotlib J, Jagasia M, Kropf P, Moore JO, Pallera A, Reddy VV, Shah NP, Smith BD, Snyder DS, Wetzler M, Gregory K, Sundar H. Chronic myelogenous leukemia, version 1.2015. J Natl Compr Canc Netw. 2014;1610;12(11):1590. doi: 10.6004/jnccn.2014.0159. [DOI] [PubMed] [Google Scholar]
- 22.Wu S, Chee D, Ugalde A, Butow P, Seymour J, Schofield P. Lack of congruence between patients’ and health professionals’ perspectives of adherence to imatinib therapy in treatment of chronic myeloid leukemia: A qualitative study. Palliat Support Care. 2015;13(2):255–263. doi: 10.1017/S1478951513001260. doi:10.1017/S1478951513001260. [DOI] [PubMed] [Google Scholar]
- 23.Guilhot F, Coombs J, Szczudlo T, Zernovak O, Paolantonio M, Bender C, Macdonald NJ, Shapiro A. The patient journey in chronic myeloid leukemia patients on tyrosine kinase inhibitor therapies: qualitative insights using a global ethnographic approach. Patient. 2013;6(2):81–92. doi: 10.1007/s40271-013-0006-3. doi:10.1007/s40271-013-0006-3. [DOI] [PubMed] [Google Scholar]
- 24.Malbasa T, Kodish E, Santacroce SJ. Adolescent adherence to oral therapy for leukemia: a focus group study. J Pediatr Oncol Nurs. 2007;24(3):139–151. doi: 10.1177/1043454206298695. [DOI] [PubMed] [Google Scholar]


