Abstract
Background
Neoplastic transformation of damaged astrocytes has been proposed as a possible pathological mechanism behind malignant astrocytic tumors. This study investigated the association between structural brain injuries causing reactive astrogliosis and long-term risk for malignant astrocytic tumors.
Methods
The cohort consisted of all individuals living in Denmark between 1978 and 2011. The personal identification number assigned to all individuals allowed retrieval of diagnoses of traumatic brain injury, cerebral ischemic infarction, and intracerebral hemorrhage from the National Patient Discharge Register. Diagnoses of anaplastic astrocytoma and glioblastoma multiforme (World Health Organization grades III and IV) were retrieved from the Danish Cancer Registry. Rate ratios (RR's) were estimated using log-linear Poisson regression.
Results
In a cohort of 8.2 million individuals, 404 812 experienced a structural brain injury and 6152 developed a malignant astrocytic tumor. No significant association was observed 1–4 years after a structural brain injury (RR = 1.14; 95% CI: 0.87–1.46), whereas the long-term (5+ y) risk for malignant astrocytic tumors was significantly reduced (RR = 0.68; 95% CI: 0.49–0.90) compared with no injury. The specific long-term risks by type of injury were: traumatic brain injury RR = 0.32 (95% CI: 0.10–0.75); cerebral ischemic infarction RR = 0.69 (95% CI: 0.47–0.96); and intracerebral hemorrhage RR = 1.39 (95% CI: 0.64–2.60).
Conclusion
We found no evidence for an association between structural brain injury and malignant astrocytic tumors within the first 5 years of follow-up. However, our study indicated a protective effect of astrogliosis-causing injuries 5 or more years after structural brain injury.
Keywords: anaplastic astrocytoma, astrogliosis, brain injury, epidemiology, glioblastoma
The highly aggressive malignant astrocytic tumors are the most common intra-axial, primary brain tumors. They typically occur in middle-aged people, with a peak incidence at 41 years for anaplastic astrocytoma (World Health Organization [WHO] grade III) and 53 years for glioblastoma multiforme (WHO grade IV).1 In 90%–95% of the cases, grade IV astrocytic tumors develop de novo without precursor lesions and with short clinical histories, whereas the rest develop more slowly due to malignant transformation/progression of lower-grade astrocytomas.2
Neoplastic transformation of damaged astrocytes has been proposed as a possible pathological mechanism based on case reports of glioblastomas occurring up to 20 years later at the site of traumatic brain injuries.3–10 A population-based study by Inskip et al11 did find a slightly increased overall incidence of intracranial tumors, whereas no association was found specifically for malignant astrocytic tumors (glioblastoma and neoplastic astrocytoma). In a later population-based cohort study by Nygren et al,12 no overall association between traumatic brain injuries and intracranial tumors was found; however, the specific risks for malignant astrocytic tumors were not reported.12 Most recently, a population-based study from Taiwan13 found an increased risk for not otherwise specified malignant brain tumors within 3 years following a traumatic brain injury (hazard ratio: 4.67; 95% CI: 1.84–11.83). Very few case-control studies specifically reported the risk for malignant astrocytoma/glioma after a head trauma, and the findings are equivocal with null associations14,15 and positive associations.16,17 No epidemiological studies could be found in the literature investigating the risk for malignant astrocytic tumors after cerebral ischemic infarction or intracerebral hemorrhage.
Experimental studies have shown that after a structural brain injury, reactive astrocytes play a major role in the localized inflammatory response leading to astrogliosis and glial scar formation.18–20 When damage to the brain parenchyma occurs, reactive astrocytes proliferate, change morphology, and migrate to the site of injury, where they may promote or inhibit the generation of neurons from neural stem cells and progenitor cells.21,22 Periventricular neural stem cells, some of which give rise to glia cells such as astrocytes and oligodendrocytes, are also activated in astrogliosis as a part of the repair mechanism in structural brain injury.19 Both reactive astrocytes and periventricular neural stem cells express glial fibrillary acidic protein,23 which is also expressed in most astocytic tumors, of which glioblastoma is by far the most common and most studied subtype. Glioblastomas are believed to arise largely from cells of astrocytic origin and from oligodendrocyte progenitor cells, dependent on the subtypes.23,24
Based on the conflicting results in the literature, we tested the hypothesis that any condition causing astrogliosis, which includes any type of structural brain injury causing damage to the brain parenchyma, may be associated with long-term risk specifically for malignant astrocytic tumors. Specifically, we investigated the potential association between structural brain injury caused by trauma, cerebral ischemic infarction, or spontaneous intracerebral hemorrhage and the subsequent risk for developing a malignant tumor of astrocytic origin (WHO grades III and IV). Access to the unique Danish registers allowed us to conduct the study in an unselected nationwide cohort with up to 34 years of follow-up.
Materials and Methods
Based on information from the Civil Registration System (CRS), we constructed a nationwide cohort consisting of all persons in Denmark between January 1, 1978 and December 31, 2011. All residents in Denmark are registered in the CRS, a computerized national civil register established in 1968.25 Records are updated daily and contain demographic information on all individuals living in Denmark. The CRS assigns each Danish resident a unique personal identification number (PIN). The PIN permits virtually complete follow-up of study subjects residing in Denmark and easy linkage between Denmark's population-based health registers.
Exposures: Structural Brain Injury
Using the PIN, information on discharge diagnoses of traumatic brain injury, intracerebral hemorrhage, and cerebral ischemic infarction was extracted by linkage to the Danish National Patient Discharge Register.26 This register contains information on discharge diagnoses for all hospital admittances since 1978.
All discharge diagnoses in Denmark are classified according to the International Classification of Diseases (ICD), using ICD-8 codes until 1993 and ICD-10 from 1994 onward. Specifically, the following diagnostic codes were used to track individuals with a structural brain injury: traumatic brain injury, ICD-8 851-3, 85409, 85419, 85499, ICD-10 DS061–69. These diagnoses cover the traumatic head injuries severe enough to cause reactive astrogliosis due to an intracerebral lesion (focal or diffuse) or due to the mass effect from a traumatic epi- or subdural hematoma. Furthermore, we included intracerebral hemorrhage ICD-8 43100, 43108, 43109, 43190, 43198, 43199, ICD-10 DI6; and ischemic stroke/cerebral infarction ICD-8 436, ICD-10 DI63–64.
We did not include patients with a diagnosis of 850/DS060 cerebral concussion as the only head trauma diagnosis. We find that it is reasonable to assume that patients who were diagnosed with a concussion only were unlikely to have astrogliosis/severe structural brain injury. By this assumption, we do not rule out that there were some signs of structural brain injury, but less severe brain damage could be detected in these patients. Notably, patients presenting with concussion/mild head injury on admission but who had really suffered a worse underlying head injury would typically progress (altered consciousness, neurologic deficits) during the observation period. This would automatically lead to a new CAT scan revealing the development or worsening of a traumatic hematoma. Notably, such patients would at the time of discharge be registered with both diagnoses, concussion and traumatic hematoma, and the latter would make them count as exposed cohort members.
Outcome: Malignant Astrocytoma
The Danish Cancer Registry has existed since 1943 and is considered very close to complete regarding all cancers diagnosed within Denmark.27 From 1978 onward, registrations have been coded according to the ICD for Oncology, third edition (ICD-O-3). The original ICD-O-1 coding from 1978–2003 of morphological diagnoses has been converted to ICD-O-3, which corresponds to the WHO classification system of intracranial tumors.28 According to a study of childhood CNS tumors, the reporting to the Danish Cancer Registry was 98%–99% complete and the validity of the morphological diagnosis was 84% for astrocytoma's.29
We included malignant tumors (WHO grades III and IV) of confirmed astrocytic origin, which correspond to the following ICD-O-3 diagnoses: 9401 anaplastic astrocytoma and 9440 glioblastoma (subtypes 9441 giant cell glioblastoma and 9442 gliosarcoma).
Statistical Analyses
Each individual was followed from January 1, 1978 or day of birth, whichever came latest, to the first of the following events: (i) diagnosis of a malignant astrocytic tumor, (ii) death, (iii) emigration, (iv) designated “missing person” in the CRS, or (v) end of follow-up (December 31, 2011). Cohort members who suffered a structural brain injury were considered as having brain injury from time of first diagnosis; that is, person-years and number of events for this group were calculated from that date. Until then, they contributed with person-years to the unexposed group.
The rate ratio (RR) of malignant astrocytic tumor by years since the structural brain injury (<1 y, 1–4 y, and 5+ y) compared with no injury was investigated using log-linear Poisson regression models with adjustment for age (5-y intervals), gender, and calendar period (5-y intervals). The estimates for the adjustment variables and characteristics of the dataset are shown in Supplementary Appendix A. The Poisson regression was applied as an approximation to Cox regression allowing for analyses on an aggregated dataset.30
In addition to the overall exposure to one or more of the structural brain injuries, we examined the effects of each type of first structural brain injury on the RR of malignant astrocytic tumors (ie, traumatic brain injury, cerebral ischemic infarction, or intracranial hemorrhage). The RRs for individuals up to 59 years of age and for individuals 60 years of age and older were estimated by adding an interaction term to the model.
In a supplementary approach, a restricted cubic spline with 3 knots located at the 0.05, 0.50, and 0.95 percentiles was used to estimate the RR of malignant astrocytic tumor by time since structural brain injury. A corresponding restricted cubic spline with 5 knots was made in a subanalysis, and can be found in Supplementary Appendix B.
Ethics Statement
The study was approved by the Danish Data Protection Agency. According to Danish law, ethical approval is not required for register-based studies in Denmark.
Results
The cohort comprised 8.2 million individuals with 197 million person-years of follow-up. Median follow-up time was 28 years (range, 0–34), and the median time from structural brain injury to end of follow-up was 3.1 years (25% fractile 0.4 y, 75% fractile 7.5 y). A total of 404 812 individuals (52% males) were diagnosed with one or more structural brain injuries, consisting of 48 194 traumatic brain injuries, 309 528 cerebral ischemic infarctions, and 44 123 intracerebral hemorrhages; 2967 persons had more than one structural brain injury diagnosis.
Of the 404 812 individuals diagnosed with one or more structural brain injuries, 444 developed a malignant astrocytic tumor. Of these 444 individuals who developed a malignant astrocytic tumor after a structural brain injury, 337 cohort members were diagnosed with the tumor within the first year of follow-up. Co-occurrence of a structural brain injury and a malignant astrocytic tumor within 1 year was interpreted as reverse causality bias (eg, the tumor presents with intracranial hemorrhage or cerebral infarction or causes head trauma such as seizures or falls due to altered consciousness) or surveillance bias (the tumor is a random finding on a brain scan performed in relation to a head trauma). Thus, 107 cohort members were diagnosed with a malignant astrocytic tumor at least 1 year after a structural brain injury (3/10 000). Among the individuals without a history of a structural brain injury, 6045 were diagnosed with a malignant astrocytic tumor (8/10 000).
The 107 malignant astrocytic tumors occurring at least 1 year after exposure were observed in 9 individuals diagnosed with anaplastic astrocytomas (WHO grade III; 8%) and 98 individuals diagnosed with glioblastomas (WHO grade IV; 92%). The 6045 malignant astrocytic tumors observed in cohort members without a history of structural brain injury consisted of 571 anaplastic astrocytomas (9%) and 5474 glioblastomas (91%).
Characteristics of exposed and nonexposed individuals diagnosed with a malignant astrocytic tumor are presented in Table 1, according to tumor type, gender, type of structural brain injury, age at structural brain injury, and age at diagnosis of malignant astrocytic tumor. We observed an abundance of males (77%) amongst the exposed cohort members who subsequently developed a malignant astrocytic tumor at least 1 year after a structural brain injury versus 58% males amongst the nonexposed cohort members who developed a malignant astrocytic tumor. Median ages at time of traumatic brain injury, cerebral infarction, and intracerebral hemorrhage were 51, 62, and 56, respectively. The corresponding median time intervals, in years, between exposure and tumor diagnosis were 3, 3, and 5, respectively.
Table 1.
Characteristics of individuals diagnosed with a malignant astrocytic tumor (MAT) (WHO grades III and IV) in a population-based cohort in Denmark 1978–2011, according to gender, type of exposure, age at exposure, and age at diagnosis of the MAT
| Person-years | Number of Individuals With MAT | Median Age (range) at Structural Brain Injury (SBI) | Median Age (range) at MAT Diagnosis | Median Number of Years (range) Between SBI and MAT | |
|---|---|---|---|---|---|
| History of SBI | 1 714 965 | 107 | 62 (0–78) | 68 (1–85) | 4 (1–26) |
| Gender | |||||
| Females | 700 329 | 25 | 61 (40–76) | 70 (25–82) | 4 (1–23) |
| Males | 834 926 (52%) | 82 (77%) | 62 (0–78) | 68 (1–85)a | 4 (1–26) |
| Type of exposure | |||||
| Traumatic brain injury | 400 115 | 14 | 51 (0–73) | 59 (1–83) | 4 (1–26) |
| Cerebral ischemic infarction | 1 167 649 | 80 | 62 (40–77) | 69 (46–85) | 3 (1–23) |
| Intracerebral hemorrhage | 136 263 | 12 | 56 (24–78) | 69 (41–81) | 5 (2–11) |
| More than one exposure | 10 938 | 1 | 59 (51–67) | 53 (53–53) | 2 (2–2) |
| No history of SBI | 194 991 946 | 6045 | – | 60 (0–93)b | – |
| Gender | |||||
| Females | 98 435 010 | 2515 | – | 61 (0–93) | – |
| Males | 96 556 935 (50%) | 3530 (58%) | – | 60 (0–92) | – |
Data for individuals diagnosed with MAT up to 1 year after SBI are not shown, assuming the vast majority of these estimates represent reverse causality bias.
aOnly 1 individual suffered a head trauma before the age of 1 year and was diagnosed with MAT at the age of 1 year.
bOnly 2 individuals were diagnosed with MAT (WHO grade III) within the first year of life.
The RR's for malignant astrocytic tumor after previous exposure to structural brain injury caused by severe head trauma, cerebral infarction, or spontaneous intracerebral hemorrhage at least 1 year after exposure are presented in Table 2. No significant association was seen after the first year and up to 5 years after the structural brain injury (RR = 1.13; 95% CI: 0.87–1.43), whereas we observed a significantly reduced long-term risk for the development of malignant astrocytic tumor 5 years or more after a structural brain injury (RR = 0.68; 95% CI: 0.49–0.90). The specific RR's 5 years or more after each of the exposures were: traumatic brain injury RR = 0.32 (95% CI: 0.10–0.75), cerebral ischemic infarction RR = 0.69 (95% CI: 0.47–0.96), and spontaneous intracerebral hemorrhage RR = 1.39 (95% CI: 0.64–2.60).
Table 2.
RR's for malignant astrocytic tumor (MAT) (WHO grades III and IV), by history of structural brain injury (SBI) caused by traumatic brain injury, cerebral ischemic infarction, or intracerebral hemorrhage in Denmark 1978–2011
| Years Since SBI | Person-years | Number of Individuals With MAT | RR | 95% CI | |
|---|---|---|---|---|---|
| No history of SBI | – | 194 991 946 | 6045 | 1 | – |
| History of SBI | 1–4 | 813 180 | 65 | 1.13 | 0.87–1.43 |
| 5+ | 901 785 | 42 | 0.68 | 0.49–0.90 | |
| Type of first exposure | |||||
| Traumatic brain injury | 1–4 | 120 435 | 10 | 1.99 | 1.00–3.50 |
| 5+ | 279 680 | 4 | 0.32 | 0.10–0.75a | |
| Cerebral ischemic infarction | 1–4 | 626 776 | 50 | 1.05 | 0.78–1.37 |
| 5+ | 540 873 | 30 | 0.69 | 0.47–0.96a | |
| Intracerebral hemorrhage | 1–4 | 60 986 | 4 | 0.90 | 0.28–2.10 |
| 5+ | 75 276 | 8 | 1.39 | 0.64–2.60a | |
All variables are adjusted for age, gender, and calendar period. Person-years and events of the 3 types of first exposure do not sum to the numbers of all exposed individuals in the time category 1–4 years because 1 individual had more than 1 exposure diagnosis and was not included.
aAccording to a test for homogeneity, P = .046.
A restricted cubic spline with 5 knots was made for traumatic brain injury (TBI) because of the relatively large difference between the RR 1–4 years after TBI and the RR 5+ years after TBI (Supplementary Appendix C).
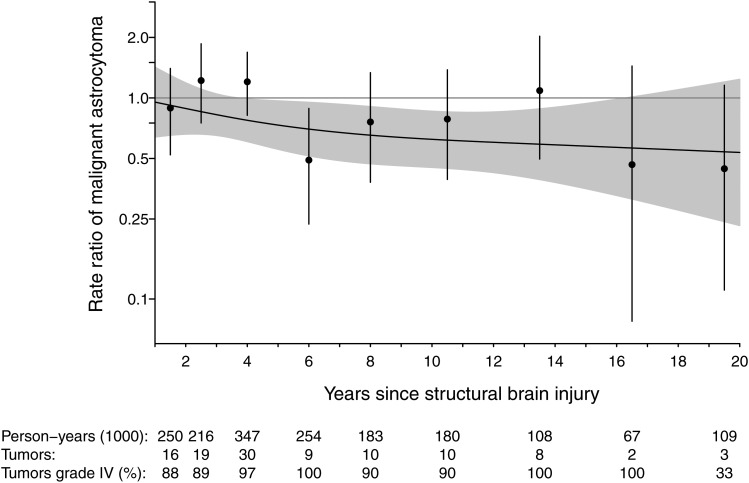
Figure 1 shows the long-term RRs for malignant astrocytic tumor as a restricted cubic spline up to 20 years or more after exposure to a structural brain injury. The reduced risk seemed to be maintained over time, but with wider CIs for the RRs a long time after exposure.
Fig. 1.
The risk (RRs) for malignant astrocytic tumor (MAT) up to 20 years after a structural brain injury (head trauma, cerebral ischemic infarction, or intracerebral hemorrhage) in Denmark 1978–2011. The number of MAT's at different time points and the percentage of WHO grade IV tumors (glioblastomas) among the MAT's are shown at the bottom of the figure. The RR curve was estimated in a supplementary approach, using a restricted cubic spline with 3 knots located at the 0.05, 0.50, and 0.95 percentiles. The shaded region represents the confidence interval of the curve, whereas the vertical lines represent the confidence intervals of the point estimates.
The malignant astrocytic tumors occurring within the first year after the structural brain injury were omitted in the main analysis due to an assumption of reverse causality and surveillance bias. As expected, we observed an increased occurrence of malignant astrocytic tumors within the first year after a structural brain injury (RR = 16.1; 95% CI: 14.4–18.0). The RR's for the specific causes of brain injury within the first year of follow-up were as follows: head trauma (4 cases; 37 794 person-years of follow-up) RR = 2.43 (95% CI: 0.75–5.64); cerebral ischemic infarction (269 cases; 234 501 person-years of follow-up) RR = 15.3 (95% CI: 13.5–17.3); and intracerebral hemorrhage (58 cases; 222 240 person-years of follow-up) RR = 36.3 (95% CI: 27.7–46.5).
Additional Analyses
In order to investigate whether the observed reduced risk for developing a malignant astrocytic tumor 5 years or more after a structural brain injury could reflect underreporting of tumors caused by reluctance to perform the invasive procedures necessary for tumor diagnosis in the elderly (eg, stereotactic biopsy, tumor resection), we estimated the risk of being diagnosed with a malignant astrocytic tumor among exposed individuals up to the age of 59 years versus the risk of diagnosis among exposed individuals 60 years of age or older. Thus, the RR 5 years or more after a structural brain injury in individuals up to 59 years of age was 0.62 (95% CI: 0.28–1.15), whereas the corresponding RR in individuals diagnosed with malignant astrocytic tumor at ≥60 years was 0.68 (95% CI: 0.48–0.94) (homogeneity test: P(<60 vs ≥60) = .80). Furthermore, we estimated the long-term risk for grade IV tumors only, which are the vast majority of malignant astrocytic tumors, 5 years or more after a structural brain injury (same exposures as the main analyses), and RR = 0.68 (95% CI: 0.48–0.92).
In contrast to other population-based studies, we did not a priori include concussion as an exposure, based on the argument that concussions most likely do not cause astrogliosis and therefore are less likely to affect the risk for subsequent development of a malignant astrocytic tumor. However, in a secondary analysis we estimated by the same approach the risk for malignant astrocytic tumors amongst the 6489 individuals diagnosed with concussions in Denmark in 1978–2011: RR up to 1 year after concussion was 4.43 (95% CI: 2.72–6.74), RR from 1 year up to 5 years after concussion was 1.20 (95% CI: 0.73–1.85), and RR 5 years or more after concussion was 0.96 (95% CI: 0.59–1.18).
In order to evaluate residual confounding, we performed additional analyses estimating interaction between age and gender, period and gender, and age and period, in which age and period were categorized in 1-year intervals. The results support that the used model is indeed adequate to evaluate the association between structural brain injuries and malignant astrocytic tumors.
Discussion
In this large nationwide cohort study we found suggestive evidence that structural brain injury (astrogliosis) may reduce the long-term risk for malignant astrocytic tumors. The risk reduction, which may be as much as 30% at 5 years or more after the structural brain injury, was maintained up to 20 years afterward. This association was observed for individuals both older and younger than 60 years. A subanalysis did not reveal a significant effect of the milder brain injury, concussion, on the long-term risk for the development of malignant astrocytic tumors.
None of 3 previously published cohort studies are directly comparable to this study due to differences in exposures (structural brain injury/astrogliosis caused by severe head trauma or stroke versus all-type head trauma)11–13 and outcomes (malignant astrocytomas versus all-type brain tumors11,12 or versus malignant brain tumors not otherwise specified).13 Two of the 3 studies did not find an increased risk for brain tumors from 1 and up to 3 years after head trauma,11–13 whereas the third study found an increased risk within 3 years after head trauma.13 However, that study was based on 9 not otherwise specified malignant tumors among head trauma patients and may be prone to surveillance bias.
In theory, low-grade lesions (WHO grades I–II astrocytic tumors) may be discovered earlier because of the structural brain injury (surveillance bias), which may lead to earlier treatment and subsequently decreased likelihood of transformation into a grade III or IV tumor. However, such a scenario would only marginally influence our results. Given that 90%–95% of grade IV astrocytic tumors arise de novo, hence without precursor lesions,2 and that the 5-year survival is <5%,31 the long-term effect should not be markedly affected. The RR for only grade IV tumors 5 years or more after exposure to a structural brain injury was 0.68 (95% CI: 0.48–0.92). If we in a “worst case” scenario assumed that the 10% of grade IV astrocytic tumors that do not arise de novo but through a precursor stage did not develop, we would instead have a crude estimate of RR = 0.68/0.9 = 0.75. Thus, the risk for grade IV tumors 5 years or more after a structural brain injury would still be markedly reduced.
One could speculate that individuals with structural brain injuries have shorter survival that leads to the observed reduced risk for these individuals. However, even though this would lead to a decreased number of malignant astrocytic tumors amongst cohort members with previous structural brain injury, it would also make the number of person-years smaller, and thus would not bias the results, at least not unless there is an unmeasured factor related to both malignant astrocytic tumors and death due to other causes. Since none of the typical lifestyle factors implies a higher risk for malignant astrocytic tumors, and since astrocytic tumors to our knowledge do not share genetic risk factors with other lethal diseases, this does not likely explain our finding of a protective effect of structural brain injury either.
The formation of glioblastomas is thought to result from a complex interplay between genetic and epigenetic factors, which may predispose some individuals.32 Although speculative, our finding of a decreased long-term risk could alternatively be due to a strong reduction of the genetically frail individuals early after exposure. Such a reduction would change the remaining population of exposed individuals after some time to be genetically less frail than the unexposed cohort members and consequently have a lower long-term risk for glioma development compared with the latter. This assumption would rely on a positive short-term association between astrogliosis and the formation of malignant astrocytic tumors, although we are much more likely to interpret this finding as reverse causality and/or surveillance bias. In line with a reverse causality interpretation, Inskip et al11 reported increased risks not only for malignant astrocytomas but for all the investigated types of brain tumors within the first year after head trauma.
Instead, we speculate that the massive inflammatory response (astrogliosis) following a structural brain injury may cause an enhanced immunologic alertness for astrocytes undergoing neoplastic transformation and thereby a clearance or reduction of premalignant astrocytes or neural stem cells, which otherwise may have developed into malignant brain tumors of astrocytic origin. Recent studies of glioblastomas support that immunologic factors play an important role in the rather complex pathogenesis of interacting gene–gene and gene–environment factors.33–35 However, the possible role of astrogliosis in the neoplastic process needs to be further elucidated.
This study has several strengths. The free and easy access to health care in Denmark enables all individuals to be referred to relevant radiologic examinations and invasive procedures independent of socioeconomic status. Because of the availability of rather detailed register information, we were able to focus on exposure diagnoses truly causing structural brain injuries compatible with damage to the brain parenchyma, hence likely to cause astrogliosis. This is important because the spectrum of head trauma diagnoses includes concussion, which mostly covers the very commonly seen short admittances for mild head traumas, a condition that is less likely to cause astrogliosis. Our finding of a nonsignificant association between concussion and malignant astrocytic tumors supported this assumption. Other studies investigated brain tumors overall as an outcome; however, brain tumors originate from a wide range of cellular origins and have very different growth patterns and therefore are likely to have different etiologies. This study was more specific, investigating the long-term impact of astrogliosis on the risk for malignant tumors of astrocytic origin with the strength of a follow-up time of up to 34 years and a median follow-up of 28 years.
A potential limitation of our study is the likely underreporting of brain tumors amongst individuals with very poor functional outcomes after structural brain injury, particularly amongst the elderly. Nevertheless, we also observed a long-term reduced risk amongst individuals with previous structural brain injury when younger than 60 years of age. Another counteracting argument for this concern is that the reduced risk seems to be maintained in long-term survivors, who supposedly have fairly good functional levels. Assuming a scenario where, say, the 10% of the long-term survivors in the poorest condition have a 50% reduced detection rate of malignant astrocytic tumors 5 years or more after structural brain injury, this would only imply a reduction of ∼5% of the RR for malignant astrocytic tumors amongst all individuals ≥5 years after exposure to a structural brain injury. We did not adjust for socioeconomic status—firstly, because socioeconomic status or lifestyle-related risk factors to our knowledge are not causatively associated with malignant astrocytomas. Furthermore, access to health care in Denmark is free and easily accessible and is thereby independent of socioeconomic status. Thus, we do not regard socioeconomic status as a confounder in this study population.
In this unselected population-based cohort study, we did not find convincing evidence for an association between structural brain injury and malignant astrocytic tumors within the first 5 years of follow-up. However, our study indicated a protective effect of astrogliosis-causing injuries 5 or more years after structural brain injury. If such an association truly exists, we speculate that it may rely on an enhanced immunologic alertness induced by the previously reactive astrocytes.
Supplementary Material
Funding
This study did not receive funding from any source.
Conflict of interest statement. No conflicts of interest declared.
Supplementary Material
References
- 1.Shafqat S, Hedley-Whyte ET, Henson JW. Age-dependent rate of anaplastic transformation in low-grade astrocytoma. Neurology. 1999;52(4):867–869. [DOI] [PubMed] [Google Scholar]
- 2.Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res. 2013;19(4):764–772. [DOI] [PubMed] [Google Scholar]
- 3.Anselmi E, Vallisa D, Berte R, et al. Post-traumatic glioma: report of two cases. Tumori. 2006;92(2):175–177. [DOI] [PubMed] [Google Scholar]
- 4.Di Trapani G, Carnevale A, Scerrati M, et al. Post-traumatic malignant glioma. Report of a case. Ital J Neurol Sci. 1996;17(4):283–286. [DOI] [PubMed] [Google Scholar]
- 5.Magnavita N, Placentino RA, Mei D, et al. Occupational head injury and subsequent glioma. Neurol Sci. 2003;24(1):31–33. [DOI] [PubMed] [Google Scholar]
- 6.Moorthy RK, Rajshekhar V. Development of glioblastoma multiforme following traumatic cerebral contusion: case report and review of literature. Surg Neurol. 2004;61(2):180–184. [DOI] [PubMed] [Google Scholar]
- 7.Mrowka R, Bogunska C, Kulesza J, et al. Grave cranio-cerebral trauma 30 years ago as cause of the brain glioma at the locus of the trauma particulars of the case. Zentralbl Neurochir. 1978;39(1):57–64. [PubMed] [Google Scholar]
- 8.Sabel M, Felsberg J, Messing-Junger M, et al. Glioblastoma multiforme at the site of metal splinter injury: a coincidence? Case report. J Neurosurg. 1999;91(6):1041–1044. [DOI] [PubMed] [Google Scholar]
- 9.Witzmann A, Jellinger K, Weiss R. [Glioblastoma multiforme developing after a gunshot injury of the brain (author’s transl)]. Neurochirurgia (Stuttg). 1981;24(6):202–206. [DOI] [PubMed] [Google Scholar]
- 10.Zhou B, Liu W. Post-traumatic glioma: report of one case and review of the literature. Int J Med Sci. 2010;7(5):248–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inskip PD, Mellemkjaer L, Gridley G, et al. Incidence of intracranial tumors following hospitalization for head injuries (Denmark). Cancer Causes Control. 1998;9(1):109–116. [DOI] [PubMed] [Google Scholar]
- 12.Nygren C, Adami J, Ye W, et al. Primary brain tumors following traumatic brain injury—a population-based cohort study in Sweden. Cancer Causes Control. 2001;12(8):733–737. [DOI] [PubMed] [Google Scholar]
- 13.Chen YH, Keller JJ, Kang JH, et al. Association between traumatic brain injury and the subsequent risk of brain cancer. J Neurotrauma. 2012;29(7):1328–1333. [DOI] [PubMed] [Google Scholar]
- 14.Zampieri P, Meneghini F, Grigoletto F, et al. Risk factors for cerebral glioma in adults: a case-control study in an Italian population. J Neurooncol. 1994;19(1):61–67. [DOI] [PubMed] [Google Scholar]
- 15.Preston-Martin S, Pogoda JM, Schlehofer B, et al. An international case-control study of adult glioma and meningioma: the role of head trauma. Int J Epidemiol. 1998;27(4):579–586. [DOI] [PubMed] [Google Scholar]
- 16.Hu J, Johnson KC, Mao Y, et al. Risk factors for glioma in adults: a case-control study in northeast China. Cancer Detect Prev. 1998;22(2):100–108. [DOI] [PubMed] [Google Scholar]
- 17.Hochberg F, Toniolo P, Cole P. Head trauma and seizures as risk factors of glioblastoma. Neurology. 1984;34(11):1511–1514. [DOI] [PubMed] [Google Scholar]
- 18.Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron. 2008;60(3):430–440. [DOI] [PubMed] [Google Scholar]
- 19.Robel S, Berninger B, Gotz M. The stem cell potential of glia: lessons from reactive gliosis. Nat Rev Neurosci. 2011;12(2):88–104. [DOI] [PubMed] [Google Scholar]
- 20.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119(1):7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buffo A, Rite I, Tripathi P, et al. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci U S A. 2008;105(9):3581–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laird MD, Vender JR, Dhandapani KM. Opposing roles for reactive astrocytes following traumatic brain injury. Neurosignals. 2008;16(2–3):154–164. [DOI] [PubMed] [Google Scholar]
- 23.Katz AM, Amankulor NM, Pitter K, et al. Astrocyte-specific expression patterns associated with the PDGF-induced glioma microenvironment. PLoS One. 2012;7(2):e32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Sage JC, Miller MR, et al. Mosaic analysis with double markers reveals tumor cell of origin in glioma. Cell. 2011;146(2):209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pedersen CB, Gotzsche H, Moller JO, et al. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–449. [PubMed] [Google Scholar]
- 26.Andersen TF, Madsen M, Jorgensen J, et al. The Danish National Hospital Register. A valuable source of data for modern health sciences. Dan Med Bull. 1999;46(3):263–268. [PubMed] [Google Scholar]
- 27.Storm HH, Michelsen EV, Clemmensen IH, et al. The Danish Cancer Registry—history, content, quality and use. Dan Med Bull. 1997;44(5):535–539. [PubMed] [Google Scholar]
- 28.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thorsteinsson R, Sorensen M, Jensen TL, et al. [Central nervous system tumours in children. An evaluation of the completeness and validity of the Cancer Registry]. Ugeskr Laeger. 2005;167(40):3782–3785. [PubMed] [Google Scholar]
- 30.Parametric regression models. In: Aalen OO, Borgan Ø, Gjessing HK, ed. Survival and Event History Analysis. New York: Springer; 2008: 223–226. [Google Scholar]
- 31.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: a clinical review. JAMA. 2013;310(17):1842–1850. [DOI] [PubMed] [Google Scholar]
- 32.Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genet. 2012;205(12):613–621. [DOI] [PubMed] [Google Scholar]
- 33.Schoemaker MJ, Robertson L, Wigertz A, et al. Interaction between 5 genetic variants and allergy in glioma risk. Am J Epidemiol. 2010;171(11):1165–1173. [DOI] [PubMed] [Google Scholar]
- 34.Lachance DH, Yang P, Johnson DR, et al. Associations of high-grade glioma with glioma risk alleles and histories of allergy and smoking. Am J Epidemiol. 2011;174(5):574–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amirian E, Liu Y, Scheurer ME, et al. Genetic variants in inflammation pathway genes and asthma in glioma susceptibility. Neuro Oncol. 2010;12(5):444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.