Abstract
Background
We sought to assess the impact of amino-acid 18F-fluoro-ethyl-tyrosine (FET) positron emission tomography (PET) on the volumetric target definition for radiation therapy of high-grade glioma versus the current standard using MRI alone. Specifically, we investigated the influence of tumor grade, MR-defined tumor volume, and the extent of surgical resection on PET positivity.
Methods
Fifty-four consecutive high-grade glioma patients (World Health Organization grades III–IV) with confirmed histology were scanned using FET-PET/CT and T1 and T2/fluid attenuated inversion recovery MRI. Gross tumor volume and clinical target volumes (CTVs) were defined in a blinded fashion based on MRI and subsequently PET, and volumetric analysis was performed. The extent of the surgical resection was reviewed using postoperative MRI.
Results
Overall, for ∼90% of the patients, the PET-positive volumes were encompassed by T1 MRI with contrast-defined tumor plus a 20-mm margin. The tumor volume defined by PET was larger for glioma grade IV (P < .001) and smaller for patients with more extensive surgical resection (P = .004). The margin required to be added to the MRI-defined tumor in order to fully encompass the FET-PET positive volume tended to be larger for grade IV tumors (P = .018).
Conclusion
With an unchanged CTV margin and by including FET-PET for gross tumor volume definition, the CTV will increase moderately for most patients, and quite substantially for a minority of patients. Patients with grade IV glioma were found to be the primary candidates for PET-guided radiation therapy planning.
Keywords: FET-PET, glioma, MRI, radiation therapy, target definition
Radiation therapy, in combination with chemotherapy (temozolomide) or as a sole treatment modality, has a proven and established role in the management of high-grade gliomas.1 The high-precision image-guided radiation therapy widely available presently warrants accurate radiation target definition. The infiltrative nature of high-grade gliomas, including glioblastoma multiforme, makes accurate estimates of macroscopic and biologically active disease extent uncertain. This problem could potentially be addressed using positron emission tomography (PET) and amino acid tracers such as [11C]methionine (MET)2 and O-(2-[18F]fluoroethyl)-l-tyrosine (FET) as part of the radiation therapy diagnostic planning procedure.3 FET-PET has been shown to have a high sensitivity and specificity for glioma tissue.4 FET is actively transported by L-type amino acid transporter 25 and correlates to the tumor cell density.4,6 The usefulness of the amino acid PET tracers has been demonstrated in a series of publications for both tracers, though in rather small patient cohorts. Both MET- and FET-positive volumes have been associated with the pattern of relapse following chemoradiation therapy.7,8 Additionally, the response on FET-PET imaging scans following chemoradiation therapy could assist in the prognostication of progression-free and overall survival.9,10 The use of amino acid tracers in neuro-oncology was recently reviewed for response assessment11 and for use in treatment planning.12,13 The problem of using only morphological imaging for the definition of tumor extent has been illustrated using MET-PET14 and FET-PET.15 The interobserver variation of the definition of the FET-PET–positive volume was found to be very small, suggesting that the diagnostic interpretation of FET-PET scans was very consistent.15
In light of the several smaller study cohorts mentioned above, the use of amino acid–based diagnostics appears warranted for radiation therapy planning target definition. However, no evidence from controlled randomized trials is available to prove or disprove clinical benefit of such use. In the present work, we wish to investigate how FET-PET impacts the target definition for radiation therapy of high-grade glioma: both the gross tumor volume (GTV) and the clinical target volume (CTV). Specifically, we wish to explore the room for a randomized trial of FET-PET–guided radiation therapy versus current standard using an MR-based target definition. Secondarily, we wish to investigate whether a subgroup of patients could be identified for which the use of FET-PET–guided radiation therapy target definition appears to be more crucial.
Methods
Patients, Diagnostic Imaging, and Treatment
FET-PET scanning was introduced as the standard procedure for high-grade glioma patients during 2012 at our institution, with an otherwise unchanged CT and MR scanning protocol. The first 54 consecutive patients with histologically verified high-grade (World Health Organization [WHO] grades III and IV) glioma intended for radiation therapy at our institution, and who were scanned using MRI and FET-PET/CT as part of the planning process, were selected. Patients' informed consent and the approval of the national board of health were obtained for the acquisition of scanning data, following the Declaration of Helsinki. The patients' scanning data were used in this retrospective analysis. Patient characteristics are presented in Table 1.
Table 1.
Patient characteristics
| N patients | 54 |
| Age, y, median (range) | 55 (21–83) |
| Histology confirmed | 54 |
| Anaplastic astrocytoma grade III | 5 |
| Oligoastrocytoma grade III | 3 |
| Oligodendroglioma grade III | 10 |
| Gliomatosis grade III | 1 |
| Glioblastoma grade IV (GBM) | 34 |
| Gliosarcoma grade IV | 1 |
| MGMT status available from histopathology | 51 |
| MGMT positive | 15 |
| MGMT negative | 36 |
| Surgery | |
| Partial resection (>5% remaining) | 31 |
| Subtotal resection (<5% remaining) | 8 |
| Complete resection (no residual tumor) | 2 |
| Stereotactic biopsy | 13 |
Abbreviations: GBM, glioblastoma multiforme; MGMT,O6-DNA methylguanine-methyltransferase.
The MRI protocol involved T1 and T2/fluid attenuated inversion recovery (FLAIR)–weighted scans including diffusion tensor imaging and tractography of the white matter tracts. Patients received an intravenous injection of contrast (Gadovist, Bayer HealthCare) prior to MR scanning. All patients fasted for at least 6 h before FET injection. A single frame static PET acquisition was performed 20–40 min post-injection of 200 MBq FET on a Biograph mCT PET/CT scanner (Siemens). An individual mold directly attached to the PET/CT scanner flat-top cradle, later used for radiation therapy, achieved head fixation. Initially, a clinical spiral CT of the head was acquired (100 kVp, 400 mAs, 1 mm slice thickness). FET-PET images were corrected for randoms, dead time, attenuation, and scatter and reconstructed with ordered subset expectation maximization 3D (6 iterations, 16 subsets, and 5 mm Gauss filter) to a matrix size of 336 × 336 × 222 (0.8 × 0.8 × 1 mm voxel size).
Image data from CT and MR were imported into the radiation therapy planning software (iPlan v4.5, BrainLab) and rigidly registered onto each other. FET-PET images were coregistered to the postcontrast T1- and FLAIR/T2-weighted MRI.
The treatment planning and delivery procedure was described previously.16 Briefly, radiation therapy was prescribed to 60 Gy in 30 fractions, where the 95% isodose contour encompassed the planning target volume. Intensity modulated arc therapy (IMAT; RapidArc, ARiA/Eclipse v10, Varian Medical Systems) typically used 2 coplanar IMAT beams. Daily imaging-guided patient position corrections were performed (ExacTrac, BrainLab).
Delineation
Delineations of the organs of risk, including brain, brainstem, eyes, lenses, optical nerves and chiasm, inner ears, and hippocampi, were performed automatically by the treatment planning software with manual edits introduced if needed. Optic tracts were generated using tractography data (FiberTracking, BrainLab).
Gross tumor volume was delineated using MRI only, referred to as “GTV(MR),” using the postcontrast T1- and FLAIR/T2-weighted MRI. The GTV(MR) contouring of all patients was done by one radiologist (J.C.) blinded from the FET-PET scan data. The GTV(MR) was defined as the contrast-enhancing lesion on the T1 MRI scans, including the surgical cavity if such was present. The GTV(PET) was auto-contoured in 3D to include voxels with uptake ≥1.6 times background value (B) according to established criteria,4 enabling the assay of tumor activity by the metabolically active tumor volume. The region of interest used to define the background value (B) was drawn in a crescent shape in a healthy-appearing cortical region in the unaffected hemisphere, contralateral to the tumor encompassing both gray and white matter. Manual edits of the uptake contour encompassing the GTV(PET) were introduced by a nuclear medicine physician (I.L.) with reference to MRI, specifically removing tissue uptake that was either reactive or physiological and not associated with the tumor. Tissue not associated with the tumor but sometimes exhibiting FET uptake included the basal ganglia, the thalami, the cerebellum, the scalp, and vascular structures. In addition, a GTV(PET with cavity) volume was defined, where the surgical cavity (if present) was included in the PET-positive volume. Clinical target volume was based on either the GTV(MR) or GTV(PET) and by adding up to a 20-mm margin to the respective GTV contours, thus creating a CTV(MR) and CTV(PET). The GTV-to-CTV margin was reduced in respect to anatomical boundaries that were reached, including bony structures. All the CTV contours were reviewed and edited by the same radiation oncologist (S.A.E.).
Volumetric Analysis and Statistics
Contour and CT data were read into MatLab v10 (IBM). The union of GTV(MR) and GTV(PET) was referred to as “GTV(MR + PET).” Similarly, the union of CTV(MR) and CTV(PET) was formed and referred to as “CTV(MR + PET).”
The margin needed to be added to the GTV(MR) contour in order to fully encompass the GTV(PET) volume was derived for all patients. This margin addition was assumed to be zero in case of a PET-negative tumor. In addition, the fraction of GTV(PET) and CTV(PET) encompassed by GTV(MR) and CTV(MR), respectively, was derived. This metric was assumed to be equal to unity in case of a PET-negative tumor. The mean and 95% confidence intervals (CIs) were derived for all volumetric data.
The GTV(PET) size, the volume fraction of GTV(PET) covered by GTV(MR), and the margin added to GTV(MR) required to fully encompass GTV(PET) were investigated for association with imaging and biological predictors. The imaging and biological predictors were glioma WHO grade III or IV, GTV(MR) size, and extent of the surgical intervention performed prior to radiotherapy simulation scanning. The extent of surgical resection was categorized as partial resection, subtotal resection, or complete resection using early postsurgical MRI scans by a radiologist (J.C.). If the postsurgical T1 contrast-enhancing region was >5% of the initial volume, it was defined as a partial resection. If the residual contrast-enhancing volume was evident but <5% of the initial volume, it was defined as a subtotal resection. With no residual contrast-enhancing tumor, the resection was considered to be complete. The rationale for choosing these covariates was that these could potentially be used to guide the selection of patients for FET-PET scanning. Statistical association was explored using a 2-tailed Spearman signed rank correlation (SPSS v22). A Bonferroni adjusted limit for significance was used, and P-values <.0042 (0.05/12) were considered statistically significant, while those <.1 were considered trends.
The probability function for encompassing the full GTV(PET) within the GTV(MR) by addition of a margin was derived in 1-mm increments from zero to 45 mm. A bootstrapping procedure was used to derive the 95% CIs for the function by drawing from all patients with replacement (1000 bootstrap samples).
The union of the CTV(MR) and CTV(PET) will naturally tend to be increased compared with the conventional CTV(MR). We derived the margin to add to the GTV(MR + PET) in order to maintain a constant CTV compared with the conventional method, where the CTV is based solely on MRI data.
We assume that the tumor has X% and zero chance of being controlled when the PET-positive volume is covered and not covered, respectively, by the prescription dose. Further, we assume that the tumor is not covered by the prescription dose if not part of the CTV. We then estimate the sample size needed to prove a benefit of PET-based planning in terms of improved tumor control in a phase III randomized trial by letting X vary between 10% and 30% (ie, the chance of tumor control). The sample size was derived for a one-sided test, power of 0.80 and with an alpha of 0.05 (R statistical package v3.0.1).
Results
An example patient is shown in Fig. 1. Five of the 54 patients were FET-PET negative. All of the PET-negative tumors were WHO grade III gliomas: 3 oligoastrocytomas (3 of 3) and 2 oligodendrogliomas (2 of 10). Incorporating the FET-positive volume into a combined MR/PET GTV caused the GTV to increase somewhat overall. The volumetric data are shown in Fig. 2. The Spearman rank sum test is shown in Table 2. The analysis revealed that the PET-positive volume size was strongly associated with WHO grade IV glioma, while GTV(MR) was not associated with a WHO glioma grade (P = .277). Further, the extent of surgical resection significantly affected the GTV(PET) size. Also, WHO grade IV glioma disease tended to require a larger margin to encompass the PET volume. Unsurprisingly, the GTV size defined on PET or MR where the cavity was included was strongly associated (P < .001).
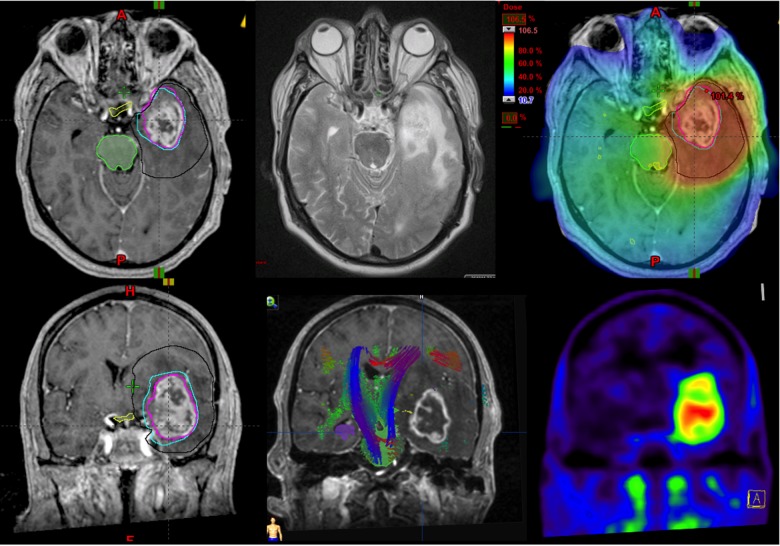
Fig. 1.
An example of a patient treatment plan with delineations of the CTV(MR) in black, GTV(PET) in light blue, and GTV(MR) in purple overlaid on the top left-right transversal slices: T1 MR, T2 MR, and dose distribution, in temperature-scale color-wash from 106.5% (red) to 10.7% (blue), where 100% is 60 Gy in 30 treatment fractions. Bottom left-right images frontal slices include: T1 MR, T2 MR, and FET-PET, respectively. The bottom-center image includes overlaid fiber tracts, where the colors indicate fiber directionality: green, red, and blue indicate that the fibers run ventrodorsally, commissurally (transversal), and superoinferiorly, respectively. The T1 contrast-enhancing region correlates well with a marked FET-PET uptake and displacement of the fiber tracts.
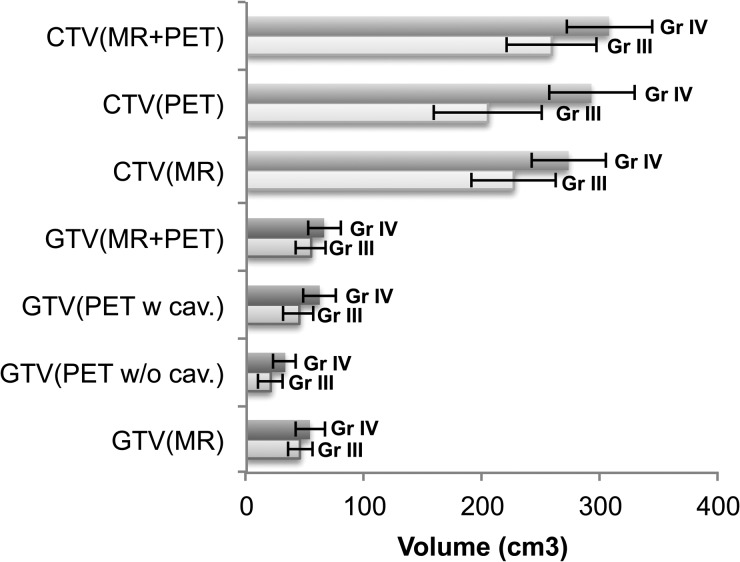
Fig. 2.
Data for grade III (Gr III) and grade IV (Gr IV) GTV or CTV based on only PET/CT with and without cavity (w cav. and w/o cav.) for MR and MR and PET, respectively. Error bars show 95% CIs.
Table 2.
Spearman signed rank correlation coefficients of volumetric data and investigated parameters (N = 54)
| Spearman's Rho | Extent of Surgery** | Grade IV | GTV(MR) | |
|---|---|---|---|---|
| GTV(PET) | Correlation coefficient | −0.412 | 0.606 | 0.296 |
| P | .002 | .000 | .030 | |
| GTV(PET with resection cavity) | Correlation coefficient | −0.070 | 0.361 | 0.774 |
| P | .614 | .007 | .000 | |
| Volume fraction of GTV(PET) included in GTV(MR) | Correlation coefficient | 0.335 | −0.315 | 0.322 |
| P | .013 | .020 | .018 | |
| Margin added to GTV(MR) to fully cover GTV(PET) | Correlation coefficient | −0.118 | 0.320 | 0.123 |
| P | .394 | .018 | .377 |
Significant (2-tailed) P-values (<.0042) are in bold type; trends are italicized (P < .1).
*WHO grade was dichotomized to 0 = grade III, and 1 = grade IV.
**Extent of surgery was dichotomized as 0 = stereotactic biopsy, 1 = partial resection, 2 = subtotal resection, and 3 = complete resection.
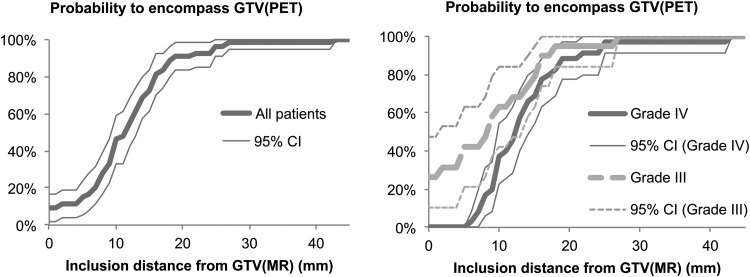
The probability of encompassing GTV(PET) with GTV(MR) with a margin added is presented in Fig. 3. With a 0- to 5-mm margin, only a small fraction of the patient's GTV was fully encompassed within the GTV(MR), and the widely used 20 mm added onto the GTV(MR) appears to be required in order to cover the physiologically active tumor for most (∼90%) of the patients.
Fig. 3.
Probability for inclusion of the complete FET-PET–positive volume as a function of distance from the GTV(MR), for all patients (left) and split for the groups of patients with grade III or IV disease (right). Most gliomas have some part of the PET-positive region outside the GTV(MR), but with a 20-mm margin from the GTV(MR), ∼90% of glioma PET-positive regions will be encompassed.
A margin of 18 mm (95% CI, ±0.8 mm) added to the GTV(MR + PET) volume produced the same overall CTV size for the study patients as if the CTV were based on MR only and with a 20-mm margin added (given that CTV was adjusted for boundaries, as described above). This GTV-CTV margin to produce the same CTV size varied for the patients from 7 to 20 mm, depending on the level of overlap of the PET- and MR-defined GTV and localization of the tumor. If 20 mm GTV-to-CTV margin were to be used, the CTV would be 8% larger (median, range: 0 to 154%).
A very large number of patients were required in order to find statistically significant difference between groups if randomizing toward use of PET-guided radiation therapy planning with an otherwise unchanged treatment protocol. The sample size required was between 2799 and 10 630 patients for the highest (0.3) and the lowest (0.1) tumor control probability (X) for each treatment arm under the stated assumptions.
Discussion
FET involves a relatively simple workflow that is comparable to the clinical use of 2-fluoro-2-deoxy-d-glucose. The 2-hour half-life of 18F-based PET makes execution of the scans more flexible than using the MET tracer with an 11C half-life of only 20 min and is better suited for large patient throughput. As FET is not metabolically trapped in the tissue, it is important to adhere to the timing of the scan, as activity in high-grade tumors could be washed out. There is slightly physiologically increased metabolic activity in the basal ganglia, the thalami, the cerebellum, the scalp, and the vascular structures, but these can easily be identified making use of coregistration to MRI. In some patients a diffuse and slightly increased uptake (T/B < 1.8) was associated with the surface and sulci of the brain on the sides of the resection cavity. These were interpreted as reactive changes secondary to postsurgical blood and debris if they were not present on the preoperative MRI scan, coincided with MRI signal changes typical of blood, and followed a distribution atypical of tumor infiltration, along the gray matter surface rather than white matter tracts.
Primarily, the data showing association of tumor cell density and FET uptake prompted us to implement the use of FET-PET routinely in our clinical practice. Amino acid PET can identify the infiltrating glioma tissue.17 Pauleit et al4 have shown an association in tumor cell density determined histologically on the tissue samples, where it was found that FET-PET yielded a sensitivity of 93% and a specificity of 94%. Further, FET-PET uptake was shown to be usable also for non-contrast-enhancing lesions.6 Also, several smaller prospective studies have indicated the specific uptake of glioma cells and the usefulness of amino acid PET tracers for radiation therapy planning and response assessment of high-grade glioma. In the study by Weber et al,15 it was shown that FET-PET target delineation was highly reproducible. No clinical evidence from a controlled randomized phase III trial in favor of the use of FET-PET–guided radiation therapy planning is, however, present. Therefore, we wished to evaluate what would arguably be the simplest approach to a clinical trial centered on FET-PET–guided radiation therapy, where the FET-PET volume is included in the GTV versus the conventional definition of radiation therapy target. We find that a limited fraction of patients (10%) had a PET-positive volume extending outside the CTV(MR). Thus, using conformal radiation therapy, this is the fraction of patients for which we can assume that part of the target will receive considerably less than the prescription dose. This is a somewhat smaller fraction compared with the experience of Weber and colleagues,15 though the data present it within the respective uncertainties of the 2 studies. In addition, we find that GTV sizes based on MR and PET were comparable if the resection cavity was included in the definition of GTV. Differences observed might also reflect differences of the tumor biology, surgical and/or scanning procedures, and the timing/delay of interventions in the respective institutional care paths.
In the present study, we also investigated the impact on CTV definition with the introduction of FET-PET. The CTV size is of interest considering that the toxicity of radiation therapy could be related to the irradiated brain volume to the prescription dose.18 We find that the CTV size if based on GTV(PET) or GTV(MR) plus 20 mm was similar. However, the union of CTV(MR) and CTV(PET) was somewhat larger than either. The 20-mm margin exists to encompass the region of the infiltration of tumor cells that cannot readily be visualized using conventional MR scanning protocols. Hence, a reduction of the CTV margin might be warranted when GTV is defined including amino acid tracer imaging. This assumption is based on PET studies with amino acid tracers demonstrating a high diagnostic accuracy for the identification of both solid and infiltrating tumor compartments in high-grade glioma that are not visualized on conventional MR scanning protocols.17,19,20 Adding a margin of 18 mm on the union of GTV(MR) and GTV(PET) produced a CTV that was the same size as CTV(MR) for the cohort.
Lee and colleagues7 found that the tumor recurred noncentrally in the case that the high-dose radiation field did not encompass the PET-positive volume.7 Several authors have found that ∼80%–90% of high-grade gliomas recur in the irradiated region.21,22 Hence, we assume unchanged tumor control probability of irradiated tumor tissue but improved probability of full radiation dose coverage by introducing FET-PET–guided planning. These 2 facts underpinned the assumption made for the power calculation in this work, in which the tumor control probability was 10%–30% or zero if the PET-positive volume was or was not encompassed, respectively, by the 60-Gy isodose. We found a limited fraction of ∼10% of patients in whom we would observe a geometrical miss of the PET-positive volume should the CTV be defined as the contrast-enhancing region on T1 MRI and the surgical cavity plus a 20-mm margin. This fact means that an unfeasibly large number of patients would need to be recruited into a clinical trial based on PET- and MR-guided versus MR-guided radiation therapy planning in order to achieve a reasonable power. This is true, however, only as long as the radiation protocol is unchanged, the prescription dose of 60 Gy is maintained, and the local control is low (10%–30%). Increasing the local control rates substantially reduces the sample size. Similarly, the required sample size was much lower and equal to approximately one tenth if we assume that one third of patients instead of one tenth have PET-positive volumes extending outside of the conventional 20 mm margin, which is what was found by Weber et al.15 Also, Rieken et al23 found a somewhat larger fraction (17%) of patients with FET-PET–positive volume extending outside the CTV(MR); however, this patient dataset included both previously irradiated patients and patients with grade II tumors and is therefore not immediately comparable to the data presented in this work.
Note that we have in this example chosen the pattern of failure to be the primary endpoint to determine sample size. If overall survival would be selected as the primary endpoint instead, the required sample size would probably have to be even larger, considering that a group of patients would presumably experience treatment failure regardless of whether radiation therapy were PET guided.
An interesting approach to integrate FET-PET into the radiation therapy workup is to escalate the radiation dose to the FET-PET–positive volume, which has been attempted by Piroth and colleagues.24 In their phase II trial, an escalation to 72 Gy was achieved to the FET-PET–positive volume. Unfortunately, no benefit in survival was observed following this treatment strategy. The lack of success with a dose escalation on the FET-PET–positive volume suggests that the radiation dose was too low, that geometrical misses were likely, or that no clinical benefit measured by progression-free and overall survival can be gained by radiation dose escalation beyond 60 Gy. Using proton therapy, Fitzek et al25 escalated the radiation dose to 90 Gy (“photon equivalent doses”) and found that the recurrence pattern was altered while long-term survival appeared promising, but severe treatment-related toxicity was prevalent. A modern approach to use proton and carbon therapy for brain tumors was recently presented by Rieken et al.26 The Radiation Therapy Oncology Group trial 93-05 used a stereotactic boost to the residual tumor mass, visible on MR (or CT), and showed it to be technically feasible, involving acceptable toxicity but with no apparent benefit to patients.27 It is evident from this work that a stereotactic boost to the tumor visible on CT and MR would fail ∼90% of the time to completely cover the FET-PET–positive volume. Using a stereotactic boost on the amino acid PET-viable part of the tumor might be a feasible path for amending the radiation therapy protocol, given that a high fraction (∼75%–85%) of recurrences within the irradiated volume were observed in several studies over ∼20 years,21,28–30 but this approach remains to be tested in patterns of failure analysis and in a clinical trial. Another very interesting treatment strategy that could be investigated in a clinical trial would be to treat the MR- and PET-positive volume plus a reasonable margin. With a small margin added, the treated volume could be dramatically reduced compared with the current standard of MR-defined GTV plus 2 cm. The proper margin to be tested in such a trial to be added to a combined PET- and MR-defined GTV may need to be determined in patterns of failure analysis first.
In conclusion, for ∼90% of the patients, the PET-positive volume can be found inside the CTV based on only MRI T1 plus contrast and with 20-mm margin. Further, we find that patients with grade IV glioma have larger FET-PET–positive volumes, which were at larger geometrical distances from the GTV(MR). Consequently, we consider patients with grade IV disease as the primary candidates for FET-PET–guided radiation therapy planning. With an unchanged CTV margin and by including FET-PET for GTV definition, the irradiated volume will tend to increase moderately for most patients, and quite substantially for a minority of patients. Prospective radiation therapy trials could determine whether the GTV-to-CTV margin can be adjusted with the introduction of FET-PET.
Funding
This work was supported by the Lundbeck Foundation.
Conflict of interest statement. None to disclose.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 2.Bergström M, Collins VP, Ehrin E, et al. Discrepancies in brain tumor extent as shown by computed tomography and positron emission tomography using [68Ga]EDTA, [11C]glucose, and [11C]methionine. J Comput Assist Tomogr. 1983;7(6):1062–1066. [DOI] [PubMed] [Google Scholar]
- 3.Wester HJ, Herz M, Weber W, et al. Synthesis and radiopharmacology of O-(2-[18F]fluoroethyl)-L-tyrosine for tumor imaging. J Nucl Med. 1999;40(1):205–212. [PubMed] [Google Scholar]
- 4.Pauleit D, Floeth F, Hamacher K, et al. O-(2-[18F]fluoroethyl)-L-tyrosine PET combined with MRI improves the diagnostic assessment of cerebral gliomas. Brain. 2005;128(3):678–687. [DOI] [PubMed] [Google Scholar]
- 5.Langen KJ, Jarosch M, Muhlensiepen H, et al. Comparison of fluorotyrosines and methionine uptake in F98 rat gliomas. Nucl Med Biol. 2003;30(5):501–508. [DOI] [PubMed] [Google Scholar]
- 6.Stockhammer F, Plotkin M, Amthauer H, et al. Correlation of F-18-fluoro-ethyl-tyrosin uptake with vascular and cell density in non-contrast-enhancing gliomas. J Neurooncol. 2008;88(2):205–210. [DOI] [PubMed] [Google Scholar]
- 7.Lee IH, Piert M, Gomez-Hassan D, et al. Association of 11C-methionine PET uptake with site of failure after concurrent temozolomide and radiation for primary glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2009;73(2):479–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weber DC, Casanova N, Zilli T, et al. Recurrence pattern after [(18)F]fluoroethyltyrosine-positron emission tomography-guided radiotherapy for high-grade glioma: a prospective study. Radiother Oncol. 2009;93(3):586–592. [DOI] [PubMed] [Google Scholar]
- 9.Piroth MD, Holy R, Pinkawa M, et al. Prognostic impact of postoperative, pre-irradiation (18)F-fluoroethyl-l-tyrosine uptake in glioblastoma patients treated with radiochemotherapy. Radiother Oncol. 2011;99(2):218–224. [DOI] [PubMed] [Google Scholar]
- 10.Piroth MD, Pinkawa M, Holy R, et al. Prognostic value of early [18F]fluoroethyltyrosine positron emission tomography after radiochemotherapy in glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2011;80(1):176–184. [DOI] [PubMed] [Google Scholar]
- 11.Dhermain FG, Hau P, Lanfermann H, et al. Advanced MRI and PET imaging for assessment of treatment response in patients with gliomas. Lancet Neurol. 2010;9(9):906–920. [DOI] [PubMed] [Google Scholar]
- 12.Grosu AL, Weber WA. PET for radiation treatment planning of brain tumours. Radiother Oncol. 2010;96(3):325–327. [DOI] [PubMed] [Google Scholar]
- 13.Niyazi M, Geisler J, Siefert A, et al. FET-PET for malignant glioma treatment planning. Radiother Oncol. 2011;99(1):44–48. [DOI] [PubMed] [Google Scholar]
- 14.Matsuo M, Miwa K, Tanaka O, et al. Impact of [11C]methionine positron emission tomography for target definition of glioblastoma multiforme in radiation therapy planning. Int J Radiat Oncol Biol Phys. 2012;82(1):83–89. [DOI] [PubMed] [Google Scholar]
- 15.Weber DC, Zilli T, Buchegger F, et al. [(18)F]Fluoroethyltyrosine- positron emission tomography-guided radiotherapy for high-grade glioma. Radiat Oncol. 2008;3(44). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Munck af Rosenschold P, Engelholm S, Ohlhues L, et al. Photon and proton therapy planning comparison for malignant glioma based on CT, FDG-PET, DTI-MRI and fiber tracking. Acta Oncol. 2011;50(6):777–783. [DOI] [PubMed] [Google Scholar]
- 17.Kracht LW, Miletic H, Busch S, et al. Delineation of brain tumor extent with [11C]L-methionine positron emission tomography: local comparison with stereotactic histopathology. Clin Cancer Res. 2004;10(21):7163–7170. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S20–S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirotte BJ, Lubansu A, Massager N, et al. Clinical impact of integrating positron emission tomography during surgery in 85 children with brain tumors. J Neurosurg Pediatr. 2010;5(5):486–499. [DOI] [PubMed] [Google Scholar]
- 20.Pauleit D, Stoffels G, Schaden W, et al. PET with O-(2–18F-fluoroethyl)-L-tyrosine in peripheral tumors: first clinical results. J Nucl Med. 2005;46(3):411–416. [PubMed] [Google Scholar]
- 21.Minniti G, Amelio D, Amichetti M, et al. Patterns of failure and comparison of different target volume delineations in patients with glioblastoma treated with conformal radiotherapy plus concomitant and adjuvant temozolomide. Radiother Oncol. 2010;97(3):377–381. [DOI] [PubMed] [Google Scholar]
- 22.Sherriff J, Tamangani J, Senthil L, et al. Patterns of relapse in glioblastoma multiforme following concomitant chemoradiotherapy with temozolomide. Br J Radiol. 2013;86(1022):20120414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rieken S, Habermehl D, Giesel FL, et al. Analysis of FET-PET imaging for target volume definition in patients with gliomas treated with conformal radiotherapy. Radiother Oncol. 2013;109(3):487–492. [DOI] [PubMed] [Google Scholar]
- 24.Piroth MD, Pinkawa M, Holy R, et al. Integrated boost IMRT with FET-PET-adapted local dose escalation in glioblastomas. Results of a prospective phase II study. Strahlenther Onkol. 2012;188(4):334–339. [DOI] [PubMed] [Google Scholar]
- 25.Fitzek MM, Thornton AF, Rabinov JD, et al. Accelerated fractionated proton/photon irradiation to 90 cobalt gray equivalent for glioblastoma multiforme: results of a phase II prospective trial. J Neurosurg. 1999;91(2):251–260. [DOI] [PubMed] [Google Scholar]
- 26.Rieken S, Habermehl D, Haberer T, et al. Proton and carbon ion radiotherapy for primary brain tumors delivered with active raster scanning at the Heidelberg Ion Therapy Center (HIT): early treatment results and study concepts. Radiat Oncol. 2012;7(41). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93–05 protocol. Int J Radiat Oncol Biol Phys. 2004;60(3):853–860. [DOI] [PubMed] [Google Scholar]
- 28.Hess CF, Schaaf JC, Kortmann RD, et al. Malignant glioma: patterns of failure following individually tailored limited volume irradiation. Radiother Oncol. 1994;30(2):146–149. [DOI] [PubMed] [Google Scholar]
- 29.Niyazi M, Schnell O, Suchorska B, et al. FET-PET assessed recurrence pattern after radio-chemotherapy in newly diagnosed patients with glioblastoma is influenced by MGMT methylation status. Radiother Oncol. 2012;104(1):78–82. [DOI] [PubMed] [Google Scholar]
- 30.Milano MT, Okunieff P, Donatello RS, et al. Patterns and timing of recurrence after temozolomide-based chemoradiation for glioblastoma. Int J Radiat Oncol Biol Phys. 2010;78(4):1147–1155. [DOI] [PubMed] [Google Scholar]