Abstract
Background
Chordoma in the spine is relatively rare, and minimal information has been published in the literature regarding this subject. Moreover, there are controversies over prognostic factors of this disease.
Methods
A retrospective analysis of chordoma in the spine was performed by survival analysis. Local relapse-free survival (LRFS) and overall survival (OS) were analyzed from the date of surgery to the date of local recurrence and death. The LRFS and OS rates were estimated using the Kaplan–Meier method to identify potential prognostic factors. Factors with P values ≤ .1 were subjected to multivariate analysis by Cox regression analysis. P values ≤ .05 were considered statistically significant.
Results
A total of 153 patients with spinal chordoma were included in the study. The mean follow-up period was 72.0 months (range, 1–279 months). Local recurrence was detected in 51 cases after initial surgery in our center, while death occurred in 42 cases. The statistical analysis suggested that tumor location of C3–L5, dedifferentiated chordoma, preoperative Frankel scores A–C, and total spondylectomy were independent prognostic factors for LRFS. In addition, total en bloc spondylectomy and Karnofsky' performance status (KPS) ≥ 80% were favorable factors for OS.
Conclusions
Total spondylectomy, by either en bloc or piecemeal method, could significantly reduce LRFS for spinal chordoma. Location of C3–L5 is a favorable factor for LRFS, while dedifferentiated subtype and preoperative Frankel scores A–C are adverse prognostic factors. In addition, total en bloc spondylectomy and KPS ≥ 80% significantly improve overall survival of patients with spinal chordoma.
Keywords: chordoma, spine tumor, surgery, survival, prognostic factor
Chordoma is a relatively rare malignant bone tumor with an incidence of 0.08 per 100 000.1,2 It is derived from embryonic rests of notochord and shows dual epithelial-mesenchymal differentiation,3,4 but its natural history is still not well known. The spine is the common site for chordoma, with an almost equal distribution in the sacrum, skull base, and mobile spine.2 Although chordoma in the spine is indolent and grows slowly,5 it exhibits strong local aggressiveness with a high rate of local recurrence and has a low to late tendency for distant metastasis.6,7 The 10-year survival rate for chordoma is considered to be nearly 40%,8 but no exact information has been reported about overall survival (OS) for spinal chordoma.
Minimal information on chordoma has been published due to its rarity, and most published information has come from case series with small sample sizes.6,9–12 We previously reported 2 case series focusing on the surgical treatment for primary or recurrent cervical chordoma, but their sample size limited statistical analysis, and the relatively short follow-up failed to provide much information.13,14 Stacchiotti et al8 reported a series of 138 cases from two referral centers. Log-rank test and Cox regression analysis were used to evaluate prognostic factors for chordoma; however, information from two referral centers increased the heterogeneity of the data so that only one prognostic factor could be identified. Moreover, tumor location was not stratified by distribution, which made this factor lack credibility. Therefore, it was necessary to perform a systematic analysis of a large case series in a single center to identify the prognostic factors for recurrence and OS of chordoma. The objective of our study was to identify the prognostic factors of chordoma in the spine by analyzing a large case series of patients undergoing surgery.
Materials and Methods
Patients
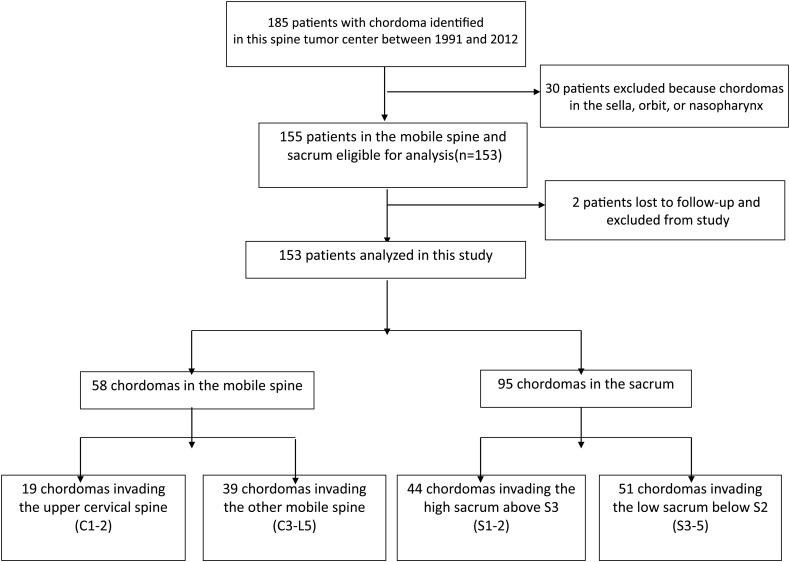
A retrospective review was performed of chordoma cases that had been treated surgically between January 1991 and December 2012. A total of 185 patients with chordomas underwent surgery at our spine tumor center in this time period. Of these patients, 155 patients had chordomas in their spine and 30 had chordomas elsewhere (Fig. 1).
Fig. 1.
Patient flow diagram.
Permission was obtained from the hospital ethics committee before commencing this study, and informed consent was obtained from all patients or their legal guardians.
Of the 155 patients, 2 patients had been lost for follow-up, and a total of 153 patients were included in this study. Medical records of all patients were retrospectively reviewed for clinical and operative reports, radiographic images, and pathology reports. Preoperative neurological status was evaluated according to the patient's Frankel score.15 The resected chordomas were diagnosed as classical, chondroid, or dedifferentiated subtype according to histological appearance.16 Chondroid chordoma (CC) is thought to be a biphasic neoplasm consisting of cartilaginous and chordoid elements in variable proportions. Microscopically, typical physaliphorous cells can be seen in the histological specimen (hematoxylin and eosin). Physaliphorous cells of CC show coexpression of vimentin, cytokeratins, epithelial membrane antigen, and S-100 protein. Cartilaginous areas are positive for S-100 protein and vimentin.
The individualized surgical strategy was decided for each patient according to Tomita classification,17 Enneking stage,18 and Weinstein-Boriani-Biagini surgical staging system.19 Surgeries were performed by posterior approach, anterior approach, or a combination.20,21
This study focused on the recurrence and death status for patients with chordoma in the spine after the initial surgery in our center. The diagnosis of recurrence was made on the basis of clinical manifestations and imaging findings at outpatient follow-up or pathological evaluation of the second surgery. Events were defined as the first evidence of local recurrence for local relapse-free survival (LRFS) or death related to any cause for OS. Event times were defined as the interval from the date of surgery to local recurrence, death, or until July 2014 for living patients. All patients were followed up on an outpatient basis at 3-month intervals for the first 6 months, then at 6-month intervals for the next 2 years, and annually for life thereafter. Death status and time of death were acquired through telephone interviews.
Statistical Method
Quantitative data are described by mean (median, range), and qualitative data are described as counts and percentages. The univariate and multivariate analyses of various clinical factors were performed to identify independent variables that could predict prognosis. Patient factors were age, sex, Karnofsky' performance status (KPS),22 treatment history, bladder and bowel function, and preoperative Frankel score. Tumor factors were tumor location, preoperative distant metastasis, Enneking stage, Tomita classification, and pathological subtype. Treatment factors were preoperative artery embolism (PAE), surgical approach, resection mode and local treatment, intraoperative blood loss, bisphosphonate treatment, and adjuvant radiotherapy. Local recurrence and distant metastasis were also evaluated as possible factors for OS.
The LRFS and OS rate were estimated by the Kaplan-Meier method, and the log-rank test was used for univariate survival analysis to identify independent variables that could predict prognosis. Clinical experience and statistical analysis were used to decide whether continuous variables should be categorized. Factors with P value ≤ .10 were subjected to multivariate analysis by Cox proportional hazards analysis. P values ≤ .05 were considered statistically significant. All statistical calculations were performed using PASW Statistics, version 19.0.
Results
The characteristics of 153 patients are described in Table 1. The population comprised 99 men and 54 women with a mean age of 54.5 years (median, 56 y; range, 15–81 y). Of these patients, 120 were admitted for primary chordoma, and the remaining 33 were recurrent one after surgical treatment in other medical institutions. Ninety-five chordomas located in sacrum, with 39 and 19 cases in C3-L5 and C1-2, respectively. One-hundred twenty-three patients were diagnosed with classical chordomas, 7 with chondroid variants, and 23 with the dedifferentiated subtype.
Table 1.
Patient characteristics and univariate analysis of the prognostic factors affecting local relapse-free survival and overall survival
| Factors | n | LRFS |
OS |
||
|---|---|---|---|---|---|
| % | P value | % | P value | ||
| Age <60/≥60, y | 109/44 | 61.5 vs 79.5 | .054* | 77.1 vs 61.4 | .109 |
| Sex, M/F | 99/54 | 70.7 vs 59.3 | .066* | 76.8 vs 64.8 | .055* |
| KPS, <60%/ 60%–80%/≥80% | 17/30/106 | 58.8 vs 63.3 vs 68.9 | .275 | 35.3 vs 70.0 vs 79.2 | <.001* |
| Treatment history, no/yes | 120/33 | 65.8 vs 69.7 | .73 | 75.8 vs 60.6 | .026* |
| Location, C1–2 / C3–L5 / S1–5 | 19/39/95 | 52.6 vs 76.9 vs 65.3 | .004* | 47.4 vs 69.2 vs 78.9 | <.001* |
| Preoperative distant metastasis, no/yes | 142/11 | 66.9 vs 63.6 | .792 | — | — |
| Bladder and bowel function, no/yes | 87/66 | 64.4 vs 69.7 | .391 | 71.3 vs 74.2 | .411 |
| Preoperative Frankel scores, A–C/D–E | 38/115 | 55.3 vs 70.4 | .016* | 63.2 vs 75.7 | .063* |
| Enneking stages, I/II–III | 121/32 | 72.7 vs 43.8 | <.001* | 76.9 vs 56.3 | .048* |
| Tomita classification, I–III/IV–VII | 47/106 | 89.4 vs 56.6 | <.001* | 80.9 vs 68.9 | .492 |
| Tumor size, ≤6 cm/>6 cm | 90/63 | 72.2 vs 58.7 | .331 | 73.3 vs 71.4 | .527 |
| Pathology grades, classical/chondroid/dedifferentiated | 123/7/23 | 71.5 vs 85.7 vs 34.8 | <.001* | 74.0 vs 100.0 vs 56.5 | .051* |
| Preoperative selective arterial embolism, no/yes | 89/64 | 60.7 vs 75.0 | .138 | 60.7 vs 89.1 | .002* |
| Surgical approach, anterior/posterior/combined | 6/112/35 | 83.3 vs 67.9 vs 60.0 | .182 | 83.3 vs 80.4 vs 45.7 | <.001* |
| Resection mode, subtotal/total piecemeal/total en bloc | 15/80/58 | 13.3 vs 58.8 vs 91.4 | <.001* | 33.3 vs 63.8 vs 94.8 | <.001* |
| Intraoperative blood loss, ≤2,000 mL/>2,000 mL | 109/44 | 71.6 vs 54.5 | .077* | 74.3 vs 68.2 | .629 |
| Local treatment, no/cisplatin or methotrexate | 46/107 | 54.3 vs 72.0 | .593 | 58.7 vs 78.5 | .775 |
| Adjuvant radiotherapy, no/yes | 122/14 | 71.3 vs 42.9 | .084* | 77.9 vs 85.7 | .224 |
| Complication, no/yes | 114/9 | — | — | 75.0 vs 33.3 | .024* |
| Postoperative recurrence | 102/51 | — | — | 79.4 vs 58.8 | .717 |
| Postoperative distant metastasis, no/brain or lung/bone or soft tissue | 128/11/14 | — | — | 77.3 vs 36.4 vs 57.1 | .001* |
Abbreviations: F, female; KPS, Karnofsky performance status; LRFS, local relapse-free survival; M, male; OS, overall survival; y, years.
*P value < .1 for the univariate analysis.
All 153 patients underwent surgical treatment; total en bloc spondylectomy, total piecemeal spondylectomy, and subtotal resection were performed in 58, 80, and 15 cases, respectively. The mean tumor diameter at diagnosis was 6.9 cm (median, 6 cm; range, 1–20 cm), with 8.4 cm (median, 8 cm; range, 3–20 cm) for sacral chordomas and 4.3 cm (median, 4 cm; range, 1–12 cm) for mobile spine chordomas.
The mean follow-up period was 72.0 months (median, 57 months; range, 1–279 months). Recurrence was detected in 51 patients after initial surgery in our center, and 42 patients died in the follow-up period. The mean time from surgery to recurrence was 46.4 months (median, 37 months; range, 4–132 months), while follow-up for the dead patients was 57.6 months (median, 52 months; range, 1–126 months).
Univariate and Multivariate Analysis of Prognostic Factors for Local Relapse-free Survival
The overall LRFS rate after surgery for primary tumor was 66.7%, with a median LRFS of 37 months (range, 4–132 months). Univariate analysis of the prognostic factors is shown in Table 1. In our series, the LRFS rate was significantly higher in patients with tumor located in C3–L5 compared with other tumor sites (P = .004). The LRFS rate was obviously different between patients with 2 different preoperative Frankel scores (A–C/D–E; P = .016). In addition, patients with Enneking stages II and III had a higher LRFS rate than those with Enneking stage I (P < .001). The LRFS rate was significantly different in patients with classical, chondroidal, and dedifferentiated chordomas (P < .001). Patients with lesions evaluated as Tomita I–III had an obviously lower LRFS rate than those evaluated as Tomita IV–VII (P < .001).
Subtotal resection was performed in 15 patients, total piecemeal spondylectomy in 80 patients, and total en bloc spondylectomy in 58 patients. LRFS rate was obviously different among patients with 3 different resection modes (P < .001). The mean intraoperative blood loss was 1734 mL (median, 1600 mL; range, 100–6000 mL). Cisplatin and methotrexate were used as local treatment for soaking the surgical field after tumor resection in 107 cases23,24 but was prohibited in cases with broken dura because of possible nerve damage.23 However, no significant difference of LRFS rate was found (P = .593).
Zoledronic acid and incadronate disodium, which are bisphosphonates, are used in our center to control osteolytic lesions of bone tumors.24 Thirty-two patients received bisphosphonate treatment. Adjuvant radiotherapy (40–55 Gy) was administered postoperatively to 14 patients. No significant difference in LRFS was observed (P = .226 for bisphosphonate treatment; P = .084 for radiotherapy). There was no significant difference in other factors (sex, KPS, treatment history, preoperative distant metastasis, bladder and bowel function, tumor size, and surgical approach).
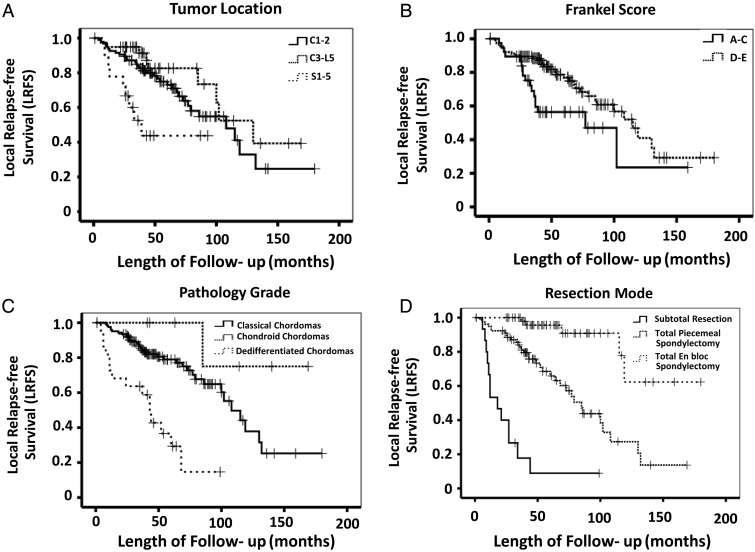
Potential prognostic factors extracted by univariate analysis were submitted to Cox proportional hazards analysis. The risk of recurrence decreased significantly for patients whose tumor was located in C3–L5 as compared with other patients (C1–2/S1–5) (The hazard ratio [HR] was 0.240, P = .007). Patients with preoperative Frankel scores of D–E had a significantly higher LRFS rate than those with preoperative Frankel scores of A–C (HR, 0.370; P = .003). Dedifferentiated chordoma significantly increased the risk of recurrence (HR, 3.194; P = .002). The risk of recurrence was significantly decreased in patients who underwent total spondylectomy (total piecemeal spondylectomy [HR, 0.275; P < .001] versus total en bloc spondylectomy [HR, 0.041; P < .001]). The Kaplan-Meier curves of LRFS for tumor location, preoperative Frankel scores, pathological grades, and resection mode for all patients are shown in Fig. 2.
Fig. 2.
Kaplan–Meier curves of local relapse-free survival (LRFS) for (A) tumor location, (B) Frankel score, (C) pathological grade, and (D) resection mode.
Multivariate analysis showed that age, sex, Enneking stages, Tomita classification, intraoperative blood loss, and adjuvant radiotherapy were not independent prognostic factors for LRFS. Details are listed in Table 2.
Table 2.
Multivariate analysis of the prognostic factors affecting local relapse-free survival
| Factor | LRFS |
||
|---|---|---|---|
| B | HR (95% CI) | P Value | |
| Age ≥60 y | .184 | ||
| Sex | .850 | ||
| Location, C3–L5 | −1.426 | 0.240 (0.085–0.677) | .007* |
| Location, S1–5 | .098 | ||
| Preoperative Frankel scores | −0.993 | 0.370 (0.191–0.719) | .003* |
| Enneking stages | .880 | ||
| Tomita classification | .066 | ||
| Pathology grades, chondroid | .122 | ||
| Pathology grades, dedifferentiated | 1.161 | 3.194 (1.522–6.700) | .002* |
| Total piecemeal spondylectomy | −1.291 | 0.275 (0.134–0.566) | <.001* |
| Total en bloc spondylectomy | −3.187 | 0.041(0.013–0.129) | <.001* |
| Intraoperative blood loss | .223 | ||
| Adjuvant radiotherapy | .791 | ||
Abbreviations: B, coefficient value; CI, confidence interval; HR, hazard ratio; y, years.
*P value < .05 for multivariate analysis.
Univariate and Multivariate Analysis of Prognostic Factors for Overall Survival
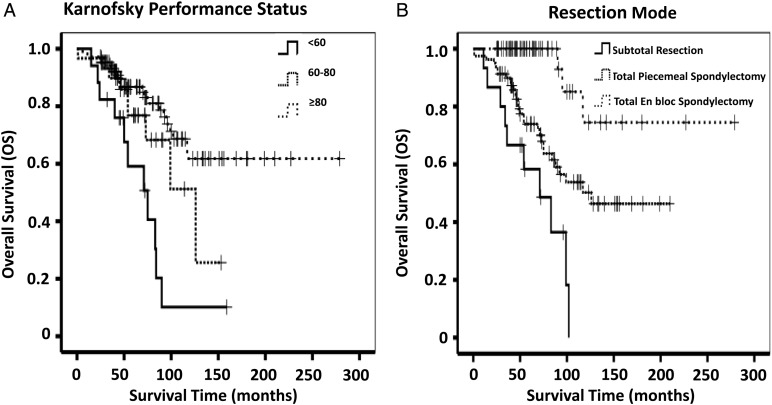
Forty-two patients (27.5%) suffered death, either directly related to chordoma (40 cases, 95.2%) or to surgical complications (2 cases, 4.8%) in our series. For the overall series, the OS was 50.1%, with a median OS of 52 months. Univariate analysis of prognostic factors affecting survival is shown in Table 1. According to statistical analysis using the Kaplan-Meier method, a significant difference was found in patients with treatment history (P = .026), Enneking stages II–III (P = .048), PAE (P = .002), and distant metastasis (P = .001). There was a significant difference of survival related to KPS (P < .001), location (P < .001), surgical approach (P < .001), and resection mode (P < .001). Complications (defined as postoperative wound infection, intracranial infection, pulmonary infection, dyspnea, or dysphagia) were also found to result in a worse outcome (P = .024).The above factors, along with sex (P = .055), preoperative Frankel scores (P = .063), and pathological grades (P = .051), were confirmed on multivariable analysis. KPS ≥ 80% and total en bloc spondylectomy remained highly significant independent predictors for OS (KPS ≥ 80: HR, 0.306; P = .004 and total en bloc spondylectomy: HR, 0.112; P = .002). Details are listed in Table 3, and the Kaplan-Meier curves of OS for KPS and resection mode are shown in Fig. 3.
Table 3.
Multivariate analysis of the prognostic factors affecting overall survival
| Factor | OS |
||
|---|---|---|---|
| B | HR (95% CI) | P Value | |
| Sex | .071 | ||
| KPS, 60%–80% | .054 | ||
| KPS, ≥80% | −1.186 | 0.306 (0.136–0.685) | .004* |
| Treatment history | .295 | ||
| Location, C3–L5 | .892 | ||
| Location, S1–5 | .524 | ||
| Preoperative Frankel scores | .160 | ||
| Enneking stages | .161 | ||
| Pathology grades, chondroid | .077 | ||
| Pathology grades, dedifferentiated | .337 | ||
| Preoperative selective arterial embolism | .054 | ||
| Surgical approach, posterior | .315 | ||
| Surgical approach, combined | .130 | ||
| Total piecemeal spondylectomy | .230 | ||
| Total en bloc spondylectomy | −2.106 | 0.112 (0.032–0.466) | .002* |
| Complication | .155 | ||
| Postoperative brain or lung metastasis | .425 | ||
| Postoperative bone or soft tissue metastasis | .458 | ||
Abbreviations: B, coefficient value; CI, confidence interval; HR, hazard ratio; KPS, Karnofsky performance status.
*P value < .05 for multivariate analysis.
Fig. 3.
Kaplan–Meier curves of overall survival for (A) KPS and (B) resection mode.
Discussion
Chordoma, a histologically low-grade neoplasm, is the most common primary spine malignancy.2,8 The special anatomical structure of the spine poses a great challenge for treatment of chordoma and increases the postoperative recurrence rate. Along with preventing recurrence, increasing OS after surgery is still an important issue that should be addressed. In our research, we performed univariate and multivariate analysis to investigate the prognostic factors affecting recurrence and OS in patients with spinal chordomas. The results suggested that total en bloc spondylectomy could significantly improve the OS and LRFS for patients with spinal chordomas. KPS ≥ 80 was an independent prognostic factor for survival, while dedifferentiated subtype, location of C3–L5, preoperative Frankel scores A–C, and total spondylectomy were closely correlated with LRFS.
In our series, mean age of 54.5 years, predominance in men, and peak incidence between 40–70 years of age were similar to the findings of previous reports.5,6,8 There are controversies about spine distribution. McMaster2 reported an almost equal distribution of mobile spine and sacrum, but Stacchiotti et al8 found that most of spinal chordomas arose from the sacrum. Our findings of sacral predominance were consistent with those of Stacchiotti et al. Moreover, we also found in our series that tumors located in C3–L5 had less risk for recurrence. In our series, age was not an independent prognostic factor for postoperative LRFS and OS of chordoma in the spine. The same results were achieved for sex, treatment history, bladder and bowel function, tumor size, Enneking stages, Tomita classification, PAE, intraoperative blood loss, surgical approach, complication, adjuvant radiotherapy, postoperative recurrence, and postoperative distant metastasis. Tumor size was considered to be an independent prognostic factor for both LRFS and OS in spinal chordomas by Stacchiotti et al8 in previous research, but we did not see the same results. Tumor sizes vary in the upper cervical spine and sacrum. We performed subgroup analysis in the mobile spine and sacrum cases and found no significant differences using the cut points of 4 cm and 9 cm, respectively.
Preoperative condition, as assessed by KPS, has been demonstrated by multiple studies to be one of the strongest prognostic indicators for survival of malignant tumors.22,25,26 However, its effect for prognosis in cases of spinal chordoma has not been evaluated before. In the present study, the median score for preoperative KPS was 80% (range, 20%–90%), and 106 patients (69.3%) were able to care for self (KPS ≥ 80%). The findings in our study suggested that preoperative KPS ≥ 80% could not affect recurrence rate but did have great influence on OS of chordoma. The Frankel score is another frequently used method for assessing preoperative condition, and a Frankel score D–E is confirmed to be an independent prognostic factor for recurrence.
Histopathologically, chordomas can be divided into 3 variant subtypes: classical (conventional), chondroid, and dedifferentiated (sarcomatous).8 The most common chordoma is the classical subtype, which exhibits relative low-grade behavior. Classical chordoma accounted for 80.4% of the cases in our study. Chondroid chordoma is a malignant, cartilage-forming tumor that shows features of both chordoma and chondrosarcoma and has a relatively favorable prognosis.16 Dedifferentiated chordoma, however, is a rare, aggressive subtype and is considered to have the poorest prognosis.5 Our finding in this study revealed that the dedifferentiated subtype was closely associated with postoperative recurrence but was not an independent prognostic factor for OS of spinal chordoma.
Surgical treatment is the standard treatment strategy for spinal chordoma,19 with the aim of preserving or even improving functionality, relieving pain, controlling local recurrence, and promising prolonged survival.6,10,13 It has been reported that radical resection might result in a better prognosis of chordoma than subtotal excision.27 The surgical procedures applicable to the spine include subtotal resection, total piecemeal spondylectomy, and total en bloc spondylectomy.19 The findings in this study suggested that total spondylectomy, by either the en bloc or piecemeal method, could significantly decrease the recurrence rate of spinal chordoma, while total en bloc spondylectomy could improve OS.
Stener and Gunterberg first introduced wide en bloc surgical resection for the treatment of sacral tumors in the 1970s,28 and it has been widely used in the surgical management of spinal chordoma ever since. Although improved local control of recurrence has been demonstrated in sacral chordomas treated by total en bloc spondylectomy,29,30 the anatomic constraints of the spine make it technically demanding and hard to reach. More importantly, a large chordoma in the upper cervical spine and sacrum cannot be excised in an ideal en bloc manner because of its proximity to vital neurovascular structures.10,14 Similarly, the patient is at risk for paralysis if the spinal cord is compressed by the tumor. Thus, this may be why tumors located at C3–L5 and preoperative Frankel score D–E,31 can provide excellent prognosis for tumor recurrence in our study. Total en bloc spondylectomy has also been reported to cause more complications than the other 2 surgical procedures.28,32,33
PAE, which is widely used before surgery, has been confirmed to reduce intraoperative blood loss and improve the excision rate and safety of surgery.24,34,35 Our results, however, have demonstrated that PAE does not improve the prognosis for both recurrence and OS. Although radiotherapy by itself has been proven to be ineffective for treating chordoma,36 it has been widely used as a postoperative adjuvant treatment for clinical management of spinal chordomas. In our study, radiotherapy was not found to be an independent prognostic factor for spinal chordomas. New advances in radiation technology, with the introduction of hadrons (high-dose protons or carbon ions), have allowed delivery of higher doses of radiation to the target volume with minimal injury to surrounding tissue and improved radiobiological effect.37,38 However, this technique was not widely used with a long follow-up in our center, and its efficacy for preventing recurrence and death needs further investigation. Bisphosphonate treatment is another adjuvant treatment that is widely used for treatment of spinal chordomas in our center.23 The pain-control effect was verified in the treatment of spinal chordomas, but bisphosphonate treatment did not significantly improve recurrence rate and OS in our research.
Although chordoma is not typically metastatic on presentation, distant metastasis is not uncommon.8,38 Twenty-five patients (16.3%) in our series experienced distant metastasis, which was slightly lower than the proportion (>20%) reported in the literature.9,39 Distant metastasis of spinal chordomas tends to run a relatively indolent course,8 and we found no direct link found between distant metastasis and prognosis of spinal chordoma in our series. Postoperative recurrence is common for chordomas, and 51 patients (33.3%) experienced recurrence in this study. Recurrence may exacerbate the neurological defects, increase the difficulty of surgery, and even lead to tumor progression and ultimate death. However, no significant association was found between postoperative recurrence and OS. The reason may be that even for the recurrent chordomas, the en-bloc spondylectomy could also be performed to reduce the recurrence rate of these patients.
To our knowledge, our study is the largest series of spinal chordomas to date. Our study is also highly homogeneous for patients coming from a single center. Nevertheless, there are some limitations. First, it is a retrospective study with all the limitations thereof. Second, as a surgical series, it only focused on patients who were underwent surgical treatment. Third, the duration of follow-up was not long enough, with the follow-up time of 55 censored patients being less than 60 months; this makes the OS appear higher than it actually was.
In conclusion, total spondylectomy, performed either en bloc or piecemeal, could significantly reduce LRFS of spinal chordoma. The location of C3–L5 is a favorable factor for LRFS, while dedifferentiated subtype and preoperative Frankel scores A–C are adverse prognostic factors. In addition, total en bloc spondylectomy and KPS ≥ 80% significantly improved OS for patients with spinal chordomas.
Funding
This study was supported in part by the National Natural Science Foundation of China (Grant No.81102036; 81402222).
Conflict of interest statement. No conflict of interest for all authors in this study.
References
- 1.Healey JH, Lane JM. Chordoma: a critical review of diagnosis and treatment. Orthop Clin North Am. 1989;20(3):417–426. [PubMed] [Google Scholar]
- 2.McMaster ML, Goldstein AM, Bromley CM, et al. Chordoma: incidence and survival patterns in the United States, 1973–1995. Cancer Causes Control. 2001;12(1):1–11. [DOI] [PubMed] [Google Scholar]
- 3.Casali PG, Stacchiotti S, Sangalli C, et al. Chordoma. Curr Opin Oncol. 2007;19(4):367–370. [DOI] [PubMed] [Google Scholar]
- 4.Horten BC, Montague SR. In vitro characteristics of a sacrococcygeal chordoma maintained in tissue and organ culture systems. Acta Neuropathol. 1976;35(1):13–25. [DOI] [PubMed] [Google Scholar]
- 5.Walcott BP, Nahed BV, Mohyeldin A, et al. Chordoma: current concepts, management, and future directions. Lancet Oncol. 2012;13(2):e69–e76. [DOI] [PubMed] [Google Scholar]
- 6.Ferraresi V, Nuzzo C, Zoccali C, et al. Chordoma: clinical characteristics, management and prognosis of a case series of 25 patients. BMC Cancer. 2010;10:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers PW, Schwinn CP. Chordoma. A clinicopathologic study of metastasis. Am J Clin Pathol. 1979;72(5):765–776. [DOI] [PubMed] [Google Scholar]
- 8.Stacchiotti S, Casali PG, Lo Vullo S, et al. Chordoma of the mobile spine and sacrum: a retrospective analysis of a series of patients surgically treated at two referral centers. Ann Surg Oncol. 2010;17(1):211–219. [DOI] [PubMed] [Google Scholar]
- 9.Baratti D, Gronchi A, Pennacchioli E, et al. Chordoma: natural history and results in 28 patients treated at a single institution. Ann Surg Oncol. 2003;10(3):291–296. [DOI] [PubMed] [Google Scholar]
- 10.Osaka S, Osaka E, Kojima T, et al. Long-term outcome following surgical treatment of sacral chordoma. J Surg Oncol. 2014;109(3):184–188. [DOI] [PubMed] [Google Scholar]
- 11.Bergh P, Kindblom LG, Gunterberg B, et al. Prognostic factors in chordoma of the sacrum and mobile spine: a study of 39 patients. Cancer. 2000;88(9):2122–2134. [DOI] [PubMed] [Google Scholar]
- 12.Prabhakaran PS, Misra S, Kannan V, et al. Sacral chordomas: a 10-year study. Australas Radiol. 1998;42(1):42–46. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Xiao J, Wu Z, et al. Primary chordomas of the cervical spine: a consecutive series of 14 surgically managed cases. J Neurosurg Spine. 2012;17(4):292–299. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Xu W, Yang X, et al. Recurrent upper cervical chordomas after radiotherapy: surgical outcomes and surgical approach selection based on complications. Spine. 2013;38(18):E1141–E1148. [DOI] [PubMed] [Google Scholar]
- 15.Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7(3):179–192. [DOI] [PubMed] [Google Scholar]
- 16.Chugh R, Tawbi H, Lucas DR, et al. Chordoma: the nonsarcoma primary bone tumor. Oncologist. 2007;12(11):1344–1350. [DOI] [PubMed] [Google Scholar]
- 17.Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine. 2001;26(3):298–306. [DOI] [PubMed] [Google Scholar]
- 18.Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. 1986;(204):9–24. [PubMed] [Google Scholar]
- 19.Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine. 1997;22(9):1036–1044. [DOI] [PubMed] [Google Scholar]
- 20.Tomita K, Kawahara N, Baba H, et al. Total en bloc spondylectomy. A new surgical technique for primary malignant vertebral tumors. Spine. 1997;22(3):324–333. [DOI] [PubMed] [Google Scholar]
- 21.Boriani S, Biagini R, De Iure F, et al. En bloc resections of bone tumors of the thoracolumbar spine. A preliminary report on 29 patients. Spine. 1996;21(16):1927–1931. [DOI] [PubMed] [Google Scholar]
- 22.Karnofsky DA. Clinical evaluation of anticancer drugs: cancer chemotherapy. Gann Monogr. 1967;2(2):223–231. [Google Scholar]
- 23.Xu W, Li X, Huang W, et al. Factors affecting prognosis of patients with giant cell tumors of the mobile spine: retrospective analysis of 102 patients in a single center. Ann Surg Oncol. 2013;20(3):804–810. [DOI] [PubMed] [Google Scholar]
- 24.Yin H, Zhou W, Meng J, et al. Prognostic factors of patients with spinal chondrosarcoma: a retrospective analysis of 98 consecutive patients in a single center. Ann Surg Oncol. 2014;2111:3572–3578. [DOI] [PubMed] [Google Scholar]
- 25.Crnalic S, Hildingsson C, Wikstrom P, et al. Outcome after surgery for metastatic spinal cord compression in 54 patients with prostate cancer. Acta Orthop. 2012;83(1):80–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju DG, Zadnik PL, Groves ML, et al. Factors associated with improved outcomes following decompressive surgery for prostate cancer metastatic to the spine. Neurosurgery. 2013;734:657–666. [DOI] [PubMed] [Google Scholar]
- 27.Schwab JH, Healey JH, Rose P, et al. The surgical management of sacral chordomas. Spine. 2009;34(24):2700–2704. [DOI] [PubMed] [Google Scholar]
- 28.Stener B, Gunterberg B. High amputation of the sacrum for extirpation of tumors. Principles and technique. Spine. 1978;3(4):351–366. [DOI] [PubMed] [Google Scholar]
- 29.Guppy KH, Chakrabarti I, Isaacs RS, et al. En bloc resection of a multilevel high-cervical chordoma involving C-2: new operative modalities: technical note. J Neurosurg Spine. 2013;19(2):232–242. [DOI] [PubMed] [Google Scholar]
- 30.Brown MJ, Kor DJ, Curry TB, et al. Sacral tumor resection: the effect of surgical staging on patient outcomes, resource management, and hospital cost. Spine. 2011;36(19):1570–1578. [DOI] [PubMed] [Google Scholar]
- 31.Kim CH, Chung CK, Jahng TA, et al. Resumption of ambulatory status after surgery for nonambulatory patients with epidural spinal metastasis. Spine J. 2011;11(11):1015–1023. [DOI] [PubMed] [Google Scholar]
- 32.Boriani S, Bandiera S, Donthineni R, et al. Morbidity of en bloc resections in the spine. Eur Spine J. 2010;19(2):231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bandiera S, Boriani S, Donthineni R, et al. Complications of en bloc resections in the spine. Orthop Clin North Am. 2009;40(1):125–131, vii. [DOI] [PubMed] [Google Scholar]
- 34.Ozkan E, Gupta S. Embolization of spinal tumors: vascular anatomy, indications, and technique. Tech Vasc Interv Radiol. 2011;14(3):129–140. [DOI] [PubMed] [Google Scholar]
- 35.Vetter SC, Strecker EP, Ackermann LW, et al. Preoperative embolization of cervical spine tumors. Cardiovasc Intervent Radiol. 1997;20(5):343–347. [DOI] [PubMed] [Google Scholar]
- 36.Boriani S, Chevalley F, Weinstein JN, et al. Chordoma of the spine above the sacrum. Treatment and outcome in 21 cases. Spine. 1996;21(13):1569–1577. [DOI] [PubMed] [Google Scholar]
- 37.Austin-Seymour M, Munzenrider J, Linggood R, et al. Fractionated proton radiation therapy of cranial and intracranial tumors. Am J Clin Oncol. 1990;13(4):327–330. [DOI] [PubMed] [Google Scholar]
- 38.Nishida Y, Kamada T, Imai R, et al. Clinical outcome of sacral chordoma with carbon ion radiotherapy compared with surgery. Int J Radiat Oncol Biol Phys. 2011;79(1):110–116. [DOI] [PubMed] [Google Scholar]
- 39.McPherson CM, Suki D, McCutcheon IE, et al. Metastatic disease from spinal chordoma: a 10-year experssience. J Neurosurg Spine. 2006;5(4):277–280. [DOI] [PubMed] [Google Scholar]