Abstract
Background
Survival outcomes for patients with glioblastoma remain poor, particularly for patients with unmethylated O6-methylguanine-DNA methyltransferase (MGMT) gene promoter. This phase II, randomized, open-label, multicenter trial investigated the efficacy and safety of 2 dose regimens of the selective integrin inhibitor cilengitide combined with standard chemoradiotherapy in patients with newly diagnosed glioblastoma and an unmethylated MGMT promoter.
Methods
Overall, 265 patients were randomized (1:1:1) to standard cilengitide (2000 mg 2×/wk; n = 88), intensive cilengitide (2000 mg 5×/wk during wk 1−6, thereafter 2×/wk; n = 88), or a control arm (chemoradiotherapy alone; n = 89). Cilengitide was administered intravenously in combination with daily temozolomide (TMZ) and concomitant radiotherapy (RT; wk 1−6), followed by TMZ maintenance therapy (TMZ/RT→TMZ). The primary endpoint was overall survival; secondary endpoints included progression-free survival, pharmacokinetics, and safety and tolerability.
Results
Median overall survival was 16.3 months in the standard cilengitide arm (hazard ratio [HR], 0.686; 95% CI: 0.484, 0.972; P = .032) and 14.5 months in the intensive cilengitide arm (HR, 0.858; 95% CI: 0.612, 1.204; P = .3771) versus 13.4 months in the control arm. Median progression-free survival assessed per independent review committee was 5.6 months (HR, 0.822; 95% CI: 0.595, 1.134) and 5.9 months (HR, 0.794; 95% CI: 0.575, 1.096) in the standard and intensive cilengitide arms, respectively, versus 4.1 months in the control arm. Cilengitide was well tolerated.
Conclusions
Standard and intensive cilengitide dose regimens were well tolerated in combination with TMZ/RT→TMZ. Inconsistent overall survival and progression-free survival outcomes and a limited sample size did not allow firm conclusions regarding clinical efficacy in this exploratory phase II study.
Keywords: cilengitide, newly diagnosed glioblastoma, randomized phase II study, unmethylated MGMT promoter
Glioblastoma is the most common high-grade, primary brain malignancy in adult patients, accounting for 45% of all malignant brain tumors.1 Glioblastoma is refractory to most chemotherapy regimens and has a poor prognosis, with a 5-year survival rate of less than 5%.1,2 Currently, the standard treatment for newly diagnosed glioblastoma is surgical resection as far as safely feasible, therapy with the oral alkylating agent temozolomide (TMZ) with concomitant radiotherapy (RT), followed by TMZ maintenance therapy (TMZ/RT→TMZ).3,4 Despite the clinically meaningful survival benefit established by the TMZ/RT→TMZ regimen, patients with glioblastoma still have poor survival, with a median survival of only 15 months and a 2-year mortality rate of over 75%.3
An important predictive factor for the beneficial effect of TMZ treatment is the methylation status of the O6-methylguanine-DNA methyltransferase (MGMT) gene promoter. Patients with tumors in which the MGMT gene is inactivated due to hypermethylation of the MGMT promoter have enhanced chemosensitivity and significantly better survival after TMZ therapy.5–7 Conversely, patients with tumors in which the MGMT gene is not methylated do not seem to benefit as much from the addition of TMZ to RT. New treatment options are therefore urgently required, particularly for patients with an unmethylated MGMT gene promoter.
The integrin family of cell adhesion molecules, which bind to extracellular ligands via an arginine-glycine-aspartate (RGD) domain,8 have been shown to have an important and complex influence on tumor cell survival, proliferation, migration and invasion, and angiogenesis.9 The integrin subtypes αvβ3 and αvβ5 are widely expressed by both glioblastoma cells and endothelial cells in tumor-associated vasculature.10 Cilengitide (EMD 121974) is a cyclic peptide with an RGD-binding domain that selectively and competitively inhibits αvβ3 and αvβ5 integrins; it potentially blocks integrin-mediated angiogenesis and tumor migration while enhancing neoplastic apoptosis.8 Phase I/II studies have previously reported that cilengitide is well tolerated with antitumor activity in patients with recurrent or newly diagnosed glioblastoma.11–15 Promising antitumor activity has been reported in phase II studies of cilengitide administered as a single agent to patients with recurrent glioblastoma, with greater responses at higher doses.12,15 In combination with TMZ/RT→TMZ in patients with newly diagnosed glioblastoma, improved overall survival (OS) at higher doses (2000 mg compared with 500 mg twice weekly) was observed,14 and cilengitide was reported to improve progression-free survival (PFS) and OS outcomes compared with historical cohorts treated with standard therapy alone.13
Subsequently, the effects of cilengitide plus TMZ/RT→TMZ were investigated in newly diagnosed glioblastoma patients with methylated MGMT gene promoter in the randomized, controlled, multicenter, phase III CENTRIC trial.16 Herein, the results of the open-label, multicenter, randomized controlled phase II CORE trial are reported, the companion trial of CENTRIC carried out to evaluate the efficacy and safety of 2 different cilengitide regimens in combination with standard TMZ/RT→TMZ in patients with newly diagnosed glioblastoma and unmethylated MGMT gene promoter.
Patients and Methods
Patient Eligibility
Eligible patients had newly diagnosed and histologically confirmed supratentorial glioblastoma (World Health Organization grade IV) with unmethylated MGMT gene promoter status (assessed as previously described16). Diagnosis of glioblastoma was based on neurosurgical resection of the tumor or on open biopsy, confirmed by central pathology review. Patients were stratified according to classifications by recursive partitioning analysis (RPA; Table 1). Additional inclusion criteria were: age ≥18 years; gadolinium-enhanced MRI <48 h after surgery or before randomization; stable or decreasing dose of steroids for ≥5 days before randomization; an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1; satisfactory laboratory blood and biochemical results within 2 weeks prior to randomization (absolute neutrophil count >500/mm3, platelets >100 000/mm3; creatinine <1.5× upper limit of normal [ULN] or creatinine clearance rate >60 mL/min; prothrombin time international normalized ratio within normal limits and partial thromboplastin time below ULN; hemoglobin >10 g/dL, total bilirubin <1.5× ULN; aspartate aminotransferase and alanine aminotransferase <2.5× ULN [except when attributable to anticonvulsants or transient increase postsurgery attributable to narcotics]; and alkaline phosphatase <2.5× ULN).
Table 1.
Patient baseline characteristics and demographics (intention-to-treat population)
| Standard Cilengitide Arm (n = 88) | Intensive Cilengitide Arm (n = 88) | Control Arm (n = 89) | |
|---|---|---|---|
| Age, y | |||
| Median (range) | 55.6 (26–77) | 56.0 (21–76) | 57.7 (21–74) |
| Gender, n (%) | |||
| Male | 50 (56.8) | 50 (56.8) | 55 (61.8) |
| ECOG performance status, n (%) | |||
| 0 | 45 (51.1) | 46 (52.3) | 40 (44.9) |
| 1 | 42 (47.7) | 41 (46.6) | 49 (55.1) |
| Missing | 1 (1.1) | 1 (1.1) | 0 (0.0) |
| RPA class, n (%) | |||
| III | 13 (14.8) | 15 (17.0) | 15 (16.9) |
| IV | 48 (54.5) | 49 (55.7) | 57 (64.0) |
| V | 26 (29.5) | 22 (25.0) | 17 (19.1) |
| Missing | 1 (1.1) | 2 (2.3) | 0 (0.0) |
| Extent of surgery, n (%) | |||
| Total resection | 44 (50.0) | 46 (52.3) | 46 (51.7) |
| Partial resection | 37 (42.0) | 37 (42.0) | 37 (41.6) |
| Biopsy | 7 (8.0) | 4 (4.5) | 6 (6.7) |
| Missing | 0 (0.0) | 1 (1.1) | 0 (0.0) |
| Time, wk, from diagnosis to randomization, n (%) | |||
| Median (range) | 4.3 (2.1–6.6) | 4.0 (2.1–7.1) | 3.7 (1.6–6.7) |
The main criteria for exclusion were: prior chemotherapy within 5 years, RT to the head, systemic antiangiogenic therapy, a history of malignancy (except for curatively treated cervical carcinoma in situ or basal cell carcinoma of the skin), and placement of Gliadel wafer at surgery.
All patients provided informed written consent prior to participating in any study procedures or treatments. The study was conducted in accordance with the Declaration of Helsinki, the International Conference on Harmonization note for good clinical practice (Topic E6, 1996), and applicable regulatory requirements. The study is registered at clinicaltrials.gov (NCT00813943).
Study Design and Treatment
The randomized phase II study was preceded by a 6-week safety run-in to determine the tolerability of intensified cilengitide dosing (2000 mg 3, 4, or 5×/wk) in combination with TMZ/RT. In the safety run-in, a standard 3 + 3 design was used for stepwise evaluation of cilengitide dose intensification in 3 patient cohorts. In each cohort, cilengitide was administered intravenously over 1 h in combination with TMZ/RT standard treatment. Patients in cohort 1 received 2000 mg cilengitide 3 times weekly; patients in cohort 2 received 2000 mg cilengitide 4 times weekly; and patients in cohort 3 received 2000 mg cilengitide 5 times weekly. Each patient was observed for dose-limiting toxicity (DLT) during the first 4 weeks of the combination therapy. If any of the 3 patients in the first dose cohort experienced a DLT, an additional 3 patients were added and evaluated in the same cohort. If 2 or fewer of the 6 patients in the expanded cohort displayed a DLT, the next dose cohort was evaluated; maximum tolerated dose (MTD) was considered to be reached if 3 or more of the 6 patients experienced DLTs.
In the randomized phase II part of the trial, patients were allocated (1:1:1 ratio) to one of the following treatments: a standard cilengitide arm of 2000 mg twice weekly, in week –1 as a single agent, during weeks 1–6 in combination with TMZ/RT standard therapy, and during weeks 7–34 in combination with TMZ maintenance therapy; an intensive cilengitide arm identical to the standard arm except that during the combination with TMZ/RT (wk 1–6), cilengitide 2000 mg was given 5 times a week; and a control arm of the standard regimen of TMZ/RT→TMZ without cilengitide.
For the intensive cilengitide arm in this trial, the frequency of days cilengitide was administered was increased rather than increasing the dose per day because cilengitide 2000 mg is considered the maximum safe dose. In addition, the intensive regimen was administered during weeks 1–6 based on in vivo preclinical data suggesting that cilengitide had synergy with radiation therapy.17 Thus, this study was designed for cilengitide intensification during the radiation therapy phase.
During RT, the 1-h cilengitide infusion was recommended to be administered 4 h before RT based on preclinical modeling studies.17 The doses and mode of administration of the standard TMZ/RT→TMZ treatment were as previously reported.3 During RT, the TMZ dose was recommended to be administered ∼1 h before RT. The TMZ maintenance phase consisted of 6 treatment cycles. Patients in the cilengitide treatment arms received cilengitide for ≥18 months. TMZ was administered for a total of 34 weeks. Treatment was stopped earlier if patients developed progressive disease (PD) or unacceptable toxicity or withdrew from the study for any other reason.
Objectives and Outcome Measures
The primary objective of the safety run-in was to evaluate the safety and tolerability of stepwise intensified cilengitide in combination with TMZ/RT. In the randomized phase II study, the primary objective was to determine OS in patients treated with either standard or intensified cilengitide regimens in combination with TMZ/RT→TMZ, compared with TMZ/RT→TMZ alone. Secondary objectives of the randomized part included PFS, the pharmacokinetic (PK) profile of the intensive cilengitide regimen (2000 mg 5 d/wk) in combination with TMZ/RT→TMZ, and the safety profile of cilengitide plus TMZ/RT→TMZ in the overall study population.
Response assessment during study therapy was based on investigator evaluation using Macdonald criteria18; however, response assessment was also determined centrally by an independent review committee (IRC) using the Response Assessment in Neuro-Oncology criteria,19 which included blinded MRI readings, assessment of neurologic status, ECOG scores, and steroid use. Adverse events (AEs) were coded according to the Medical Dictionary for Regulatory Activities and their severity graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events version 3.0. For cilengitide PK, blood samples on day 1 and day 5 were taken pre-dose (0 h) and at 1 h, 2 h, 3 h, 4 h, 8 h, and 24 h after the start of cilengitide infusion. The parameters measured included maximum plasma concentration as observed (Cmax), area under the plasma concentration-time curve from zero to last sampling time (AUC0−t), plasma concentration at the end of infusion (EOI) as observed, apparent terminal half-life (t1/2), clearance (CL), apparent volume of distribution during the terminal phase, and apparent volume of distribution at steady state (Vss).
Statistical Analyses
All efficacy analyses were carried out on the intention-to-treat population, and safety analyses were performed on the safety population, defined as patients who received any study treatment. Patient characteristics and safety variables were summarized using descriptive statistics. Efficacy outcomes were evaluated using Kaplan–Meier methodology. OS was measured from randomization to death; PFS was determined from randomization to the first sign of PD or death due to any cause. Hazard ratios (HRs), including 95% confidence intervals (CIs), for both cilengitide arms versus the control arm were calculated using a Cox' proportional hazards model stratified by patient RPA classification. No confirmatory hypothesis testing was planned for this phase II study and no adjustment for multiple testing was performed. Cilengitide PK parameters were analyzed using standard descriptive statistics; a noncompartmental analysis was performed using data from a subset of 11 subjects in the intensive cilengitide regimen after single (day 1) and repeated dosing (day 5) in week 1 of the study.
Results
Patient Characteristics
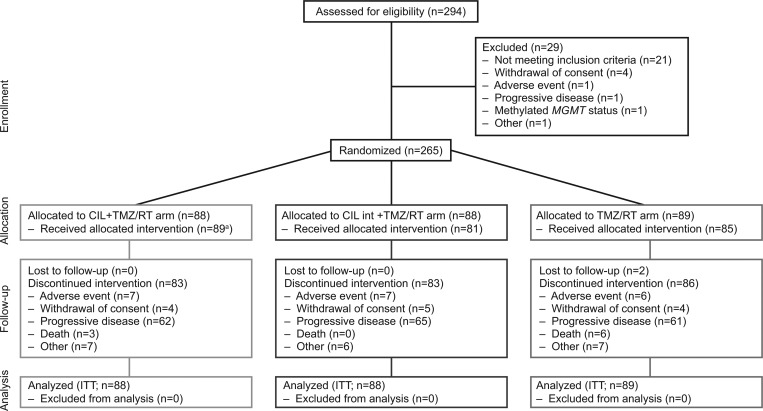
Patients were first enrolled for the safety run-in part of the trial on March 6, 2009, and the cutoff date for the primary analysis was February 7, 2013. Twelve patients were enrolled in the safety run-in phase; in the randomized part, a total of 265 patients were enrolled and allocated to either the standard cilengitide arm (n = 88), the intensive cilengitide arm (n = 88), or the control arm (n = 89) (Fig. 1). Of these, 89 patients received standard cilengitide plus TMZ/RT→TMZ (including 3 patients who were randomized to cilengitide intensive treatment but received cilengitide standard treatment), 81 patients received intensive cilengitide plus TMZ/RT→TMZ, and 85 patients were treated in the control arm. Patient baseline characteristics and demographics were similar across treatment arms and are summarized in Table 1. All patients underwent histology review: 94.7% of patients had glioblastoma, 3.8% of patients had gliosarcoma, and 1.1% of patients had a giant-cell glioblastoma.
Fig. 1.
CONSORT diagram. aIncludes 3 patients who were randomized to cilengitide intensive (CIL int) treatment but actually received cilengitide standard treatment.
In total, 252 patients (95.0%) discontinued treatment. PD was the most common reason for discontinuation: 62 patients (70.0%) in the standard cilengitide arm, 65 patients (73.9%) in the intensive cilengitide arm, and 61 patients (68.5%) in the control arm (Fig. 1). Overall, 165 patients (62.3%) received anticancer follow-up therapy. The most frequent follow-up treatments were cytotoxic chemotherapy for 119 patients, surgery for 47 patients, and treatment targeting pathways of vascular endothelial growth factor for 39 patients. Of the patients receiving follow-up antineoplastic agents, 29.5% in the standard cilengitide arm, 33% in the intensive cilengitide arm, and 22.5% in the control arm received bevacizumab.
Safety of Intensive Cilengitide Dosing (Safety Run-in)
Twelve patients completed the safety run-in; no MTD for cilengitide was identified. No DLTs were observed in cohorts 1 and 2; 1 patient experienced a DLT of hyperbilirubinemia in cohort 3. This cohort was then expanded to 6 patients. No further DLTs were observed. Four serious AEs considered related to cilengitide were observed in 2 patients (hyperbilirubinemia, elevated alanine aminotransferase, and elevated aspartate aminotransferase in 1 patient; and pulmonary embolism in another). No AEs leading to death were reported during the safety run-in. The overall safety profile observed in this safety run-in was consistent with the underlying malignancy of the patients and/or reflected AEs associated with the TMZ/RT→TMZ regimen. The safety monitoring committee recommended cilengitide 2000 mg administered 5 times weekly for evaluation as intensive regimen in the randomized part of the CORE study.
Treatment Exposure (Randomized Study)
Patients in the intensive cilengitide arm received cilengitide for a median of 34.3 weeks compared with a median of 28.3 weeks for patients in the standard cilengitide arm. Similarly, patients in the intensive cilengitide arm received TMZ for a median of 34.0 weeks compared with a median duration of 28.0 weeks in the standard cilengitide arm. In the control arm, TMZ was administered for a median of 30.0 weeks.
Overall Survival
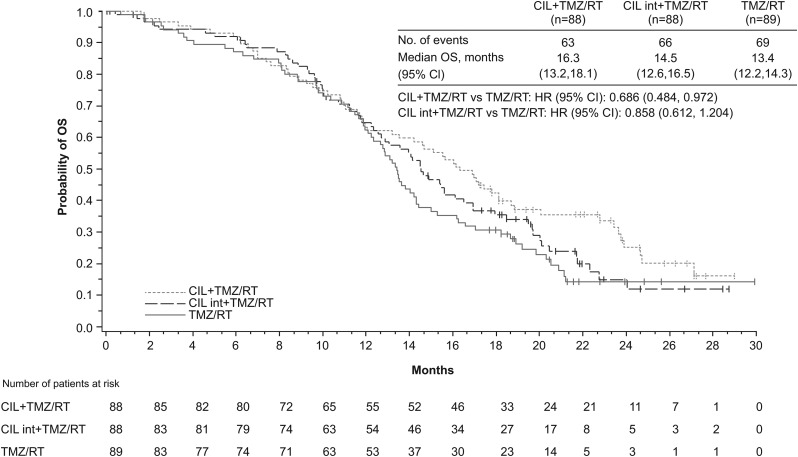
The median OS was 13.4 months (range, 0–30 mo) in the control arm, 16.3 months (range, 0–29) in the standard cilengitide arm, and 14.5 months (range, 0–29) in the intensive cilengitide arm (Fig. 2). The HR (stratified for RPA classes III/IV–V) in the standard cilengitide arm versus the control arm was 0.686 (95% CI: 0.484, 0.972; P = .0328). The HR (stratified for RPA classes III/IV–V) for the intensive cilengitide versus the control arm was 0.858 (95% CI: 0.612, 1.204; P = .3771). Forest plots of OS in the standard cilengitide versus control and the intensive cilengitide versus control arms according to patient baseline characteristics are provided in Supplementary Fig. S1.
Fig. 2.
Kaplan–Meier estimate for OS in the 3 treatment arms of the CORE phase II study. CIL, cilengitide; int, intensive.
Progression-Free Survival
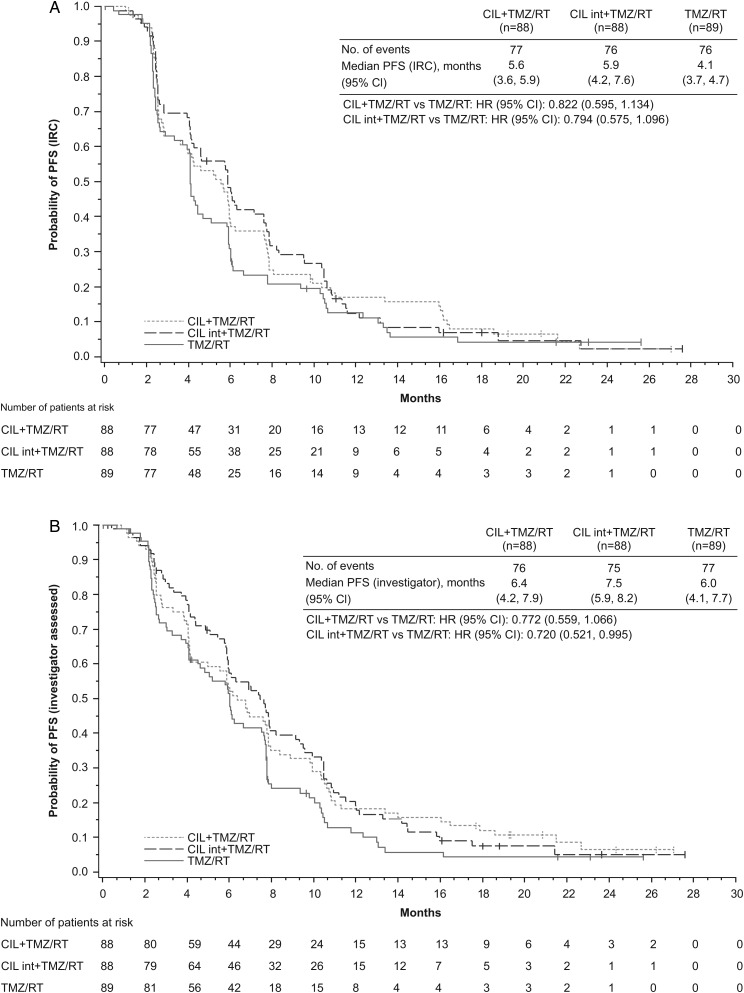
Based on the IRC assessment, median PFS was 4.1 months (range, 0–26) in the control arm, 5.6 months (range, 0–27) in the standard cilengitide arm, and 5.9 months (range, 0–28) in the intensive cilengitide arm (Fig. 3A). The HR (stratified for RPA classes III/IV–V) for the standard cilengitide versus the control arm was 0.822 (95% CI: 0.595, 1.134); for the intensive cilengitide versus control arm the HR (stratified for RPA classes III/IV–V) was 0.794 (95% CI: 0.575, 1.096). Forest plots illustrating the PFS per IRC assessment in the standard cilengitide versus control and the intensive cilengitide versus control arms based on patient baseline characteristics are provided in Supplementary Fig. S2.
Fig. 3.
Kaplan–Meier estimate for PFS assessed (A) by IRC and (B) by investigators. CIL, cilengitide; int, intensive.
The median PFS as assessed by investigators was 6.0 months (range, 0–26) in the control arm, 6.4 months (range, 0–27) in the standard cilengitide arm, and 7.5 months (range, 0–28) in the intensive cilengitide arm (Fig. 3B). The HR (stratified for RPA classes III/IV–V) for the standard cilengitide versus the control arm was 0.772 (95% CI: 0.559, 1.066). For the comparison of the intensive cilengitide versus the control arm the HR (stratified for RPA classes III/IV–V) was 0.720 (95% CI: 0.521, 0.995). Forest plots describing investigator assessed PFS in the standard cilengitide versus control and the intensive cilengitide versus control arms according to patient baseline characteristics are shown in Supplementary Fig. S3.
Pharmacokinetic Profile
On day 1, after a single dose, exposure to cilengitide as defined by AUC0−t was 259 526 h*ng/mL (geometric mean). The Cmax of 105 336 ng/mL (geometric mean) was reached a median of 1.02 h after the start of cilengitide infusion in concordance with the EOI. The median t1/2 was 2.00 h and the geometric mean CL was 122.28 mL/min. The geometric mean volume of distribution was 23.98 L. On day 5 (after 5 repeated doses of cilengitide), exposure to cilengitide was 297 961 h*ng/mL (geometric mean AUC0−t). The Cmax of 128 670 ng/mL (geometric mean) was reached a median of 1.02 h after the start of cilengitide infusion, again in concordance with the EOI. The median t1/2 was 2.35 h and the geometric mean CL was 104.15 mL/min. The Vss (geometric mean) was 17.39 L. Cilengitide did not accumulate after 5 daily infusions of cilengitide 2000 mg daily, most likely as a consequence of the short t1/2 of the drug. Overall, the observed exposure to cilengitide in patients in this study was comparable to that observed for cilengitide in previous clinical studies in glioblastoma patients.11–13
Safety
Treatment-emergent adverse events (TEAEs) occurred in 82 patients (96.5%) in the control arm, 88 patients (98.9%) in the standard cilengitide arm, and 80 patients (98.8%) in the intensive cilengitide arm (Table 2). The most commonly observed TEAEs are listed in Table 3. Serious TEAEs were reported in 30 patients (35.3%) in the control arm, 47 patients (52.8%) in the standard cilengitide arm, and 36 patients (44.4%) in the intensive cilengitide arm. Death due to a TEAE occurred in 5 patients (5.9%) in the control arm, 8 patients (9.0%) in the standard cilengitide arm, and 8 patients (9.9%) in the intensive cilengitide arm. Of these, 5 deaths were considered as related to study treatment (cilengitide, TMZ, or RT): 2 cases of pancytopenia (1 patient each in the intensive cilengitide and control arms), 1 case of thrombocytopenia in the standard cilengitide arm, and 2 cases of pulmonary embolism (1 patient in each of the 2 cilengitide-containing treatment arms).
Table 2.
Treatment-emergent adverse events (safety population)
| Standard Cilengitide Arm (n = 89a) | Intensive Cilengitide Arm (n = 81) | Control Arm (n = 85) | |
|---|---|---|---|
| TEAEs, n (%) | |||
| All | 88 (98.9) | 80 (98.8) | 82 (96.5) |
| Study treatmentb–related | 70 (78.7) | 64 (79.0) | 56 (65.9) |
| Serious TEAEs, n (%) | |||
| All | 47 (52.8) | 36 (44.4) | 30 (35.3) |
| Study treatment*–related | 13 (14.6) | 4 (4.9) | 5 (5.9) |
| NCI-CTCAE grade 3 or 4 TEAEs, n (%) | |||
| All | 57 (64.0) | 47 (58.0) | 45 (52.9) |
| Study treatmentb–related | 25 (28.1) | 19 (23.5) | 17 (20.0) |
| TEAEs leading to death, n (%) | |||
| All | 8 (9.0) | 8 (9.9) | 5 (5.9) |
| Study treatmentb–related | 2 (2.2) | 2 (2.5) | 1 (1.2) |
Abbreviation: NCI-CTCAE, National Cancer Institute's Common Terminology Criteria for Adverse Events.
aIncludes 3 patients who were randomized to cilengitide intensive treatment but actually received cilengitide standard treatment; they were therefore allocated to the cilengitide standard treatment group for the safety population.
bCilengitide, radiotherapy, or temozolomide.
Table 3.
Most common TEAEsa by system organ class and preferred term (safety population)
| System Organ Class | Standard Cilengitide Arm (n = 89b) |
Intensive Cilengitide Arm (n = 81) |
Control Arm (n = 85) |
|||
|---|---|---|---|---|---|---|
| Preferred Term, n (%) | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | Any Grade | Grade ≥3 |
| Blood and lymphatic system disorders | 27 (30.3) | 13 (14.6) | 31 (38.3) | 14 (17.3) | 28 (32.9) | 11 (12.9) |
| Anemia | 6 (6.7) | 3 (3.4) | 9 (11.1) | 1 (1.2) | 1 (1.2) | 1 (1.2) |
| Lymphopenia | 9 (10.1) | 6 (6.7) | 7 (8.6) | 2 (2.5) | 7 (8.2) | 5 (5.9) |
| Neutropenia | 12 (13.5) | 5 (5.6) | 10 (12.3) | 1 (1.2) | 8 (9.4) | 2 (2.4) |
| Thrombocytopenia | 11 (12.4) | 4 (4.5) | 15 (18.5) | 9 (11.1) | 17 (20.0) | 7 (8.2) |
| Gastrointestinal disorders | 56 (62.9) | 3 (3.4) | 52 (64.2) | 3 (3.7) | 51 (60.0) | 2 (2.4) |
| Constipation | 26 (29.2) | 0 (0.0) | 27 (33.3) | 1 (1.2) | 27 (31.8) | 0 (0.0) |
| Diarrhea | 9 (10.1) | 0 (0.0) | 6 (7.4) | 1 (1.2) | 2 (2.4) | 0 (0.0) |
| Nausea | 33 (37.1) | 1 (1.1) | 31 (38.3) | 1 (1.2) | 30 (35.3) | 1 (1.2) |
| Vomiting | 19 (21.3) | 1 (1.1) | 18 (22.2) | 1 (1.2) | 21 (24.7) | 2 (2.4) |
| General disorders and administration site conditions | 66 (74.2) | 13 (14.6) | 57 (70.4) | 9 (11.1) | 42 (49.4) | 10 (11.8) |
| Asthenia | 22 (24.7) | 2 (2.2) | 25 (30.9) | 4 (4.9) | 12 (14.1) | 3 (3.5) |
| Fatigue | 28 (31.5) | 5 (5.6) | 21 (25.9) | 2 (2.5) | 21 (24.7) | 4 (4.7) |
| Gait disturbance | 3 (3.4) | 0 (0.0) | 7 (8.6) | 0 (0.0) | 11 (12.9) | 2 (2.4) |
| Peripheral edema | 9 (10.1) | 1 (1.1) | 5 (6.2) | 0 (0.0) | 8 (9.4) | 0 (0.0) |
| Pyrexia | 17 (19.1) | 0 (0.0) | 14 (17.3) | 0 (0.0) | 11 (12.9) | 1 (1.2) |
| Infections and infestations | 43 (48.3) | 6 (6.7) | 42 (51.9) | 2 (2.5) | 25 (29.4) | 2 (2.4) |
| Nasopharyngitis | 10 (11.2) | 1 (1.1) | 5 (6.2) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Upper respiratory tract infection | 6 (6.7) | 0 (0.0) | 10 (12.3) | 0 (0.0) | 5 (5.9) | 0 (0.0) |
| Urinary tract infection | 6 (6.7) | 0 (0.0) | 10 (12.3) | 0 (0.0) | 6 (7.1) | 0 (0.0) |
| Injury, poisoning, and procedural complications | 24 (27.0) | 2 (2.2) | 16 (19.8) | 1 (1.2) | 16 (18.8) | 0 (0.0) |
| Radiation skin injury | 11 (12.4) | 0 (0.0) | 3 (3.7) | 0 (0.0) | 3 (3.5) | 0 (0.0) |
| Investigations | 18 (20.2) | 7 (7.8) | 23 (28.4) | 5 (6.2) | 21 (24.7) | 9 (10.6) |
| Platelet count decreased | 3 (3.4) | 2 (2.2) | 1 (1.2) | 0 (0.0) | 5 (5.9) | 4 (4.8) |
| Metabolism and nutrition disorders | 31 (34.8) | 5 (5.6) | 31 (38.3) | 7 (8.6) | 26 (30.6) | 3 (3.5) |
| Decreased appetite | 21 (23.6) | 1 (1.1) | 18 (22.2) | 0 (0.0) | 18 (21.2) | 1 (1.2) |
| Musculoskeletal and connective tissue disorders | 33 (37.1) | 4 (4.5) | 36 (44.4) | 1 (1.2) | 16 (18.8) | 2 (2.4) |
| Muscular weakness | 10 (11.2) | 3 (3.4) | 19 (23.5) | 1 (1.2) | 4 (4.7) | 1 (1.2) |
| Pain in extremity | 9 (10.1) | 1 (1.1) | 3 (3.7) | 0 (0.0) | 6 (7.1) | 0 (0.0) |
| Nervous system disorders | 67 (75.3) | 26 (29.2) | 54 (66.7) | 27 (33.3) | 54 (63.5) | 20 (23.5) |
| Convulsion | 13 (14.6) | 5 (5.6) | 14 (17.3) | 6 (7.4) | 12 (14.1) | 5 (5.9) |
| Epilepsy | 6 (6.7) | 4 (4.5) | 4 (4.9) | 2 (2.5) | 2 (2.4) | 1 (1.2) |
| Headache | 39 (43.8) | 4 (4.5) | 33 (40.7) | 4 (4.9) | 28 (32.9) | 4 (4.7) |
| Hemiparesis | 8 (9.0) | 5 (5.6) | 7 (8.6) | 5 (6.2) | 9 (10.6) | 6 (7.1) |
| Neurologic status worsening | 0 (0.0) | 0 (0.0) | 6 (7.4) | 5 (6.2) | 0 (0.0) | 0 (0.0) |
| Psychiatric disorders | 32 (36.0) | 3 (3.4) | 33 (40.7) | 1 (1.2) | 24 (28.2) | 2 (2.4) |
| Depression | 12 (13.5) | 0 (0.0) | 9 (11.1) | 0 (0.0) | 2 (2.4) | 0 (0.0) |
| Insomnia | 12 (13.5) | 0 (0.0) | 15 (18.5) | 0 (0.0) | 12 (14.1) | 1 (1.2) |
| Respiratory, thoracic, and mediastinal disorders | 23 (25.8) | 13 (14.6) | 18 (22.2) | 4 (4.9) | 18 (21.2) | 3 (3.6) |
| Cough | 9 (10.1) | 1 (1.1) | 7 (8.6) | 0 (0.0) | 3 (3.5) | 0 (0.0) |
| Pulmonary embolism | 10 (11.2) | 10 (11.2) | 2 (2.5) | 2 (2.5) | 1 (1.2) | 1 (1.2) |
| Skin and subcutaneous tissue disorders | 44 (49.4) | 3 (3.4) | 39 (48.1) | 2 (2.5) | 26 (30.6) | 0 (0.0) |
| Alopecia | 14 (15.7) | 0 (0.0) | 18 (22.2) | 2 (2.5) | 11 (12.9) | 0 (0.0) |
| Pruritus | 9 (10.1) | 0 (0.0) | 11 (13.6) | 0 (0.0) | 5 (5.9) | 0 (0.0) |
| Rash | 11 (12.4) | 1 (1.1) | 8 (9.9) | 0 (0.0) | 3 (3.5) | 0 (0.0) |
| Vascular disorders | 19 (21.3) | 12 (13.5) | 17 (21.0) | 4 (4.9) | 13 (15.3) | 4 (4.7) |
| Deep vein thrombosis | 7 (7.9) | 7 (7.9) | 2 (2.5) | 2 (2.5) | 4 (4.7) | 3 (3.5) |
aIn either treatment arm ≥10% TEAE of any grade or ≥4% TEAE of grade 3.
bIncludes 3 patients who were randomized to cilengitide intensive treatment but actually received cilengitide standard treatment; they were therefore allocated to the cilengitide standard treatment group for the safety population.
Discussion
The CORE trial was conducted to investigate the safety and efficacy of standard and intensive cilengitide dosing in combination with TMZ/RT→TMZ in patients with newly diagnosed glioblastoma and an unmethylated MGMT gene promoter. Prior to the actual phase II part of the trial, a safety run-in was performed because of the lack of prior clinical experience with 3, 4, or 5 times weekly intensive cilengitide dosing as a single agent or in combination regimens for glioblastoma treatment. The safety run-in did not identify an MTD and demonstrated that the intensive regimen of cilengitide 2000 mg administered 5 times weekly was well tolerated and suitable for further investigation.
The primary objective of the randomized phase II trial was OS in the cilengitide treatment arms compared with the control arm that received standard TMZ/RT→TMZ alone. There were no major imbalances in prognostic factors between the treatment arms in this study. Median OS was increased in both the standard twice-weekly and in the intensive 5-times-weekly cilengitide treatment arms, compared with the control arm. However, in contrast to the synergy between cilengitide and RT previously demonstrated in orthotopic in vivo models of glioblastoma,17 the observed increase in OS was more pronounced in the standard cilengitide arm compared with the intensive cilengitide arm. Conversely, the observed increase in median PFS over the control arm, as assessed by both the IRC and investigator, was greater in the intensive cilengitide arm compared with the standard cilengitide arm. These contradictory results may, in part, be related to compartmental expression of the target integrins by proliferating endothelial cells or tumor cells, which can enable cilengitide to have antiangiogenic and antitumor effects. The paradox that with standard-dose cilengitide a greater improvement in OS was observed than with the intensive dose can currently not be explained. Of note, the median OS and PFS observed for the control arm of the CORE study were similar to those previously reported for patients with newly diagnosed glioblastoma and unmethylated MGMT promoter treated with TMZ/RT→TMZ.6 The determination of progression remains a challenge in the postradiation glioblastoma population, and this may have contributed to observed inconsistencies for this endpoint. Further questions about the relative efficacy of the treatment arms were raised by the late separation of the OS curves, which did not diverge in the 3 different treatment arms until 1 year postrandomization. The significance of this observation is unclear. However, a possible explanation could be that tumor molecular heterogeneity resulted in a subset of patients having a survival advantage due to tumors that may be more dependent on integrin signaling.
The multicenter, randomized, open-label, phase III companion study CENTRIC investigated efficacy and safety of twice-weekly infusions of 2000 mg cilengitide added to TMZ/RT→TMZ in the complementary population of patients with newly diagnosed glioblastoma and methylated MGMT gene promoter.16 Independent of treatment, MGMT promoter methylation has been shown to be a favorable prognostic factor, associated with an OS of 18.2 months, compared with 12.2 months for patients with unmethylated MGMT promoter status (P < .001).6 In the CENTRIC study, the addition of cilengitide to standard TMZ/RT→TMZ treatment failed to prolong OS (26 mo in both cilengitide and control arms; HR = 1.02, P = .86), and only a slight increase in PFS was observed in the cilengitide versus the control arm (13.5 vs 10.7 mo, HR 0.93, P = .48).16
The limited efficacy benefit observed in the CORE trial together with the negative outcomes of CENTRIC suggest that the addition of cilengitide as administered in these studies does not improve outcome when added to TMZ/RT→TMZ for newly diagnosed glioblastoma patients. In addition, due to the relatively small sample size, CORE was not powered to detect statistically significant differences between the cilengitide treatment arms compared with the control arm in terms of the efficacy outcomes.
The PK analysis revealed a PK profile in line with previously reported PK analyses of cilengitide12,13 characterized by a short elimination half-life, leading to a lack of accumulation after repeated dosing. However, the short half-life may mean that constant infusion is required or a modification in the drug structure/formulation may be needed. Maximum plasma concentrations were reached shortly after the end of treatment infusion, as in previous studies.12,13 In the current study, both cilengitide regimens (standard and intensive) were well tolerated and no new safety concerns for cilengitide administered in combination with TMZ/RT→TMZ were observed. The incidence of pulmonary embolism was higher in the cilengitide arms in this trial. Thromboembolic events associated with cilengitide have also been reported in previous cilengitide studies,11,14,16 although in another phase I/II cilengitide study the incidence did not appear to increase following the addition of cilengitide.13 Overall, the AE profile observed in the cilengitide treatment arms was as expected for patients with glioblastoma and similar to that previously reported in clinical trials of cilengitide.11–13,16
In conclusion, the addition of either standard regimen of twice-weekly 2000 mg/d infusions of cilengitide or an intensified regimen of 2000 mg/d 5 times per week to the standard of care with TMZ/RT→TMZ was well tolerated in patients with newly diagnosed glioblastoma with an unmethylated MGMT gene promoter. However, modest improvement in OS and PFS outcomes between the cilengitide arms of this exploratory phase II study together with the negative efficacy outcome of cilengitide in the phase III CENTRIC trial16 indicate that cilengitide as dosed in these studies is ineffective when added to TMZ/RT→TMZ for newly diagnosed glioblastoma patients. Further clinical investigations may be warranted if a potentially predictive biomarker of response to cilengitide is identified, such as αvβ3 and αvβ5 expression on tumor tissue or proliferating endothelial cells. Although results of the CORE and CENTRIC trials are disappointing, integrins are critically involved in several facets of glioblastoma biology and thus remain attractive therapeutic targets.
Supplementary Material
Funding
The trial was sponsored by Merck KGaA, Darmstadt, Germany.
Supplementary Material
Acknowledgments
Editorial and medical writing assistance was provided by Eva Polk, PhD CMPP, TRM Oncology, The Hague, The Netherlands, funded by Merck KGaA, Darmstadt, Germany.
Conflict of interest statement. L.B.N. serves on a noncompensated advisory board for Merck. R.T. received investigator fees as principal investigator during the study from Merck KGaA. D.H.N. received a research grant from Merck Serono. M.M. received investigator fees as principal investigator from Merck KGaA. C.H. has stock options in and is an employee of Merck KGaA. M.P. filed patents for and is an employee of Merck KGaA. D.A.R. is a paid advisor for Merck/Schering and EMD Serono and a paid consultant for Merck/Schering. M.E.H. has a service contract from EMD Serono and receives advisory fees from Merck/Schering, Roche, and MDxHealth. All other authors have nothing to declare.
References
- 1.Ostrom QT, Gittleman H, Farahet P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(suppl 2):ii1–ii56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haar CP, Hebbar P, Wallace GC, 4th, et al. Drug resistance in glioblastoma: a mini review. Neurochem Res. 2012;37(6):1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. 2014. NCCN clinical practice guidelines in oncology (NCCN Guidelines): central nervous system cancers. Version 2.2014, Comprehensive Cancer Network, Fort Washington, PA Available at http://www.nccn.org/ Accessed March 5, 2015. [Google Scholar]
- 5.Hegi ME, Diserens AC, Godard S, et al. Clinical trial substantiates the predictive value of O-6-methylguanine-DNA methyltransferase promoter methylation in glioblastoma patients treated with temozolomide. Clin Cancer Res. 2004;10(6):1871–1874. [DOI] [PubMed] [Google Scholar]
- 6.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. [DOI] [PubMed] [Google Scholar]
- 7.Nagane M, Kobayashi K, Ohnishi A, et al. Prognostic significance of O6-methylguanine-DNA methyltransferase protein expression in patients with recurrent glioblastoma treated with temozolomide. Jpn J Clin Oncol. 2007;37(12):897–906. [DOI] [PubMed] [Google Scholar]
- 8.Smith JW. Cilengitide Merck. Curr Opin Investig Drugs. 2003;4(6):741–745. [PubMed] [Google Scholar]
- 9.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 2010;10(1):9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bello L, Francolini M, Marthyn P, et al. Alpha(v)beta3 and alpha(v)beta5 integrin expression in glioma periphery. Neurosurgery. 2001;49(2):380–389. [DOI] [PubMed] [Google Scholar]
- 11.Nabors LB, Mikkelsen T, Rosenfeld SS, et al. Phase I and correlative biology study of cilengitide in patients with recurrent malignant glioma. J Clin Oncol. 2007;25(13):1651–1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reardon DA, Fink KL, Mikkelsen T, et al. Randomized phase II study of cilengitide, an integrin-targeting arginine-glycine-aspartic acid peptide, in recurrent glioblastoma multiforme. J Clin Oncol. 2008;26(34):5610–5617. [DOI] [PubMed] [Google Scholar]
- 13.Stupp R, Hegi ME, Neyns B, et al. Phase I/IIa study of cilengitide and temozolomide with concomitant radiotherapy followed by cilengitide and temozolomide maintenance therapy in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(16):2712–2718. [DOI] [PubMed] [Google Scholar]
- 14.Nabors LB, Mikkelsen T, Hegi ME, et al. A safety run-in and randomized phase 2 study of cilengitide combined with chemoradiation for newly diagnosed glioblastoma (NABTT 0306). Cancer. 2012;118(22):5601–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilbert MR, Kuhn J, Lamborn KR, et al. Cilengitide in patients with recurrent glioblastoma: the results of NABTC 03–02, a phase II trial with measures of treatment delivery. J Neurooncol. 2012;106(1):147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stupp R, Hegi ME, Gorlia T, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1100–1108. [DOI] [PubMed] [Google Scholar]
- 17.Mikkelsen T, Brodie C, Finniss S, et al. Radiation sensitization of glioblastoma by cilengitide has unanticipated schedule-dependency. Int J Cancer. 2009;124(11):2719–2727. [DOI] [PubMed] [Google Scholar]
- 18.Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 19.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.