Abstract
While the use of targeted therapies, particularly radiosurgery, has broadened therapeutic options for CNS metastases, patients respond minimally and prognosis remains poor. The inability of many systemic chemotherapeutic agents to penetrate the blood-brain barrier (BBB) has limited their use and allowed brain metastases to become a burgeoning clinical challenge. Adequate preclinical models that appropriately mimic the metastatic process, the BBB, and blood-tumor barriers (BTB) are needed to better evaluate therapies that have the ability to enhance delivery through or penetrate into these barriers and to understand the mechanisms of resistance to therapy. The heterogeneity among and within different solid tumors and subtypes of solid tumors further adds to the difficulties in determining the most appropriate treatment approaches and methods of laboratory and clinical studies. This review article discusses therapies focused on prevention and treatment of CNS metastases, particularly regarding the BBB, and the challenges and opportunities these therapies present.
Keywords: brain metastases, CNS sanctuary, imaging, prevention, therapy
Brain metastases are the most common intracranial tumor in adults and are 10 times more common than primary malignant CNS tumors, with at least 170 000 new cases reported in the United States each year.1 Up to 10% of all patients with advanced cancer (particularly lung and breast) will develop CNS involvement, with HER2-positive breast cancer patients having as high as a 30% incidence of brain metastases.2 Importantly, the incidence of CNS metastasis is increasing.2,4 While this is due in part to increased diagnoses from improved imaging techniques and the widespread availability of MRI, it also likely reflects improved overall survival (OS) in patients with better control of peripheral disease.1 Unfortunately, limited treatment strategies exist for management of sanctuary disease in the brain.2–5 Chemotherapy, radiation therapy, and surgery can result in small improvements in survival for a subset of patients, but many patients with CNS involvement die from neurological progression despite controlled systemic disease.6 The increased rate of diagnosis and lack of effective therapies are of significant concern.
The neurovascular unit (NVU), which consists of blood-brain barrier (BBB) endothelial cells and surrounding pericytes, astrocytes, and neurons as well as basement membrane and extracellular matrix, is heterogeneous in brain metastases. Tumor neovasculature has disorganized structure, basement membrane abnormalities, and dysfunctional tight junctions.7,8 Conversely, infiltrating metastatic cells can make use of the existing brain vasculature with a largely intact BBB.7 Inflammatory reactions to infiltrating tumor cells can also alter the permeability and function of the NVU.9 Heterogeneous NVU function and inflammation within the tumor mass at the proliferating edge of the tumor and peritumoral brain around tumor result in inconsistent delivery of imaging and therapeutic agents.10 This review discusses novel approaches for imaging, treatment, and prevention of CNS metastases.
Imaging of Brain Metastases
Magnetic Resonance Imaging
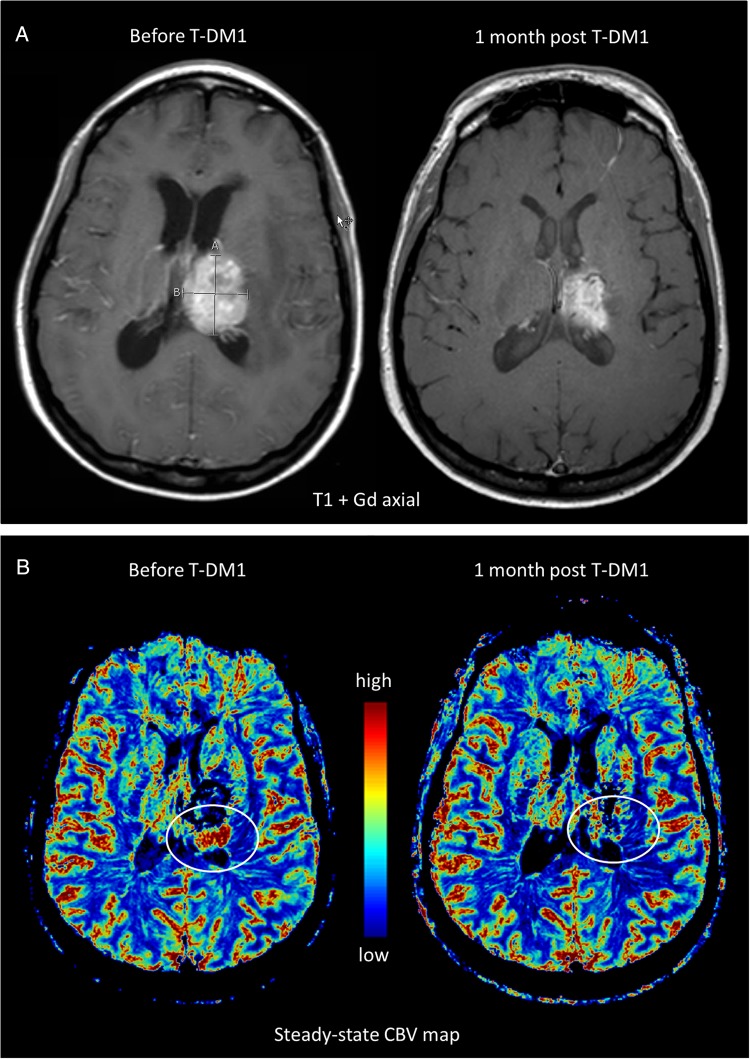
Contrast-enhanced MRI is the current gold standard for diagnosing CNS metastases (Fig. 1). However, 2 main challenges exist with regard to imaging CNS metastases. First is the differentiation between primary malignancies and solitary metastases. High-resolution gadolinium (Gd)-enhanced T1 and T2 fluid-attenuated inversion recovery imaging is highly sensitive, but only modestly specific, and is therefore not ideal for differentiation. The second challenge is determining tumor response to therapy and then differentiating response from recurrence or treatment-related changes. Radiological guidelines such as RECIST and Macdonald criteria for response assessment in solid tumors are inadequate for the CNS due to the unique anatomy of brain vasculature, including the BBB and the microenvironment of the NVU, as well as the inherent heterogeneity of brain metastases.10 Currently the updated Response Assessment in Neuro-Oncology (RANO) criteria are used for evaluation of brain metastases11 but remain controversial, particularly for assessing neuroinflammatory processes and the impact of vascular targeting agents. Various advanced MRI techniques have been tested to overcome these challenges. A case example describing these issues is shown in Fig. 1. This case is not atypical and illustrates a number of inherent challenges in the treatment of patients with brain metastases including drug delivery, effective imaging, and distinguishing response from progression or radiation-induced necrosis.
Fig. 1.
Relative cerebral blood volume (rCBV) to evaluate response in a brain metastasis. A 38-year-old woman with HER2-overexpressing, node-positive breast cancer was found to have an asymptomatic left frontal brain metastasis during screening MRI. The patient received multiple treatments including stereotactic radiosurgery, systemic therapy with lapatinib and docetaxel, whole brain radiation therapy, and i.v. trastuzumab and lapatinib, followed by recurrence each time. (A) Conventional T1-weighted MRI with gadolinium-based contrast (T1+Gd) showed a large mass. (B) Subsequent steady-state MRI with ferumoxytol showed increased rCBV along the superior and posterior margins of the thalamic lesion, indicative of active tumor (arrow). The patient was then started on trastuzumab-emtansine (T-DM1) an antibody drug conjugate. Three weeks later, there was a partial response with 50% decrease of the enhancing area on MRI and a marked decrease in CBV on steady-state MRI.
Neuroimaging techniques to assess peritumoral edema may be useful for differentiating metastases from gliomas and for assessing the impact of the infiltrative phenotype. While brain metastases show microscopic infiltration in the brain around tumor,12 the peritumoral areas comprise predominantly vasogenic edema.13 In contrast, gliomas show malignant cell infiltration throughout the peritumoral edematous region. This difference can be detected with proton magnetic resonance spectroscopy to assess tissue metabolites using single voxel or multivoxel chemical shift-imaging techniques.13 Minimal peritumoral edema in brain metastases may actually be associated with elevated levels of brain infiltration and decreased survival.14 Diffusion-weighted MRI to assess water molecule mobility can demonstrate changes in the apparent diffusion coefficient at the infiltrating edge of brain metastases and peritumoral region that can provide a biomarker to assess response to radiation therapy15 and predict patient outcomes.16
Dynamic susceptibility contrast-enhanced (DSC) perfusion-weighted imaging, when used to estimate relative cerebral blood volume (rCBV) in tumor and surrounding brain, has shown value for distinguishing primary from metastatic cerebral malignancies and assessing response to therapy. DSC adds little to no extra cost to a Gd-enhanced MRI scan while adding significant prognostic value.17 A number of studies have reported higher rCBV values in the enhancing component of high-grade gliomas than in metastasis, although there is high variability with some investigators showing no difference in rCBV in the tumor mass.18 In contrast, examination of the peritumoral edema region shows an even higher rCBV difference between gliomas and metastasis (1.31 vs 0.39, respectively [P = .001]) that may be diagnostic.18 These differences likely reflect the infiltrating angiogenic tumor cells in the peritumoral region of gliomas versus pure vasogenic edema in metastatic disease.
Relative cerebral blood volume analysis is also being used to differentiate progression from radiation injury or inflammatory responses to therapy (pseudoprogression). This is an important diagnostic dilemma that impacts prognosis and the decision to change therapies. Using a lesion rCBV threshold of 1.5–2.5 correlated with survival in the differentiation of tumor progression from chemoradiation injury.17,19,20 Further improvements in rCBV analysis may be obtained using preload or leakage correction algorithms with Gd-based contrast agents or ferumoxytol iron oxide nanoparticles that have no early leakage.17–19,21
Varallyay et al recently described the use of ferumoxytol MRI17,19 to obtain a high-resolution, steady-state-CBV image that differentiates regions of high vascularity and active tumor growth (Fig. 1B). Routine imaging following ferumoxytol administration can be limited by residual T1 and possibly T2 enhancement in the first week following administration and may persist for 4 weeks or longer in rare instances. The signal alterations are similar to common residual blood products in the subacute and chronic postsurgical patient. With pre-Gd T1 studies and foreknowledge of prior ferumoxytol administration, there is minimal issue with misdiagnosis in the follow-up imaging.22,23
Positron Emission Tomography
Positron emission tomography (PET) to detect localized concentrations of tracers containing radionuclides such as 18F, 11C, or 13N can be used to diagnose and monitor CNS metastases. Standard PET imaging with the prototypical fluorodeoxyglucose (FDG) tracer is limited by high background glucose uptake in the brain, loss of PET avidity in previously irradiated areas in the brain over time, and high metabolic activity that is indistinguishable from active metastases in recently treated areas.24 A number of strategies are being investigated to overcome these limitations. The use of dual time point FDG-PET imaging is particularly useful for distinguishing malignancy from more benign causes of enhancement. Using dual-phase FDG-PET, the standardized uptake values of metastases, when compared with gray matter ratios as a function of time, was found to be >95% accurate for distinguishing between the lesion versus radiation necrosis (n = 25 patients).24 Integrated PET-MRI has been used in glioma but has not yet been reported in brain metastases.25
Amino acid PET tracers may better differentiate normal brain from tumors because these regions show low and high amino acid uptake, respectively. Sensitivity and specificity upwards of 90% have been described with this method.26,27 PET may also be able to distinguish between treatment-related changes and disease recurrence. Using O-(2-18F-fluoroethyl)-L-tyrosine (18F-FET) tumor-to-brain ratios, in combination with time uptake curves, correctly identified tumor versus necrosis (n = 21) versus metastatic tumor growth (n = 19) in 93% of cases.28 Similarly, 18-F-DOPA, with low physiological uptake but high tumor-to-normal-brain uptake, may have greater accuracy than 18F-FDG for identifying recurrence versus radiation necrosis.29 Specific PET tracers may be used to detect membrane changes consistent with apoptosis as an indication of therapeutic response.30
PET-radiolabeled pharmaceuticals may potentially be used for both imaging and treat brain metastases. For example, imaging (89Zr)-trastuzumab in the brain metastases of patients with HER2-positive breast cancers showed specific tumor localization. Uptake by brain metastases are 18-fold higher than in normal brain.31
There are many challenges inherent to imaging brain metastases, but there are also many opportunities for improvement as well. New techniques can be helpful for distinguishing the etiology of CNS lesions and assessing the response to therapy, but there have been difficulties implementing these techniques in national clinical trials. Collaboration between institutions involved in brain metastases imaging trials will be the key to moving these imaging strategies forward.
Radiation Therapy for Brain Metastases
Whole brain radiotherapy (WBRT) is a successful strategy for both primary prevention (eg, prophylactic cranial irradiation for small cell lung cancer [SCLC])32 and secondary prevention (eg, after surgical resection or stereotactic radiosurgery (SRS) for limited metastases) of CNS metastases.3,33
Over the past decade, management of CNS metastases has shifted from universal use of WBRT to techniques that allow targeting individual lesions, such as MRI SRS techniques, which allow application of high doses of radiation with appropriate safety to surrounding brain. Use of SRS without WBRT was a controversial concept because it was believed that there would be multiple, diffuse areas of spread once tumor cells had breached the BBB, even if it had not been detected. Randomized trials have confirmed that survival is similar for patients with 1–4 lesions (treated with SRS with or without additional WBRT3), and a recent meta-analysis has indicated that addition of WBRT is more effective for controlling metastatic recurrence than SRS alone (but there was no survival benefit).33 In contrast, patients receiving WBRT showed decreased quality of life and cognitive function.33
SRS can provide significant tumor control, but patients can still progress despite this treatment. Laser interstitial thermal therapy (LITT), using image-guided, localized hyperthermia to kill tumor cells, may provide a novel treatment strategy for primary and metastatic CNS disease. There appear to be advantages for LITT over SRS in glioma patients, including potential for better treatment planning and efficacy.34 Pilot studies suggest the feasibility of LITT in the control of brain metastases.35
Future research may be directed at utilizing the effects of a modest dose of radiation on the BBB to enhance the outcome of therapy by improving drug delivery10,36,37 and potentially improving an immune response in the CNS. This speculative use could be investigated with technologies such as a computed tomography-guided small animal irradiator, which can administer partial brain treatment such that drug penetration and other markers can be assessed in both the irradiated and non-irradiated areas of the same animal. Several preclinical and clinical studies suggest that the nature of the postradiation immune response may impact tumor control38,39 despite the immune-privileged properties of the brain. More information about the extent, dose-effect, and timing of BBB and immune effects is needed to inform the design of a clinical study that would test this approach.
Novel Chemotherapy and Targeted Therapy Approaches for Brain Metastases
There is a compelling rationale for studying systemic therapies that may sufficiently penetrate the BBB to elicit a clinical response in the CNS that matches the response elsewhere in the body. Although it is unlikely that current chemotherapeutic or targeted agents will replace radiotherapy and surgery as the primary means for obtaining durable control, use of such agents may allow delay of radiation or may improve lesion response magnitude or duration when used in conjunction with SRS or WBRT. More importantly, an agent capable of inducing response in gross lesions may have sufficient activity to sterilize micrometastatic disease in some patients, thereby reducing the incidence of brain metastasis when administered earlier in the disease course or of additional lesions in patients receiving SRS.
Advances in diagnosis, chemotherapy, radiation, and surgery have improved, and will continue to improve, the long-term survival for individuals with CNS metastases. Cognitive deficits are common among individuals with CNS cancers, and perceived cognitive dysfunction (“chemo brain”) can directly affect the quality of life for cancer survivors. In addition to the neuropathology itself, neurotoxicity can occur after WBRT.33 Whether used in treatment and/or prophylaxis for CNS metastases, the long-term cognitive and emotional consequences of radiotherapy have likely been underestimated. Cognitive decrements can also accompany some chemotherapeutic treatments,40 and both cognitive and affective side effects have been noted with adjuvant treatments (eg, steroids, antiepileptic drugs). These results underscore the necessity of evaluating, monitoring, and addressing cognitive impairment in this population.
Lung Cancer Central Nervous System Metastases
Lung cancer is the most common cancer to metastasize to the brain.1 Pemetrexed is a folate antimetabolite that is commonly used for the treatment of non-small cell lung cancer (NSCLC). Despite some reports showing low cerebrospinal fluid (CSF) concentration and limited efficacy,41 a number of studies have suggested benefit from pemetrexed in combination with other chemotherapies and radiation.42,43 In combination with WBRT, which could increase permeability of the BBB, intracranial response rates with pemetrexed and cisplatin combinations were nearly 70%, with extended progression free survival (PFS) and OS. Larger studies with pemetrexed, both in combination with radiation and as a single agent, are warranted to further explore this potential activity. Pemetrexed treatment is also associated with nearly 50% lower odds of subsequent brain metastases over non–pemetrexed-containing regimens in the first line setting of NSCLC.44
There are promising data using tyrosine kinase inhibitors (TKIs) of the epidermal growth factor receptor (EGFR), which is constitutively active in 10%–15% of lung cancers due to a mutation45,46 Erlotinib and gefitinib cross the BBB with sufficient CSF concentrations for potential efficacy.47 Retrospective studies in Asian nonsmokers, who have a higher incidence of EGFR mutations, have demonstrated intracranial response rates of 10%–70%.48 Patients with brain metastases from NSCLC demonstrated an overall response rate of 86% with erlotinib in combination with WBRT and a median survival of 11.8 months.49 Of interest, EGFR-mutations may be associated with a higher incidence of brain metastases.50 A retrospective analysis on the outcome of patients with known EGFR mutations and brain metastases reported a lower risk of CNS progression in patients who had received either gefitinib or erlotinib.51 In contrast, a phase 3 trial of unselected patients did not show any benefit from adding erlotinib to radiation, and there was a suggestion of a potential detriment on outcome.52
Melanoma Central Nervous System Metastases
Malignant melanoma will frequently metastasize to the brain, with overall incidence of 5%–8% and involvement in 40%–50% of patients with advanced disease.1 Historically, the treatment of melanoma brain metastases has been difficult because these tumors are less radiosensitive, and systemic therapy options for melanomas have been scarce until recently.53 Harnessing the patient's own immune system to fight cancer is showing promise in the treatment of melanoma patients, including those with CNS involvement.54–56
Immune modulation approaches include monoclonal antibodies (mAbs) to the cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) (eg, ipilimumab) and the programmed death receptor (PD-1) (eg, nivolumab, pembrolizumab).57,58 These mAbs enhance the T cell immune response but have differing response rates and toxicity. In a phase 2 trial of ipilimumab in metastatic melanoma, the CNS disease control rate was 24% in neurologically asymptomatic patients with 7.0 month OS, similar to that seen in patients without brain metastases.59 In contrast, disease control was 5%, and OS was 3.7 months for patients whose CNS lesions induced neurological symptoms that required the use of steroids,. Another phase 2 trial added fotemustine to ipilimumab for patients with melanoma brain metastases and showed a 50% intracranial control rate, a 40% objective CNS response rate, and an OS of 13.4 months.55 The results of these studies are promising, and further trials with additional combinations are anticipated.
Another recent breakthrough in the treatment of metastatic melanoma is the use of TKIs targeting the BRAF pathway. Approximately 50% of melanoma patients have activating mutations in either Val600Glu or Val600Lys of the BRAF oncogene that confer sensitivity to the BRAF inhibitors dabrafenib and vemurafenib.60,61 In a large study of 172 patients, the intracranial response rate was 39% in Val600Glu patients with no previous local treatment (OS > 8 months) and 31% in patients with progression after local treatment.60 In contrast, those patients with Val600Lys had much lower response rates (6.7% in previously untreated and 22% in progressive tumors [OS, 4 months]). Notably, 2 patients with a Val600Glu mutation achieved an intracranial complete response.60 BRAF inhibitors appear safe used in conjunction with radiotherapy in melanoma brain metastases.62
Breast Cancer Central Nervous System Metastases
There is disparity in outcome for CNS metastases depending on the subtype of primary breast cancer.63 As such, recent clinical trials researching breast cancer brain metastases have been subtype specific, with particular focus on HER2-positive disease. The TKI lapatinib has undergone extensive clinical evaluation in breast cancer brain metastases. Typical phase 2 results are PFS of approximately 5 months and CNS objective responses ranging from 21%–31%.64 Lapatinib is often used in conjunction with capecitabine chemotherapy.64–66 Both agents penetrate the BBB, although delivery is variable, and response may be partially dose limited.67–69 In patients who had progressive brain metastases (despite prior radiation and trastuzumab-based therapies, n = 242), lapatinib alone resulted in 21% of patients having a CNS volumetric reduction response of ≥20%, while patients who received capecitabine plus lapatinib had a CNS volumetric response of ≥ 40%.64 In previously untreated HER2-positive brain metastases receiving combination capecitabine and lapatinib, 66% of patients achieved a CNS partial response defined as > 50% volumetric reduction.65 Newer brain-penetrating TKIs (eg, neratinib and afatinib) are currently being evaluated in HER2-positive brain metastases.70,71
The anti-HER2 mAb trastuzumab has not shown effectiveness against HER2-postive breast cancer brain metastases, but trastuzumab emtansine (T-DM1), an antibody drug conjugate of trastuzumab linked to a microtubule-disrupting agent, may be beneficial for a subset of patients. Case reports have shown responses to T-DM1 in radiation-naïve CNS lesions72 and in heavily pretreated patients (Fig. 1). In the recently reported TH3RESA trial, relative to physicians' choice of chemotherapy, T-DM1 improved outcomes in patients with history of CNS metastases, (median PFS improved from 2.4 to 5.8 months).73 Similarly, subset analysis of the EMILIA trial that compared T-DM1 to the lapatinib/capecitabine combination (a typical standard for HER2+ CNS metastases) showed similar rates of CNS progression in both trial arms. In patients with history of treated and stable brain metastases at study entry, T-DM1 improved survival over lapatinib/capecitabine.74 Preclinical studies suggest that combination of HER2-targeted TKIs with mAbs or mAb conjugates may further improve efficacy in CNS disease.
Triple negative breast cancer (TNBC) also has a propensity for CNS metastasis. Unlike HER2-positive disease, there is no specific chemotherapy or biological target for TNBC, resulting in limited systemic regimens and few therapies targeting TNBC brain metastases. The combination of irinotecan topoisomerase inhibitor with iniparib (a putative PARP inhibitor that modulates reactive oxygen species metabolism) produced an intracranial response rate of 12% and an intracranial clinical benefit rate of 27% in a study of 34 TNBC patients with brain metastases.75 Iniparib has been discontinued, but the concept remains viable. Another potential approach is antiangiogenic therapy, such as bevacizumab anti-VEGF mAb, in combination with chemotherapy or radiotherapy.76 In a study of HER2-positive and TNBC brain metastases, bevacizumab plus carboplatin produced a CNS composite response rate of 63%. PFS remained short at 3.7 months; however, there was a subset of patients with prolonged clinical benefit who remained on therapy for up to 19 cycles.
Recommendations and Future Directions
Strategies to advance the study of chemotherapeutics in the treatment of brain metastases must include collaborative efforts to conduct trials. First, patients with brain metastases should be allowed to enroll in phase 1 trials, which have traditionally excluded patients with CNS disease. This inclusion does not lead to higher toxicities or premature closure of such trials but does provide a wider range of clinical research opportunities for evaluating the CNS activity of novel agents. Second, working groups are needed that focus on the management of brain metastases; an example of such a team is the US National Cancer Institute (NCI)-sponsored Brain Metastasis in Breast Cancer Working Group, which combines translational and clinical investigators from the NCI and academic institutions. Third, cooperative groups must form novel collaborations. The recent realignment of the NCI cooperative groups provides an opportunity for new collaborations, which may increase the number of studies of chemotherapy in brain metastases.
Preclinical Models of Brain Metastases
While the process of brain metastasis is poorly understood, we do know that it is an extremely selective, inefficient, multistep process that involves (i) breaking away of individual cells from the primary carcinoma, (ii) arrest or seeding of cells in the cerebrovasculature, (iii) extravasation of the cancer cell into the brain parenchyma, and (iv) colonization and growth in brain parenchyma.77 In vitro assays of migration or invasion fail to replicate the complexity of the NVU, hemodynamic variables, or the role of the immune system. In order to develop alternative therapies targeted to the different steps in brain metastasis, preclinical and translational research must develop more and better models.46,78,79
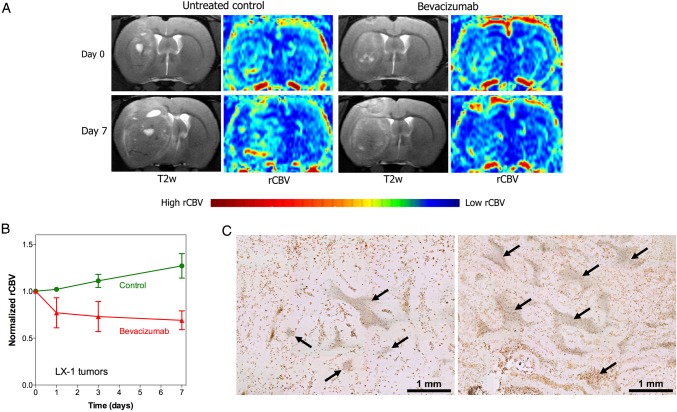
The majority of brain metastasis studies are based upon direct intracerebral injection of tumor lines that were isolated and established years ago. Orthotopic models are useful for assessing therapies and vascular targeting in established metastases. Figure 2 shows a rat model of intracerebral SCLC xenograft used to assess the impact of bevacizumab on rCBV (as determined by DSC-MRI) and the cerebrovasculature (as determined by histology).80 However, xenograft models bear little relationship to natural in vivo processes involved in brain metastasis, and their predictive accuracy is open to question.81
Fig. 2.
Effect of bevacizumab on relative cerebral blood volume (rCBV) in lung cancer brain metastases. Rats with intracerebral xenografts of human LX-1 small cell lung cancer SCLC were treated with or without bevacizumab (45 mg/kg IV, single dose). (A) MRI before and 7 days after treatment show brain tumor growth with increased rCBV in control tumor and decreased rCBV in the bevacizumab-treated rat. (B) Quantification of changes in rCBV over time. (C) CD68 immunohistochemistry for macrophages shows areas of necrosis (arrows) in untreated control tumor (left) and bevacizumab treated tumor (right), bar indicates 1 mm. (Adapted from Muldoon et al 2011, and used with permission of Oxford University Press).80
There are models of spontaneous and induced brain metastases in rodents for melanoma, lung cancer, and mammary cancers (Table 1). Spontaneous models, such as seeding from orthotopic or subcutaneous xenografts, can provide information about the process of intravasation and organotropism.82–85 The advantage of syngeneic models is that they allow for studying immune system interaction within the animal, as well as the role of the intact BBB and BTB; however, the applicability of these models for studying human disease is unclear. Model systems that utilize ectopic (bloodstream) injection of human cancer cells produce hematogenous brain metastases.7,10,86–90 Ectopic models have the advantage of allowing visualization of the fate of individual cancer cells as they bind to brain vasculature and infiltrate into brain parenchyma.7,91,92 A recent review describes new animal models to investigate mechanisms of brain metastasis.81
Table 1.
Selected brain metastasis preclinical models
| Cell line | Origin | Route of Administration | Reference |
|---|---|---|---|
| A549 | Human lung adenocarcinoma | Orthotopic lung xenograft, spontaneous brain metastases | 84 |
| A549 | Human lung adenocarcinoma | i.v., tail vein | 87 |
| EBC-1 | Human squamous cell NSCLC | i.v., tail vein | 87 |
| PC14-PE6 | Human lung adenocarcinoma | Carotid artery | 7 |
| H460 (HTB177) | Human large cell NSCLC | Carotid artery | 7 |
| MCF-7 variants | Human breast cancer | Subcutaneous, spontaneous brain metastases | 83 |
| MCF-7 | Human breast cancer | Intracardiac or carotid artery | 86,90 |
| 4T1 | Mouse breast carcinoma | Mammary fat pad, spontaneous brain metastases. | 85 |
| 4T1 variants | Mouse breast carcinoma | Intracardiac or carotid artery | 10,86 |
| MDA-MB231 variants | Human breast cancer | Intracardiac or carotid artery | 10,86,88–91 |
| WM239A variants | Human melanoma | Orthotopic xenograft, spontaneous brain metastases | 82 |
| A2058 | Human melanoma | Carotid artery | 7 |
Abbreviation: NSCLC, non-small cell lung cancer
After intracardiac or intracarotid infusion, brain-seeking metastatic breast cancer sublines (MDA-MB231BR) circulate in the peripheral vasculature, arrest in brain capillaries, extravasate across the BBB, and develop multiple metastatic lesions predominantly in brain (Figs. 3 and 4).10,89 Pretreatment with cyclophosphamide to further decrease host immune response may enhance hematogenous brain metastasis formation in athymic rats.89,93 When MDA-MB231BR contrast-enhancing brain metastases in mice were compared with resected human brain metastases of breast cancer (n = 16 human cases), they were found to have nearly equal rates of apoptosis, cellular growth, and neuroinflammatory response.88
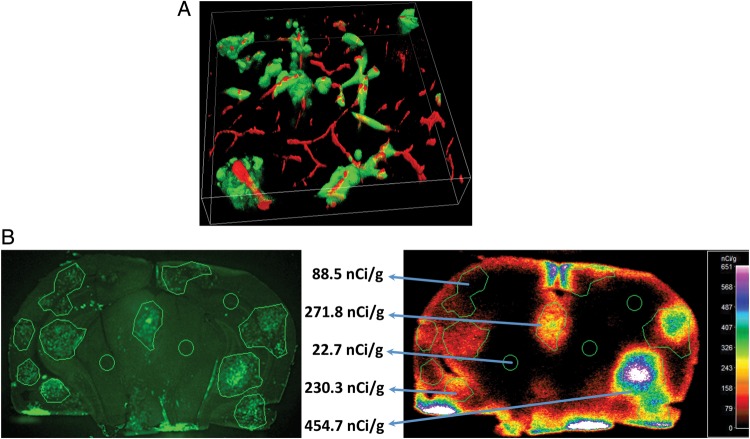
Fig. 3.
(A) 3-dimensional multiphoton image of preclinical brain metastases of breast cancer in a brain slice is shown. Brain metastases are green (eGFP), and blood vessels are red (red; animal underwent a 2 min vascular perfusion with Texas red 70 kDa dextran,15 mg/mL) prior to being euthanized. Image is 100 microns deep. Lesion vessel co-option is the predominant formation of the metastases in this model. (Paul Lockman, personal communication). (B) Experimental brain metastases of breast cancer exhibit heterogeneous passive permeability with weak correlation to lesion size. Left image shows representative images of metastases of eGFP-transfected 231-BR-Her2. Right image of the same brain section shows metastases and brain accumulation of 3-kDa 14C-AIB with vascular washout. (Modified from Lockman et al 2010, and used with permission of Clinical Cancer Research).10
Fig. 4.
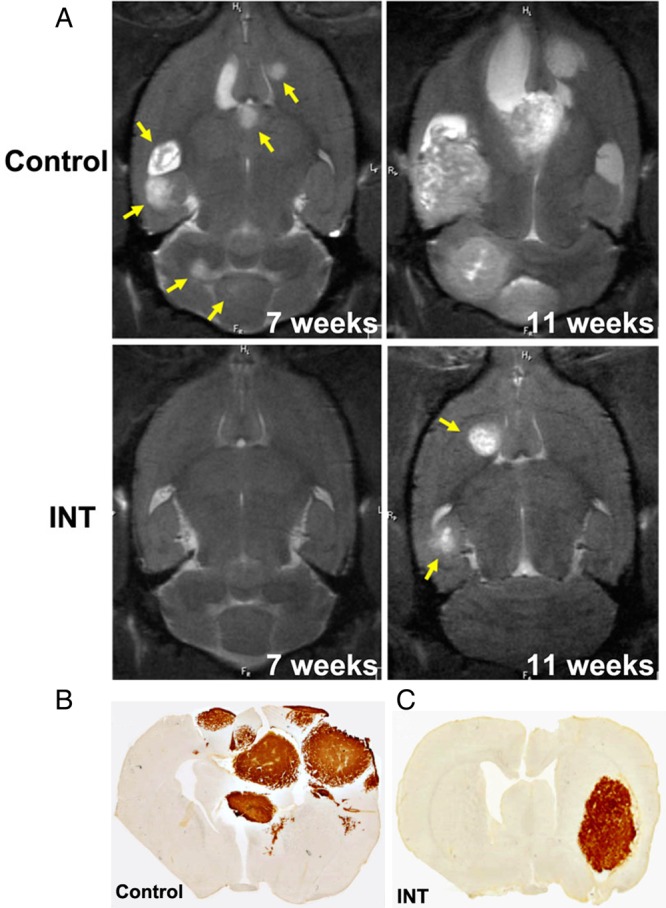
Effect of Intetumumab on breast cancer brain metastases. (A) T2-weighted MRI is shown for a control and weekly IV intetumumab (INT) 7 and 11 weeks after intracarotid tumor cell infusion. Representative micrographs of human mitochondrial antigen immunohistochemistry are shown for a control rat (B) and intetumumab-treated rat (C) at 11 weeks.(Modified from Wu et al, 2012 and used with permission of Springer).89
Model systems such as these94 may provide significant insight into potential targets for the prevention of brain metastases of breast cancer. The drawback of rodent models of human tumor metastasis is that immunodeficient rodents must be used. Therefore, the influence of the immune system, which is likely a major influence in human systems, is lost. Of interest, even though these cell lines reliably produce brain metastases in mice, only a small percentage of injected cells actually form measurable tumors. Most cells fail to bind to or transmigrate the cerebrovasculature or remain as dormant single cells once they enter the brain parenchyma, which suggests that the development of CNS metastasis is an inefficient process overall.91
Use of Preclinical Models in Drug Pharmacokinetics
Current drug development programs typically demonstrate proof of concept in vitro and then dose to the maximum level for drug efficacy studies in vivo, when such concentration can rarely be obtained in humans. Standard pharmacokinetic practices focus on the total drug level in plasma, with minimal assessment of distribution of the agent in brain metastases or whole brain (an agent is often said to either cross or not cross the BBB). Brain drug levels, when assessed, are rarely expressed in terms of free drug concentration or correlated in pharmacokinetic-pharmacodynamic (PK-PD) models to better predict drug efficacy. The majority of drug delivery and sensitivity studies have used intracerebrally implanted brain metastasis models that poorly replicate the variety of human brain metastases.
To improve predictive accuracy of chemotherapeutic drug assays and models, greater attention must be placed on the actual concentration of drug that reaches key sites within the tumor and the extent to which this concentration is effective. Surgical efforts to quantitate drug entry into metastases suffer from artefact introduced by the surgical disruption of the BBB and BTB.67 Thus, drug-delivery imaging methods are needed that will accurately report the extent of brain metastasis drug exposure (and not simply the shape of the tumor or the extent of its barrier integrity). Care must be taken to account for the heterogeneity of drug delivery within tumors due to BBB compromise. In hematogenous models of metastatic breast cancer, the dysfunctional BTB still limits drug delivery of taxanes, anthracyclines, and lapatinib to 2%–15% of that in systemic metastases.10,68 In only a small subset of brain metastases (<20%) did chemotherapeutic drug concentrations approach those of systemic tumors and show signs of drug-induced efficacy. A clinical study documented inconsistently cytotoxic concentrations of lapatinib and capecitabine in breast cancer brain metastases of women treated with those agents preoperatively.
It is important to consider interventions that raise the concentrations of drugs targeting brain metastases to an appreciable degree (ie, >10 fold).10,68 Close correlation of drug concentration with in vivo biomarkers of efficacy will permit development of rational PK-PD models that better demonstrate the limiting factors in drug efficacy and the extent to which limited drug delivery must be overcome. For many agents, the delivery deficit is not 2–3 fold, but rather 10–50 fold, underscoring the need for better focus and creative thinking toward targeted, novel drug delivery solutions. Potential strategies include BBB disruption, inhibition, or modulation of active efflux transport and design of agents that are membrane permeable and not substrates to limiting efflux transporters.
Brain Metastases Prevention
Given the current failure rates of chemotherapy, surgery and radiation, the prevention of brain metastases (particularly in diseases such as HER2-positive breast cancer) is under active investigation. While the risk of brain metastases is only 5% for all women with a primary breast cancer, women who have stage IV HER2-positive or TNBC disease have a 20%–40% risk of developing brain metastases.95
An ideal prevention study would first need to randomize asymptomatic patients with high-risk disease (TNBC, HER2-positive breast cancer, high-grade melanoma, and lung cancer) to serial screening MRI (or other imaging) as compared with those for whom CNS imaging was only done in the setting of neurological symptoms to confirm that early diagnosis (presumably of limited metastatic disease) would lead to improved outcomes. Once there was a historical control rate of development of metastatic disease and the early adoption of limited SRS or surgery, then alternatives to WBRT could be tested as a mechanism for preventing further macroscopic metastatic disease.96 The use of a surveillance strategy was evaluated in 80 patients with neurologically asymptomatic HER2-positive metastatic breast cancer without known CNS involvement, who were screened every 3 months with MRI for the development of brain metastases.4 These patients were compared with a separate cohort who had received WBRT for symptomatic brain metastases. In this study, the OS of the 2 groups was similar (53 vs 51 months), but death from CNS involvement was significantly reduced (16% in the treated asymptomatic group vs 48% in the treated symptomatic group). None of the patients in this study received surgery or SRS, so the applicability of the findings to patients treated with those modalities is limited and does not reflect current US practice patterns.4 Larger studies and trials with a goal to intervene with novel systemic agents are needed. These trials require thoughtful design and multidisciplinary endorsement to avoid early closure due to poor accrual of these trials as noted in Table 2.
Table 2.
Selected trials of chemoprevention for brain metastasis
| Title | Sponsor | ClinicalTrials.gov Identifier | Comment | |
|---|---|---|---|---|
| Primary prevention | Nab-paclitaxel and temozolomide plus oblimersen (melanoma) | Genta Incorporated | NCT00409383 | Active, not recruiting |
| Trastuzumab-radiotherapy | Centre Oscar Lambret | NCT01613482 | Stopped due to poor accrual | |
| Lapatinib/capecitabine vs trastuzumab/capecitabine (Her2+ breast) | GlaxoSmithKline | NCT00820222 | Stopped early; very small number of events in both arms | |
| Temozolomide (metastatic breast cancer) | Schering-Plough Italy | NCT00638963 | Stopped due to poor accrual | |
| Maintenance temozolomide vs observation (stage III/IV non-small cell lung cancer) | Schering-Plough Italy | NCT00632203 | Completed | |
| Secondary prevention | Stereotactic radiosurgery with sunitinib for brain metastases | University Health Network, Toronto | NCT00981890 | Recruiting |
| Stereotactic radiosurgery → sunitinib (1-3 newly diagnosed brain metastases) | Cleveland Clinic | NCT00910039 | Stopped due to poor accrual | |
| Stereotactic radiosurgery → temozolomide (1–3 brain metastases) | University of Florida | NCT00717275 | Stopped due to poor accrual |
The CEREBEL trial highlights some of the important considerations with a CNS metastases prevention trial.97 This trial was designed with a primary endpoint of the CNS as a site of first relapse. The comparison was between a regimen with proven efficacy to cross the BBB (capecitabine/lapatinib)67 with a regimen that would not cross into the CNS (capecitabine/trastuzumab) but was otherwise a standard in HER2-positive systemic disease. Upfront screening for CNS involvement with a pre-enrollment MRI disqualified 20% of potential patients due to occult brain metastases. The incidence of developing HER2-positive CNS disease was low in both arms: 3% in the lapatinib/capecitabine arm and 5% in the trastuzumab/capecitabine arm. The low incidence, combined with better PFS in the trastuzumab-treated arm (HR, 1.30), prompted early termination of the study. The CEREBEL study demonstrated the need for relatively equal efficacy against systemic disease in the treatment arms. The combination of lapatinib with a taxane was inferior to trastuzumab and taxane in the EORTC 10054 study.98 Similarly, in the CEREBEL trial, the lapatinib-only arm was inferior from an overall efficacy standpoint, which likely limited the ability to show any improvement in the CNS endpoint. In the CEREBEL trial, 40% of patients had not received prior trastuzumab. Another potential consideration is that with a number of new, improved therapies, such as pertuzumab anti-HER2 mAb, and T-DM1 mAb-drug conjugate controlling systemic disease longer in the metastatic setting, the best setting in which to study CNS metastases prevention may be later in the disease course. Perhaps initially studying CNS prevention, in the more advanced third line and beyond setting, would be more appropriate.
Alternative prevention strategies include blocking tumor cell adhesion to brain vasculature or infiltration across the BBB. Integrin cell adhesion dimers are basic components of cell cytoskeleton and signaling architecture and are essential for cell-cell and cell-ECM adhesion in the NVU.99 The αv integrins are associated with lung cancer brain metastasis100 and in the MDA-MB231BR hematogenous breast cancer brain metastasis model.89 Intetumumab, a monoclonal antibody targeting αV integrins, delayed the onset of breast cancer brain metastases in rats with the MDA-MB231BR hematogenous metastasis model as determined by MRI (Fig. 4).89 Rats receiving intetumumab i.v. before or after cell infusion, or mixed with the cells prior to cell infusion (n = 24), developed significantly fewer metastases than untreated controls, with 7 rats (32%) showing no detectable brain tumors. The difference in brain metastasis incidence correlated with improved survival. These animal studies suggest that intetumumab can act directly on tumor cells and/or vasculature to prevent one or more of the steps in metastasis. Of note, there is not efficacy of this agent in terms of treatment of existing brain metastases. We feel that a clinical trial for the prevention of brain metastases with intetumumab should be considered.
Summary, Conclusions and Recommendations
As our understanding of cancer pathogenesis and treatment progresses, the need to understand the alterations and that confer a predisposition to CNS metastases becomes great. Evaluation of CNS metastases for metastatic homing signatures will allow researchers and clinicians to better understand the somatic mutations that may add in the pathogenesis or the predilection of CNS metastasis. Three important challenges currently need to be addressed. The first is the development of preclinical models that more accurately recapitulate CNS metastases for both biological and therapeutic studies. The second involves the sequencing of SRS and WBRT during radiotherapy and determining how local therapies can be delayed in order to allow assessment of new systemic treatments. Finally, the clinical trial challenges include image standardization to assess response, as well as inclusion of novel imaging paradigms. We wholeheartedly endorse the continued research evaluating systemic therapy options specifically for CNS metastases. We also strongly support the inclusion of patients with CNS metastases in clinical trials. We are optimistic that continued efforts toward understanding chemotherapeutic drug delivery, short- and long-term therapy toxicities, and the underlying biology of CNS metastasis will lead to new and improved therapeutic options for patients with CNS metastases.
Funding
Grants from the National Institutes of Health NIH/NIGMS U54GM104942, and R13 NS076353 (E.N.) and the Walter S. and Lucienne Driskill Foundation (E.N.). This report was based on presentations at the 19th Annual Meeting of the Blood-Brain Barrier Consortium (March 21-23, 2013, Stevenson, Washington), which was partially funded by the R13 grant.
Conflict of interest statement. The authors have no conflicts of interest to disclose.
References
- 1.Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5–14. [DOI] [PubMed] [Google Scholar]
- 2.Brufsky AM, Mayer M, Rugo HS, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–4843. [DOI] [PubMed] [Google Scholar]
- 3.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295(21):2483–2491. [DOI] [PubMed] [Google Scholar]
- 4.Niwinska A, Tacikowska M, Murawska M. The effect of early detection of occult brain metastases in HER2-positive breast cancer patients on survival and cause of death. Int J Radiat Oncol Biol Phys. 2010;77(4):1134–1139. [DOI] [PubMed] [Google Scholar]
- 5.Platta CS, Khuntia D, Mehta MP, et al. Current treatment strategies for brain metastasis and complications from therapeutic techniques: a review of current literature. Am J Clin Oncol. 2010;33(4):398–407. [DOI] [PubMed] [Google Scholar]
- 6.Pesce GA, Klingbiel D, Ribi K, et al. Outcome, quality of life and cognitive function of patients with brain metastases from non-small cell lung cancer treated with whole brain radiotherapy combined with gefitinib or temozolomide. A randomised phase II trial of the Swiss Group for Clinical Cancer Research (SAKK 70/03). Eur J Cancer. 2012;48(3):377–384. [DOI] [PubMed] [Google Scholar]
- 7.Kienast Y, von Baumgarten L, Fuhrmann M, et al. Real-time imaging reveals the single steps of brain metastasis formation. Nat Med. 2010;16(1):116–122. [DOI] [PubMed] [Google Scholar]
- 8.Kodack DP, Chung E, Yamashita H, et al. Combined targeting of HER2 and VEGFR2 for effective treatment of HER2-amplified breast cancer brain metastases. Proc Natl Acad Sci USA. 2012;109(45):E3119–E3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muldoon LL, Alvarez JI, Begley DJ, et al. Immunologic privilege in the central nervous system and the blood-brain barrier. J Cereb Blood Flow Metab. 2013;33(1):13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lockman PR, Mittapalli RK, Taskar KS, et al. Heterogeneous blood-tumor barrier permeability determines drug efficacy in experimental brain metastases of breast cancer. Clin Cancer Res. 2010;16(23):5664–5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quant EC, Wen PY. Response assessment in neuro-oncology. Curr Oncol Rep. 2011;13(1):50–56. [DOI] [PubMed] [Google Scholar]
- 12.Berghoff AS, Rajky O, Winkler F, et al. Invasion patterns in brain metastases of solid cancers. Neuro Oncol. 2013;15(12):1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee EJ, ter Brugge K, Mikulis D, et al. Diagnostic value of peritumoral minimum apparent diffusion coefficient for differentiation of glioblastoma multiforme from solitary metastatic lesions. AJR Am J Roentgenol. 2011;196(1):71–76. [DOI] [PubMed] [Google Scholar]
- 14.Spanberger T, Berghoff AS, Dinhof C, et al. Extent of peritumoral brain edema correlates with prognosis, tumoral growth pattern, HIF1a expression and angiogenic activity in patients with single brain metastases. Clin Exp Metastasis. 2013;30(4):357–368. [DOI] [PubMed] [Google Scholar]
- 15.Farjam R, Tsien CI, Feng FY, et al. Investigation of the diffusion abnormality index as a new imaging biomarker for early assessment of brain tumor response to radiation therapy. Neuro Oncol. 2014;16(1):131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zakaria R, Das K, Radon M, et al. Diffusion-weighted MRI characteristics of the cerebral metastasis to brain boundary predicts patient outcomes. BMC Med Imaging. 2014;14:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gahramanov S, Muldoon LL, Varallyay CG, et al. Pseudoprogression of glioblastoma after chemo- and radiation therapy: diagnosis by using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging with ferumoxytol versus gadoteridol and correlation with survival. Radiology. 2013;266(3):842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Law M, Cha S, Knopp EA, et al. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology. 2002;222(3):715–721. [DOI] [PubMed] [Google Scholar]
- 19.Varallyay CG, Nesbit E, Fu R, et al. High-resolution steady-state cerebral blood volume maps in patients with central nervous system neoplasms using ferumoxytol, a superparamagnetic iron oxide nanoparticle. J Cereb Blood Flow Metab. 2013;33(5):780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boxerman JL, Schmainda KM, Weisskoff RM. Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR. Am J Neuroradiol. 2006;27(4):859–867. [PMC free article] [PubMed] [Google Scholar]
- 21.Nasseri M, Gahramanov S, Netto JP, et al. Evaluation of pseudoprogression in patients with glioblastoma multiforme using dynamic magnetic resonance imaging with ferumoxytol calls RANO criteria into question. Neuro Oncol. 2014;16(8):1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schieda N. Parenteral ferumoxytol interaction with magnetic resonance imaging: a case report, review of the literature and advisory warning. Insights Imaging. 2013;4(4):509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCullough BJ, Kolokythas O, Maki JH, et al. Ferumoxytol in clinical practice: implications for MRI. J Magn Reson Imaging. 2013;37(6):1476–1479. [DOI] [PubMed] [Google Scholar]
- 24.Horky LL, Hsiao EM, Weiss SE, et al. Dual phase FDG-PET imaging of brain metastases provides superior assessment of recurrence versus post-treatment necrosis. J Neurooncol. 2011;103(1):137–146. [DOI] [PubMed] [Google Scholar]
- 25.Navarria P, Reggiori G, Pessina F, et al. Investigation on the role of integrated PET/MRI for target volume definition and radiotherapy planning in patients with high grade glioma. Radiother Oncol. 2014;112(3):425–429. [DOI] [PubMed] [Google Scholar]
- 26.Terakawa Y, Tsuyuguchi N, Iwai Y, et al. Diagnostic accuracy of 11C-methionine PET for differentiation of recurrent brain tumors from radiation necrosis after radiotherapy. J Nucl Med. 2008;49(5):694–699. [DOI] [PubMed] [Google Scholar]
- 27.Weckesser M, Langen KJ, Rickert CH, et al. O-(2-[18F]fluorethyl)-L-tyrosine PET in the clinical evaluation of primary brain tumours. Eur J Nucl Med Mol Imaging. 2005;32(4):422–429. [DOI] [PubMed] [Google Scholar]
- 28.Galldiks N, Stoffels G, Filss CP, et al. Role of O-(2-(18)F-fluoroethyl)-L-tyrosine PET for differentiation of local recurrent brain metastasis from radiation necrosis. J Nucl Med. 2012;53(9):1367–1374. [DOI] [PubMed] [Google Scholar]
- 29.Calabria F, Chiaravalloti A, Di Pietro B, et al. Molecular imaging of brain tumors with 18F-DOPA PET and PET/CT. Nucl Med Commun. 2012;33(6):563–570. [DOI] [PubMed] [Google Scholar]
- 30.Allen AM, Ben-Ami M, Reshef A, et al. Assessment of response of brain metastases to radiotherapy by PET imaging of apoptosis with (1)(8)F-ML-10. Eur J Nucl Med Mol Imaging. 2012;39(9):1400–1408. [DOI] [PubMed] [Google Scholar]
- 31.Dijkers EC, Oude Munnink TH, Kosterink JG, et al. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin Pharmacol Ther. 2010;87(5):586–592. [DOI] [PubMed] [Google Scholar]
- 32.Meert AP, Paesmans M, Berghmans T, et al. Prophylactic cranial irradiation in small cell lung cancer: a systematic review of the literature with meta-analysis. BMC Cancer. 2001;1:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hasan S, Shah AH, Bregy A, et al. The role of whole-brain radiation therapy after stereotactic radiation surgery for brain metastases. Pract Radiat Oncol. 2014;4(5):306–315. [DOI] [PubMed] [Google Scholar]
- 34.Elder JB, Chiocca EA. Editorial: Glioblastoma multiforme and laser interstitial thermal therapy. J Neurosurg. 2013;118(6):1199–1201. [DOI] [PubMed] [Google Scholar]
- 35.Mohammadi AM, Schroeder JL. Laser interstitial thermal therapy in treatment of brain tumors--the NeuroBlate System. Expert Rev Med Devices. 2014;11(2):109–119. [DOI] [PubMed] [Google Scholar]
- 36.Percy DB, Ribot EJ, Chen Y, et al. In vivo characterization of changing blood-tumor barrier permeability in a mouse model of breast cancer metastasis: a complementary magnetic resonance imaging approach. Invest Radiol. 2011;46(11):718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cao Y, Tsien CI, Shen Z, et al. Use of magnetic resonance imaging to assess blood-brain/blood-glioma barrier opening during conformal radiotherapy. J Clin Oncol. 2005;23(18):4127–4136. [DOI] [PubMed] [Google Scholar]
- 38.Szeifert GT, Kondziolka D, Levivier M, et al. Histopathology of brain metastases after radiosurgery. Prog Neurol Surg. 2012;25:30–38. [DOI] [PubMed] [Google Scholar]
- 39.Finkelstein SE, Timmerman R, McBride WH, et al. The confluence of stereotactic ablative radiotherapy and tumor immunology. Clin Dev Immunol. 2011;2011:439752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wen P. “Central Nervous System Complications of Cancer Therapy.” In: Schiff D, Wen P, eds. Cancer Neurology in Clinical Practice. Totowa, NJ: Humana Press; 2003:215–231. [Google Scholar]
- 41.Kumthekar P, Grimm SA, Avram MJ, et al. Pharmacokinetics and efficacy of pemetrexed in patients with brain or leptomeningeal metastases. J Neurooncol. 2013;112(2):247–255. [DOI] [PubMed] [Google Scholar]
- 42.Chargari C, Pacaut C, Le Moulec S, et al. First assessment of whole-brain radiation therapy combined with pemetrexed-based chemotherapy in non-small-cell lung carcinoma: data on safety and efficacy. Anticancer Drugs. 2013;24(7):736–742. [DOI] [PubMed] [Google Scholar]
- 43.Dinglin XX, Huang Y, Liu H, et al. Pemetrexed and cisplatin combination with concurrent whole brain radiotherapy in patients with brain metastases of lung adenocarcinoma: a single-arm phase II clinical trial. J Neurooncol. 2013;112(3):461–466. [DOI] [PubMed] [Google Scholar]
- 44.Ortuzar W, Hanna N, Pennella E, et al. Brain metastases as the primary site of relapse in two randomized phase III pemetrexed trials in advanced non-small-cell lung cancer. Clin Lung Cancer. 2012;13(1):24–30. [DOI] [PubMed] [Google Scholar]
- 45.Rosell R, Moran T, Queralt C, et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med. 2009;361(10):958–967. [DOI] [PubMed] [Google Scholar]
- 46.Zhang J, Yu J, Sun X, et al. Epidermal growth factor receptor tyrosine kinase inhibitors in the treatment of central nerve system metastases from non-small cell lung cancer. Cancer Lett. 2014;351(1):6–12. [DOI] [PubMed] [Google Scholar]
- 47.Kodaira H, Kusuhara H, Fujita T, et al. Quantitative evaluation of the impact of active efflux by p-glycoprotein and breast cancer resistance protein at the blood-brain barrier on the predictability of the unbound concentrations of drugs in the brain using cerebrospinal fluid concentration as a surrogate. J Pharmacol Exp Ther. 2011;339(3):935–944. [DOI] [PubMed] [Google Scholar]
- 48.Kim JE, Lee DH, Choi Y, et al. Epidermal growth factor receptor tyrosine kinase inhibitors as a first-line therapy for never-smokers with adenocarcinoma of the lung having asymptomatic synchronous brain metastasis. Lung Cancer. 2009;65(3):351–354. [DOI] [PubMed] [Google Scholar]
- 49.Welsh JW, Komaki R, Amini A, et al. Phase II trial of erlotinib plus concurrent whole-brain radiation therapy for patients with brain metastases from non-small-cell lung cancer. J Clin Oncol. 2013;31(7):895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumoto S, Takahashi K, Iwakawa R, et al. Frequent EGFR mutations in brain metastases of lung adenocarcinoma. Int J Cancer. 2006;119(6):1491–1494. [DOI] [PubMed] [Google Scholar]
- 51.Heon S, Yeap BY, Britt GJ, et al. Development of central nervous system metastases in patients with advanced non-small cell lung cancer and somatic EGFR mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2010;16(23):5873–5882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sperduto PW, Wang M, Robins HI, et al. A phase 3 trial of whole brain radiation therapy and stereotactic radiosurgery alone versus WBRT and SRS with temozolomide or erlotinib for non-small cell lung cancer and 1 to 3 brain metastases: Radiation Therapy Oncology Group 0320. Int J Radiat Oncol Biol Phys. 2013;85(5):1312–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gorantla V, Kirkwood JM, Tawbi HA. Melanoma brain metastases: an unmet challenge in the era of active therapy. Curr Oncol Rep. 2013;15(5):483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Berghoff AS, Ricken G, Widhalm G, et al. Tumour-infiltrating lymphocytes and expression of programmed death ligand 1 (PD-L1) in melanoma brain metastases. Histopathology. 2015;66(2):289–299. [DOI] [PubMed] [Google Scholar]
- 55.Di Giacomo AM, Ascierto PA, Pilla L, et al. Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol. 2012;13(9):879–886. [DOI] [PubMed] [Google Scholar]
- 56.Jang S, Atkins MB. Which drug, and when, for patients with BRAF-mutant melanoma? Lancet Oncol. 2013;14(2):e60–e69. [DOI] [PubMed] [Google Scholar]
- 57.Azijli K, Stelloo E, Peters GJ, et al. New developments in the treatment of metastatic melanoma: immune checkpoint inhibitors and targeted therapies. Anticancer Res. 2014;34(4):1493–1505. [PubMed] [Google Scholar]
- 58.Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Margolin K, Ernstoff MS, Hamid O, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–465. [DOI] [PubMed] [Google Scholar]
- 60.Long GV, Trefzer U, Davies MA, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(11):1087–1095. [DOI] [PubMed] [Google Scholar]
- 61.Robinson SD, O'Shaughnessy JA, Cowey CL, et al. BRAF V600E-mutated lung adenocarcinoma with metastases to the brain responding to treatment with vemurafenib. Lung Cancer. 2014;85(2):326–330. [DOI] [PubMed] [Google Scholar]
- 62.Gaudy-Marqueste C, Carron R, Delsanti C, et al. On demand Gamma-Knife strategy can be safely combined with BRAF inhibitors for the treatment of melanoma brain metastases. Ann Oncol. 2014;25(10):2086–2091. [DOI] [PubMed] [Google Scholar]
- 63.Sperduto PW, Kased N, Roberge D, et al. Effect of tumor subtype on survival and the graded prognostic assessment for patients with breast cancer and brain metastases. Int J Radiat Oncol Biol Phys. 2012;82(5):2111–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lin NU, Dieras V, Paul D, et al. Multicenter phase II study of lapatinib in patients with brain metastases from HER2-positive breast cancer. Clin Cancer Res. 2009;15(4):1452–1459. [DOI] [PubMed] [Google Scholar]
- 65.Bachelot T, Romieu G, Campone M, et al. Lapatinib plus capecitabine in patients with previously untreated brain metastases from HER2-positive metastatic breast cancer (LANDSCAPE): a single-group phase 2 study. Lancet Oncol. 2013;14(1):64–71. [DOI] [PubMed] [Google Scholar]
- 66.Metro G, Foglietta J, Russillo M, et al. Clinical outcome of patients with brain metastases from HER2-positive breast cancer treated with lapatinib and capecitabine. Ann Oncol. 2011;22(3):625–630. [DOI] [PubMed] [Google Scholar]
- 67.Morikawa A, Peereboom DM, Thorsheim HR, et al. Capecitabine and lapatinib uptake in surgically resected brain metastases from metastatic breast cancer patients: a prospective study. Neuro Oncol. 2015;17(2):289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Taskar KS, Rudraraju V, Mittapalli RK, et al. Lapatinib distribution in HER2 overexpressing experimental brain metastases of breast cancer. Pharm Res. 2012;29(3):770–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin NU, Carey LA, Liu MC, et al. Phase II trial of lapatinib for brain metastases in patients with human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2008;26(12):1993–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Freedman R, Gelman R, Wefel J, et al. TBCRC 022: Phase II Trial of Neratinib for Patients with Human Epidermal Growth Factor Receptor 2 (HER2)-Positive Breast cancer and Brain Metastases. Cancer Res. 2012;72(24 Supplement):OT1-1-11. [Google Scholar]
- 71.Joensuu H, Ould-Kaci M, the TG LUX-Breast 3: Randomized Phase II trial of afatinib (BIBW 2992) alone or with vinorelbine versus investigator's choice of treatment in patients (pts) with HER2-positive breast cancer (BC) with progressive brain metastases after trastuzumab and/or lapatinib-based therapy*. Cancer Res. 2012;72(24 Supplement):OT1–1–15. [Google Scholar]
- 72.Bartsch R, Berghoff AS, Preusser M. Breast cancer brain metastases responding to primary systemic therapy with T-DM1. J Neurooncol. 2014;116(1):205–206. [DOI] [PubMed] [Google Scholar]
- 73.Wildiers H, Kim SB, Gonzalez-Martin A, et al. T-DM1 for HER2-postive metastatic breast cancer (MBC): Primary results from the TH3RESA, a phase 3 study of T-DM1 vs treatment of physician's choice. Paper presented at: European Cancer Congress, 2013; Amsterdam. 27 Sept-1 Oct. [Google Scholar]
- 74.Krop IE, Lin NU, Blackwell K, et al. Trastuzumab emtansine (T-DM1) versus lapatinib plus capecitabine in patients with HER2-positive metastatic breast cancer and central nervous system metastases: a retrospective, exploratory analysis in EMILIA. Annals of Oncology. 2015;26(1):113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anders C, Deal AM, Abramson V, et al. TBCRC 018: phase II study of iniparib in combination with irinotecan to treat progressive triple negative breast cancer brain metastases. Breast Cancer Res Treat. 2014;146(3):557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Levy C, Allouache D, Lacroix J, et al. REBECA: a phase I study of bevacizumab and whole-brain radiation therapy for the treatment of brain metastasis from solid tumours. Ann Oncol. 2014;25(12):2351–2356. [DOI] [PubMed] [Google Scholar]
- 77.Palmieri D, Chambers AF, Felding-Habermann B, et al. The biology of metastasis to a sanctuary site. Clin Cancer Res. 2007;13(6):1656–1662. [DOI] [PubMed] [Google Scholar]
- 78.Fidler IJ. The role of the organ microenvironment in brain metastasis. Semin Cancer Biol. 2011;21(2):107–112. [DOI] [PubMed] [Google Scholar]
- 79.Kienast Y, Klein C, Scheuer W, et al. Ang-2-VEGF-A CrossMab, a novel bispecific human IgG1 antibody blocking VEGF-A and Ang-2 functions simultaneously, mediates potent antitumor, antiangiogenic, and antimetastatic efficacy. Clin Cancer Res. 2013;19(24):6730–6740. [DOI] [PubMed] [Google Scholar]
- 80.Muldoon LL, Gahramanov S, Li X, et al. Dynamic magnetic resonance imaging assessment of vascular targeting agent effects in rat intracerebral tumor models. Neuro Oncol. 2011;13(1):51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Daphu I, Sundstrom T, Horn S, et al. In vivo animal models for studying brain metastasis: value and limitations. Clin Exp Metastasis. 2013;30(5):695–710. [DOI] [PubMed] [Google Scholar]
- 82.Cruz-Munoz W, Man S, Xu P, et al. Development of a preclinical model of spontaneous human melanoma central nervous system metastasis. Cancer Res. 2008;68(12):4500–4505. [DOI] [PubMed] [Google Scholar]
- 83.Kurebayashi J, McLeskey SW, Johnson MD, et al. Quantitative demonstration of spontaneous metastasis by MCF-7 human breast cancer cells cotransfected with fibroblast growth factor 4 and LacZ. Cancer Res. 1993;53(9):2178–2187. [PubMed] [Google Scholar]
- 84.Mathieu A, Remmelink M, D'Haene N, et al. Development of a chemoresistant orthotopic human nonsmall cell lung carcinoma model in nude mice: analyses of tumor heterogenity in relation to the immunohistochemical levels of expression of cyclooxygenase-2, ornithine decarboxylase, lung-related resistance protein, prostaglandin E synthetase, and glutathione-S-transferase-alpha (GST)-alpha, GST-mu, and GST-pi. Cancer. 2004;101(8):1908–1918. [DOI] [PubMed] [Google Scholar]
- 85.Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res. 1998;58(7):1486–1493. [PubMed] [Google Scholar]
- 86.Lorger M, Felding-Habermann B. Capturing changes in the brain microenvironment during initial steps of breast cancer brain metastasis. Am J Pathol. 2010;176(6):2958–2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shintani Y, Higashiyama S, Ohta M, et al. Overexpression of ADAM9 in non-small cell lung cancer correlates with brain metastasis. Cancer Res. 2004;64(12):4190–4196. [DOI] [PubMed] [Google Scholar]
- 88.Song HT, Jordan EK, Lewis BK, et al. Rat model of metastatic breast cancer monitored by MRI at 3 tesla and bioluminescence imaging with histological correlation. J Transl Med. 2009;7:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wu YJ, Muldoon LL, Gahramanov S, et al. Targeting alphaV-integrins decreased metastasis and increased survival in a nude rat breast cancer brain metastasis model. J Neurooncol. 2012;110(1):27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yoneda T, Williams PJ, Hiraga T, et al. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res. 2001;16(8):1486–1495. [DOI] [PubMed] [Google Scholar]
- 91.Heyn C, Ronald JA, Ramadan SS, et al. In vivo MRI of cancer cell fate at the single-cell level in a mouse model of breast cancer metastasis to the brain. Magn Reson Med. 2006;56(5):1001–1010. [DOI] [PubMed] [Google Scholar]
- 92.Palmieri D, Bronder JL, Herring JM, et al. Her-2 overexpression increases the metastatic outgrowth of breast cancer cells in the brain. Cancer Res. 2007;67(9):4190–4198. [DOI] [PubMed] [Google Scholar]
- 93.Wu YJ, Muldoon LL, Dickey DT, et al. Cyclophosphamide enhances human tumor growth in nude rat xenografted tumor models. Neoplasia. 2009;11(2):187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Daphu I, Pedersen PH, et al. A novel brain metastases model developed in immunodeficient rats closely mimics the growth of metastatic brain tumours in patients. Neuropathol Appl Neurobiol. 2011;37(2):189–205. [DOI] [PubMed] [Google Scholar]
- 95.Trinh VA, Hwu WJ. Chemoprevention for brain metastases. Curr Oncol Rep. 2012;14(1):63–69. [DOI] [PubMed] [Google Scholar]
- 96.Steeg PS. Perspective: The right trials. Nature. 2012;485(7400):S58–S59. [DOI] [PubMed] [Google Scholar]
- 97.Pivot X, Semiglazov V, Zurawski B, et al. CEREBEL (EFG111438): An open label randomized phase III study comparing the incidence of CNS metastases in patients (pts) with HER2+ metastatic breast cancer, treated with lapatinib plus capecitabine versus trastuzumab plus capecitabine. Paper presented at: 37th ESMO Conference, 2012. Vienna Austria, 28 September-2 October 2012. [Google Scholar]
- 98.Bonnefoi H, Jacot W, Saghatchian M, et al. Neoadjuvant treatment with docetaxel plus lapatinib, trastuzumab, or both followed by an anthracycline based chemotherapy in HER2-positive breast cancer: results of the randomised phase II EORTC 10054 study. Ann Oncol. 2015;26(2):325–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.McCarty JH. Cell adhesion and signaling networks in brain neurovascular units. Curr Opin Hematol. 2009;16(3):209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Berghoff AS, Kovanda AK, Melchardt T, et al. alphavbeta3, alphavbeta5 and alphavbeta6 integrins in brain metastases of lung cancer. Clin Exp Metastasis. 2014;31(7):841–851. [DOI] [PubMed] [Google Scholar]