Abstract
Effective treatment of glioblastoma (GBM) remains a formidable challenge. Survival rates remain poor despite decades of clinical trials of conventional and novel, biologically targeted therapeutics. There is considerable evidence that most of these therapeutics do not reach their targets in the brain when administered via conventional routes (intravenous or oral). Hence, direct delivery of therapeutics to the brain and to brain tumors is an active area of investigation. One of these techniques, convection-enhanced delivery (CED), involves the implantation of catheters through which conventional and novel therapeutic formulations can be delivered using continuous, low–positive-pressure bulk flow. Investigation in preclinical and clinical settings has demonstrated that CED can produce effective delivery of therapeutics to substantial volumes of brain and brain tumor. However, limitations in catheter technology and imaging of delivery have prevented this technique from being reliable and reproducible, and the only completed phase III study in GBM did not show a survival benefit for patients treated with an investigational therapeutic delivered via CED. Further development of CED is ongoing, with novel catheter designs and imaging approaches that may allow CED to become a more effective therapeutic delivery technique.
Keywords: blood-brain barrier, CED infusates, convection-enhanced delivery (CED), delivery vehicles, glioblastoma
Effective treatment of glioblastoma (GBM), the most common and most malignant glioma, represents one of the most formidable challenges in oncology. Despite the use of extensive surgery, radiation therapy, and chemotherapy, the prognosis for patients with GBM remains poor, with a median survival of 12–15 months.1 Following recurrence, salvage treatment provides only about 6–8 months of additional survival.2 It is the infiltrative nature of these tumors that eliminates the possibility of curative surgical resection. Even at the time of initial presentation, there is infiltration of tumor cells that extend at least 2 cm away from the radiographical contrast-enhancing mass.3 Efforts to treat this residual disease with conventional and targeted chemotherapies delivered via oral or intravenous routes have been rendered minimally effective by the presence of the blood-brain barrier (BBB), and the partially functional blood-tumor-barrier, which prevent the effective delivery of potentially active chemotherapeutic compounds.4 Strategies have been investigated for enhancing drug delivery to brain tumor cells by methods that do not rely on the circulatory system. These techniques circumvent the BBB completely in order to improve drug delivery to the brain parenchyma. Furthermore, these intraparenchymal delivery approaches result in locally high, but systemically low, concentrations of chemotherapies known to have activity against gliomas, thereby eliminating the systemic toxicity that has limited their dosing. Direct intraparenchymal delivery also provides a means for delivering newer, tumor-selective molecules that are often largely excluded from the CNS.5–9
Delivery of Therapeutics Directly into the Central Nervous System
Drug delivery directly into the CNS has been an actively investigated field of study for the treatment of primary brain tumors. This form of administration for therapeutics requires consideration of multiple variables that affect the extent of delivery (including diffusion gradients, infusion rates, physical properties of the therapeutic itself, tumor cellular architecture, interstitial architecture, and the physical characteristics of the device[s] used to produce delivery) when applicable. Approaches to local drug delivery have included the use of implantable, controlled-release polymer systems,10 delivery into the cerebrospinal fluid (CSF) or a cyst cavity (often using an implanted reservoir),11–13 and catheter-based convection-enhanced delivery (CED).5,14 Experience with implantable controlled-release polymer systems will be discussed in another paper in this volume.
Intracavitary/Cerebrospinal Fluid Delivery
The use of an intraventricular or intracavitary system, such as an Ommaya reservoir, allows delivery of intermittent bolus injections of anticancer drugs directly into a tumor cyst or cavity.11 Other systems are capable of delivering drugs over a prolonged period of time at a desired constant infusion rate to the tumor resection cavity by means of a catheter. Drugs such as nitrosoureas12 and methotrexate13 have been used in various clinical trials; however, infection, catheter obstruction, and inadequate drug distribution have limited the success of these delivery methods. Utilization of the CSF space for delivering chemotherapeutic agents has been a valuable method of treatment for some patients with CNS malignancies, particularly those who have disease localized to the leptomeningeal space. However, there is little bulk flow between the intercellular space of the brain and the CSF space. Hence, scant drug is able to diffuse from the CSF space into the parenchyma, which prohibits this delivery system from being effective for treating intraparenchymal brain tumors such as GBM.15 Intrathecal delivery is therefore a technique that is typically reserved for leptomeningeal spread of malignant cells, as is often seen in primary CNS lymphoma and various types of metastatic carcinomas.16
Convection-enhanced Delivery of Therapeutics
CED was first proposed by Bobo et al in 1994.14 CED involves continuous positive-pressure infusion of a solute containing a therapeutic agent. It relies on pressure-driven bulk flow of infusate as a means for delivering therapeutic agents to the CNS. The bulk flow mechanism is created by a small pressure gradient from a pump that pushes solute through a catheter targeted within the CNS. There are several potential advantages of CED as compared with traditional delivery methods17: (i) CED bypasses the BBB and can be used to infuse therapeutic agents with large or small molecular weights via bulk interstitial flow; (ii) CED provides targeted delivery to the region into which the catheter is placed and the potential for real-time monitoring of distribution, which would allow intelligent adjustment of flow rates18; (iii) and unlike diffusion-limited delivery, CED provides pressure-driven delivery that enhances interstitial drug distribution.14
This form of localized delivery limits the potential for neurotoxicity because the infused doses do not need to be as high as those needed for diffusion-mediated delivery (ie, there is not the same issue with steep concentration gradients requiring a potentially toxic initial dose).17 Therefore, CED may not produce the same elevated risk of neurologic injury when compared with implantable polymers.5 Neurological symptoms in the eloquent areas during both animal19 and human CED studies20–22 are most often reported to be transient and reversible with reduction of infusion rates. While some concern may be raised about the need for placing a catheter into the brain in order to perform CED, histological studies have shown that inflammation adjacent to the catheter tract and at the catheter tip is typically limited to within a 50-µm radius.23
Basic Principles of Convection-enhanced Delivery
To perform CED, one or more catheters are stereotactically implanted through a burr hole into or adjacent to the enhancing portion of a tumor and/or the nonenhancing infiltrative surrounding tissue. Pressure-driven flow of drug is achieved via an infusion pump, and the agent is infused directly into the target tissue at a predetermined concentration, rate, and duration. Infusate distribution delivered via CED relies on the bulk flow of interstitial fluid, which occurs due to pressure gradients, and therefore relies less on the concentration of the infusate. Infusion rate and volume of infusion (Vi) are key components that impact infusate distribution and are also important variables to be considered in terms of risk of backflow. It has been observed that the volume of distribution (Vd) of an infusate will initially correlate in a linear fashion with Vi, even large (80 kDa) molecules.14 However, this relationship is very dependent upon the infusion rate because significant backflow occurs around the infusion catheter above certain rates, thereby rendering the Vd independently of the Vi.24 It is anticipated that improvements to the devices used for CED will result in a reduced propensity for backflow and that a more predictable relationship between Vd and Vi is likely to be achieved, thereby facilitating higher rates of infusion.14,23
The Vd achieved via CED may also vary depending upon the cytoarchitecture of the tissue being treated. For example, CED in GBM produces a markedly different distribution from that observed in normal brain.25–27 The central portion of a GBM is generally marked by poor vascularity surrounded by unique, heterogeneous cytoarchitecture that is conversely rich in vascularity with a pathologic interstitial composition.26 This leaky cytoarchitecture and outward pressure gradient result in higher infusate clearance rates.26,27 Furthermore, infusates may preferentially move in accordance with regional anisotropy and paths of pre-existing white matter edema.28 Recent studies have also shown the ability of CED to penetrate infiltrating tumor cells at the margin, with distribution at the margin improved in the absence of preceding craniotomy and in the setting of prior steroid use.29 Although the obstacles produced by tissue characteristics and catheter-related backflow have likely affected early clinical trials, changes in infusate and cannula characteristics,30,31 development of catheter placement guidelines,32 mathematical modeling to predict infusate spatial distribution,33 and monitoring of infusate delivery in real-time23,32 have been considered in order to optimize this drug delivery technique.
Initial Clinical Development of Convection-enhanced Delivery for Glioblastoma
CED clinical trials have been carried out with various agents including conventional chemotherapies,34 cytotoxin-ligand conjugates targeting cell surface receptors,6–8,35 monoclonal antibodies with36 or without9 radioactive isotope conjugates, antisense oligonucleotides,37 and liposomal vectors engineered to deliver gene therapies.38 Phase I–III trials for GBM were performed, beginning in the 1990s, that demonstrated adequate safety profiles for a number of convection-delivered agents. However, these early CED trials involved the use of catheters not designed for CED. As noted above, early experience with these “off-the-shelf” catheters provided hints that they were likely prone to backflow. Despite a lack of definitive data evaluating the delivery characteristics of the catheters in the clinical setting, and fueled by apparently promising results from the small phase I and II trials, larger CED trials moved forward using off-label catheters that had FDA clearance for other uses (eg, peritoneal and ventricular catheters).
Two phase III trials were initiated in participants with GBM. One trial utilizing Tf-CRM107 was aborted, and the most recent data published about Tf-CRM107 pertained to a phase II trial from 2003.6 The other phase III trial, the PRECISE trial, compared the infusion of citredekin besudotox (CB; a chimeric pseudomonas exotoxin with recombinant human interleukin-13) delivered by CED with chemotherapy wafers placed in the surgical resection cavity. The study completed its accrual but unfortunately did not reveal statistically significant improvement in survival for patients with recurrent GBM.5 Although no survival benefit for the CED experimental arm was found, the study was impaired by its statistical design requiring a >50% survival benefit over the active control arm; no other agents, even those approved by the FDA, have even come close to this mark for recurrent GBM. Furthermore, the authors of the study noted that only 68% of catheter placements were performed per protocol specifications. Despite these limitations, there was a statistically significant improvement in progression-free survival (17.7 vs 11.4 weeks; P = .0008), although this was not a prespecified analysis and hence was not considered to be acceptable evidence of efficacy.5
A phase I clinical trial studied the use of CB in newly diagnosed malignant glioma patients who were also being treated with standard-of-care external beam radiation therapy and concurrent temozolomide.39 This study seemed to indicate that the dose of CB used in the recurrent GBM setting was also safe when used in combination with chemoradiation. No further development of CB for this indication has been performed.
Monoclonal antibodies have also been utilized in clinical trials. (131)I-chTNT-1/B mAb, a radioactive isotope-conjugated monoclonal antibody, was well tolerated in phase I and II trials.36 However, no phase III study of a monoclonal antibody used in this manner has been completed to date.
CED has also been used to deliver conventional chemotherapies directly into GBM tumors and/or surrounding tumor-infiltrated brain. Clinical trials utilizing CED have investigated carboplatin40 and topotecan.34 Topotecan has shown promising results with favorable progression-free and overall survival rates of 23 weeks and 60 weeks, respectively.34 In a report of 2 participants also enrolled in the topotecan study, Anderson et al showed that it was possible to infuse topotecan into the brainstem of 2 pediatric patients with diffuse intrinsic pontine gliomas.20 The infusion rates and drug concentrations had to be lowered because the brainstem is more sensitive than other parts of the brain to side effects from local delivery of chemotherapy and/or fluid infusion rate. Nevertheless, the tolerability of this treatment when using lower infusion rates demonstrates the treatment's possible value for treating brainstem lesions, albeit cautiously (particularly for those with mass effect).20
Newer Agents and Infusates Investigated for CED in Preclinical and Early Clinical Investigation
Multiple other classes of therapeutic agents have been investigated as potential CED infusates for glioma therapy. These include gene therapies,38,40,41 oligonucleotides,37,42 nanoparticle conjugates,43–46 liposomes,38,47,48 and viral particles.40,41 One approach that has generated substantial interest involves the use of liposomal encapsulation. Liposomes have been used to encapsulate a multitude of therapeutics and prolong their half-life systemically; they may have particularly advantageous properties when used to deliver therapeutics via CED.24 In the CNS, liposomal encapsulation can potentially reduce unwanted early drug-tissue interaction, allowing for greater volumes of distribution and reduced tissue clearance rates, consequently providing a longer drug exposure to target tissue.47 Liposomal encapsulation also provides a vector for gene therapy delivery.38 Liposomes can carry MRI contrast agents themselves, as has been shown in animal models.47
While liposomes are promising as carrier agents for therapeutic CED, nanoparticles are emerging as smaller, potentially more efficient vehicles.43–46,49 For example, magnetic nanoparticles, such as maghemite (15–80 nanometer [nm]), can be delivered via CED and be loaded with bioactive molecules (which would normally have high tissue clearance or reactivity rates) and utilized as MRI contrast agents.44 Polymeric nanoparticles offer similar advantages in that they can be conjugated to numerous chemotherapies in addition to a contrast agent and can be fabricated for optimal convection characteristics (<100 nm).43 While there are many permutations being investigated in animal models, no particular vehicle has been proven to be reliably better, and few have been tested in clinical trials.
The visualization of infusions in real time has emerged as an important potential means for identifying reflux as soon as it happens, thereby allowing adjustments to be made in the infusions accordingly. Visualization of infusions also provides a basis for more routine measurement of Vd and Vi. Most of these studies have been performed by mixing the infusate containing the investigational therapeutic with MRI contrast agents and performing the infusion in an operative MRI suite using an MRI-compatible catheter (Fig. 1), thus allowing serial MRIs to be performed throughout the infusion.21,50
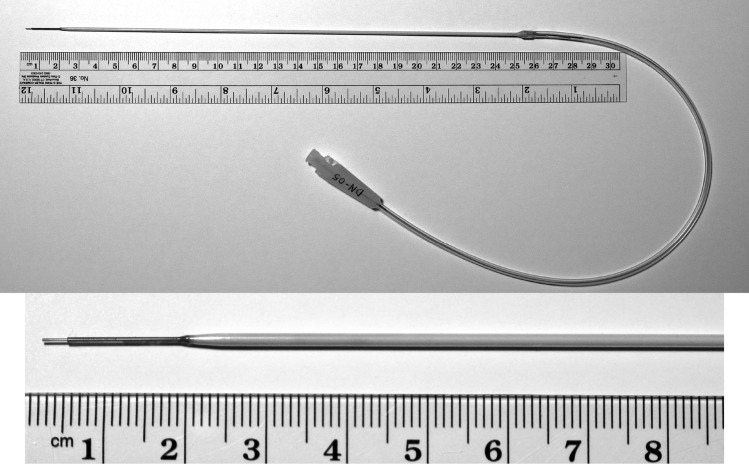
Fig. 1.
MRI-compatible cannula for convection-enhanced delivery. This ClearFlow cannula (MRI Interventions) has a gradually tapered tip designed to prevent reflux, is MRI compatible, and can be used with an MRI-compatible stereotactic guidance system.
Recent Advances in Convection-enhanced Delivery for Glioblastoma
Advances in CED catheter design have included a step-design cannula that was first described by Fiandaca et al.51 The original design of this cannula was a 0.2 mm needle with a glued-in silica tubing (0.168 mm external diameter) extending beyond the end of the needle by 5–10 mm. Even with this technical improvement, which allows CED at flow rates as high as 5.0 µL/min, the step-design cannula is not reflux free. Real-time MRI has documented that reflux can be seen along the insertion tract in up to 20% of catheter placements, albeit at higher achievable infusion flow rates. More recently, and with use of a 16-gauge catheter that gradually narrows in diameter from 0.063 inches to 0.028 inches to 0.014 inches at its tip, the step design has been used to infuse replicating retrovirus into recurrent glioblastoma (ClinicalTrials.gov, NCT01156584) (Figs. 1 and 2).
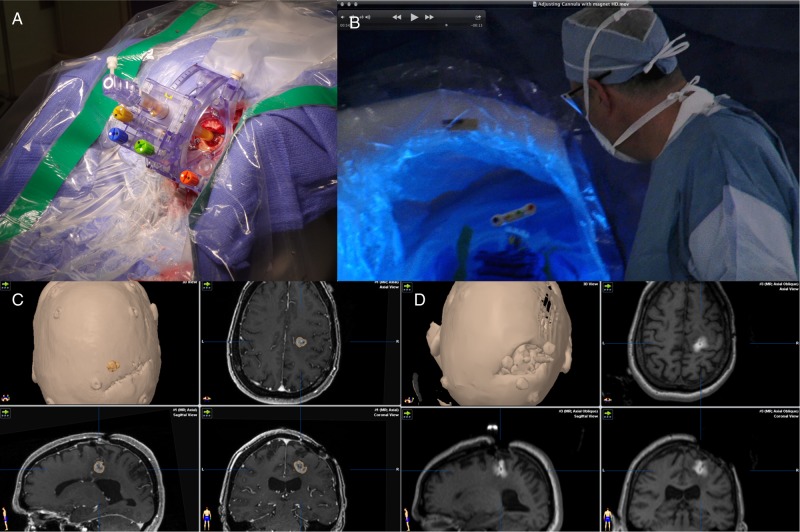
Fig. 2.
Real-time imaging of convection-enhanced delivery of an investigational therapy. (A) Placement of the SmartFrame (MRI Interventions) MRI-compatible stereotactic guidance system over the site of a burr hole. (B) Access to the port of the SmartFrame is maintained while the patient is in the imaging position within the intraoperative MRI. (C) Preinfusion MRI showing the target lesion, which is a recurrent high-grade glioma. (D) Intraoperative MRI during infusion of an experimental therapy, to which gadolinium was added as a tracer.
Notable improvements had been made in catheter placement. Considerable variability between neurosurgeons in terms of surgical technique and placement accuracy was noted in the PRECISE trial, and work has been done to develop software algorithms to define optimal trajectories that can be incorporated into the intraoperative navigation system typically used by neurosurgeons.52
Another area of early investigation relates to the fact that CED can deliver a therapeutic only during a fixed period of time, that being when a temporarily implanted catheter is in place. It is believed that more durable glioblastoma treatment might require ongoing treatment with a chronically infused agent. This creates a need for implantable catheters with ports of access under the scalp that can be left inside the brain and brain tumors for extended periods of time. This concept has been explored in primate models53 and is being developed for human use as well.
Conclusion
By bypassing the BBB and delivering treatments to large volumes of brain tumor and tumor-infiltrated brain, CED may serve as a platform for translating the molecular understanding of glioblastoma achieved in the laboratory into effective clinical treatments. Additional developments in technology will be needed to ensure reliable and reproducible high-volume therapeutic delivery to substantial volumes of involved brain tissue. Advances in imaging also will be required to resolve questions about dose-response relationships and regional dosimetry associated with CED.
Acknowledgments
This paper is one of three review articles for a supplement entitled “Local delivery of cytoreductive agents for the treatment of glioblastoma.” The supplement is supported by Arbor Pharmaceuticals. The physician editor of the supplement is Steven N. Kalkanis, MD, Department of Neurosurgery, Henry Ford Hospital, Detroit, MI, who has contributed an introduction to the supplement. The two other review articles in the supplement cover polymeric drug delivery and gene therapy and delivery systems.
Conflict of interest statement. M.A.V has stock options in Infuseon Therapeutics, Inc., which is commercializing a CED catheter that he invented; he is Chair of the Scientific Advisory Board for Infuseon Therapeutics, Inc., which is commercializing a CED catheter that he invented; he is serving as Chief Medical Officer for Infuseon Therapeutics, Inc., which is commercializing a CED catheter that he invented, and he has a US patent for a CED catheter and several other patents pending. M.K.A has no conflict of interest.
References
- 1.Ostrom QT, Gittleman H, Farah P, et al. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15(Suppl 2):ii1–i56. doi: 10.1093/neuonc/not151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oppenlander ME, Wolf AB, Snyder LA, et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg. 2014;120(4):846–853. doi: 10.3171/2013.12.JNS13184. [DOI] [PubMed] [Google Scholar]
- 3.Watanabe M, Tanaka R, Takeda N. Magnetic resonance imaging and histopathology of cerebral gliomas. Neuroradiology. 1992;34(6):463–469. doi: 10.1007/BF00598951. [DOI] [PubMed] [Google Scholar]
- 4.Muldoon LL, Soussain C, Jahnke K, et al. Chemotherapy delivery issues in central nervous system malignancy: a reality check. J Clin Oncol. 2007;25(16):2295–2305. doi: 10.1200/JCO.2006.09.9861. [DOI] [PubMed] [Google Scholar]
- 5.Kunwar S, Chang S, Westphal M, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12(8):871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weaver M, Laske DW. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol. 2003;65(1):3–13. doi: 10.1023/a:1026246500788. [DOI] [PubMed] [Google Scholar]
- 7.Weber F, Asher A, Bucholz R, et al. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J Neurooncol. 2003;64(1–2):125–137. doi: 10.1007/BF02700027. [DOI] [PubMed] [Google Scholar]
- 8.Weber FW, Floeth F, Asher A, et al. Local convection enhanced delivery of IL4-Pseudomonas exotoxin (NBI-3001) for treatment of patients with recurrent malignant glioma. Acta Neurochir Suppl. 2003;88:93–103. doi: 10.1007/978-3-7091-6090-9_15. [DOI] [PubMed] [Google Scholar]
- 9.Wersall P, Ohlsson I, Biberfeld P, et al. Intratumoral infusion of the monoclonal antibody, mAb 425, against the epidermal-growth-factor receptor in patients with advanced malignant glioma. Cancer Immunol Immunother. 1997;44(3):157–164. doi: 10.1007/s002620050368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brem H, Mahaley MS, Jr., Vick NA, et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J Neurosurg. 1991;74(3):441–446. doi: 10.3171/jns.1991.74.3.0441. [DOI] [PubMed] [Google Scholar]
- 11.Pollina J, Plunkett RJ, Ciesielski MJ, et al. Intratumoral infusion of topotecan prolongs survival in the nude rat intracranial U87 human glioma model. J Neurooncol. 1998;39(3):217–225. doi: 10.1023/a:1005954121521. [DOI] [PubMed] [Google Scholar]
- 12.Boiardi A, Silvani A, Pozzi A, et al. Interstitial chemotherapy plus systemic chemotherapy for glioblastoma patients: improved survival in sequential studies. J Neurooncol. 1999;41(2):151–157. doi: 10.1023/a:1006119505170. [DOI] [PubMed] [Google Scholar]
- 13.Nierenberg D, Harbaugh R, Maurer LH, et al. Continuous intratumoral infusion of methotrexate for recurrent glioblastoma: a pilot study. Neurosurgery. 1991;28(5):752–761. doi: 10.1097/00006123-199105000-00023. [DOI] [PubMed] [Google Scholar]
- 14.Bobo RH, Laske DW, Akbasak A, et al. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci U S A. 1994;91(6):2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blasberg RG, Patlak CS, Shapiro WR. Distribution of methotrexate in the cerebrospinal fluid and brain after intraventricular administration. Cancer Treat Rep. 1977;61(4):633–641. [PubMed] [Google Scholar]
- 16.Walter KA, Tamargo RJ, Olivi A, et al. Intratumoral chemotherapy. Neurosurgery. 1995;37(6):1128–1145. [PubMed] [Google Scholar]
- 17.Bidros D, Vogelbaum MA. Barriers to delivery of therapeutics to brain tumors. In: Winn RH, editor. Youman's Neurological Surgery. 6th ed. Philadelphia: Elsevier Saunders; 2011. pp. 1172–1178. [Google Scholar]
- 18.Varenika V, Dickinson P, Bringas J, et al. Detection of infusate leakage in the brain using real-time imaging of convection-enhanced delivery. J Neurosurg. 2008;109(5):874–880. doi: 10.3171/JNS/2008/109/11/0874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lonser RR, Gogate N, Morrison PF, et al. Direct convective delivery of macromolecules to the spinal cord. J Neurosurg. 1998;89(4):616–622. doi: 10.3171/jns.1998.89.4.0616. [DOI] [PubMed] [Google Scholar]
- 20.Anderson RC, Kennedy B, Yanes CL, et al. Convection-enhanced delivery of topotecan into diffuse intrinsic brainstem tumors in children. J Neurosurg Pediatr. 2013;11(3):289–295. doi: 10.3171/2012.10.PEDS12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lonser RR, Schiffman R, Robison RA, et al. Image-guided, direct convective delivery of glucocerebrosidase for neuronopathic Gaucher disease. Neurology. 2007;68(4):254–261. doi: 10.1212/01.wnl.0000247744.10990.e6. [DOI] [PubMed] [Google Scholar]
- 22.Shahar T, Ram Z, Kanner AA. Convection-enhanced delivery catheter placements for high-grade gliomas: complications and pitfalls. J Neurooncol. 2012;107(2):373–378. doi: 10.1007/s11060-011-0751-x. [DOI] [PubMed] [Google Scholar]
- 23.Lonser RR, Walbridge S, Garmestani K, et al. Successful and safe perfusion of the primate brainstem: in vivo magnetic resonance imaging of macromolecular distribution during infusion. J Neurosurg. 2002;97(4):905–913. doi: 10.3171/jns.2002.97.4.0905. [DOI] [PubMed] [Google Scholar]
- 24.Chen PY, Ozawa T, Drummond DC, et al. Comparing routes of delivery for nanoliposomal irinotecan shows superior anti-tumor activity of local administration in treating intracranial glioblastoma xenografts. Neuro Oncol. 2013;15(2):189–197. doi: 10.1093/neuonc/nos305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall WA, Sherr GT. Convection-enhanced delivery: targeted toxin treatment of malignant glioma. Neurosurg Focus. 2006;20(4):E10. [PubMed] [Google Scholar]
- 26.Jain RK. Barriers to drug delivery in solid tumors. Sci Am. 1994;271(1):58–65. doi: 10.1038/scientificamerican0794-58. [DOI] [PubMed] [Google Scholar]
- 27.Vogelbaum MA. Convection enhanced delivery for treating brain tumors and selected neurological disorders: symposium review. J Neurooncol. 2007;83(1):97–109. doi: 10.1007/s11060-006-9308-9. [DOI] [PubMed] [Google Scholar]
- 28.Geer CP, Grossman SA. Interstitial fluid flow along white matter tracts: a potentially important mechanism for the dissemination of primary brain tumors. J Neurooncol. 1997;32(3):193–201. doi: 10.1023/a:1005761031077. [DOI] [PubMed] [Google Scholar]
- 29.Yang X, Saito R, Nakamura T, et al. Peri-tumoral leakage during intra-tumoral convection-enhanced delivery has implications for efficacy of peri-tumoral infusion before removal of tumor. Drug Deliv. 2014;0:1–6. doi: 10.3109/10717544.2014.914987. [DOI] [PubMed] [Google Scholar]
- 30.Guarnieri M, Carson BS, Khan A, et al. Flexible versus rigid catheters for chronic administration of exogenous agents into central nervous system tissues. J Neurosci Methods. 2005;144(2):147–152. doi: 10.1016/j.jneumeth.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Stukel JM, Caplan MR. Targeted drug delivery for treatment and imaging of glioblastoma multiforme. Expert Opin Drug Deliv. 2009;6(7):705–718. doi: 10.1517/17425240902988470. [DOI] [PubMed] [Google Scholar]
- 32.Sampson JH, Brady M, Raghavan R, et al. Colocalization of gadolinium-diethylene triamine pentaacetic acid with high-molecular-weight molecules after intracerebral convection-enhanced delivery in humans. Neurosurgery. 2011;69(3):668–676. doi: 10.1227/NEU.0b013e3182181ba8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Linninger AA, Somayaji MR, Mekarski M, et al. Prediction of convection-enhanced drug delivery to the human brain. J Theor Biol. 2008;250(1):125–138. doi: 10.1016/j.jtbi.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 34.Bruce JN, Fine RL, Canoll P, et al. Regression of recurrent malignant gliomas with convection-enhanced delivery of topotecan. Neurosurgery. 2011;69(6):1272–1279. doi: 10.1227/NEU.0b013e3182233e24. discussion 1279–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3(12):1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 36.Patel SJ, Shapiro WR, Laske DW, et al. Safety and feasibility of convection-enhanced delivery of Cotara for the treatment of malignant glioma: initial experience in 51 patients. Neurosurgery. 2005;56(6):1243–1252. doi: 10.1227/01.neu.0000159649.71890.30. discussion 1252–1243. [DOI] [PubMed] [Google Scholar]
- 37.Bogdahn U, Hau P, Stockhammer G, et al. Targeted therapy for high-grade glioma with the TGF-beta2 inhibitor trabedersen: results of a randomized and controlled phase IIb study. Neuro Oncol. 2011;13(1):132–142. doi: 10.1093/neuonc/noq142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voges J, Reszka R, Gossmann A, et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54(4):479–487. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 39.Vogelbaum MA, Sampson JH, Kunwar S, et al. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: phase 1 study of final safety results. Neurosurgery. 2007;61(5):1031–1037. doi: 10.1227/01.neu.0000303199.77370.9e. discussion 1037–1038. [DOI] [PubMed] [Google Scholar]
- 40.White E, Bienemann A, Pugh J, et al. An evaluation of the safety and feasibility of convection-enhanced delivery of carboplatin into the white matter as a potential treatment for high-grade glioma. J Neurooncol. 2012;108(1):77–88. doi: 10.1007/s11060-012-0833-4. [DOI] [PubMed] [Google Scholar]
- 41.Barua NU, Woolley M, Bienemann AS, et al. Convection-enhanced delivery of AAV2 in white matter--a novel method for gene delivery to cerebral cortex. J Neurosci Methods. 2013;220(1):1–8. doi: 10.1016/j.jneumeth.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Carpentier A, Metellus P, Ursu R, et al. Intracerebral administration of CpG oligonucleotide for patients with recurrent glioblastoma: a phase II study. Neuro Oncol. 2010;12(4):401–408. doi: 10.1093/neuonc/nop047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bernal GM, LaRiviere MJ, Mansour N, et al. Convection-enhanced delivery and in vivo imaging of polymeric nanoparticles for the treatment of malignant glioma. Nanomedicine. 2014;10(1):149–157. doi: 10.1016/j.nano.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Corem-Salkmon E, Ram Z, Daniels D, et al. Convection-enhanced delivery of methotrexate-loaded maghemite nanoparticles. Int J Nanomedicine. 2011;6:1595–1602. doi: 10.2147/IJN.S23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hadjipanayis CG, Machaidze R, Kaluzova M, et al. EGFRvIII antibody-conjugated iron oxide nanoparticles for magnetic resonance imaging-guided convection-enhanced delivery and targeted therapy of glioblastoma. Cancer Res. 2010;70(15):6303–6312. doi: 10.1158/0008-5472.CAN-10-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sawyer AJ, Saucier-Sawyer JK, Booth CJ, et al. Convection-enhanced delivery of camptothecin-loaded polymer nanoparticles for treatment of intracranial tumors. Drug Deliv Transl Res. 2011;1(1):34–42. doi: 10.1007/s13346-010-0001-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickinson PJ, LeCouteur RA, Higgins RJ, et al. Canine model of convection-enhanced delivery of liposomes containing CPT-11 monitored with real-time magnetic resonance imaging: laboratory investigation. J Neurosurg. 2008;108(5):989–998. doi: 10.3171/JNS/2008/108/5/0989. [DOI] [PubMed] [Google Scholar]
- 48.MacKay JA, Deen DF, Szoka FC., Jr Distribution in brain of liposomes after convection enhanced delivery; modulation by particle charge, particle diameter, and presence of steric coating. Brain Res. 2005;1035(2):139–153. doi: 10.1016/j.brainres.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Rosca EV, Stukel JM, Gillies RJ, et al. Specificity and mobility of biomacromolecular, multivalent constructs for cellular targeting. Biomacromolecules. 2007;8(12):3830–3835. doi: 10.1021/bm700791a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Murad GJ, Walbridge S, Morrison PF, et al. Image-guided convection-enhanced delivery of gemcitabine to the brainstem. J Neurosurg. 2007;106(2):351–356. doi: 10.3171/jns.2007.106.2.351. [DOI] [PubMed] [Google Scholar]
- 51.Fiandaca MS, Varenika V, Eberling J, et al. Real-time MR imaging of adeno-associated viral vector delivery to the primate brain. Neuroimage. 2009;47(Suppl 2):T27–T35. doi: 10.1016/j.neuroimage.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenbluth KH, Martin AJ, Mittermeyer S, et al. Rapid inverse planning for pressure-driven drug infusions in the brain. PLoS One. 2013;8(2):e56397. doi: 10.1371/journal.pone.0056397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sonabend AM, Stuart RM, Yun J, et al. Prolonged intracerebral convection-enhanced delivery of topotecan with a subcutaneously implantable infusion pump. Neuro Oncol. 2011;13(8):886–893. doi: 10.1093/neuonc/nor051. [DOI] [PMC free article] [PubMed] [Google Scholar]