Abstract
Gene therapy offers a multidimensional set of approaches intended to treat and cure glioblastoma (GBM), in combination with the existing standard-of-care treatment (surgery and chemoradiotherapy), by capitalizing on the ability to deliver genes directly to the site of neoplasia to yield antitumoral effects. Four types of gene therapy are currently being investigated for their potential use in treating GBM: (i) suicide gene therapy, which induces the localized generation of cytotoxic compounds; (ii) immunomodulatory gene therapy, which induces or augments an enhanced antitumoral immune response; (iii) tumor-suppressor gene therapy, which induces apoptosis in cancer cells; and (iv) oncolytic virotherapy, which causes the lysis of tumor cells. The delivery of genes to the tumor site is made possible by means of viral and nonviral vectors for direct delivery of therapeutic gene(s), tumor-tropic cell carriers expressing therapeutic gene(s), and “intelligent” carriers designed to increase delivery, specificity, and tumoral toxicity against GBM. These vehicles are used to carry genetic material to the site of pathology, with the expectation that they can provide specific tropism to the desired site while limiting interaction with noncancerous tissue. Encouraging preclinical results using gene therapies for GBM have led to a series of human clinical trials. Although there is limited evidence of a therapeutic benefit to date, a number of clinical trials have convincingly established that different types of gene therapies delivered by various methods appear to be safe. Due to the flexibility of specialized carriers and genetic material, the technology for generating new and more effective therapies already exists.
Keywords: delivery vehicles, gene therapy, glioblastoma, immunomodulatory therapy, oncolytic virotherapy
The aggressive biology and highly invasive nature of glioblastoma (GBM) make the prognosis poor for patients with this tumor. Despite a decade's worth of advances in surgery and chemoradiotherapy, patients diagnosed with GBM today have a mean life expectancy of only 14.6 months.1 Because of the difficulties inherent in treating diseases of the brain, therapeutic options for GBM are disconcertingly limited. Advances in the field of neuro-oncology have certainly made the management of GBM more hopeful. Nevertheless, the neoplasm remains an irreversible catalyst for mortality. The gene therapy modality has afforded new therapeutic options that might yield more successful treatment of GBM.
There are 4 types of gene therapy currently being investigated for potential use in treating GBM: (i) suicide genes, which induce the localized generation of cytotoxic compounds; (ii) immunomodulatory genes, which induce or augment an enhanced antitumoral immune response; (iii) tumor-suppressor genes, which induce apoptosis in cancer cells; and (4) oncolytic virotherapy, which causes lysis of tumor cells while also delivering any or all of the aforementioned other types of gene therapy. Used alone or in combination, each type of gene therapy capitalizes on some factor of the genetic hyperplastic deregulation in GBM.
The delivery of genes to the tumor site is made possible by means of (i) vectors for direct delivery of therapeutic gene(s), (ii) tumor-tropic cell carriers expressing therapeutic gene(s), and (iii) intelligent carriers. These vehicles are used to carry genetic material to the site of pathology, with the expectation that they can provide specific tropism to the desired site while limiting interaction with noncancerous tissue. Currently, viral vector-based methods are more commonly used than nonviral vector-based methods, which are still considered experimental.2
Here we provide a review of the types of gene therapy and the corresponding delivery systems. We then highlight prospective gene-therapy clinical trials of interest for GBM.
The Types of Gene Therapy
Gene therapy, which was developed as the horizontal transfer of genetic material for treating an array of genetic diseases, was initially established in the early 1970s to restore the function of defective genes.3 However, gene therapy became a prevailing part of cancer research because of increasing interest in the role of gene function in the regulation of cancer. Clearly, a therapy that can specifically treat the neoplasm with minimal effects on the surrounding brain is very appealing, especially given the currently limited therapeutic options. Considering the unique challenges that GBM poses in neuro-oncology, specialized genes from all strata of cancer biology have been explored as potential therapeutic means.
Suicide Gene Therapy
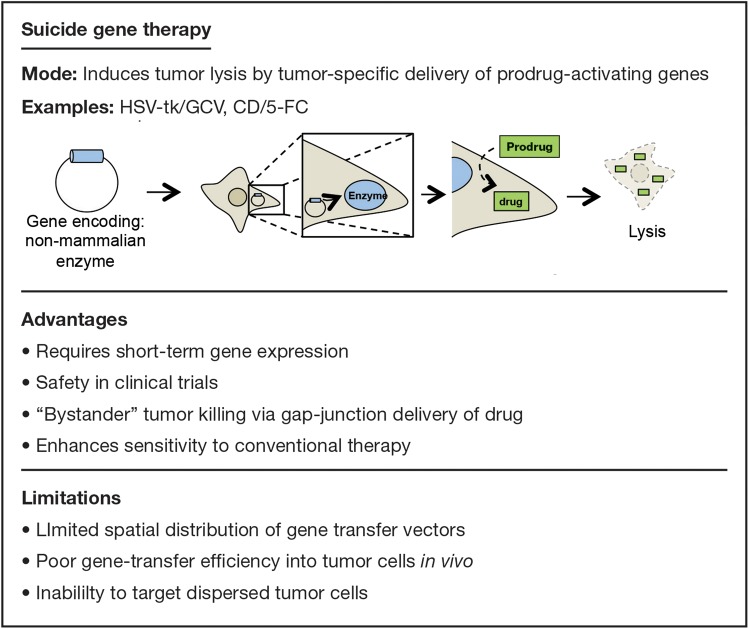
The use of genes that encode enzymes able to convert a temporarily inert prodrug into an active cytotoxic compound is one of the most extensively studied types of gene therapy. The encoding genes are termed “suicide genes.” The advantages and limitations of suicide gene therapy are summarized in Fig. 1.
Fig. 1.
Examples, advantages, and limitations of suicide gene therapy.
Two well-studied suicide gene therapies are the herpes simplex virus (HSV) type 1 thymidine kinase (tk)/ganciclovir (GCV) system (HSV-tk/GCV) and the cytosine deaminase (CD)/5-fluorocytosine (5-FC) system (CD/5-FC).4 In each of these combinations, delivery of a gene (tk or CD) to the tumor causes a systemically injected prodrug (GCV or 5-FC, respectively) to be converted to an activated chemotherapeutic agent (ganciclovir triphosphate [GCV 3-P] or 5-fluorouracil [5-FU], respectively). To date, suicide gene therapy has demonstrated limited clinical efficacy for treatment of malignant glioma.5 In a large phase III study, Rainov et al randomized 248 patients with newly diagnosed GBM to receive either standard chemotherapy and radiotherapy or standard therapy with adjuvant HSV-tk/GCV mediated through a retroviral vector. Although the gene therapy was safe, there was no significant difference between the groups in 1-year survival rates (55% for the control group vs 50% for the gene-therapy group).6
Despite the absence of a demonstrated therapeutic effect for suicide gene therapy for GBM, exciting new developments in enhanced delivery7 and synergistic addition of other chemotherapeutics8–10 have sustained interest in this therapeutic approach. By utilizing the tumor-tropic properties of mesenchymal stem cells, HSV-tk expressing MSCs can migrate to glioma tissue and exert enhanced antitumor activity. One such example is a study in which HSV-tk MSC treatment, in conjunction with valproic acid administration, significantly enhanced the antitumor response of suicide gene therapy by enhancing the bystander effect.10
Immunomodulatory Gene Therapy
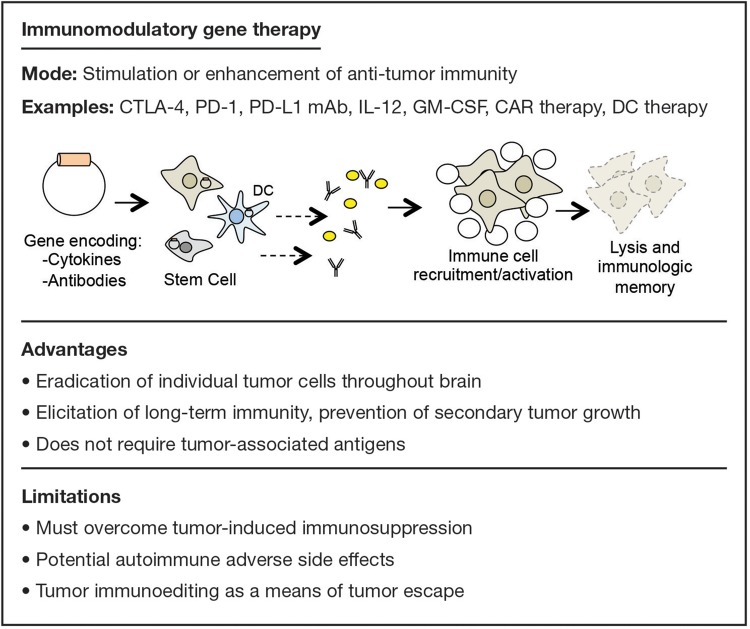
Advantages and limitations of immunomodulatory gene therapy are summarized in Fig. 2. Since recognition of tumor immunosurveillance, it has become widely accepted that growing tumors actively evade the immune system. Overcoming tumor-induced immunosuppression by enhancing the immune system is the overarching goal of immunotherapy, and successful therapies have been generated for solid and hematological malignancies in the clinical setting.11 Primarily due to the belief that immune cells cannot penetrate the blood-brain barrier (BBB), immunomodulatory gene therapy has only recently been suggested as an approach for GBM.12 However, there is substantial evidence of tumor-induced immunosuppression in malignant gliomas.13–15 While the brain may be an immune-privileged site, it is clear that this privilege is not absolute.
Fig. 2.
Examples, advantages, and limitations of immunomodulatory gene therapy.
Many immunotherapeutic approaches have been tested including blocking inhibitors of the immune response,16 pulsing dendritic cells with tumor lysates,17 and depleting suppressive cell types.18 These therapies are demonstrably potent, for such combinatorial immunotherapies can completely eradicate GBM in mouse models.19 For example, vom Berg et al demonstrated that combining interleukin-12 (IL-12) with cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) blockade significantly reduced regulatory T cells and increased effector T cells, resulting in extended survival compared with mice treated with either IL-12 or CTLA-4 blockade alone.20 Future work should explore various gene combinations aimed at producing immune stimulation via different pathways. Furthermore, the use of immunomodulatory therapies in conjunction with standard care promises to yield therapeutic benefits.12 For example, Zeng et al demonstrated that combining anti–programmed-cell death (PD)-1 antibodies with stereotactic radiation worked synergistically to greatly improve survival in a mouse model of glioma.21
Tumor-suppressor Gene Therapy
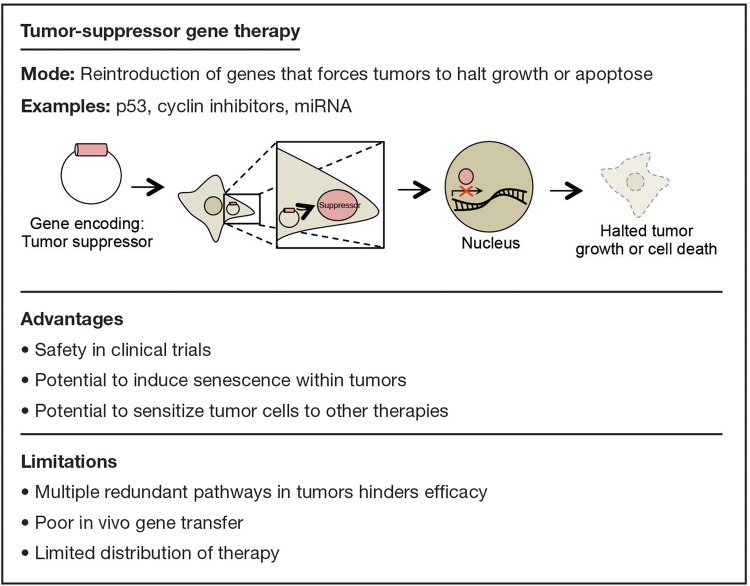
Tumor-suppressor genes are critical for the prevention of oncogenesis. In GBM, all patients have at least one tumor-suppressor gene that is either mutated or deleted; in 91% of patients, 2 or more of these tumor-suppressor genes are inactivated.22
Therapies have been devised to deliver genes encoding functional tumor suppressors to the site of neoplasia in order to restore their function and directly impede unregulated growth (Fig. 3). For instance, the delivery of genes encoding p53,23 cyclin inhibitors24 and, more recently, miRNAs25,26 has been shown to increase survival significantly in animal models. To date, clinical trials have not shown the same efficacy as preclinical animal models, possibly due to the lack of proper delivery systems. For example, Lang et al used an adenovirus vector to transfer genes encoding p53 into 12 patients with recurrent glioma. Although toxicity was minimal, widespread distribution of the agent was not achieved.27
Fig. 3.
Examples, advantages, and limitations of tumor-suppressor gene therapy.
Another recently developed methodology involves RNA-guided use of chimeric nucleases to disrupt, remove, or even replace mutated DNA in target cells. These include zinc-finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeat (CRISPR)/Cas-based endonucleases.28,29 In the context of GBM, delivery of these nucleases could potentially be used to replace mutated tumor suppressors (eg, p53, pRB, or PTEN) with functional gene versions. While this approach has not yet been used to treat cancers in vivo, doing so appears to be an inevitable next step in the development of this technology.
Enhancing Gene Therapy by Targeting the Tumor Microenvironment
Apart from targeting the neoplastic cells directly, another strategy is introducing genes that may alter the tumor stroma in order to create unfavorable conditions for tumor growth or enhance the efficacy of therapy. One such approach targets the tumor extracellular matrix (ECM) proteins with proteases that degrade and remodel the ECM to augment the spread of a therapeutic virus throughout the tumor site. Dmitrieva et al demonstrated that this approach could be clinically beneficial, showing that an oncolytic virus expressing an ECM-degrading enzyme had improved spread throughout the tumor and greater therapeutic efficacy than a virus without the ECM-degrading enzyme.30
Oncolytic Virotherapy
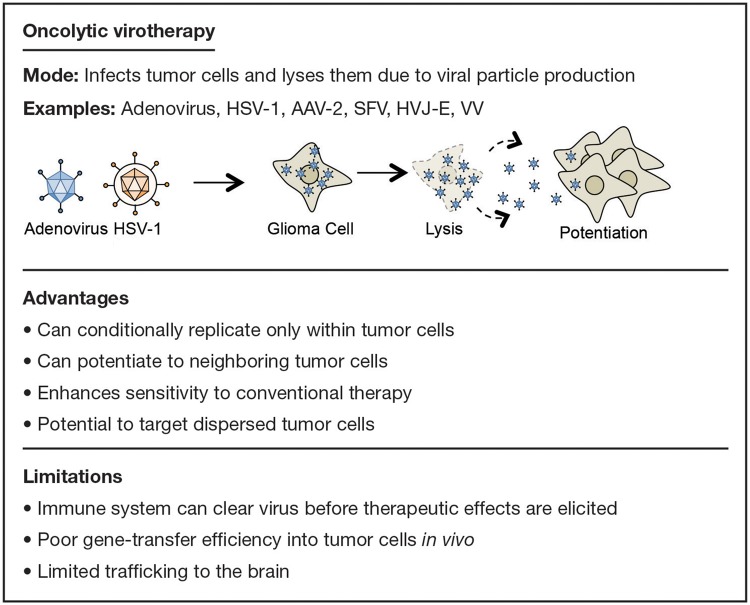
While viruses are the most efficient vectors for delivering a therapeutic gene to tumor cells, oncolytic virotherapy itself can also be considered a mode of gene therapy for treating GBM (Fig. 4). The implementation of viruses to induce the lysis of tumor cells is an attractive avenue of therapy since its effects can also be broadened to neighboring cells through what is aptly termed the “bystander effect.” Furthermore, oncolytic viruses have also been demonstrated to promote an effective antitumoral immune response.31 These observations, along with the potential for applied modification and generation of these viral particles, suggest that oncolytic virotherapy can be an exceptional resource for potential GBM treatment.
Fig. 4.
Examples, advantages, and limitations of oncolytic virotherapy.
Many viruses have the capability to induce tumor-cell lysis in GBM models, although the 2 most widely studied oncolytic viruses are adenoviruses (Ads) and HSV-1 viruses.32 Both of these double-stranded DNA viruses allow for extensive modification in directing their tropism and ability to carry therapeutic genes. Additionally, a number of other viruses have been tested,33 and many are in phase I and II clinical trials. These viruses must be inherently replication competent to induce lysis. As such, further modification is required to limit their toxicity to surrounding nonneoplastic tissue, making them tumor tropic or by limiting their replication to cancerous cells.34 The imposing problem concerning oncolytic virotherapy, however, is that the host immune system might effectively clear the oncolytic viruses before they can provide any notable benefit.35 Because of this complication, suppressing the immune system, augmenting the immunogenicity of the virus, or a different method of delivery is required for this therapy to be effective in the clinical setting.
Summary
Researchers are utilizing these types of gene therapy, often in combination with one another, to elicit a potent antitumor response in preclinical studies. More experimental studies are presently in development that exhibit distinct potential to further promote this marked antitumoral effect. Although limited data are available to support the effectiveness of gene therapy for GBM, the safety of its usage is now well established. Another hurdle that persistently plagues all of these therapeutic modalities is their delivery. In the clinical setting, gene therapy will only be as efficacious as its ability to be delivered. In the following sections, we will evaluate the recent advances that have resulted in more efficient delivery of these therapeutic genes.
Delivery Methods for Gene Therapy
Direct Delivery of Therapeutic Gene(s) into the Tumor Site: Virus Mediated
Modification of viruses to infect and alter the fate of glioma cells offers a unique opportunity for GBM therapy. Many viral vectors have been developed to eliminate glioma, either in combination with conventional therapy or with other novel therapeutic regimens. In the following sections, we discuss the viral vectors that have been best studied for gene delivery and most commonly used.
Adenovirus
Among many human Ads, the human adenovirus serotype 5 (HAd5) is the most commonly used for gene therapies.36 The primary receptor of HAd5 is coxsackievirus and adenovirus receptor (CAR), which is poorly expressed in GBM. Therefore, the fiber of HAd5 (ie, its receptor-binding motif) is modified to achieve higher infectivity in glioma cells. This effect can be achieved by incorporating a polylysine or RGD domain on the fiber to infect the glioma efficiently and deliver the therapeutic agent.36,37 These genetically modified HAd5 vectors have been previously designed to secrete cancer-specific cytotoxic proteins (eg, TRAIL38) or suicide genes (eg, HSV-tk) in conjunction with an immunostimulatory cytokine (Flt3L).39
As mentioned above, mutations in tumor-suppressor proteins such as p53 and absence of functional apoptosis are well documented in a considerable subset of GBM patients. Therefore, researchers have utilized HAd5 vectors to express WT-p53 or proapoptotic Bax, causing glioma cells to undergo apoptosis (with the latter also sensitizing glioma cells to radiation).40,41 Furthermore, oncolytic HAd5 vectors have been modified to produce antiangiogenic agents such as Vstat120 for glioma therapy, which improved survival in preclinical rodent models.42
Herpes Simplex Virus-1
HSV-1 has been widely employed as a potential therapeutic agent for GBM because of its neurotropism and large packaging capability for therapeutic genes (∼160 Kb of DNA). HSV-1 is also well suited for gene therapy in the CNS because of its capacity for long-lasting gene expression in neurons.43
Multiple potentially therapeutic genes have been investigated for glioma using HSV-1 as the vector. For example, Ho et al modified HSV-1 to express cytotoxic FasL and FADD (pG8-FasL/FADD), which caused cytotoxicity to glioma cells. Further, the pG8-FasL/FADD virus, when inoculated intracranially into a ΔGli36 model of human glioma, in combination with temozolomide, improved survival.44 Zhang et al generated oncolytic HSVs that encoded either the antiangiogenic angiostatin or the immunostimulatory IL-12. When these separate viruses were inoculated together in 2 different models of GBM (U87MG, MGG4), the experimental findings demonstrated significant survival benefit compared with that achieved using either individual virus alone.45 To date, multiple clinical trials have used oncolytic HSV-1 as a vector for therapeutic genes in participants with malignant gliomas, thus paving the way for development of a more advanced generation of viruses that may provide more significant clinical benefit.
Adeno-associated Virus-2
Adeno-associated virus-2 (AAV-2) has also been utilized for viral gene delivery, although less commonly than other viruses because (i) only small genes (<4 Kb) can be inserted into the AAV-2 genome and (ii) the virus exhibits a limited cell-targeting ability. However, AAV-2 still presents a clinically viable option as a vector based on the low immunogenicity and minimal side effects noted in clinical trials.46 In a recent report, Ma et al used an AAV-2 vector that had been modified to express the anticancer gene decorin in an animal model of GBM and demonstrated marked regression of tumor.47 In another report, Ma et al modified the AAV-2 vector to express the angiostatin gene that, combined with Ad-carrying HSV-tk, improved survival in glioma-bearing rats.48 Interestingly, these researchers also reported that a single intramuscular injection of AAV-2 expressing angiostatin inhibited angiogenesis and improved survival in a preclinical mouse model.48
Other Viral Vectors
To identify better viral vectors for gene delivery, many different viruses have been explored. For example, when Masuda et al used hemagglutinating virus of Japan envelope (HVJ-E) as a viral vector injected intratumorally into mice, they found improvement in tumor cytotoxicity and survival.49 Additionally, Yamanka et al found that Semliki Forest virus (SFV), used as a viral vector expressing IL-18, increased antitumoral immunity when injected intracranially into a B16 brain-tumor model.50 Moreover, when Timiryasova et al used replication-deficient vaccinia virus (VV) as a viral vector expressing WT-p53 in combination with mild psoralen and ultraviolet light, they found that apoptosis was induced in an animal model and that tumor growth in nude mice was significantly reduced.51 Finally, Tanaka et al found that using a retrovirus as a viral vector expressing a secretable form of the antiangiogenic protein platelet factor 4 inhibited endothelial cell proliferation and improved animal survival in an orthotopic glioma model.52
Direct Delivery of Therapeutic Gene(s) into the Tumor Site: Nonviral Vehicle Based
Aside from the use of viruses as carriers of therapeutic genes, other carriers capable of crossing the BBB have been developed to combat GBM as well.
Nanoparticles
Nanoparticles represent a burgeoning fleet of delivery vehicles for the treatment of GBM. Nanoparticles can be broadly defined as small subcellular objects (<100 nm). They can be further classified based on their composition or physical properties and can be generated in the laboratory in a number of ways to induce tumor-targeting specificity. Nanoparticles tend to accumulate within tumors. A number of explanations have been proposed to explain this phenomenon, although conclusive support for any one explanation has not yet been established.53–55
A wide variety of nanoparticles have been devised, and some are currently being tested in the clinical setting.54 Silver and gold nanoparticles have been particularly favorable because they are inert, nonimmunogenic, and capable of passing the BBB while also promoting chemo- and radiosensitization to cancer cells.56 In another promising class of nanoparticles, multi-walled carbon nanotubes can be carriers of plasmids or chemotherapeutics and can be designed to have tumor-targeting capabilities.57,58 Carbon nanotubes can have polyethylene glycol (PEG) chains attached as well and have been shown to accumulate preferentially within the tumor.58
Liposomes and Micelles
Liposomes and micelles are lipid nanoparticles designed in similarity to the lipid bilayers of cells. A liposome is a complete lipid bilayer, and a micelle is a lipid monolayer. A liposome has a hydrophilic core, and a micelle has a hydrophobic core, thereby allowing for packaging of a wide variety of therapeutic genes. By incorporating PEG chains onto the surface of these carriers, different molecules can be added to increase their targeting to specific tissues. Adding PEG chains also prevents uptake by phagocytic immune cells, significantly increasing half-life in vivo.55 The BBB endothelium also expresses high levels of transferrin receptor, and many studies have shown that adding transferrin to the surface of liposomes and micelles allows more efficient delivery of chemotherapeutics to the glioma tissue.59–61 In some instances, researchers have placed 2 different targets on the surface of a liposome (one to cross the BBB and one to target the glioma); this strategy has successfully delivered chemotherapeutics in a selective manner, with potential reduction in toxicity to nonneoplastic tissues.59,62 In an in vivo study, Gao et al attached transferrin and folate to the surface of liposomes due to high expression of the folate receptor on glioma cells. By loading these liposomes with doxorubicin, they observed specific and potent antitumor effects.59 In an in vitro and in vivo study, Yang et al used angiopep-1 and the neuropilin-1 receptor to cross the BBB and target glioma. By loading these targeting liposomes with VEGF siRNA or docetaxel, they elicited an effective antitumor response.62
Although initial clinical trials utilizing lipid nanoparticles have not demonstrated very substantial therapeutic efficacy,61 the development of better liposomal targeting and conditional drug-releasing liposomal carriers (discussed in the section below on intelligent carriers) has potential for better outcomes in the future.
Tumor-tropic Cell Carriers Expressing Therapeutic Gene(s) in the Tumor Site
Neural Stem Cells
Neural stem cells (NSCs) have a natural tropism toward brain tumor tissue.63 NSCs are the progenitors to most cells of the CNS and can be isolated and expanded in vitro from both humans and mice.64 The tumor-tropic capabilities of NSCs and their ability to stably express introduced genes make them ideal cell-based carriers.63 Another fascinating aspect of NSCs is that they can be delivered not only through systemic injection but also by an intranasal route, which also allows them to reach the intracranial tumor site.65
Experimentally, NSCs have been loaded with a variety of genes and delivered successfully to the tumor site. For example, NSCs loaded with oncolytic adenovirus have been found in vivo to travel efficiently to the tumor and reduce tumor growth.66 NSCs have also been engineered to transport an array of cytokines, nanoparticles, and enzymes for converting inert prodrugs into chemotherapeutics.67 Also, NSCs have been engineered to express TRAIL, and their delivery can reduce glioma tumor burden significantly in mice.68,69 By utilizing the CD/5-FU system described above, Aboody et al recently found that human NSCs expressing CD reduced glioma burden significantly in immunocompetent mice injected with 5-FC.70 In the future, more effective means of isolation and propagation will be required to make NSCs more useful for clinical applications.
Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are relatively easier to isolate than NSCs. They can be obtained autologously from the bone marrow and then be manipulated and inserted back into the same patient, thereby preventing an allogeneic response to the carrier.71 Like NSCs, MSCs can be loaded with therapeutic genes and delivered to the tumor. MSCs have also been engineered to express TRAIL72 and CD73 with potent antitumor effects. When umbilical–cord-derived MSCs expressing IL-12 were injected into glioma-bearing mice, the tumors were rejected; the mice were also resistant to rechallenge, which is a hallmark for immunological memory.55 For reasons not yet completely elucidated, MSCs and NSCs have similar glioma tropism and thus have great potential as carriers.74 Understanding the properties that allow NSCs and MSCs to migrate efficiently to the tumor will be critical for enhancing their tumor tropism and improving their use as stem-cell carriers for gene therapy.
Intelligent Carriers
As technologies evolve, many laboratories have begun to develop new intelligent techniques designed to increase delivery, specificity, and tumoral toxicity against GBM.
The acidic intratumoral pH of glioma is important for brain tumor maintenance; hence, pH-sensitive therapies are being developed as attractive options for treating GBM.75 Many groups have devised pH-sensitive molecules that only deliver their therapeutic cargo at a low pH.76–79 Our group has recently explored this approach and shown that it is possible to enhance the delivery of doxorubicin specifically to the glioma microenvironment by loading NSCs with pH-sensitive mesoporous nanoparticles,.79
While pegylating liposomes and micelles is beneficial to their stability and targeting capabilities, it also makes them resistant to intracellular degradation, thus preventing the release of their genetic cargo and hindering their efficacy. By adding pH-sensitive components to lipid nanoparticles, more efficient and directed delivery of genetic cargo can be achieved.76
Furthermore, liposomes and micelles can be used to encapsulate many nanoparticles to aid their targeted delivery to the tumor.80 The approach of enhancing the specificity of stimuli-responsive therapies has shown therapeutic potential in the clinical setting. These particles respond to a number of external stimuli—including, but not limited to, infrared radiation,81 magnetic fields,82 and ultrasound83—to induce tumor cell death through the release of chemotherapeutic compounds or RNA interference, physical disruption, and thermolysis.84,85
There is an unlimited potential for developing future therapies by coupling these stimuli with other delivery systems, thereby creating multifunctional carriers. While several of these multifunctional therapies have not yet been explored in GBM, we can expect to see them in the near future with hopefully better outcomes for patients suffering from this highly lethal malignancy.
Summary of Delivery Systems
The delivery systems for gene therapy are as varied as the cargo they carry. Each carrier has its advantages and limitations, which are summarized in Table 1. Using viruses as delivery systems is advantageous because they can be extensively manipulated for tumor-specific tropism and have been shown to be safe in the clinical setting. Furthermore, conditionally replicative viruses can be used to both lyse the tumor cells they target and carry therapeutic genetic material. Such viruses have the potential to synergistically spread the virus to the entirety of the tumor. However, the fact that they are recognized by the immune system, and as such have limited tissue distribution capability, hinders their use. Conversely, stem cells have intrinsic tumor tropism with the potential to allow greater tumor targeting and distribution of therapeutic cargo; however, their use is hampered by the fact that they have tumorigenic potential, can be killed by the therapy they are carrying, and can be rejected if they are not autologously acquired.
Table 1.
Delivery methods for gene therapy
| Type of Delivery Method | Advantages | Limitations |
|---|---|---|
| Direct Delivery of Therapeutic Genes into the Tumor Site | ||
| Virus mediated Adenovirus Herpes simplex virus-1 Adeno-associated virus-2 |
Conditional replication allows for potentiation of therapy specifically within tumor cells Intrinsic tumor cell death capabilities, synergism with cargo Demonstrated safety in the clinic |
Targeted for destruction by immune system Tumor targeting capabilities are limited Distribution within tumor limited |
| Nonviral vector based Nanoparticles Liposomes and micelles |
Engineered to survive in vivo Extensive modification possible Can carry therapeutics across the BBB Can passively accumulate within tumors |
Can be toxic to surrounding tissues Trafficking to the tumor tissue can be inefficient Delivery of genetic material can be inefficient |
| Tumor-tropic Cell Carriers Expressing Therapeutic Gene(s) in the Tumor Site | ||
| Neural stem cells Mesenchyal stem cells |
Multiple administration routes possible Traffic efficiently to brain Can carry therapeutics, including viruses, across the BBB |
Genetic material can be toxic to stem cells Can be rejected by immune system if not autologous Risk for tumor formation |
| Intelligent Carriers | ||
| pH-sensitive drug release pH-sensitive liposomal carriers Stimuli-responsive particles |
Temporal release of therapeutics prevents toxicity to surrounding tissues Extensive modification possible Can carry therapeutics across the BBB |
Research in its infancy Efficiency of intelligent release in vivo still uncertain |
Abbreviations: BBB, blood-brain barrier.
Nanoparticles represent a broad and diverse set of gene therapy carriers. They can be metals, liposomes, polymers, and often times a mixture of these. With their virtually unlimited potential for modification, tendency to accumulate passively within tumors, and engineered stability in vivo, they present some of the most exciting carrier options to date. Furthermore, researchers have modified nanoparticles to become intelligent. By responding to intratumoral pH or external stimuli, nanoparticles can selectively release cargo and specifically exert their antitumor effects. This can prevent toxicity to surrounding tissue and has the potential to deliver even stronger therapeutics to the tumor. While their capacity for tumor targeting and intelligent release has been demonstrated in preclinical research, their efficacy in the clinical setting has not yet been evaluated. Extensive research will undoubtedly uncover new and exciting ways to manipulate these different carriers for therapeutic benefit.
Current Studies of Interest
Encouraging preclinical results using gene therapies for GBM have led to a series of human clinical trials. Table 2 lists current clinical trials using gene therapy for GBM. Highlighted below are current and recently completed clinical trials of interest.
Table 2.
Clinical trials of gene therapy for glioblastoma and other malignant brain tumors in the United States*
| Clinical Trial Identifier | Trial Phase | Therapeutic Agent | Delivery Mechanism | Therapeutic Strategy | Type(s) of Cancer |
|---|---|---|---|---|---|
| NCT01156584 | I/II | Toca-511 carrying CD + 5-FC | RRV | Suicide gene + viral oncolysis | Recurrent HGG |
| NCT01470794 | I | Toca-511 carrying CD + 5-FC | RRV | Suicide gene + viral oncolysis | Resection cavity of recurrent or progressive grade III or IV astrocytic tumors |
| NCT01985256 | I | Toca-511 carrying CD + 5-FC | RRV given intravenously | Suicide gene + viral oncolysis | Recurrent or progressive grade III or IV astrocytic tumors |
| NCT01174537 | I/II | Newcastle Disease Virus | Replicating virus | Viral oncolysis | GBM, gliosarcoma, and neuroblastoma |
| NCT01301430 | I/II | H-1 parvovirus (ParvOryx-01) | Replicating virus | Viral oncolysis | GBM |
| NCT01491893 | I | Engineered chimeric poliovirus (PVS-RIPO) | Replicating virus | Viral oncolysis + immune activation | GBM and recurrent supratentorial GBM |
| NCT00390299 | I | Measles virus derivative (MV-CEA) | Replicating virus | Viral oncolysis + immune activation | Recurrent GBM |
| NCT02062827 | I | HSV-1 expressing IL-12 (M032) | Replicating virus | Viral oncolysis + antiangiogenesis | Recurrent/progressive GBM, anaplastic astrocytoma, or gliosarcoma |
| NCT01582516 | I/II | AdV-delta-24-RGD | Replicating virus delivered via CED | Viral oncolysis | Recurrent GBM |
| NCT00805376 | I | DNX2401 (formerly known as Delta-24-RGD-4C) | Replicating virus | Viral oncolysis | Recurrent malignant gliomas |
| NCT01956734 | I | DNX2401 (formerly known as Delta-24-RGD-4C) + TMZ | Replicating virus | Viral oncolysis | First GBM recurrence |
| NCT02031965 | I | HSV-1716 | Replicating virus | Viral oncolysis | Recurrent childhood GBM and malignant glioma |
| NCT00589875 | IIa | AdV-tk + valacyclovir + radiation therapy | Nonreplicating virus | Suicide gene | Newly diagnosed malignant gliomas |
| NCT00634231 | I | AdV-tk + valacyclovir + radiation therapy | Nonreplicating virus | Suicide gene | Children with malignant glioma |
| NCT00751270 | Ib | AdV-tk + valacyclovir + radiation therapy | Nonreplicating virus | Suicide gene | Malignant gliomas |
| NCT01811992 | I | AdV-hCMV-TK & AdV-hCMV-Flt3L | Nonreplicating virus | Suicide gene + immune stimulation | Malignant glioma and GBM |
| NCT01260506 | I/II | VB-111 + bevacizumab | Nonreplicating virus given intravenously | Antiangiogenic | Relapsed GBM |
| NCT02026271 | I | Adenovirus vector expressing IL-12 (INXN-2001) + veledimex | Nonreplicating virus | Immune-mediated cell death | Recurrent/progressive GBM and grade III astrocytic tumor |
| NCT01172964 | Pilot | CD + 5-FC | Neural stem cells | Suicide gene | Recurrent HGG |
*The trials have active status (open, recruiting, or ongoing) as of September 2014.
Abbreviations: 5-FC, fluorocytosine; AdV, adenovirus; ALT, autologous lymphocyte transfer; CD, cytosine deaminase; CEA, carcinoembryonic antigen; CED, convection-enhanced delivery; CMV, cytomegalovirus promoter; Flt3L, FMS-like tyrosine kinase 3 ligand; GBM, glioblastoma; HER, human epidermal growth factor receptor 2; HGG, high-grade glioma; HSV, herpes simplex virus; IL, interleukin; MV, measles virus; RGD, Arg-Gly-Asp motif; RRV, retroviral replicating vector; TK, thymidine kinase; TMZ, temozolomide; VB-111, nonreplicating adenovector targeting Fas-Chimera transgene to angiogenic tumor blood vessels.
A current phase II trial (NCT00589875), which employs AdV-tk and valacyclovir together with standard surgery and chemoradiotherapy, has generated interest. This study expands on a phase IB trial conducted by Chiocca et al,86 in which the gene therapy showed potential efficacy without side effects and was independent of the patient's MGMT promoter methylation status. In this trial, 12 participants with newly diagnosed malignant gliomas received intratumoral injections of AdV-tk vector particles at the time of surgery. The participants then received valacyclovir followed by radiation therapy. After 14 days of valacyclovir treatment, temozolomide was administered. Twenty-five percent of the participants survived for 3 years, and post-treatment histological analyses showed significant CD3+ T-cell and CD68+ macrophage infiltrate present in tumors 22 months after the AdV-tk injection. This suggests that the treatment has potential for promoting productive immunity against the tumor.86
A recent phase III clinical trial completed by Ark Therapuetics Ltd. employed a nonreplicating adenoviral vector that encodes the HSV-tk followed by ganciclovir administration (Cerepro or sitimagene ceradenovec) in participants with supratentorial GBM.87 Although this trial was considered unsuccessful, a number of factors have emerged that potentially hampered the efficacy of the treatment. There is still a belief that this therapy could yield a small yet clinically significant benefit, particularly if gene delivery to the tumor is improved or if the gene therapy is combined with other treatments.88
One promising virotherapy, which has progressed to phase I clinical trials (NCT00805376 and NCT01956734), uses an oncolytic adenovirus called DNX-2401 (previously referred to as Delta-24-RGD-4C) for the treatment of recurrent malignant glioma. This virus has been modified so that it can efficiently infect cells through interaction between the RGD motif of the adenovirus fibers and the integrins on cells (ie, the viral infectivity no longer relies on poor CAR expression by the glioma cells) and has been made conditionally replicative so that it only replicates in cells with inactive retinoblastoma protein such as cancer cells. This modified virus has demonstrated robust efficacy in preclinical experiments.89
Based on encouraging preclinical experiments,70,90 preliminary findings reported by Tocagen, Inc., from 2 ongoing clinical trials (NCT01156584 and NCT01470794) have been largely positive. The studied treatment, which used a replicating retroviral vector (Toca 511) in conjunction with 5-FC, was well tolerated in 68 participants, with higher survival rates at 6 and 12 months. Moreover, after Toca 511 treatment, there appeared to be evidence of immune activation against the residual tumor. These results are encouraging with regard to future trials that aim to combine antitumor immune activation with another therapeutic agent such as oncolytic viruses or drug-loaded nanoparticles.
Another clinical trial (NCT01172964) is attempting to improve therapeutic efficacy in participants with recurrent high-grade glioma by modifying NSCs to express CD.63 Should this stem-cell carrier approach prove to be reasonably safe, it can be used to deliver other types of gene therapy such as AdV-tk or oncolytic viruses. Other stem-cell carriers such as MSCs, which have also delivered enzyme-prodrug therapy successfully in animal models, warrant investigation in future clinical trials.91,92
The majority of completed clinical trials have relied on the administration of a single therapeutic agent. However, as evidence from preclinical and clinical studies has accumulated, it appears that a combination of targets allowing for a multifaceted eradication of the tumor might prove to be more effective.19 Today, finding the right combination of gene therapy, virotherapy, and immunotherapy, as well as the best way to delivery these platforms to the tumor, is a dominant theme concerning the use of gene therapy for treating GBM. A series of upcoming preclinical and clinical trials will explore the effects of combinations of oncolytic virotherapy and immunomodulation in an attempt to produce synergistic tumor subtraction. In the meantime, more clinical trials are urgently needed to explore the efficacy of different vehicles for gene therapy delivery as they are developed.
Conclusion
The ineffectiveness of current treatments for GBM provides researchers and clinicians alike with compelling reasons to test highly unconventional therapies for this disease. Although there is limited evidence of a therapeutic benefit to date, a number of clinical trials have convincingly established that different types of gene therapies delivered by various methods appear to be safe.93
The efficacy of current glioma therapies can be enhanced by more efficiently and specifically designed approaches (eg, tumor-specific killing, manipulating the tumor microenvironment, and maneuvering the immune system) to tilt the balance toward antitumor immunity. In combination with intelligently designed, tumor-specific viral and nonviral delivery systems, these novel therapeutic strategies could yield profound improvements in GBM patient survival.
New and exciting means for gene therapy are already on the horizon. As described above, the CRISPR/Cas system is a recently developed approach that allows directed insertion of genetic material anywhere in a target cell using guide DNA. With this technique, mutated genes are disrupted and replaced with functional ones.28,29 The technique has enormous potential for use in gene therapy for GBM.
With regard to carriers, new discoveries are being made on a continuing basis. Recent studies have dramatically enhanced the loading capabilities of magnetic nanoparticles while maintaining their multi-targeted approach.94 Ma et al have devised nanoparticle carriers that cause photosensitization by x-rays, a potential way to target deeply-rooted tumors using photodynamic therapy.95
Due to the flexibility of specialized carriers and genetic material, the technology for generating new and more effective therapies already exists. The field of gene therapy is exploding with new advances occurring on a rapid basis, and the possibilities for the future treatment of GBM are virtually unlimited. With a basic understanding of the current status of GBM research, both in preclinical and clinical settings, we hope this review can provide a basis for other researchers to develop their own novel and innovative therapies.
Funding
This study was supported by NIH grants R01CA122930, U01NS069997, R01CA138587, and R01NS077388 to MSL.
Acknowledgments
This paper is one of three review articles for a supplement entitled “Local delivery of cytoreductive agents for the treatment of glioblastoma.” The supplement is supported by Arbor Pharmaceuticals. The physician editor of the supplement is Steven N. Kalkanis, MD, Department of Neurosurgery, Henry Ford Hospital, Detroit, MI, who has contributed an introduction to the supplement. The two other articles in the supplement cover convection-enhanced delivery and polymeric drug delivery.
Conflict of interest statement: None of the authors have any conflict of interest.
References
- 1.Grossman SA, Ye X, Piantadosi S, et al. Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16(8):2443–2449. doi: 10.1158/1078-0432.CCR-09-3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kay MA, Glorioso JC, Naldini L. Viral vectors for gene therapy: the art of turning infectious agents into vehicles of therapeutics. Nat Med. 2001;7(1):33–40. doi: 10.1038/83324. [DOI] [PubMed] [Google Scholar]
- 3.Friedmann T, Roblin R. Gene therapy for human genetic disease? Science. 1972;175(4025):949–955. doi: 10.1126/science.175.4025.949. [DOI] [PubMed] [Google Scholar]
- 4.Fischer U, Steffens S, Frank S, et al. Mechanisms of thymidine kinase/ganciclovir and cytosine deaminase/5-fluorocytosine suicide gene therapy-induced cell death in glioma cells. Oncogene. 2005;24(7):1231–1243. doi: 10.1038/sj.onc.1208290. [DOI] [PubMed] [Google Scholar]
- 5.Kroeger KM, Muhammad AK, Baker GJ, et al. Gene therapy and virotherapy: novel therapeutic approaches for brain tumors. Discov Med. 2010;10(53):293–304. [PMC free article] [PubMed] [Google Scholar]
- 6.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11(17):2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 7.Matuskova M, Hlubinova K, Pastorakova A, et al. HSV-tk expressing mesenchymal stem cells exert bystander effect on human glioblastoma cells. Cancer Lett. 2010;290(1):58–67. doi: 10.1016/j.canlet.2009.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Kim SW, Kim SJ, Park SH, et al. Complete regression of metastatic renal cell carcinoma by multiple injections of engineered mesenchymal stem cells expressing dodecameric TRAIL and HSV-TK. Clin Cancer Res. 2013;19(2):415–427. doi: 10.1158/1078-0432.CCR-12-1568. [DOI] [PubMed] [Google Scholar]
- 9.McBride WH. Integration of adenovirus thymidine kinase suicide-gene therapy with surgery and radiation therapy for malignant glioma. Future Oncol. 2012;8(1):17–20. doi: 10.2217/fon.11.126. [DOI] [PubMed] [Google Scholar]
- 10.Ryu CH, Park KY, Kim SM, et al. Valproic acid enhances anti-tumor effect of mesenchymal stem cell mediated HSV-TK gene therapy in intracranial glioma. Biochem Biophys Res Commun. 2012;421(3):585–590. doi: 10.1016/j.bbrc.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 11.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480(7378):480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heimberger AB, Sampson JH. Immunotherapy coming of age: what will it take to make it standard of care for glioblastoma? Neuro Oncol. 2011;13(1):3–13. doi: 10.1093/neuonc/noq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wainwright DA, Dey M, Chang A, et al. Targeting Tregs in Malignant Brain Cancer: Overcoming IDO. Front Immunol. 2013;4:4116. doi: 10.3389/fimmu.2013.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wei J, Wu A, Kong LY, et al. Hypoxia potentiates glioma-mediated immunosuppression. PLoS One. 2011;6(1):e16195. doi: 10.1371/journal.pone.0016195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu S, Shao QQ, Sun JT, et al. Synergy between the ectoenzymes CD39 and CD73 contributes to adenosinergic immunosuppression in human malignant gliomas. Neuro Oncol. 2013;15(9):1160–1172. doi: 10.1093/neuonc/not067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fecci PE, Ochiai H, Mitchell DA, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13(7):2158–2167. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 17.Prins RM, Soto H, Konkankit V, et al. Gene expression profile correlates with T-cell infiltration and relative survival in glioblastoma patients vaccinated with dendritic cell immunotherapy. Clin Cancer Res. 2011;17(6):1603–1615. doi: 10.1158/1078-0432.CCR-10-2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maes W, Rosas GG, Verbinnen B, et al. DC vaccination with anti-CD25 treatment leads to long-term immunity against experimental glioma. Neuro Oncol. 2009;11(5):529–542. doi: 10.1215/15228517-2009-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wainwright DA, Chang AL, Dey M, et al. Durable therapeutic efficacy utilizing combinatorial blockade against IDO, CTLA-4 and PD-L1 in mice with brain tumors. Clin Cancer Res. 2014;20:5290–5301. doi: 10.1158/1078-0432.CCR-14-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vom Berg J, Vrohlings M, Haller S, et al. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J Exp Med. 2013;210(13):2803–2811. doi: 10.1084/jem.20130678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng J, See AP, Phallen J, et al. Anti-PD-1 blockade and stereotactic radiation produce long-term survival in mice with intracranial gliomas. Int J Radiat Oncol Biol Phys. 2013;86(2):343–349. doi: 10.1016/j.ijrobp.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishii N, Maier D, Merlo A, et al. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol. 1999;9(3):469–479. doi: 10.1111/j.1750-3639.1999.tb00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badie B, Drazan KE, Kramar MH, et al. Adenovirus-mediated p53 gene delivery inhibits 9L glioma growth in rats. Neurol Res. 1995;17(3):209–216. doi: 10.1080/01616412.1995.11740314. [DOI] [PubMed] [Google Scholar]
- 24.Inoue R, Moghaddam KA, Ranasinghe M, et al. Infectious delivery of the 132 kb CDKN2A/CDKN2B genomic DNA region results in correctly spliced gene expression and growth suppression in glioma cells. Gene Ther. 2004;11(15):1195–1204. doi: 10.1038/sj.gt.3302284. [DOI] [PubMed] [Google Scholar]
- 25.Katakowski M, Buller B, Zheng X, et al. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013;335(1):201–204. doi: 10.1016/j.canlet.2013.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Papagiannakopoulos T, Friedmann-Morvinski D, Neveu P, et al. Pro-neural miR-128 is a glioma tumor suppressor that targets mitogenic kinases. Oncogene. 2012;31(15):1884–1895. doi: 10.1038/onc.2011.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lang FF, Bruner JM, Fuller GN, et al. Phase I trial of adenovirus-mediated p53 gene therapy for recurrent glioma: biological and clinical results. J Clin Oncol. 2003;21(13):2508–2518. doi: 10.1200/JCO.2003.21.13.2508. [DOI] [PubMed] [Google Scholar]
- 28.Gaj T, Gersbach CA, Barbas CF. ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 2013;31(7):397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang HY, Yang H, Shivalila CS, et al. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 2013;153(4):910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dmitrieva N, Yu L, Viapiano M, et al. Chondroitinase ABC I-mediated enhancement of oncolytic virus spread and antitumor efficacy. Clin Cancer Res. 2011;17(6):1362–1372. doi: 10.1158/1078-0432.CCR-10-2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Melcher A, Parato K, Rooney CM, et al. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol Ther. 2011;19(6):1008–1016. doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakimoto H, Kesari S, Farrell CJ, et al. Human glioblastoma-derived cancer stem cells: establishment of invasive glioma models and treatment with oncolytic herpes simplex virus vectors. Cancer Res. 2009;69(8):3472–3481. doi: 10.1158/0008-5472.CAN-08-3886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wollmann G, Ozduman K, van den Pol AN. Oncolytic virus therapy for glioblastoma multiforme: concepts and candidates. Cancer J. 2012;18(1):69–81. doi: 10.1097/PPO.0b013e31824671c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leber MF, Bossow S, Leonard VH, et al. MicroRNA-sensitive oncolytic measles viruses for cancer-specific vector tropism. Mol Ther. 2011;19(6):1097–1106. doi: 10.1038/mt.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikeda K, Ichikawa T, Wakimoto H, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5(8):881–887. doi: 10.1038/11320. [DOI] [PubMed] [Google Scholar]
- 36.Nandi S, Lesniak MS. Adenoviral virotherapy for malignant brain tumors. Expert Opin Biol Ther. 2009;9(6):737–747. doi: 10.1517/14712590902988451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim JW, Glasgow JN, Nakayama M, et al. An adenovirus vector incorporating carbohydrate binding domains utilizes glycans for gene transfer. PLoS One. 2013;8(2):e55533. doi: 10.1371/journal.pone.0055533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jeong M, Kwon YS, Park SH, et al. Possible novel therapy for malignant gliomas with secretable trimeric TRAIL. PLoS One. 2009;4(2):e4545. doi: 10.1371/journal.pone.0004545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Puntel M, Ghulam Muhammad AKM, Farrokhi C, et al. Safety profile, efficacy, and biodistribution of a bicistronic high-capacity adenovirus vector encoding a combined immunostimulation and cytotoxic gene therapy as a prelude to a phase I clinical trial for glioblastoma. Toxicol Appl Pharmacol. 2013;268(3):318–330. doi: 10.1016/j.taap.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowers G, He J, Schulz K, et al. Efficacy of adenoviral p53 delivery with SCH58500 in the intracranial 9 l and RG2 models. Front Biosci. 2003;8:8a54–8a61. doi: 10.2741/946. [DOI] [PubMed] [Google Scholar]
- 41.Arafat WO, Buchsbaum DJ, Gomez-Navarro J, et al. An adenovirus encoding proapoptotic Bax synergistically radiosensitizes malignant glioma. Int J Radiat Oncol Biol Phys. 2003;55(4):1037–1050. doi: 10.1016/s0360-3016(02)04488-7. [DOI] [PubMed] [Google Scholar]
- 42.Hardcastle J, Kurozumi K, Dmitrieva N, et al. Enhanced antitumor efficacy of vasculostatin (Vstat120) expressing oncolytic HSV-1. Mol Ther. 2010;18(2):285–294. doi: 10.1038/mt.2009.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gatson NN, Chiocca EA, Kaur B. Anti-angiogenic gene therapy in the treatment of malignant gliomas. Neurosci Lett. 2012;527(2):62–70. doi: 10.1016/j.neulet.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ho IA, Ng WH, Lam PY. FasL and FADD delivery by a glioma-specific and cell cycle-dependent HSV-1 amplicon virus enhanced apoptosis in primary human brain tumors. Mol Cancer. 2010;9:9270. doi: 10.1186/1476-4598-9-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang W, Fulci G, Wakimoto H, et al. Combination of oncolytic herpes simplex viruses armed with angiostatin and IL-12 enhances antitumor efficacy in human glioblastoma models. Neoplasia. 2013;15(6):591–599. doi: 10.1593/neo.13158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coura Rdos S, Nardi NB. The state of the art of adeno-associated virus-based vectors in gene therapy. Virol J. 2007;4:499. doi: 10.1186/1743-422X-4-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma HI, Hueng DY, Shui HA, et al. Intratumoral decorin gene delivery by AAV vector inhibits brain glioblastomas and prolongs survival of animals by inducing cell differentiation. Int J Mol Sci. 2014;15(3):4393–4414. doi: 10.3390/ijms15034393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma HI, Lin SZ, Chiang YH, et al. Intratumoral gene therapy of malignant brain tumor in a rat model with angiostatin delivered by adeno-associated viral (AAV) vector. Gene Ther. 2002;9(1):2–11. doi: 10.1038/sj.gt.3301616. [DOI] [PubMed] [Google Scholar]
- 49.Matsuda M, Yamamoto T, Matsumura A, et al. Highly efficient eradication of intracranial glioblastoma using Eg5 siRNA combined with HVJ envelope. Gene Ther. 2009;16(12):1465–1476. doi: 10.1038/gt.2009.99. [DOI] [PubMed] [Google Scholar]
- 50.Yamanaka R, Tsuchiya N, Yajima N, et al. Induction of an antitumor immunological response by an intratumoral injection of dendritic cells pulsed with genetically engineered Semliki Forest virus to produce interleukin-18 combined with the systemic administration of interleukin-12. J Neurosurg. 2003;99(4):746–753. doi: 10.3171/jns.2003.99.4.0746. [DOI] [PubMed] [Google Scholar]
- 51.Timiryasova TM, Chen B, Fodor I. Replication-deficient vaccinia virus gene therapy vector: evaluation of exogenous gene expression mediated by PUV-inactivated virus in glioma cells. J Gene Med. 2001;3(5):468–477. doi: 10.1002/jgm.205. [DOI] [PubMed] [Google Scholar]
- 52.Tanaka T, Manome Y, Wen P, et al. Viral vector-mediated transduction of a modified platelet factor 4 cDNA inhibits angiogenesis and tumor growth. Nat Med. 1997;3(4):437–442. doi: 10.1038/nm0497-437. [DOI] [PubMed] [Google Scholar]
- 53.Mitra S, Gaur U, Ghosh PC, et al. Tumour targeted delivery of encapsulated dextran-doxorubicin conjugate using chitosan nanoparticles as carrier. J Control Release. 2001;74(1–3):317–323. doi: 10.1016/s0168-3659(01)00342-x. [DOI] [PubMed] [Google Scholar]
- 54.Albanese A, Tang PS, Chan WC. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu Rev Biomed Eng. 2012;14:141–116. doi: 10.1146/annurev-bioeng-071811-150124. [DOI] [PubMed] [Google Scholar]
- 55.Frieboes HB, Wu M, Lowengrub J, et al. A computational model for predicting nanoparticle accumulation in tumor vasculature. PLoS One. 2013;8(2):e56876. doi: 10.1371/journal.pone.0056876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Orza A, Soritau O, Tomuleasa C, et al. Reversing chemoresistance of malignant glioma stem cells using gold nanoparticles. Int J Nanomedicine. 2013;8:8689–8702. doi: 10.2147/IJN.S37481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang CH, Chiou SH, Chou CP, et al. Photothermolysis of glioblastoma stem-like cells targeted by carbon nanotubes conjugated with CD133 monoclonal antibody. Nanomedicine. 2011;7(1):69–79. doi: 10.1016/j.nano.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Bai Y, Yan B. Functionalized carbon nanotubes for potential medicinal applications. Drug Discov Today. 2010;15(11–12):428–435. doi: 10.1016/j.drudis.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao JQ, Lv Q, Li LM, et al. Glioma targeting and blood-brain barrier penetration by dual-targeting doxorubincin liposomes. Biomaterials. 2013;34(22):5628–5639. doi: 10.1016/j.biomaterials.2013.03.097. [DOI] [PubMed] [Google Scholar]
- 60.Eavarone DA, Yu X, Bellamkonda RV. Targeted drug delivery to C6 glioma by transferrin-coupled liposomes. J Biomed Mater Res. 2000;51(1):10–14. doi: 10.1002/(sici)1097-4636(200007)51:1<10::aid-jbm2>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 61.Ananda S, Nowak AK, Cher L, et al. Phase 2 trial of temozolomide and pegylated liposomal doxorubicin in the treatment of patients with glioblastoma multiforme following concurrent radiotherapy and chemotherapy. J Clin Neurosci. 2011;18(11):1444–1448. doi: 10.1016/j.jocn.2011.02.026. [DOI] [PubMed] [Google Scholar]
- 62.Yang ZZ, Li JQ, Wang ZZ, et al. Tumor-targeting dual peptides-modified cationic liposomes for delivery of siRNA and docetaxel to gliomas. Biomaterials. 2014;35(19):5226–5239. doi: 10.1016/j.biomaterials.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 63.Aboody KS, Brown A, Rainov NG, et al. Neural stem cells display extensive tropism for pathology in adult brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA. 2000;97(23):12846–12851. doi: 10.1073/pnas.97.23.12846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yuan SH, Martin J, Elia J, et al. Cell-surface marker signatures for the isolation of neural stem cells, glia and neurons derived from human pluripotent stem cells. PLoS One. 2011;6(3):e17540. doi: 10.1371/journal.pone.0017540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reitz M, Demestre M, Sedlacik J, et al. Intranasal delivery of neural stem/progenitor cells: a noninvasive passage to target intracerebral glioma. Stem Cells Transl Med. 2012;1(12):866–873. doi: 10.5966/sctm.2012-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tyler MA, Ulasov IV, Sonabend AM, et al. Neural stem cells target intracranial glioma to deliver an oncolytic adenovirus in vivo. Gene Ther. 2009;16(2):262–278. doi: 10.1038/gt.2008.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Young JS, Kim JW, Ahmed AU, et al. Therapeutic cell carriers: a potential road to cure glioma. Expert Rev Neurother. 2014;14(6):651–660. doi: 10.1586/14737175.2014.917964. [DOI] [PubMed] [Google Scholar]
- 68.Balyasnikova IV, Ferguson SD, Han Y, et al. Therapeutic effect of neural stem cells expressing TRAIL and bortezomib in mice with glioma xenografts. Cancer Letters. 2011;310(2):148–159. doi: 10.1016/j.canlet.2011.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hingtgen S, Ren X, Terwilliger E, et al. Targeting multiple pathways in gliomas with stem cell and viral delivered S-TRAIL and Temozolomide. Mol Cancer Ther. 2008;7(11):3575–3585. doi: 10.1158/1535-7163.MCT-08-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aboody KS, Najbauer J, Metz MZ, et al. Neural stem cell-mediated enzyme/prodrug therapy for glioma: preclinical studies. Sci Transl Med. 2013;5(184):184ra159. doi: 10.1126/scitranslmed.3005365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418(6893):41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 72.Choi SA, Hwang SK, Wang KC, et al. Therapeutic efficacy and safety of TRAIL-producing human adipose tissue-derived mesenchymal stem cells against experimental brainstem glioma. Neuro Oncol. 2011;13(1):61–69. doi: 10.1093/neuonc/noq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang DY, Yoo SW, Hong Y, et al. The growth of brain tumors can be suppressed by multiple transplantation of mesenchymal stem cells expressing cytosine deaminase. Int J Cancer. 2010;127(8):1975–1983. doi: 10.1002/ijc.25383. [DOI] [PubMed] [Google Scholar]
- 74.Lee DH, Ahn Y, Kim SU, et al. Targeting rat brainstem glioma using human neural stem cells and human mesenchymal stem cells. Clin Cancer Res. 2009;15(15):4925–4934. doi: 10.1158/1078-0432.CCR-08-3076. [DOI] [PubMed] [Google Scholar]
- 75.Honasoge A, Sontheimer H. Involvement of tumor acidification in brain cancer pathophysiology. Front Physiol. 2013;4:4316. doi: 10.3389/fphys.2013.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torchilin VP. Targeted pharmaceutical nanocarriers for cancer therapy and imaging. AAPS J. 2007;9(2):E128–E147. doi: 10.1208/aapsj0902015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li Y, He H, Jia X, et al. A dual-targeting nanocarrier based on poly(amidoamine) dendrimers conjugated with transferrin and tamoxifen for treating brain gliomas. Biomaterials. 2012;33(15):3899–3908. doi: 10.1016/j.biomaterials.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 78.Bellavance MA, Poirier MB, Fortin D. Uptake and intracellular release kinetics of liposome formulations in glioma cells. Int J Pharm. 2010;395(1–2):251–259. doi: 10.1016/j.ijpharm.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 79.Cheng Y, Morshed R, Cheng SH, et al. Nanoparticle-programmed self-destructive neural stem cells for glioblastoma targeting and therapy. Small. 2013;9(24):4123–4129. doi: 10.1002/smll.201301111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng Z, Al Zaki A, Hui JZ, et al. Multifunctional nanoparticles: cost versus benefit of adding targeting and imaging capabilities. Science. 2012;338(6109):903–910. doi: 10.1126/science.1226338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng Y, Doane TL, Chuang CH, et al. Near infrared light-triggered drug generation and release from gold nanoparticle carriers for photodynamic therapy. Small. 2014;10(9):1799–1804. doi: 10.1002/smll.201303329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thomas CR, Ferris DP, Lee JH, et al. Noninvasive remote-controlled release of drug molecules in vitro using magnetic actuation of mechanized nanoparticles. J Am Chem Soc. 2010;132(31):10623–10625. doi: 10.1021/ja1022267. [DOI] [PubMed] [Google Scholar]
- 83.Rapoport NY, Kennedy AM, Shea JE, et al. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J Control Release. 2009;138(3):268–276. doi: 10.1016/j.jconrel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jensen SA, Day ES, Ko CH, et al. Spherical nucleic acid nanoparticle conjugates as an RNAi-based therapy for glioblastoma. Sci Transl Med. 2013;5(209):209ra152. doi: 10.1126/scitranslmed.3006839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 86.Chiocca EA, Aguilar LK, Bell SD, et al. Phase IB study of gene-mediated cytotoxic immunotherapy adjuvant to up-front surgery and intensive timing radiation for malignant glioma. J Clin Oncol. 2011;29(27):3611–3619. doi: 10.1200/JCO.2011.35.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Westphal M, Yla-Herttuala S, Martin J, et al. Adenovirus-mediated gene therapy with sitimagene ceradenovec followed by intravenous ganciclovir for patients with operable high-grade glioma (ASPECT): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(9):823–833. doi: 10.1016/S1470-2045(13)70274-2. [DOI] [PubMed] [Google Scholar]
- 88.Castro MG, Lowenstein PR. Neuro-oncology: The long and winding road--gene therapy for glioma. Nat Rev Neurol. 2013;9(11):609–610. doi: 10.1038/nrneurol.2013.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang H, Fueyo J. Eradication of brain tumor stem cells with an oncolytic adenovirus. Discov Med. 2010;10(50):24–28. [PubMed] [Google Scholar]
- 90.Ostertag D, Amundson KK, Lopez Espinoza F, et al. Brain tumor eradication and prolonged survival from intratumoral conversion of 5-fluorocytosine to 5-fluorouracil using a nonlytic retroviral replicating vector. Neuro Oncol. 2012;14(2):145–159. doi: 10.1093/neuonc/nor199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Altaner C, Altanerova V, Cihova M, et al. Complete regression of glioblastoma by mesenchymal stem cells mediated prodrug gene therapy simulating clinical therapeutic scenario. Int J Cancer. 2014;134(6):1458–1465. doi: 10.1002/ijc.28455. [DOI] [PubMed] [Google Scholar]
- 92.Yong RL, Shinojima N, Fueyo J, et al. Human bone marrow-derived mesenchymal stem cells for intravascular delivery of oncolytic adenovirus Delta24-RGD to human gliomas. Cancer Res. 2009;69(23):8932–8940. doi: 10.1158/0008-5472.CAN-08-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaufmann JK, Chiocca EA. Glioma virus therapies between bench and bedside. Neuro Oncol. 2014;16(3):334–351. doi: 10.1093/neuonc/not310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guan YQ, Zheng Z, Huang Z, et al. Powerful inner/outer controlled multi-target magnetic nanoparticle drug carrier prepared by liquid photo-immobilization. Sci Rep. 2014;4:1–9. doi: 10.1038/srep04990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ma L, Zou XJ, Chen W. A New X-Ray Activated Nanoparticle Photosensitizer for Cancer Treatment. J Biomed Nanotechnol. 2014;10(8):1501–1508. doi: 10.1166/jbn.2014.1954. [DOI] [PubMed] [Google Scholar]