Abstract
Glioblastoma (GBM) remains an almost universally fatal diagnosis. The current therapeutic mainstay consists of maximal safe surgical resection followed by radiation therapy (RT) with concomitant temozolomide (TMZ), followed by monthly TMZ (the “Stupp regimen”). Several chemotherapeutic agents have been shown to have modest efficacy in the treatment of high-grade glioma (HGG), but blood-brain barrier impermeability remains a major delivery obstacle. Polymeric drug-delivery systems, developed to allow controlled local release of biologically active substances for a variety of conditions, can achieve high local concentrations of active agents while limiting systemic toxicities. Polymerically delivered carmustine (BCNU) wafers, placed on the surface of the tumor-resection cavity, can potentially provide immediate chemotherapy to residual tumor cells during the standard delay between surgery and chemoradiotherapy. BCNU wafer implantation as monochemotherapy (with RT) in newly diagnosed HGG has been investigated in 2 phase III studies that reported significant increases in median overall survival. A number of studies have investigated the tumoricidal synergies of combination chemotherapy with BCNU wafers in newly diagnosed or recurrent HGG, and a primary research focus has been the integration of BCNU wafers into multimodality therapy with the standard Stupp regimen. Overall, the results of these studies have been encouraging in terms of safety and efficacy. However, the data must be qualified by the nature of the studies conducted. Currently, there are no phase III studies of BCNU wafers with the standard Stupp regimen. We review the rationale, biochemistry, pharmacokinetics, and research history (including toxicity profile) of this modality.
Keywords: BCNU, carmustine, chemotherapy, glioblastoma, high-grade glioma, polymeric delivery
Glioblastoma (GBM) is the most common malignant primary brain tumor in adults.1 An annual incidence of 4–5 cases per 100 000 persons results in about 12 000 to 15 000 new cases each year in the United States.2,3 Median survival measurements continue to be reported in months (12–15 months) despite decades of intensive work on multiple therapeutic targets.4,5 Overall prognosis remains dismal for patients diagnosed with high-grade glioma (HGG), of which GBM is the most aggressive, with virtually all patients succumbing to the disease. For the last 10 years, the mainstay of standard therapy has remained maximal safe surgical resection followed by external beam radiation with concomitant administration of temozolomide (TMZ), followed by monthly TMZ (a multimodality approach commonly referred to as the “Stupp regimen”).5,6
Multiple advances are being investigated on the surgical front. Several innovations have surfaced that have shown modest utility for improving the quality and safety of surgical resection. These include awake intraoperative mapping, intraoperative MRI, stereotactic guided resection, fluorescence-guided resection, stereotactic-fused functional MRI-guided resection, and minimally invasive techniques such as laser ablation.7–9 Unfortunately, none of these surgical advances are likely to produce substantial survival improvements independently due to the infiltrative nature of HGGs.
Achieving advances in the treatment of most HGGs requires the development of therapies with the potential to address the residual disease that invariably remains after surgical therapies. Modifications of fractionated and single-fraction radiation therapy (RT), with or without concurrent systemic therapies, have been investigated.4,10 By and large, efforts to increase fractionated RT dose, boost residual disease with stereotactic radiosurgery, or apply novel fractionation regimens have been negative or have only been studied in uncontrolled single arm series.11–13 Central failure (within the high-dose RT region and proximate to the tumor cavity and residual disease) remains the primary pattern of failure with this disease.14
Various systemic chemotherapies have been utilized for treating HGG.15 Several agents have been shown to have modest efficacy in the treatment of HGG—including alkylating agents such as carmustine (BCNU [1,3-bis(2-chloroethyl)-1-nitrosurea]), carboplatin, procarbazine, cisplatin, and antiangiogenesis inhibitors (bevacizumab).16–21 Vaccines and targeted systemic agents are under investigation, but none has yet been approved for widespread use.22
Blood-brain barrier (BBB) impermeability remains a major obstacle to delivering systemic (ie, intravenous) agents to tumor cells within the CNS. BBB disruption has shown mixed results for treating HGG, and this technique is not commonly used.23 Additional limitations of various systemic agents have included short half-lives, significant hematologic toxicity, and limited effectiveness at systemically tolerated concentrations. The most commonly employed systemic agent in current use, TMZ, appears better tolerated than previous alkylating agents and has better availability and greater penetration of the BBB.24
When given concurrently with radiation, TMZ has been found to confer a significant survival advantage compared with RT alone.5 Despite its relative advantages, the efficacy of TMZ is constrained by extra-CNS toxicities and biological limits to achieving sustained tumoricidal tissue concentrations, like most other systemic therapies.25 Therapy intensification with TMZ, in the form of dose-dense adjuvant TMZ, was also found to have significantly increased high-grade toxicity without any survival benefit compared with the standard Stupp regimen for GBM.26
Bevacizumab, which had shown promise in recurrent disease, has been studied in 2 large prospective trials as a portion of initial therapy in concert with RT and TMZ but was shown not to confer a survival advantage.27,28 In interim results from a multicenter randomized trial in newly diagnosed GBM patients presented at the 2014 meeting of the Society for Neuro-Oncology, the addition of treatment with the NovoTTF100A (tumor-treating fields) device (Novocure Ltd) prolonged survival by several months compared with treatment of radiation and TMZ alone.29
Given the limitations of existing systemically delivered therapies, along with the biological realities inherent in treating infiltrative CNS tumors, various investigators have examined the effectiveness of introducing antineoplastic therapies directly into CNS tumors. This review will evaluate the work performed to date on polymeric chemotherapy delivery systems, with a description of the rationale for this approach, preclinical pharmacokinetic analyses, efficacy data, and likely future directions of this treatment strategy.
HGGs, even when gross-totally resected, recur because of the infiltrative nature of the disease. Most recurrences are local, occurring within 2 cm of the tumor bed.30 These factors form the biological rationale for employing enhanced local treatments for gliomas with therapies in addition to maximal safe resection followed by RT and concurrent as well as adjuvant TMZ. Many such additional local approaches have been evaluated, such as direct introduction of chemotherapeutic agents by polymeric delivery of wafers placed against the wall of the resection cavity (reviewed here), direct infusion of toxin conjugates into the tumor, direct infusion of chemotherapy, and application of virus-producing cells for suicide gene therapy.31–40 Convection-enhanced delivery (CED) of tumoricidal agents and brachytherapy to the tumor cavity have also been evaluated.
Polymeric Drug Delivery for Malignant Primary Brain Tumors
Introduction and Rationale
Polymeric drug delivery systems have been developed to allow controlled local release of biologically active substances for a variety of conditions. Their primary advantages relate to their ability to achieve high local concentrations of active agents while limiting or eliminating systemic toxicities.
At the time the first polymeric delivery system was being developed for primary malignant brain tumors, BCNU was widely considered to be the most effective systemic chemotherapy for HGG.41–43 For this reason, BCNU was chosen as the best candidate for locally delivered chemotherapy. BCNU is a nitrosourea that has shown activity against gliomas and other solid tumors. As a group, nitrosoureas are highly lipid soluble, nonionized, cell-cycle-nonspecific agents that have good BBB penetration compared with other systemic chemotherapies. They decompose spontaneously into 2 active intermediates: an isocyanate group and chloroethyldiazohydroxide. DNA alkylation leads to the formation of DNA-DNA and DNA-protein crosslinks, which are mediated by the chloroethyldiazohydroxide intermediate. The isocyanate intermediate produces carbamoylation of amino groups, which depletes glutathione, inhibits DNA repair, and interferes with RNA synthesis.
Systemic BCNU is delivered intravenously and has a half-life of 15–30 minutes. Its systemic hematologic toxicity is such that it often takes weeks for patient cell counts to recover before another dose can be given. Subsequently, tumor cells are typically exposed to intermittent, brief doses of systemically delivered BCNU.
BCNU wafers (poly carboxyphenoxy-propane/sebacic acid anhydride [PCPP:SA] wafers containing 3.85% BCNU) were designed to release BCNU slowly after being placed on the surface of the tumor-resection cavity. The advantages gained over systemic BCNU relate to the ability of these wafers to concentrate BCNU locally, avoid systemic toxicity, and provide durable drug delivery over several days to weeks.40,44,45 BCNU wafers also provide immediate chemotherapy to residual tumor cells during the standard delay between surgery and chemoradiotherapy.
Over the years, a number of other antitumor agents have been investigated for local delivery using PCPP:SA wafer technology. In almost all instances, the agents had been used successfully in preclinical studies, but there has been no translation of these results to clinical care. For various reasons, the surge of research in PCPP:SA wafer technology for delivering antitumor drugs abated when the TMZ standard-of-care model emerged, perhaps because of growing interest in other local delivery systems such as convection-enhanced delivery of drugs and gene transfer. Before 2008, antitumor agents that were incorporated into PCPP:SA wafers and studied in rodent glioma models included paclitaxel,46 camptothecin,47,48 hydroperoxycyclophosphamide,49 tirapazamine,50 and 5-iodo-2V deoxyuridine.51,52 It was even found by Brem et al that local delivery of TMZ by means of PCPP:SA wafers in a rodent glioma model was superior to oral TMZ in terms of median survival.53
A few recent studies, also in animal models, have again underscored the potential of the core technology of PCPP:SA wafers for carrying antitumor agents. Bow et al incorporated the angiogenesis inhibitor minocycline into PCPP:SA wafers for treatment, combined with either radiation therapy or oral TMZ, of a 9L glioma rat model.54 The minocycline wafers were implanted 2 days before radiation therapy. Compared with radiation therapy alone, the combination of minocycline wafers and radiotherapy increased median survival by 139%. Compared with treatment of oral TMZ alone, the combination of minocycline wafers and oral TMZ increased median survival by 38%. Local delivery of the angiogenesis inhibitor minocycline thus appeared to potentiate the effects of radiotherapy and oral TMZ. In a similar study, Wicks et al incorporated 3-bromopyruvate (3-BrPA) and dichloroacetate (DCA) into separate PCPP:SA wafers for treatment of a rodent glioma model.55 These drugs are inhibitors of cancer-cell-specific aerobic glycolysis. The use of oral 3-BrPA for glioma has been limited by its ability to cross the BBB, and the use of oral DCA for glioma has been limited by toxicity. In this study, both the 3-BrPA wafer and the DCA wafer significantly increased survival compared with controls. Moreover, the 3-BrPA wafer in combination with oral TMZ significantly increased survival compared with either therapy alone.
Polymeric Drug-delivery Systems: Biochemistry
Initially, non-biodegradable polymers were used to deliver substances locally to tissue cavities; however, these systems were not optimal due to a variety of factors including the slowing of drug-release rates over time.56 Most early clinically available polymeric drug-delivery systems consisted of hydrophilic matrices that absorb water and undergo homogeneous degradation. Such systems would result in rapid, uncontrolled, inactivation of agents such as BCNU. The ideal polymeric drug-delivery system for agents such as BCNU would be hydrophobic and would degrade from the surface, layer by layer, thus maintaining the drug until the eroding border of the dissolving polymer reached the BCNU molecule.
In the early 1980s, biodegradable, hydrophobic polymers were introduced into medicine in the form of absorbable sutures.57 This advance eliminated the rapid-degradation problems associated with earlier polymers and allowed the development of more controlled, long-lasting drug-delivery systems.
PCPP:SA wafers were chosen for use with BCNU based on a variety of preclinical experiments that demonstrated controlled, sustained BCNU release in experimental brain models.58,59 After placement into the aqueous environment of the brain, hydrolysis of the anhydride polymer bonds of current BCNU wafers causes slow degradation of the polymer matrix, which releases carmustine at a controlled and nearly constant rate. Current, commercially available BCNU wafers degrade by surface erosion (as opposed to bulk erosion) from the outside in because the aqueous environment breaks down successive layers of anhydride bonds of the matrix. Once released from the matrix, carmustine diffuses away from the wafer and into the local interstitial fluids, down its concentration gradient.60
Other biodegradable polymeric delivery technologies have been, or are currently being, explored besides PCPP:SA wafer technology. A few studies using different polymeric technologies to carry agents have been conducted in humans. Biodegradable copolymer polylactic-coglycolic acid (PLGA) microspheres, loaded with an antitumor agent and delivered by stereotaxic and computer-assisted approaches to precise areas of the brain, appear to be safe. Compared with the PCPP:SA macroscopic wafer, these microspheres could be implanted into brain tissue for intraparenchymal delivery; their very small size could enable multiple and spatially distributed implantations.61 To date, the use of PLGA microspheres has been explored for HGG, pain, and spasticity. In an open-label phase II study, Menei et al randomized 77 participants with newly diagnosed HGG (1) to injection of 40 μg PLGA microspheres incorporating radiosensitizing 5-fluorouracil after tumor resection followed by radiotherapy (n = 38), or (2) to radiotherapy alone after the resection (n = 39).62 The PLGA microspheres were delivered by multiple stereotaxic injections into the walls of the resection cavity to a depth of 2 cm and spaced 1 cm apart. Unfortunately, there was no significant difference in median overall survival between the treatment arms, although the trend favored PLGA microspheres (15.2 months vs 13.5 months). Animal research with PLGA microspheres continues.
In another approach after tumor resection, Sheleg et al implanted biodegradable cisplatin-incorporated 6-carboxycellulose polymer depots (CDDP-D), delivering 45 mg of radiosensitizing cisplastin, in 11 participants with newly diagnosed GBM (with whom 21 control patients were compared).63 At 2 or 3 weeks after resection, both groups received radiotherapy. The implantation of CDDP-D was well tolerated and significantly increased median survival compared with the control group (427.5 days vs 211 days, P < .001). Further research on the implantation of CDDP-D for HGG has not been pursued.
Currently, Rahman et al have been evaluating a novel formulation of PLGA/poly(ethylene glycol) (PEG) microparticle-based matrices.64 These matrices can be molded into any size or shape desired, therefore making possible local drug delivery when there is a partial or irregular brain tumor resection. In preclinical models to date, the investigators have loaded the PLGA/PEG matrices with trichostatin A, etoposide, and methotrexate and measured release characteristics.
Preclinical Work on BCNU-containing Polymers for Treatment of Malignant Brain Tumors
Tamargo et al published the first paper on BCNU-embedded wafers in experimental animals.65 Rats with implanted 9L gliosarcomas that were treated with local wafers survived longer than those treated with systemic chemotherapy or distantly implanted wafers. These investigators used 2 different preparations of biodegradable polymers: ethylene-vinyl acetate copolymer (EVAc) and PCPP:SA. They ultimately settled on PCPP:SA, known as polifeprosan, because of its superior ability to protect BCNU from prerelease degradation.66
Initial pharmacokinetic data were derived using radiolabeled BCNU wafers implanted in rabbit brain models. Grossman et al (at Johns Hopkins University) demonstrated that BCNU diffused up to 12 mm away from the wafers.67 The area of spread and concentration were largest within 7 days post implantation and fell to <10% of the original dose at days 14 and 21. The area of detectable BCNU concentration at days 14 and 21 was <3 mm from the wafer implantation site. They also compared directly injected BCNU with that released from wafers and found that wafer placement led to considerably longer periods of local distribution of BCNU than direct injection, confirming the controlled-release property of the wafers. Subsequent studies in monkeys documented BCNU concentration at the site of implantation on day 1 of 3.5 mmol/L, diminishing to 1 mmol/L at a distance of 3 mm. By 1 week post implantation, only the tissue within 0.5 mm of the wafers had a BCNU concentration >1 mmol/L.67 These measured drug concentrations are in excess of the known inhibitory concentrations of BCNU on human glioma lines in vitro, which are as low as 15 to 300 μmol/L.68,69 These data indicate that tissue adjacent to the BCNU wafer receives effective BCNU drug concentrations for at least 1 week post implantation, with rapid concentration falloff thereafter. The Johns Hopkins group also investigated BCNU wafer pharmacokinetics in the brains of rats and monkeys.70,71 In the rat experiments, they found that 10% of the implanted dose concentration of BCNU extended to 5 mm of the wafer/brain interface on postimplantation day 1. This penetration reduced significantly to 1 mm for days 3–14. They hypothesized that the rapid and distant penetration in the first day was due to increased edema assisting the normal diffusion of the drug.
Subsequent trials in monkey models demonstrated the safety of combining external beam RT with BCNU wafer placement. Brem et al conducted a trial in which 18 monkeys were randomly assigned to one of 4 groups: (i) no intervention control, (ii) bland placebo polymer implantation, (iii) BCNU-loaded polymer implantation, and (iv) a group with implantation of placebo polymer in the right hemisphere and BCNU-loaded polymer in the left hemisphere, followed by RT.33 No significant neurological toxicities were noted in any of the animals. The authors concluded that interstitial delivery of BCNU via BCNU wafer was safe in the primate brain and that concomitant RT did not lead to any excessive adverse effects. These studies paved the way for initiating BCNU wafer trials in humans.
Human Trials with BCNU-impregnated Wafers
BCNU-impregnated Wafers as Monochemotherapy for Recurrent and Newly Diagnosed High-grade Glioma
Studies of BCNU-impregnated wafers as monochemotherapy for recurrent HGG are summarized in Table 1, and studies of BCNU-impregnated wafers as monochemotherapy for newly diagnosed HGG are summarized in Table 2. Brem et al performed the initial work demonstrating the safety and efficacy of BCNU for HGG in humans.44,72,73 The dose-escalation study was performed in participants with recurrent HGG and compared 2 concentrations of BCNU (3.85% and 6.35%).72 There was no difference in adverse events; however, the lower-dose group had longer survival, possibly because that treatment group was unbalanced due to having more grade III tumors. Nevertheless, 3.85% was selected as the standard for ongoing evaluation. A multicenter single-arm study by the same group demonstrated that delivering RT to the postoperative bed in the presence of BCNU wafers was safe and resulted in improved survival compared with historical controls for newly diagnosed GBM.73 Subsequently, the group published their double-blind, multicenter, placebo-controlled trial of 222 participants with recurrent HGG, which demonstrated improved 6-month mortality comparing implanted BCNU wafers with placebo (40% vs 53%; P = .06) and a median survival increase of 8 weeks (31 weeks vs 23 weeks; P = .06).44 Approximately two-thirds of the participants in this trial had GBM. BCNU wafer placement was associated with significantly reduced 6-month mortality in the GBM patient subset (44% vs 64%; P = .02). Multivariate adjusted models demonstrated a significant overall survival benefit in the intention-to-treat (ITT) population with BCNU wafer (hazard ratio, 0.67; P = .006). The US Food and Drug Administration subsequently approved BCNU wafer implantation for use in recurrent HGG.
Table 1.
BCNU-impregnated wafers as monochemotherapy for recurrent high-grade glioma
| Study | Year | Study Phase | Description | Design | Results | Conclusion/Comments/Complications |
|---|---|---|---|---|---|---|
| Brem et al72 | 1991 | Phase I/II | 21 patients with recurrent HGG treated with surgery and 1 of 3 concentrations of BCNU wafers | Dose escalation, single-arm, multicenter | Mean postimplant survival: 65 weeks (1.93% BCNU) 64 weeks (3.85% BCNU) 32 weeks (6.35% BCNU) |
Mid-concentration dose (3.85% BCNU) chosen for further study No complications More GBMs in 6.35% group |
| Brem et al44 | 1995 | Phase III | 222 patients with recurrent HGG treated with surgery and either BCNU wafers or placebo wafers | Multicenter, placebo-controlled, double-blind | Median postimplant survival (P = .006): 31 weeks (BCNU wafers) 23 weeks (placebo wafers) |
Safe and effective for recurrent HGG Similar postop seizure rates for each group Nonsignificant increase in intracranial infection in BCNU group (3.6% vs 0.89% |
| Olivi et al114 | 2003 | Phase I | 44 patients with recurrent HGG treated with surgery and escalating BCNU wafer doses | Multicenter, dose-escalation trial measuring 5 BCNU doses (6.5%, 10%, 14.5%, 20%, and 28%) | Median overall survival: 251 days Maximum tolerated dose: 20% BCNU |
Seizures, infections, CSF leak, and brain edema common in 28% BCNU concentration (3/4 participants), less common in 20% BCNU concentration (13.6%), 1 participant had wound infection in 6.5%, 10%, and 14.5% groups combined (5.6%) |
Abbreviations: BCNU, 1,3-bis(2-chloroethyl)-1-nitrosurea; HGG, high-grade glioma.
Table 2.
BCNU-impregnated wafers as monochemotherapy for newly diagnosed high-grade glioma
| Study | Year | Study Phase | Description | Design | Results | Conclusion/Comments/Complications |
|---|---|---|---|---|---|---|
| Brem et al73 | 1995 | Phase I | 22 patients with newly diagnosed HGG treated with surgery and BCNU wafers | Multicenter, single-arm designed to evaluate safety of wafers and XRT | Median overall survival: 40 weeks | BCNU wafers and XRT safe in combination No increase in neurotoxicity when compared with historical controls. No wound infection or systemic toxicity. |
| Valtonen et al45 | 1997 | Phase III | 32 patients with newly diagnosed HGG treated with surgery and BCNU wafers or placebo wafers | Multicenter, placebo-controlled, double-blind | Median overall survival: BCNU 58.1 weeks Placebo 39.9 weeks P = .012 |
BCNU wafers effective for prolonging survival Infection noted in treatment group; frequency not reported |
| Westphal et al40,74 | 2003/ 2006 | Phase III | 240 patients with newly diagnosed HGG treated with surgery and BCNU wafers or placebo wafers | Multicenter, placebo-controlled, double-blind | Median intent-to-treat overall survival: BCNU 13.9 months Placebo 11.6 months |
BCNU wafers significantly effective for prolonging overall survival in intent-to-treat analysis BCNU wafers effective for prolonging overall survival in GBM group after applying Cox proportional hazards model CSF leak (5% vs 0.8%) and intracranial hypertension (9.1% vs 1.7%) were more common in the BCNU group |
Abbreviations: BCNU, 1,3-bis(2-chloroethyl)-1-nitrosurea; CSF, cerebrospinal fluid; GBM, glioblastoma; HGG, high-grade glioma; XRT, external beam radiation therapy.
The role of BCNU wafer implantation in newly diagnosed HGG was investigated in 2 phase III multicenter, double-blind, placebo-controlled trials (Table 2).40,45,74 The authors of both studies reported a significant increase in median survival comparing participants implanted with BCNU wafers with those implanted with control wafers (58.1 vs 39.9 weeks, P = .012, for Valtonen et al45; 13.9 vs 11.6 months, P = .03, for Westphal et al40). All participants in both trials underwent adjuvant RT. The study by Westphal et al reported more adverse events such as cerebrospinal fluid (CSF) leak (5% vs 0.8%) and symptomatic intracranial hypertension (9.1% vs 1.7%) with BCNU wafer placements. Several criticisms of the Westphal et al study have been aired. First, the study was designed as an ITT analysis. The placebo group had more cases of GBM (106/120 vs 101/120), possibly contaminating the findings. When the GBM participants were analyzed separately, there was a nonsignificant (P = .1) increase in survival of 2.1 months (11.4 vs 13.5 months). When the groups were reanalyzed using a Cox proportional hazards model, the survival became significant with a 31% risk reduction of death (P = .04). Second, the placebo arm of the study did not receive any adjuvant chemotherapy until recurrence. Today TMZ would be standard of care for both groups.
BCNU-Impregnated Wafers as Combination Chemotherapy
Combination chemotherapies using BCNU-impregnated wafers are attractive for a number of reasons. Chemotherapy has been shown to improve overall survival in HGG, and further attempts at improved regimens and combinations are potentially fruitful areas for ongoing research. One theoretical advantage of combination chemotherapies with BCNU wafers is that immediate postoperative chemotherapy delivery is possible, in contrast to most existing treatment paradigms that typically involve a 3–6 week window between surgery and initiation of concurrent chemoradiation. In addition, combination chemotherapy seeks to synergize the tumoricidal effects of the chemotherapeutic agents.
TMZ, an orally administered alkylating agent, has a number of desirable characteristics for combination with BCNU wafers, including good bioavailability, good CNS penetration, demonstrated antitumor efficacy as a single agent and in combination with RT, and antitumor synergy in preclinical analyses with BCNU.75–77 Therapeutic synergy between TMZ and alkylating agents such as BCNU has been attributed to the ability of TMZ to suppress the activity of methylguanine-DNA methyltransferase (MGMT), a DNA-mismatch repair enzyme that is upregulated in glioma cells. Unlike nitrosoureas, which can actually induce production of MGMT (and promote tumor resistance), TMZ can deplete MGMT levels in tumor cells, thereby (at least in laboratory settings) improving the efficacy of alkylating agents.78
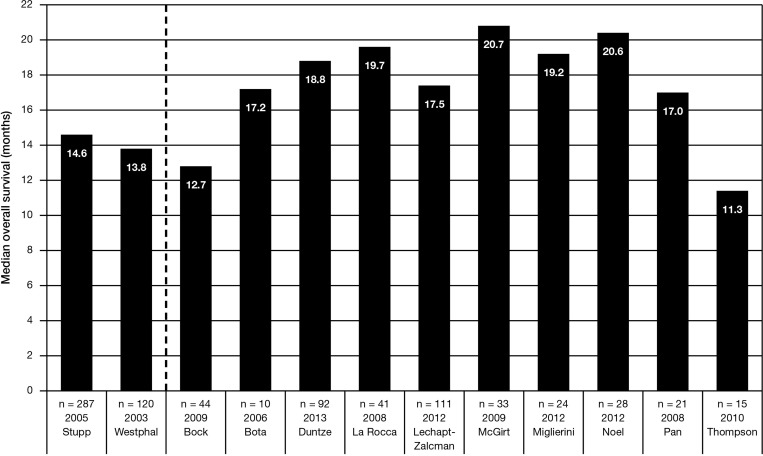
Through 2013, 14 reported clinical trials have investigated the role of BCNU wafer implantation in combination with the standard Stupp regimen of RT and concurrent and adjuvant TMZ (Table 3). Of these, the majority (10) were retrospective,76,79–87 while 4 were prospective.88–91 Overall results have been encouraging in terms of safety and efficacy. Figure 1 displays the median overall survival available for 10 of these combination-therapy trials in comparison with the active treatments arms available in the large phase III randomized clinical trial that established the Stupp regimen5,6 and the use of BCNU wafers.40,74
Table 3.
Summary of articles describing the use of concomitant therapy of BCNU-impregnated wafers and the standard Stupp regimen
| Study | Year | Study Phase | Description | Design | N (GBM) | Median OS (mo) | Conclusion/Comments/Complications |
|---|---|---|---|---|---|---|---|
| Bock et al79 | 2010 | Retrospective | Newly diagnosed | Resection + BCNU wafer → Stupp | 44 (44) | 12.7 | 19 patients (43%) experienced grade 3 or grade 4 adverse events |
| Bota et al80 | 2006 | Retrospective | Newly diagnosed | (1) Resection + BCNU wafer → Stupp (2) Resection/biopsy → Stupp |
10 (10) 13 (13) |
17.2a 19.2a |
No difference in survival or toxicity between the 2 treatments |
| Duntze et al90 | 2013 | Prospective observational | Newly diagnosed | Resection + BCNU wafer → Stupp | 92 (74) | 18.8 | 20.6% of patients presented with perioperative adverse events potentially attributable to BCNU wafer, including 5 severe infections |
| La Rocca et al89 | 2008 | Phase II single arm | Newly diagnosed | Resection + BCNU wafer → Stupp | 41 (40) | 19.7 | Any grade nausea/vomiting 56.1%, DVT 22%, pulmonary embolism 14.6% |
| Lechapt-Zalcman et al81 | 2012 | Retrospective | Newly diagnosed | Resection + BCNU wafer → Stupp | 111 (111) | 17.5 | Patients with tumors that were MGMT methylated had a significantly longer OS compared with patients who had wild-type MGMT (21.7 months vs 15.1 months; P = .025) |
| McGirt et al82 | 2009 | Retrospective | Newly diagnosed | (1) Resection + BCNU wafer → Stupp (2) Resection + BCNU wafer → RT (3) Resection/biopsy → Stupp regimen |
33 (33) 78 (78) 45 (45) |
20.7b 12.4 14.7 |
BCNU wafer + Stupp regimen was not associated with an increase in perioperative morbidity in comparison with BCNU wafer + RT alone Incorporation of TMZ w/or w/o BCNU wafer had better OS compared with BCNU wafer + RT |
| Menei et al83 | 2010 | Retrospective | Newly diagnosed | (1) Resection + BCNU wafer → Stupp (2) Resection + BCNU wafer → other regimens |
83 (72) | 17 (all patients receiving BCNU wafers) | 4 cases of seizure and 9 cases of intracranial hypertension |
| Miglierini et al84 | 2012 | Retrospective | Newly diagnosed | Resection + BCNU wafer → Stupp | 24 (22) | 19.2 | 3 cases of grade 3 thrombocytopenia 10 patients stopped TMZ early due to toxicity or early progression |
| Noel et al85 | 2012 | Retrospective | Newly diagnosed | (1) Resection + BCNU wafer → Stupp (2) Resection/biopsy → Stupp regimen |
28 (20) 37 (16) |
20.6 20.8 |
No difference in outcomes between groups 4 cases of grade 3 thrombocytopenia occurred, all in the BCNU wafer group |
| Pan et al76 | 2008 | Retrospective | Newly diagnosed | Resection + BCNU wafer → Stupp | 21 (21) | 17 | Grade 3 cerebritis 9.5%, altered mental status 5%; no grade 4 toxicities observed |
| Ryken et al91 | 2011 | Phase II, single arm | Newly diagnosed | Resection + BCNU wafer → Stupp | 21 (21) | 18.2 (average) | 9 grade 3–5 toxicities in 5 patients, including 1 grade 5 |
| Salvati et al86 | 2011 | Retrospective | Newly diagnosed | Resection + BCNU wafer → Stupp | 32 | – | Through 15 months, 4 patients showed progression of the disease, and 1 patient died at 14 months No postsurgical complications but a single case of fever |
| Silvani et al88 | 2011 | Phase II | Newly diagnosed | Resection + BCNU wafer → Stupp | 35 | – | Median PFS was 12.5 months 7 patients prematurely stopped TMZ because of toxicities |
| Thompson et al87 | 2010 | Retrospective | Newly diagnosed | (1) Resection + BCNU wafer → Stupp (2) Resection + BCNU wafer → RT (3) Resection/biopsy → Stupp regimen |
15 (15) 9 (9) 22 (22) |
11.3 13 12.4 |
No difference in outcomes between groups Only patients receiving BCNU wafer, TMZ, and RT experienced grade 4 complications (1 seizure, 1 wound healing, and 2 DVT/PE) |
Abbreviations: BCNU, 1,3-bis(2-chloroethyl)-1- nitrosurea; DVT, deep-vein thrombosis; HGG, high-grade glioma; MGMT, O(6)-methylguanine-DNA methyltransferase; OS, overall survival; PE, pulmonary embolism; PFS, progression-free survival; RT, radiation therapy; TMZ, temozolomide.
a = days or weeks converted to months.
b=P < .05.
Fig. 1.
Reported median overall survival (months) in studies of the combination of BCNU wafers with the standard Stupp regimen for the treatment of high-grade gliomas compared with the active treatment arm of the phase III studies of temozolomide and radiotherapy5,6 and the active treatment arm of the phase II study of BCNU wafers and radiotherapy.40,74
Notably, in a prospective observational multicenter study of 92 participants with newly diagnosed malignant glioma treated with BCNU wafers and the standard Stupp regimen, Duntze et al reported a median progression-free survival of 10.5 months and a median overall survival of 18.8 months. The investigators concluded that the survival rates were better than those usually described when BCNU or TMZ is used alone independently from one another, and that these outcomes were obtained without increased BCNU or TMZ toxicity compared with their use as monotherapies.90
There have also been a number of reports of BCNU wafers employed in combination with other treatments, including modifications of the standard Stupp regimen (Table 4).92–103 For example, it has been shown that agents such as O6-benzylguanine (O6-BG) or TMZ can potentiate the activity of BCNU by blocking the activity of DNA repair enzymes and could increase the effectiveness of existing therapies. 06-BG suppresses tumor O6-alkylguanine-DNA alkyltransferase (AGT) levels, while TMZ has been shown to potentially deplete levels of O(6)-MGMT.104 Weingart et al and Quinn et al separately reported phase I trials, followed by phase II trials, on the use of BCNU wafers with subsequent O6-BG infusions in participants with recurrent GBM.98,103 The phase II study of 52 participants showed a 6-month survival rate of 82%, which compared favorably with 56% for a historical control arm of BCNU wafer therapy alone. However, these investigators did report increased adverse event rates, primarily CSF leak (19.2%) and infection (13.4%).98
Table 4.
Summary of articles describing the use of concomitant therapy of BCNU-impregnated wafers and other treatments, including modifications of the Stupp regimen
| Study | Year | Study Phase | Description | Design | N (GBM) | Median OS (mo) | Conclusion/Comments/Complications |
|---|---|---|---|---|---|---|---|
| Affronti et al92 | 2009 | Retrospective | Newly diagnosed | (1) Resection + BCNU wafer → RT + TMZ + rotational chemotherapy (2) Resection → RT + TMZ + rotational chemotherapy |
36 (36) 49 (49) |
16.7 20.6 |
31% grade 3 or 4 hematological toxicity 16% grade 3 or 4 hematological toxicity |
| Asher et al93 | 2007 | Phase II, single arm | Newly diagnosed | Resection + BCNU wafer → early TMZ (day 4) → Stupp regimen | 46 (43) | 18.2 | 33% of patients who received TMZ 200 mg/m2 experienced grade 3–4 thrombocytopenia compared with 5% who received 150 mg/m2 |
| Ewelt et al94 | 2008 | Retrospective | Recurrent | Resection + BCNU wafer → TMZ 1 week on/1 week off | 25 (25) | 18 (from initial diagnosis) | Ten patients with grade 3 and 6 patients with grade 4 toxicity |
| Gururangan et al95 | 2001 | Phase I | Recurrent | Resection + BCNU wafer → TMZ up to 12 cycles | 10 (7) | – | Median of 3 TMZ cycles administered 1 patient experienced grade 3 thrombocytopenia at the highest dose level |
| Limentani et al96 | 2005 | Phase I | Newly diagnosed | Resection + BCNU wafer → early (day 3 or 4) carboplatin → RT | 16 (14) | 22.3a | No grade 3 or 4 toxicities occurred |
| McPherson et al97 | 2012 | Phase I | Newly diagnosed | Resection + I-125 seeds (3000 cGy) + BCNU wafers → RT + adjuvant TMZ | 5 (5) | 12 | Dose-escalation study closed because of radiation toxicity |
| Quinn et al98 | 2009 | Phase II single arm | Recurrent | GTR + BCNU wafer → O6-BG | 52 (52) | 11.6a | Treatment-related toxicity included grade 3 hydrocephalus (9.6%), grade 3 CSF leak (19.2%), and grade 3 CSF/brain infection (13.4%) |
| Rezazadeh et al102 | 2011 | Phase II | Newly diagnosed | Resection + BCNU wafer → dense-dose RT + TMZ → bevacizumab every other week for 12 weeks | 10 (10) | – | 2 patients experienced intracranial hemorrhage and 1 experienced neutropenic fever Through median follow-up of 9 months, 6 patients experienced progression of disease and 6 patients died |
| Salmaggi et al99 | 2013 | Phase II, single arm | Newly diagnosed | Resection + BCNU wafer → RT + TMZ → daily metronomic TMZ 75 mg/m2 up to 6 months | 35 (35) | 17.8 | 7 patients had to prematurely stop TMZ due to toxicity DVT (n = 4) and/or PE (n = 1) occurred in 4 cases |
| Sampath et al100 | 2005 | Phase I | Recurrent | GTR + BCNU wafer → Irinotecan up to 6 cycles | 10 (10) | 20 | Mean number of Irinotecan cycles: 2.7 Median survival from the time of the second surgery was 13.5 months |
| Smith et al101 | 2008 | Phase II | Newly diagnosed | Resection + BCNU wafer → Gamma knife surgery → RT | 27 (27) | 11.5a | No acute early toxicity or complications within the first 3 months |
| Weingart et al103 | 2007 | Phase I | Recurrent | GTR + BCNU wafer → O6-BG | 32 (32) | 11.6a | No treatment-related grade 4 events 10 patients experienced grade 3 CSF leak and 7 patients experienced grade 3 CSF infection |
Abbreviations: BCNU, 1,3-bis(2-chloroethyl)-1- nitrosurea; DVT, deep-vein thrombosis; GTR, gross total resection; HGG, high-grade glioma; MGMT, O(6)-methylguanine-DNA methyltransferase; OS, overall survival; PE, pulmonary embolism; PFS, progress-free survival; RT, radiation therapy; TMZ, temozolamide.
a = days or weeks converted to months.
In a prospective observational report of 35 newly operated patients with GBM who received BCNU wafers in combination with 6-month metronomic TMZ and RT, Salmaggi et al found a median progression-free survival of 12.5 months and median overall survival of 17.8 months.99 The progression-free survival was longer than that for any of the previously mentioned retrospective studies, in which the highest rate of 12-month survivors was 40%.
For all of the studies cited, there is a heterogeneity of effect, with substantial limitations due to patient selection bias, largely retrospective analyses, temporal bias with comparison to historical controls, and various combinations of agents used in addition to TMZ such as bevacizumab,102 brachytherapy,97 or rotational multiagent chemotherapy.92
There has also been heterogeneity in terms of prognostic and predictive factors for the efficacy of the BCNU wafer in combination with the standard Stupp regimen. In one retrospective study that did demonstrate an overall survival benefit with the addition of BCNU wafers, there was no benefit in the subset of 41 participants who underwent gross total resection (overall survival of 21.5 months for participants who received combination therapy including the BCNU wafer vs 19.8 months for those with the standard Stupp regimen, P = .3).82 MGMT promoter methylation is known to be a prognostic and predictive factor for TMZ benefit.105 However, there is disagreement in the literature about the role of MGMT methylation status in patients treated with BCNU wafers and the Stupp regimen. In a phase II trial, LaRocca et al found no difference in overall survival based on MGMT methylation status for 41 participants treated with this regimen (median overall survival was 23.3 months for participants with methylated MGMT vs 19.7 months for those with unmethylated MGMT, P = .29).89 On the other hand, in a retrospective study of 111 participants treated with the combination regime, Lechapt-Zalcman et al found a significant difference in median overall survival based on MGMT status (21.7 months methylated vs 15.1 months unmethylated, P = .025).81
Toxicity and Adverse Events
A variety of adverse events associated with the use of BCNU have been reported in the studies previously mentioned in this review. Recently, 3 published review articles have performed combined analysis of the adverse events from multiple studies.16,106,107 Complications including seizure (5%–16%), CSF leak (0%–11%), local infection (6%–12%), delayed wound healing (2%–16%), cerebral edema (2%–25%), malignant cerebral hypertension or hydrocephalus (7%–10%), and new neurological deficit (6%–16%) have been reported.107 Bregy et al summarized adverse events combining 2 reports on BCNU wafers as monochemotherapy with 14 reports on the use of concomitant delayed (weeks) systemic chemotherapy.16 They reported a combined total estimate of toxicity/adverse events of 42.7%.
It should be noted that the majority of these reports described all adverse events experienced by patients in the BCNU wafer arm of the study under examination, not just those that were thought to be directly attributable to the addition of BCNU wafers in combination treatments including radiation and other systemic chemotherapy. Many of the adverse events—including venous thromboembolism, pneumonitis, systemic infections, nephrotoxicity, fatigue, gastrointestinal complications, myelosuppression—are almost certainly the result of adjuvant systemic chemotherapy and are unrelated to BCNU released by the implanted wafers since that is virtually undetectable in the systemic circulation.
In 2008, Attenello et al summarized the institutional experience with implantation of BNCU wafers at Johns Hopkins University School of Medicine.108 Between 1996 and 2006, 1013 patients underwent resection for HGG, of whom 288 (28%) were implanted with the BCNU wafer (166 for primary HGG and 122 for recurrent HGG). In comparing morbidity between participants who received the BCNU wafer and those who did not, respectively, the investigators found no statistically significant differences in the incidence of pulmonary embolism (4.9% vs 3.7%, P = .41), deep vein thrombosis (6.3% vs 5.2%, P = .53), surgical site infection (2.8% vs 1.8%, P = .33), CSF leak (2.8% vs 1.8%, P = .33), seizure (14.6% vs 15.7%, P = .65), wound-healing problems (0.7% vs 0.4%, P = .63), or symptomatic malignant edema (4.9% vs 3.7%, P = 0.41).
The reported toxicities/adverse events remain one concern that may have limited the use of BCNU wafers despite evidence of improved outcomes in some patients with HGG. Despite the institutional experience at Johns Hopkins University, there appears to be an increased incidence of seizures, mass effect, and local/wound infection/CSF leak in patients undergoing BCNU wafer implantation, congruent with the local nature of the treatment. Once combination systemic chemotherapy is instituted (most commonly TMZ), the rate of toxicity/adverse events rises with the inherent toxicities of the associated therapies. There is no good evidence of increased toxicity with the combination of BCNU wafers and systemic chemotherapy. Also, as commonly used, the vast majority of BCNU has been delivered locally well before the initiation of systemic therapy. There is an increase in seizures and wound healing issues when BCNU wafers are used for recurrent GBM, as these are high-risk patients who have undergone multiple previous interventions including RT. In a placebo-controlled trial of BCNU wafers in the treatment setting of recurrent GBM, Brem et al found that seizures (37.3% vs 28.6%, P = .199) were increased in the BCNU wafer group but were within the expected frequency for postoperative seizures; likewise, the incidence of serious intracranial infections (3.6% vs 0.9%) was nonsignificantly more common with BCNU treatment but was well within the reported range (9% to 13%) for recurrent glioma surgery.44
Imaging Characteristics of BCNU Wafer Implantation
BCNU wafers themselves—as well as potential edema, mass effect, and local enhancement after BCNU wafer implantation—can pose a challenge when interpreting immediate, postimplantation, and distant postoperative MRI. Contrast enhancement, diffusion restriction, and T2/fluid-attenuated inversion recovery (FLAIR) signal are evident in the immediate (postoperative day 1) period. Contrast enhancement peaks at 1 month post implantation and diffusion restriction may remain for up to 1 year. The T2/FLAIR signal peaks within the first 2 months post implantation and resolves thereafter.109,110 Given these known imaging changes, one should view postoperative imaging of patients with implanted BCNU wafers with caution. Early postoperative changes classified as recurrence might actually reflect pseudoprogression from the BCNU wafer's local effect and/or radiotherapy. Changes in the early portion of therapy based on imaging alone should be weighed carefully with the known imaging changes of the therapy and potential early response.
Future Directions
A primary goal of current research efforts is to determine the optimal method for integrating BCNU wafers into the standard Stupp regimen. Two pharmacokinetic observations regarding BCNU wafers are particularly relevant with respect to the optimal timing of administration of potentially synergistic systemic agents. First, tumoricidal concentrations are highest within 1 cm of implantation and fall off over time (2–3 weeks) and distance from implantation. Second, the majority of BCNU is liberated from the wafers during the first week after implantation.70,71 With the understanding that local failure of therapy can often occur at a moderate distance from the immediate tumor resection margin, and assuming that high local tissue concentrations of BCNU would increase the potential for additive or synergistic effects with other agents, the kinetics of BCNU dispersion into surrounding brain tissue suggest that systemic agents may be optimally administered in the early postoperative setting.
A phase I proof of concept evaluation of carboplatin administered on days 3–4 following surgical resection of malignant glioma with intraoperative placement of BCNU wafers was published by Limentani et al in 2005.96 Standard RT was started on days 14–36. Carboplatin was well tolerated in the initial postoperative setting, and no grade 3 or 4 toxicities were observed. Median time to progression was 159 days, and median survival was 590 days. The authors concluded that their study had demonstrated the safety of administering chemotherapy in the early postoperative setting after BCNU wafer placement.
In a phase II trial of 46 participants with newly diagnosed HGG (43 GBM, 3 anaplastic astrocytoma), Asher et al studied the addition of early TMZ to BCNU wafer implantation and the adjuvant Stupp regimen.93 After resection and BCNU wafer placement, TMZ was begun on day 4 postoperatively. Radiation and concomitant TMZ were then administered, followed by monthly TMZ at 200 mg/m2 for the first 26 participants (which was subsequently reduced to 150 mg/m2 for the remaining 20 participants). The treatment was well tolerated at the TMZ 150 mg/m2 dose level. Median progression-free survival was 8.3 months, and median overall survival was 18.2 months. The 1-year overall survival rate was 76%, representing a significant improvement (P = .02) compared with the 1-year overall survival of 59.2% reported by Westphal et al40 for the experimental arm of the randomized phase III trial using BCNU wafers with RT (without TMZ).
There has been no prospective, multicenter, double-blind trial comparing the combination of BCNU wafers and RT/TMZ therapy with either therapy alone. The retrospective and prospective single-arm data are encouraging in terms of the safety and efficacy of the combination approach, primarily compared with the overall outcomes of the large phase III randomized clinical trial that established the Stupp regimen,5,6 although weighting of the data is qualified by the nature of the studies. A phase III trial comparing the standard-of-care Stupp regimen with and without BCNU wafer therapy is unlikely to be conducted. Larger prospective or database trials are needed to determine a potential subgroup of benefit for this approach, such as the potential of overcoming the detrimental effects of unmethylated MGMT with the inclusion of BCNU wafers. There are indications of this potential in the literature, but larger and confirmatory studies are needed.
Other novel concurrent chemotherapeutic agents are being explored, but recent studies have yielded negative results for dose-dense adjuvant TMZ and the addition of bevacizumab in newly diagnosed GBM and for cedirinib alone or in combination with lomustine in recurrent GBM.27,111,112 It is unlikely that these agents would have additional benefit in combination with BCNU wafers. Future studies investigating the optimal timing and incorporation of known agents (eg, TMZ) and novel agents currently being investigated (eg, immunomodulatory drugs including ipilimumab and PD-1/PDL-1 inhibitors) are needed to better characterize the potential and future role of BNCU wafers in the multimodal care of GBM.
BCNU wafer implantation served as the control therapy for a phase III randomized trial investigating the effect of CED of cintredekin besudotox (IL13-PE38QQR) for recurrent GBM.113 Because the investigational therapy was an adjunct to treatment, the BCNU wafer (the only other approved local treatment) was used as the comparator. The 296 enrolled participants were randomized 2 : 1 to receive CED or BCNU wafers, with no significant differences between the 2 groups. Median survival was 9.1 months for the CED group and 8.8 months for the BCNU wafer group. The occurrence of adverse events was similar for both groups, except the incidence of pulmonary embolism was higher in the CED group (8% vs 1%, P = .014). Noting the need for further assessment of CED drug distribution, the investigators concluded that there was no survival advantage in using CED of IL13 conjugated toxin compared with the implantation of BCNU wafers.
Conclusion
HGG remains a vexing problem, with an almost universal failure to cure patients. In the last 2 decades, the introductions of TMZ and BCNU wafers represent the only significant advances that have been confirmed in phase III trials. In a systematic review and evidence-based clinical practice guideline on the role of cytotoxic chemotherapy for managing progressive glioblastoma (published in 2014 by Olson et al), there were no level I recommendations; level II recommendations were limited to TMZ (noted as superior to procarbazine) and BCNU wafers (as a surgical adjunct when cytoreductive surgery was indicated, “taking into account the associated toxicities”).17 The systematic review noted that neither agent was curative. Indeed, either agent alone in combination with RT improved survival by roughly 2 months. As this review has shown, when BCNU wafers and TMZ are employed together sequentially, there appears to be an additive or synergistic effect, and there are encouraging data suggesting additional weeks to months of survival.
The available literature fails to clearly delineate which patients would benefit the most from the addition of BCNU wafers. Inexplicably, previous implantation of BCNU wafers often disqualified patients from participating in novel HGG trials.17 While the imaging changes noted earlier may be thought to increase the difficulty of defining radiographic progression in trials of novel chemotherapeutics and immunotherapy, investigators still could—and should—easily include BCNU wafer use as a stratification factor to allow patients access to studies and investigative treatment for HGG.
Currently, it is doubtful that combining any of the existing therapies and/or manipulating their doses and timing is going to improve overall survival outcomes by more than a few weeks or months. Even so, such incremental advances with local therapies might well be seen as securing the best stage for our patients with HGG until the occurrence of future treatment breakthroughs.
Acknowledgments
This paper is one of three review articles for a supplement entitled “Local delivery of cytoreductive agents for the treatment of glioblastoma.” The supplement is supported by Arbor Pharmaceuticals. The physician editor of the supplement is Steven N. Kalkanis, MD, Department of Neurosurgery, Henry Ford Hospital, Detroit, MI, who has contributed an introduction to the supplement. The two other review articles in the supplement cover convection-enhanced delivery and gene therapy and delivery systems.
Conflict of interest statement: None of the authors have any conflict of interest.
References
- 1.DeAngelis LM. Brain tumors. N Engl J Med. 2001;344(2):114–123. doi: 10.1056/NEJM200101113440207. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Bauchet L, Davis FG, et al. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol. 2014;16(7):896–913. doi: 10.1093/neuonc/nou087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 4.Wilson TA, Karajannis MA, Harter DH. Glioblastoma multiforme: State of the art and future therapeutics. Surg Neurol Int. 2014;0:564. doi: 10.4103/2152-7806.132138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 6.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 7.D'Amico RS, Kennedy BC, Bruce JN. Neurosurgical oncology: advances in operative technologies and adjuncts. J Neurooncol. 2014;119(3):451–463. doi: 10.1007/s11060-014-1493-3. [DOI] [PubMed] [Google Scholar]
- 8.Duffau H. The necessity of preserving brain functions in glioma surgery: the crucial role of intraoperative awake mapping. World Neurosurg. 2011;76(6):525–527. doi: 10.1016/j.wneu.2011.07.040. [DOI] [PubMed] [Google Scholar]
- 9.Norred SE, Johnson JA. Magnetic resonance-guided laser induced thermal therapy for glioblastoma multiforme: a review. Biomed Res Int. 2014;76:1312. doi: 10.1155/2014/761312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ryu S, Buatti JM, Morris A, et al. The role of radiotherapy in the management of progressive glioblastoma : a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118(3):489–499. doi: 10.1007/s11060-013-1337-6. [DOI] [PubMed] [Google Scholar]
- 11.Souhami L, Seiferheld W, Brachman D, et al. Randomized comparison of stereotactic radiosurgery followed by conventional radiotherapy with carmustine to conventional radiotherapy with carmustine for patients with glioblastoma multiforme: report of Radiation Therapy Oncology Group 93–05 protocol. Int J Radiat Oncol Biol Phys. 2004;60(3):853–860. doi: 10.1016/j.ijrobp.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 12.Chan JL, Lee SW, Fraass BA, et al. Survival and failure patterns of high-grade gliomas after three-dimensional conformal radiotherapy. J Clin Oncol. 2002;20(6):1635–1642. doi: 10.1200/JCO.2002.20.6.1635. [DOI] [PubMed] [Google Scholar]
- 13.Nieder C, Andratschke N, Wiedenmann N, et al. Radiotherapy for high-grade gliomas. Does altered fractionation improve the outcome? Strahlenther Onkol. 2004;180(7):401–407. doi: 10.1007/s00066-004-1220-7. [DOI] [PubMed] [Google Scholar]
- 14.McDonald MW, Shu HK, Curran WJ, Jr., et al. Pattern of failure after limited margin radiotherapy and temozolomide for glioblastoma. Int J Radiat Oncol Biol Phys. 2011;79(1):130–136. doi: 10.1016/j.ijrobp.2009.10.048. [DOI] [PubMed] [Google Scholar]
- 15.Olson JJ. Glioblastoma at progression: therapy of a challenging problem addressed candidly with evidence-based techniques. J Neurooncol. 2014;118(3):427–428. doi: 10.1007/s11060-014-1431-4. [DOI] [PubMed] [Google Scholar]
- 16.Bregy A, Shah AH, Diaz MV, et al. The role of Gliadel wafers in the treatment of high-grade gliomas. Expert Rev Anticancer Ther. 2013;13(12):1453–1461. doi: 10.1586/14737140.2013.840090. [DOI] [PubMed] [Google Scholar]
- 17.Olson JJ, Nayak L, Ormond DR, et al. The role of cytotoxic chemotherapy in the management of progressive glioblastoma: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2014;118(3):501–555. doi: 10.1007/s11060-013-1338-5. [DOI] [PubMed] [Google Scholar]
- 18.Capdevila L, Cros S, Ramirez JL, et al. Neoadjuvant cisplatin plus temozolomide versus standard treatment in patients with unresectable glioblastoma or anaplastic astrocytoma: a differential effect of MGMT methylation. J Neurooncol. 2014;117(1):77–84. doi: 10.1007/s11060-013-1352-7. [DOI] [PubMed] [Google Scholar]
- 19.Parney IF, Chang SM. Current chemotherapy for glioblastoma. Cancer J. 2003;9(3):149–156. doi: 10.1097/00130404-200305000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Ajaz M, Jefferies S, Brazil L, et al. Current and investigational drug strategies for glioblastoma. Clin Oncol (R Coll Radiol) 2014;26(7):419–430. doi: 10.1016/j.clon.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Xu LW, Chow KK, Lim M, et al. Current vaccine trials in glioblastoma: a review. J Immunol Res. 2014 doi: 10.1155/2014/796856. 2014:796856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bidros DS, Vogelbaum MA. Novel drug delivery strategies in neuro-oncology. Neurotherapeutics. 2009;6(3):539–546. doi: 10.1016/j.nurt.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery. 1996;39(2):235–250. doi: 10.1097/00006123-199608000-00001. discussion 250–232. [DOI] [PubMed] [Google Scholar]
- 24.Ostermann S, Csajka C, Buclin T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10(11):3728–3736. doi: 10.1158/1078-0432.CCR-03-0807. [DOI] [PubMed] [Google Scholar]
- 25.Newlands ES, Stevens MF, Wedge SR, et al. Temozolomide: a review of its discovery, chemical properties, pre-clinical development and clinical trials. Cancer Treat Rev. 1997;23(1):35–61. doi: 10.1016/s0305-7372(97)90019-0. [DOI] [PubMed] [Google Scholar]
- 26.Gilbert MR, Wang M, Aldape KD, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–4091. doi: 10.1200/JCO.2013.49.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 28.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stupp R, Wong E, Scott C. Interim analysis of the EF-14 trial: a prospective, multicenter trial of NovoTTF-100A together with temozolomide compared to temozolomide alone in patients with newly diagnosed GBM; November 13–16; Miami, FL. 2014. Presented at: the Society for Neuro-Oncology; [Google Scholar]
- 30.Hochberg FH, Pruitt A. Assumptions in the radiotherapy of glioblastoma. Neurology. 1980;30(9):907–911. doi: 10.1212/wnl.30.9.907. [DOI] [PubMed] [Google Scholar]
- 31.Bigner DD, Brown MT, Friedman AH, et al. Iodine-131-labeled antitenascin monoclonal antibody 81C6 treatment of patients with recurrent malignant gliomas: phase I trial results. J Clin Oncol. 1998;16(6):2202–2212. doi: 10.1200/JCO.1998.16.6.2202. [DOI] [PubMed] [Google Scholar]
- 32.Culver KW, Ram Z, Wallbridge S, et al. In vivo gene transfer with retroviral vector-producer cells for treatment of experimental brain tumors. Science. 1992;256(5063):1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 33.Brem H, Tamargo RJ, Olivi A, et al. Biodegradable polymers for controlled delivery of chemotherapy with and without radiation therapy in the monkey brain. J Neurosurg. 1994;80(2):283–290. doi: 10.3171/jns.1994.80.2.0283. [DOI] [PubMed] [Google Scholar]
- 34.Kramm CM, Sena-Esteves M, Barnett FH, et al. Gene therapy for brain tumors. Brain Pathol. 1995;5(4):345–381. doi: 10.1111/j.1750-3639.1995.tb00615.x. [DOI] [PubMed] [Google Scholar]
- 35.Laske DW, Youle RJ, Oldfield EH. Tumor regression with regional distribution of the targeted toxin TF-CRM107 in patients with malignant brain tumors. Nat Med. 1997;3(12):1362–1368. doi: 10.1038/nm1297-1362. [DOI] [PubMed] [Google Scholar]
- 36.Lesser GJ, Grossman S. The chemotherapy of high-grade astrocytomas. Semin Oncol. 1994;21(2):220–235. [PubMed] [Google Scholar]
- 37.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11(17):2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 38.Mesnil M, Piccoli C, Tiraby G, et al. Bystander killing of cancer cells by herpes simplex virus thymidine kinase gene is mediated by connexins. Proc Natl Acad Sci USA. 1996;93(5):1831–1835. doi: 10.1073/pnas.93.5.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ram Z, Culver KW, Oshiro EM, et al. Therapy of malignant brain tumors by intratumoral implantation of retroviral vector-producing cells. Nat Med. 1997;3(12):1354–1361. doi: 10.1038/nm1297-1354. [DOI] [PubMed] [Google Scholar]
- 40.Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5(2):79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stewart LA. Chemotherapy in adult high-grade glioma: a systematic review and meta-analysis of individual patient data from 12 randomised trials. Lancet. 2002;359(9311):1011–1018. doi: 10.1016/s0140-6736(02)08091-1. [DOI] [PubMed] [Google Scholar]
- 42.Black PM. Brain tumors. Part 1. N Engl J Med. 1991;324(21):1471–1476. doi: 10.1056/NEJM199105233242105. [DOI] [PubMed] [Google Scholar]
- 43.Black PM. Brain tumor. Part 2. N Engl J Med. 1991;324(22):1555–1564. doi: 10.1056/NEJM199105303242205. [DOI] [PubMed] [Google Scholar]
- 44.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345(8956):1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 45.Valtonen S, Timonen U, Toivanen P, et al. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41(1):44–48. doi: 10.1097/00006123-199707000-00011. discussion 48–49. [DOI] [PubMed] [Google Scholar]
- 46.Walter KA, Cahan MA, Gur A, et al. Interstitial taxol delivered from a biodegradable polymer implant against experimental malignant glioma. Cancer Res. 1994;54(8):2207–2212. [PubMed] [Google Scholar]
- 47.Sampath P, Amundson E, Wall ME, et al. Camptothecin analogs in malignant gliomas: comparative analysis and characterization. J Neurosurg. 2003;98(3):570–577. doi: 10.3171/jns.2003.98.3.0570. [DOI] [PubMed] [Google Scholar]
- 48.Storm PB, Moriarity JL, Tyler B, et al. Polymer delivery of camptothecin against 9L gliosarcoma: release, distribution, and efficacy. J Neurooncol. 2002;56(3):209–217. doi: 10.1023/a:1015003232713. [DOI] [PubMed] [Google Scholar]
- 49.Judy KD, Olivi A, Buahin KG, et al. Effectiveness of controlled release of a cyclophosphamide derivative with polymers against rat gliomas. J Neurosurg. 1995;82(3):481–486. doi: 10.3171/jns.1995.82.3.0481. [DOI] [PubMed] [Google Scholar]
- 50.Yuan X, Tabassi K, Williams JA. Implantable polymers for tirapazamine treatments of experimental intracranial malignant glioma. Radiat Oncol Investig. 1999;7(4):218–230. doi: 10.1002/(SICI)1520-6823(1999)7:4<218::AID-ROI3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 51.Williams JA, Dillehay LE, Tabassi K, et al. Implantable biodegradable polymers for IUdR radiosensitization of experimental human malignant glioma. J Neurooncol. 1997;32(3):181–192. doi: 10.1023/a:1005704913330. [DOI] [PubMed] [Google Scholar]
- 52.Yuan X, Dillehay LE, Williams JR, et al. Synthetic, implantable polymers for IUdR radiosensitization of experimental human malignant glioma. Cancer Biother Radiopharm. 1999;14(3):187–202. doi: 10.1089/cbr.1999.14.187. [DOI] [PubMed] [Google Scholar]
- 53.Brem S, Tyler B, Li K, et al. Local delivery of temozolomide by biodegradable polymers is superior to oral administration in a rodent glioma model. Cancer Chemother Pharmacol. 2007;60(5):643–650. doi: 10.1007/s00280-006-0407-2. [DOI] [PubMed] [Google Scholar]
- 54.Bow H, Hwang LS, Schildhaus N, et al. Local delivery of angiogenesis-inhibitor minocycline combined with radiotherapy and oral temozolomide chemotherapy in 9L glioma. J Neurosurg. 2014;120(3):662–669. doi: 10.3171/2013.11.JNS13556. [DOI] [PubMed] [Google Scholar]
- 55.Wicks RT, Azadi J, Mangraviti A, et al. Local delivery of cancer-cell glycolytic inhibitors in high-grade glioma. Neuro Oncol. 2015;17(1):70–80. doi: 10.1093/neuonc/nou143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;263(5580):797–800. doi: 10.1038/263797a0. [DOI] [PubMed] [Google Scholar]
- 57.Wang PP, Frazier J, Brem H. Local drug delivery to the brain. Adv Drug Deliv Rev. 2002;54(7):987–1013. doi: 10.1016/s0169-409x(02)00054-6. [DOI] [PubMed] [Google Scholar]
- 58.Dang W, Daviau T, Brem H. Morphological characterization of polyanhydride biodegradable implant gliadel during in vitro and in vivo erosion using scanning electron microscopy. Pharm Res. 1996;13(5):683–691. doi: 10.1023/a:1016035229961. [DOI] [PubMed] [Google Scholar]
- 59.Domb AJ, Rock M, Schwartz J, et al. Metabolic disposition and elimination studies of a radiolabelled biodegradable polymeric implant in the rat brain. Biomaterials. 1994;15(9):681–688. doi: 10.1016/0142-9612(94)90166-x. [DOI] [PubMed] [Google Scholar]
- 60.Brem H. Polymers to treat brain tumours. Biomaterials. 1990;11(9):699–701. doi: 10.1016/0142-9612(90)90030-t. [DOI] [PubMed] [Google Scholar]
- 61.Menei P, Montero-Menei C, Venier MC, et al. Drug delivery into the brain using poly(lactide-co-glycolide) microspheres. Expert Opin Drug Deliv. 2005;2(2):363–376. doi: 10.1517/17425247.2.2.363. [DOI] [PubMed] [Google Scholar]
- 62.Menei P, Capelle L, Guyotat J, et al. Local and sustained delivery of 5-fluorouracil from biodegradable microspheres for the radiosensitization of malignant glioma: a randomized phase II trial. Neurosurgery. 2005;56(2):242–248. doi: 10.1227/01.neu.0000144982.82068.a2. discussion 242–248. [DOI] [PubMed] [Google Scholar]
- 63.Sheleg SV, Korotkevich EA, Zhavrid EA, et al. Local chemotherapy with cisplatin-depot for glioblastoma multiforme. J Neurooncol. 2002;60(1):53–59. doi: 10.1023/a:1020288015457. [DOI] [PubMed] [Google Scholar]
- 64.Rahman CV, Smith SJ, Morgan PS, et al. Adjuvant chemotherapy for brain tumors delivered via a novel intra-cavity moldable polymer matrix. PLoS One. 2013;8(10):e77435. doi: 10.1371/journal.pone.0077435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tamargo RJ, Myseros JS, Epstein JI, et al. Interstitial chemotherapy of the 9L gliosarcoma: controlled release polymers for drug delivery in the brain. Cancer Res. 1993;53(2):329–333. [PubMed] [Google Scholar]
- 66.Fleming AB, Saltzman WM. Pharmacokinetics of the carmustine implant. Clin Pharmacokinet. 2002;41(6):403–419. doi: 10.2165/00003088-200241060-00002. [DOI] [PubMed] [Google Scholar]
- 67.Grossman SA, Reinhard C, Colvin OM, et al. The intracerebral distribution of BCNU delivered by surgically implanted biodegradable polymers. J Neurosurg. 1992;76(4):640–647. doi: 10.3171/jns.1992.76.4.0640. [DOI] [PubMed] [Google Scholar]
- 68.Gu B, DeAngelis LM. Enhanced cytotoxicity of bioreductive antitumor agents with dimethyl fumarate in human glioblastoma cells. Anticancer Drugs. 2005;16(2):167–174. doi: 10.1097/00001813-200502000-00008. [DOI] [PubMed] [Google Scholar]
- 69.Hunter KJ, Deen DF, Pellarin M, et al. Effect of alpha-difluoromethylornithine on 1,3-bis(2-chloroethyl)-1-nitrosourea and cis-diamminedichloroplatinum(II) cytotoxicity, DNA interstrand cross-linking, and growth in human brain tumor cell lines in vitro. Cancer Res. 1990;50(9):2769–2772. [PubMed] [Google Scholar]
- 70.Fung LK, Ewend MG, Sills A, et al. Pharmacokinetics of interstitial delivery of carmustine, 4-hydroperoxycyclophosphamide, and paclitaxel from a biodegradable polymer implant in the monkey brain. Cancer Res. 1998;58(4):672–684. [PubMed] [Google Scholar]
- 71.Fung LK, Shin M, Tyler B, et al. Chemotherapeutic drugs released from polymers: distribution of 1,3-bis(2-chloroethyl)-1-nitrosourea in the rat brain. Pharm Res. 1996;13(5):671–682. doi: 10.1023/a:1016083113123. [DOI] [PubMed] [Google Scholar]
- 72.Brem H, Mahaley MS, Jr., Vick NA, et al. Interstitial chemotherapy with drug polymer implants for the treatment of recurrent gliomas. J Neurosurg. 1991;74(3):441–446. doi: 10.3171/jns.1991.74.3.0441. [DOI] [PubMed] [Google Scholar]
- 73.Brem H, Ewend MG, Piantadosi S, et al. The safety of interstitial chemotherapy with BCNU-loaded polymer followed by radiation therapy in the treatment of newly diagnosed malignant gliomas: phase I trial. J Neurooncol. 1995;26(2):111–123. doi: 10.1007/BF01060217. [DOI] [PubMed] [Google Scholar]
- 74.Westphal M, Ram Z, Riddle V, et al. Gliadel wafer in initial surgery for malignant glioma: long-term follow-up of a multicenter controlled trial. Acta Neurochir (Wien) 2006;148(3):269–275. doi: 10.1007/s00701-005-0707-z. discussion 275. [DOI] [PubMed] [Google Scholar]
- 75.Newlands ES, Blackledge GR, Slack JA, et al. Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856) Br J Cancer. 1992;65(2):287–291. doi: 10.1038/bjc.1992.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pan E, Mitchell SB, Tsai JS. A retrospective study of the safety of BCNU wafers with concurrent temozolomide and radiotherapy and adjuvant temozolomide for newly diagnosed glioblastoma patients. J Neurooncol. 2008;88(3):353–357. doi: 10.1007/s11060-008-9576-7. [DOI] [PubMed] [Google Scholar]
- 77.Plowman J, Waud WR, Koutsoukos AD, et al. Preclinical antitumor activity of temozolomide in mice: efficacy against human brain tumor xenografts and synergism with 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res. 1994;54(14):3793–3799. [PubMed] [Google Scholar]
- 78.Cankovic M, Mikkelsen T, Rosenblum ML, et al. A simplified laboratory validated assay for MGMT promoter hypermethylation analysis of glioma specimens from formalin-fixed paraffin-embedded tissue. Lab Invest. 2007;87(4):392–397. doi: 10.1038/labinvest.3700520. [DOI] [PubMed] [Google Scholar]
- 79.Bock HC, Puchner MJ, Lohmann F, et al. First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience. Neurosurg Rev. 2010;33(4):441–449. doi: 10.1007/s10143-010-0280-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bota D, Taylor S. Retrospective analysis of temozolomide (TMZ) plus BCNU wafers and TMZ alone in the primary treatment of glioblastoma multiforme. J Clin Oncol. 2006;24:3644–3650. [Google Scholar]
- 81.Lechapt-Zalcman E, Levallet G, Dugue AE, et al. O(6) -methylguanine-DNA methyltransferase (MGMT) promoter methylation and low MGMT-encoded protein expression as prognostic markers in glioblastoma patients treated with biodegradable carmustine wafer implants after initial surgery followed by radiotherapy with concomitant and adjuvant temozolomide. Cancer. 2012;118(18):4545–4554. doi: 10.1002/cncr.27441. [DOI] [PubMed] [Google Scholar]
- 82.McGirt MJ, Than KD, Weingart JD, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110(3):583–588. doi: 10.3171/2008.5.17557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Menei P, Metellus P, Parot-Schinkel E, et al. Biodegradable carmustine wafers (Gliadel) alone or in combination with chemoradiotherapy: the French experience. Ann Surg Oncol. 2010;17(7):1740–1746. doi: 10.1245/s10434-010-1081-5. [DOI] [PubMed] [Google Scholar]
- 84.Miglierini P, Bouchekoua M, Rousseau B, et al. Impact of the per-operatory application of GLIADEL wafers (BCNU, carmustine) in combination with temozolomide and radiotherapy in patients with glioblastoma multiforme: efficacy and toxicity. Clin Neurol Neurosurg. 2012;114(9):1222–1225. doi: 10.1016/j.clineuro.2012.02.056. [DOI] [PubMed] [Google Scholar]
- 85.Noel G, Schott R, Froelich S, et al. Retrospective comparison of chemoradiotherapy followed by adjuvant chemotherapy, with or without prior gliadel implantation (carmustine) after initial surgery in patients with newly diagnosed high-grade gliomas. Int J Radiat Oncol Biol Phys. 2012;82(2):749–755. doi: 10.1016/j.ijrobp.2010.11.073. [DOI] [PubMed] [Google Scholar]
- 86.Salvati M, D'Elia A, Frati A, et al. Safety and feasibility of the adjunct of local chemotherapy with biodegradable carmustine (BCNU) wafers to the standard multimodal approach to high grade gliomas at first diagnosis. J Neurosurg Sci. 2011;55(1):1–6. [PubMed] [Google Scholar]
- 87.Thompson D, Lazio B, Lee S, et al. Retrospective review evaluating safety and survival of surgery, Gliadel, radiation, and temozolomide for glioblastoma multiforme; May 1–5; Philadelphia, PA. 2010. Presented at: the American Association of Neurological Surgeons; Abstract 64831. [Google Scholar]
- 88.Silvani A, Gaviani P, Lamperti E, et al. Phase II study: carmustine implant (Gliadel Wafer) plus adjuvant and concomitant temozolomide in combination with radiotherapy in primary glioblastoma patients; November 17–20; Orange County, CA. 2011. Presented at: the Society for Neuro-Oncology November 17–20, 2011. Neuro Oncol. 2011;13(11 suppl 3):Abstract NO-24. [Google Scholar]
- 89.LaRocca R, Vitaz T, Villanueva W, et al. A Phase 2 study of multimodal therapy with surgery, carmustine (BCNU) wafer, radiation therapy, and temozolomide in patients with newly diagnosed supratentorial malignant glioma. Presented at: the Eighth Congress of the European Association for Neuro-Oncology (EANO); September 12–14, 2008; Barcelona, Spain. Neuro Oncol. 2008;10(6):1172. Abstract P172] [Google Scholar]
- 90.Duntze J, Litre CF, Eap C, et al. Implanted carmustine wafers followed by concomitant radiochemotherapy to treat newly diagnosed malignant gliomas: prospective, observational, multicenter study on 92 cases. Ann Surg Oncol. 2013;20(6):2065–2072. doi: 10.1245/s10434-012-2764-x. [DOI] [PubMed] [Google Scholar]
- 91.Ryken T. Prospective multi-modality therapy for newly diagnosed glioblastoma with surgical resection, implantable BCNU chemotherapy and combination radiotherapy and temozolomide: long-term follow-up; November 17–20; Orange County, CA. 2011. Presented at: the Society for Neuro-Oncology Neuro Oncol. 2011;13(11 suppl 3):Abstract OT-13. [Google Scholar]
- 92.Affronti ML, Heery CR, Herndon JE, 2nd, et al. Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer. 2009;115(15):3501–3511. doi: 10.1002/cncr.24398. [DOI] [PubMed] [Google Scholar]
- 93.Asher A. Prospective analysis of temozolomide as adjuvant to Gliadel and radiation in newly diagnosed malignant glioma. Presented at: the Annual Meeting of the American Association of Neurological Surgeons; April 14–19; Washington, DC. 2007. Abstract 42860. [Google Scholar]
- 94.Ewelt C, Schroeteler J, Stummer W, et al. Therapeutic efficacy and toxicity of local (Gliadel Wafer) and systemic intensified chemotherapy 1 Week on/1 Week off in recurrent gliomas after surgical resection; September 12–14; Barcelona, Spain.. 2008. Presented at: the Eighth Congress of the European Association for Neuro-Oncology (EANO) Neuro Oncol. 2008;10(6):1063. Abstract O05. [Google Scholar]
- 95.Gururangan S, Cokgor L, Rich JN, et al. Phase I study of Gliadel wafers plus temozolomide in adults with recurrent supratentorial high-grade gliomas. Neuro Oncol. 2001;3(4):246–250. doi: 10.1093/neuonc/3.4.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Limentani SA, Asher A, Heafner M, et al. A phase I trial of surgery, Gliadel wafer implantation, and immediate postoperative carboplatin in combination with radiation therapy for primary anaplastic astrocytoma or glioblastoma multiforme. J Neurooncol. 2005;72(3):241–244. doi: 10.1007/s11060-004-2339-1. [DOI] [PubMed] [Google Scholar]
- 97.McPherson CM, Gerena-Lewis M, Breneman JC, et al. Results of phase I study of a multi-modality treatment for newly diagnosed glioblastoma multiforme using local implantation of concurrent BCNU wafers and permanent I-125 seeds followed by fractionated radiation and temozolomide chemotherapy. J Neurooncol. 2012;108(3):521–525. doi: 10.1007/s11060-012-0854-z. [DOI] [PubMed] [Google Scholar]
- 98.Quinn JA, Jiang SX, Carter J, et al. Phase II trial of Gliadel plus O6-benzylguanine in adults with recurrent glioblastoma multiforme. Clin Cancer Res. 2009;15(3):1064–1068. doi: 10.1158/1078-0432.CCR-08-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Salmaggi A, Milanesi I, Silvani A, et al. Prospective study of carmustine wafers in combination with 6-month metronomic temozolomide and radiation therapy in newly diagnosed glioblastoma: preliminary results. J Neurosurg. 2013;118(4):821–829. doi: 10.3171/2012.12.JNS111893. [DOI] [PubMed] [Google Scholar]
- 100.Sampath P, Sungarian A, Alderson L, et al. BCNU impregnated wafers (Gliadel) plus irinotecan (Camptosar) in combination treatment for patients with recurrent glioblastoma multiforme [abstract]; October 8–13, 2005; Boston, MA. Presented at: the Congress of Neurological Surgeons. [Google Scholar]
- 101.Smith KA, Ashby LS, Gonzalez LF, et al. Prospective trial of gross-total resection with Gliadel wafers followed by early postoperative Gamma Knife radiosurgery and conformal fractionated radiotherapy as the initial treatment for patients with radiographically suspected, newly diagnosed glioblastoma multiforme. J Neurosurg. 2008;109(Suppl):106–117. doi: 10.3171/JNS/2008/109/12/S17. [DOI] [PubMed] [Google Scholar]
- 102.Rezazadeh A, LaRocca R, Vitaz T, et al. A phase II study of multimodal therapy employing surgery, carmustine (BCNU) wafer, concomitant temozolomide and radiation, followed by dose dense therapy with temozolomide plus bevacizumab for newly diagnosed glioblastoma (GBM); November 17–20; Orange County, CA. 2011. Presented at: the Society for Neuro-Oncology Neuro Oncol. 2011;13(11 suppl 3):Abstract OT-22. [Google Scholar]
- 103.Weingart J, Grossman SA, Carson KA, et al. Phase I trial of polifeprosan 20 with carmustine implant plus continuous infusion of intravenous O6-benzylguanine in adults with recurrent malignant glioma: new approaches to brain tumor therapy CNS consortium trial. J Clin Oncol. 2007;25(4):399–404. doi: 10.1200/JCO.2006.06.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu L, Gerson SL. Targeted modulation of MGMT: clinical implications. Clin Cancer Res. 2006;12(2):328–331. doi: 10.1158/1078-0432.CCR-05-2543. [DOI] [PubMed] [Google Scholar]
- 105.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 106.Dixit S, Hingorani M, Achawal S, et al. The sequential use of carmustine wafers (Gliadel®) and post-operative radiotherapy with concomitant temozolomide followed by adjuvant temozolomide: a clinical review. Br J Neurosurg. 2011;25(4):459–469. doi: 10.3109/02688697.2010.550342. [DOI] [PubMed] [Google Scholar]
- 107.Sabel M, Giese A. Safety profile of carmustine wafers in malignant glioma: a review of controlled trials and a decade of clinical experience. Curr Med Res Opin. 2008;24(11):3239–3257. doi: 10.1185/03007990802508180. [DOI] [PubMed] [Google Scholar]
- 108.Attenello FJ, Mukherjee D, Datoo G, et al. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;15(10):2887–2893. doi: 10.1245/s10434-008-0048-2. [DOI] [PubMed] [Google Scholar]
- 109.Ulmer S, Spalek K, Nabavi A, et al. Temporal changes in magnetic resonance imaging characteristics of Gliadel wafers and of the adjacent brain parenchyma. Neuro Oncol. 2012;14(4):482–490. doi: 10.1093/neuonc/nos003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Colen RR, Zinn PO, Hazany S, et al. Magnetic resonance imaging appearance and changes on intracavitary Gliadel wafer placement: A pilot study. World J Radiol. 2011;3(11):266–272. doi: 10.4329/wjr.v3.i11.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Batchelor TT, Mulholland P, Neyns B, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–3218. doi: 10.1200/JCO.2012.47.2464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Armstrong TS, Wefel JS, Wang M, et al. Net clinical benefit analysis of radiation therapy oncology group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2013;31(32):4076–4084. doi: 10.1200/JCO.2013.49.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kunwar S, Chang S, Westphal M, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12(8):871–881. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Olivi A, Grossman SA, Tatter S, et al. Dose escalation of carmustine in surgically implanted polymers in patients with recurrent malignant glioma: a New Approaches to Brain Tumor Therapy CNS Consortium trial. J Clin Oncol. 2003;21(9):1845–1849. doi: 10.1200/JCO.2003.09.041. [DOI] [PubMed] [Google Scholar]