Abstract
Background
Diagnosis of WHO grade III anaplastic gliomas does not always correspond to its clinical outcome because of the isocitrate dehydrogenase (IDH) gene status. Anaplastic gliomas without IDH mutation result in a poor prognosis, similar to grade IV glioblastomas. However, the malignant features of anaplastic gliomas without IDH mutation are not well understood. The aim of this study was to examine anaplastic gliomas, in particular those without IDH mutation, with regard to their malignant features, recurrence patterns, and association with glioma stem cells.
Methods
We retrospectively analyzed 86 cases of WHO grade III anaplastic gliomas. Data regarding patient characteristics, recurrence pattern, and prognosis were obtained from medical records. We examined molecular alterations such as IDH mutation, 1p19q loss, TP53 mutation, MGMT promoter methylation, Ki67 labeling index, and CD133, SOX2, and NESTIN expression.
Results
Of the 86 patients with anaplastic gliomas, 58 carried IDH mutation, and 40 experienced recurrence. The first recurrence was local in 25 patients and distant in 15. Patients without IDH mutation exhibited significantly higher CD133 and SOX2 expression (P = .025 and .020, respectively) and more frequent distant recurrence than those with IDH mutation (P = .022).
Conclusions
Patients with anaplastic gliomas without IDH mutation experienced distant recurrence and exhibited glioma stem cell markers, indicating that this subset may share some malignant characteristics with glioblastomas.
Keywords: anaplastic gliomas, CD133, distant recurrence, grade III gliomas, IDH
Diagnosis of gliomas is based on histological features. Glioblastomas, WHO grade IV tumors, are highly malignant tumors of the brain characterized by the presence of microvascular proliferation and/or necrosis. Anaplastic gliomas, WHO grade III tumors, are less malignant than glioblastomas but show anaplasia and mitotic activity1 and are histologically subdivided into anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), and anaplastic oligoastrocytoma (AOA). Somatic mutations in isocitrate dehydrogenase (IDH) 1 and 22–4 genes occur at a very early stage of gliomagenesis;5 thus, patients with gliomas can be subdivided into those with and without IDH mutation.6–8 Overall, 59%–83% of patients with anaplastic gliomas carry IDH mutation, whereas 17%–41% do not.3,8 Patients with anaplastic gliomas with IDH mutation can be further subdivided into those carrying TP53 mutation and those carrying 1p19q loss,3,7–10 and their prognosis is favorable.3,9–11 On the other hand, those without IDH mutation carry a 7p (EGFR) gain3,10 and a 10q (PTEN) loss,3,10,11 and their prognosis is poor. Therefore, the question arises whether anaplastic gliomas with and without IDH mutation should be classified as the same grade III tumor.6,10,12,13 The clinical course of these 2 subtypes of anaplastic gliomas is not well understood; thus, their progression and recurrence mechanisms need to be elucidated.
The origin of glioblastomas is a controversial topic, but the presence of glioma stem cells has gained wide acceptance as a factor contributing to development of glioblastoma.14,15 Glioma stem cells are small subsets of tumor cells characterized by their potential for self-renewal that drives tumorigenesis.16 Among several markers reported, the cell surface protein CD133,14,15 SOX2,17 and NESTIN18 are cited frequently. Previous studies reported that high CD133 expression is associated with poor prognosis,19,20 early distant recurrence,21 dissemination,22 malignant progression,20 and a higher grade glioma.23 Furthermore, SOX2 and NESTIN are associated with poor prognosis and a higher grade glioma.18,24–26 Although most of these reports indicated that stem cell markers are often found in glioblastomas, their expression was also found in some anaplastic gliomas.17,18,20,23–27 Nonetheless, the correlation between stem cell markers and anaplastic gliomas, in particular anaplastic gliomas with and without IDH mutation, is not fully understood.
These findings led us to hypothesize that anaplastic gliomas may show a different recurrence pattern and a different stem cell marker expression pattern, depending upon their IDH status. In this study, we examined the recurrence pattern and stem cell marker expression pattern in anaplastic gliomas with and without IDH mutation to further elucidate the characteristics of anaplastic gliomas.
Materials and Methods
Patients and Samples
We previously reported the IDH status of 115 cases with grade III anaplastic gliomas.10 Among these, data from patients with protein of good quality were included in this study. Thus, all cases were reported previously. All patients were treated in the Department of Neurosurgery at Tohoku University. Clinical profiles and radiological features of each case were obtained from medical records. Tumor specimens were immediately frozen in liquid nitrogen and stored at −80°C until the extraction of genomic DNA and protein. The initial gadolinium (Gd)-enhanced MRI was used to determine radiological appearance. Tumors with well-demarcated borders were defined as focal, whereas those with diffuse appearance were defined as diffuse. Tumors exhibiting partial or strong Gd enhancement were defined as Gd-enhanced tumors. This retrospective study was conducted with the approval of the ethics committee of Tohoku University School of Medicine, and written informed consent was obtained from all patients.
Definition of Recurrence Patterns
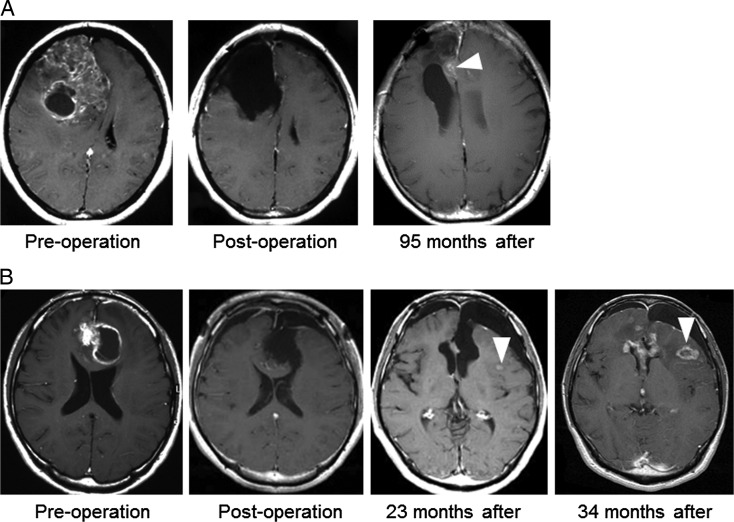
All patients underwent Gd-enhanced MRI within 3 days of their initial surgical procedure. Patient observation was performed using Gd-enhanced MRI every 2 months for the first 2 years, and every 3–6 months thereafter. Those with emergence of any new enhanced lesions on Gd-enhanced MRI were followed every 1–2 months thereafter. Some patients underwent 11C-methionine PET; once the enlargement of an enhanced lesion was confirmed, we recorded the first day of recurrence. We excluded from the list of recurrence cases those patients who underwent a second surgical procedure with a histopathological diagnosis of radiation necrosis or those in which there was no evidence of recurrence. If after repeated follow-up, regression of the enhanced lesion was observed, we also excluded such patients from recurrence cases. Recurrence patterns were subdivided into local recurrence and distant recurrence. Modifying the previously reported criteria for recurrence patterns,28,29 local recurrence was defined as a new enhanced lesion at, adjacent to, or contiguous with the primary site resection cavity (Fig. 1A), whereas distant recurrence was defined as a new enhanced lesion at a site remote from the resection cavity of the primary site, at a site not contiguous with the initial tumor location, or more than one lesion site with each lesion having a well-defined border and normal brain signal on imaging (Fig. 1B). The radiation field was also examined to determine whether a recurring lesion was infield or outfield.
Fig. 1.
Definition of a recurrence pattern. Representative gadolinium-enhanced MRI of anaplastic gliomas. (A) Local recurrence is illustrated in this case of anaplastic oligoastrocytoma with IDH mutation. Ninety-five months after the surgical procedure, local recurrence was found adjacent to the resection cavity (arrow). (B) Distant recurrence is illustrated in this case of anaplastic oligoastrocytoma without IDH mutation. Twenty-three months after the surgical procedure, an enhanced lesion was observed at a location distant from the initial lesion (arrow). Thirty-four months after, the enhanced lesion showed enlargement; thus, the case was confirmed as distant recurrence.
Molecular Analysis: IDH1 and IDH2, MGMT Promoter Methylation, TP53 Mutation, and 1p19q Loss
Genomic DNA was extracted using the QIAamp DNA Mini Kit (Qiagen Science) according to the manufacturer's protocol. Data on molecular changes in IDH1 and IDH2, exons 4–9 of TP53, MGMT promoter methylation, and 1p19q loss were obtained from our previous study of grade III gliomas.10 In brief, PCR amplification, methylation-specific PCR, and multiple-ligation–dependent probe amplification were performed to assess molecular alterations. The detailed methods were previously reported,10,30–32 and all primer sequences are shown in Supplementary Table 1.
CD133 Expression: Western Blotting
CD133 expression was analyzed using Western blotting, as reported previously.21 In brief, protein extracted from fresh-frozen tissue specimens was visualized on 8% polyacrylamide gels (Invitrogen) using the primary antibodies anti-CD133 (Miltenyi Biotec) and anti–β-actin (Santa Cruz Technology). The intensity of bands on Western blots was analyzed using ImageJ software (National Institutes of Health), and the ratio of CD133 to β-actin was determined for each tumor sample.
Immunohistochemical Analysis: CD133, SOX2, and NESTIN Expression and Ki-67 Labeling Index
To validate CD133 expression as analyzed using Western blotting, we performed parallel immunohistochemical staining for CD133, SOX2, and NESTIN. The results were compared with those of CD133 expression obtained using Western blotting. The methods for immunostaining were as reported previously.18,21,25 In brief, paraffin-embedded 2 μm-thick sections of human grade III gliomas were stained with an anti-CD133 antibody (CD133/1 AC133; Miltenyi Biotec), anti-SOX2 antibody (Millipore), or anti-NESTIN antibody (Millipore). All staining data were evaluated by 2 neuropathologists who were unaware of the clinical information and IDH status. The entire tissue section, excluding the healthy brain tissue, was semiquantitatively reviewed, and the percentage of CD133-positive cells was calculated.20 A semiquantitative score for SOX2 and NESTIN expression was calculated, all cell nuclei were counted in 3 randomly selected 400× magnification (high power) visual fields in the tumor core, and the proportion of cells positive for immunoreactivity in the nuclei (SOX2) or the cell membrane/cytoplasm (NESTIN) was calculated.17,24 Ki-67 labeling index was established by counting the percentage of immunoreactive nuclei stained by an anti–Ki-67 antibody (Dako) in a manner similar to the SOX2 and NESTIN assays described above.
Outcome
The start point for the overall survival (OS) was the day of the surgical procedure, and the endpoint was the last follow-up or death. As reported previously,21 the interval between the day of first surgical procedure and the day of recurrence detection on MRI was defined as time to recurrence. In particular, time to recurrence was further subdivided into time to distant recurrence (TTD) and time to local recurrence (TTL). To set the cutoff value for differentiating high and low CD133 expression in univariate survival analysis, the CD133/β-actin ratio from Western blotting was graded as ≥0.1 or <0.1, ≥0.2 or <0.2, ≥0.3 or <0.3, ≥0.4 or <0.4, and ≥0.5 or <0.5. Each cutoff was analyzed using logistic regression analyses, and the odds ratios (ORs) were compared.
Statistical Analysis
A relationship between 2 variables was evaluated using the Mann–Whitney U test and Fisher' exact test. Probability of TTD, TTL, and OS was calculated according to the Kaplan–Meier method and compared with the log-rank test. For multivariate analysis, factors achieving P < .10 in univariate analysis were used in backward stepwise Cox regression analysis for estimating hazard ratios (HRs) and 95% confidence intervals (CIs). Distant and local recurrences were considered competing events; thus, Fine-Gray proportional hazard models were used to analyze competing risks. All calculations were performed using Prism (GraphPad Software) and R version 3.0.2. Differences with P < .05 were considered significant.
Results
Patients
Protein of good quality was obtained from 86 participants with anaplastic gliomas, and all of them were enrolled in this study. This cohort comprised 46 males and 40 females with a median age of 46 years (range, 10–77 years) and a median preoperative KPS score of 90% (range, 20%–100%). Median follow-up was 56 months (range, 4–262 months). The number of participants with AA was 40, AO was 34, and AOA was 12. Among these, paraffin-embedded sections for immunohistochemical analysis were obtained from 54 participants. Table 1 summarizes participant characteristics. Postoperative treatment consisted of radiation alone in 7 participants, nimustine hydrochloride–based chemotherapy alone in 4, a combination of radiation and nimustine hydrochloride–based chemotherapy in 63, and a combination of radiation, and temozolomide-based chemotherapy in 8; 4 participants did not receive any chemotherapy.
Table 1.
Summary of patient characteristics based on IDH gene status
| Clinical Factor | Total | Mutated IDH | Wild-type IDH | P |
|---|---|---|---|---|
| n = 86 | n = 58 (67%) | n = 28 (33%) | ||
| Median age at diagnosis, year | 46 | 41 | 52 | .22a |
| Sex, female, n (%) | 40 (46) | 30 (52) | 10 (34) | .18b |
| Preoperative KPS ≥80%, n (%) | 69 (80) | 49 (84) | 20 (72) | .16b |
| Radiological pattern, diffuse: focal | 26:60 | 14:44 | 12:16 | .086b |
| Gd enhancement, yes: no | 63:23 | 44:14 | 19:9 | .44b |
| Number of recurrences, n (%) | 40 (47) | 18 (31) | 22 (79) | <.0001b |
| Recurrence pattern, local: distant | 25:15 | 15:3 | 10:12 | .022b |
| Recurrence area, infield: outfield | 30:10 | 17:1 | 13:9 | .013b |
| Ki-67 labeling index, mean (%) | 19.1 ± 14.5 | 16.4 ± 11.4 | 25.6 ± 18.6 | .020a |
| CD133/β-actin ratio (Western blots) | 0.32 ± 0.79 | 0.12 ± 0.19 | 0.74 ± 1.3 | .025a |
| Percentage of CD133 (IHC) | 5.8% ± 6.9% | 3.0% ± 4.4% | 11% ± 8.7% | .0014a |
| Sox2 expression (IHC) | 13.3% ± 17.3% | 10.5% ± 15.3% | 19.4% ± 39.6% | .020a |
| Nestin expression (IHC) | 10.0% ± 7.9% | 9.8% ± 8.0% | 10.5% ± 8.0% | .50a |
| MGMT methylation, n (%) | 62 (72) | 53 (91) | 9 (32) | <.0001b |
| 1p19q loss, n (%) | 24 (28) | 22 (38) | 2 (7.4) | .004b |
| TP53 mutation, n (%) | 37 (43) | 26 (45) | 11 (39) | .65b |
aMann-Whitney test,
bFisher' exact test.
Abbreviations: IHC, immunohistochemistry KPS; Karnofsky performance status.
IDH Mutation Frequency
Table 1 summarizes the frequency of clinical profiles based on the IDH status. IDH mutation was detected in 58 (67%; 53 IDH1 and 5 IDH2 mutations) of the 86 participants with anaplastic gliomas. The frequency of mutation in each tumor type was 67.5% (AA), 59% (AO), and 92% (AOA). Characteristics such as age, sex, and preoperative KPS did not differ between participants with IDH mutation and those without. Median follow-up was 69 months (range, 4–262 months) for participants with IDH mutation and 22 months (range, 7–134 months) for those without.
Molecular Alterations
MGMT promoter methylation, 1p19q loss, and TP53 mutation were detected in 62, 24, and 37 of the 86 participants, respectively (Table 1). MGMT promoter methylation and 1p19q loss were significantly associated with IDH mutation (P < .0001 and P = .004, respectively).
Recurrence Pattern
Of the 86 participants, 40 experienced tumor recurrence; tumors in 22 of 28 (79%) participants carried wild-type IDH, and tumors in 18 of 58 (31%) participants harbored mutated IDH, indicating that tumors with wild-type IDH had a significantly higher recurrence rate (P < .0001, Table 1). The pattern was local recurrence in 25 participants and distant recurrence in 15. Of the 15 participants with distant recurrence, 12 had a tumor with wild-type IDH (P = .022, Table 1). The remaining 46 participants did not experience recurrence. Of the 40 participants with recurrence, 30 showed infield recurrence, and 10 showed outfield recurrence. Among tumors with mutated IDH, only 1 of 18 participants experienced outfield recurrence, whereas 9 out of 22 participants having tumors with wild-type IDH experienced outfield recurrence; the difference was significant (P = .013, Table 1). Among tumors with wild-type IDH, MGMT methylation was observed in 4 of the 13 participants with infield recurrence and 3 of the 9 participants with outfield recurrence (P = 1.0, data not shown). Based on MRI, diffuse appearance was observed in 26 and Gd enhancement in 63 out of the 86 patients. Tumors with wild-type IDH tended to have a diffuse appearance, but this difference did not reach significance (P = .086, Table 1).
Expression of Stem Cell Markers
Figure 2A shows the representative Western blots for CD133 staining, and Fig. 2B–D shows the representative immunohistochemical staining for CD133, SOX2, and NESTIN, respectively. The mean CD133/β-actin ratio was 0.32 ± 0.79. When this ratio was combined with the results of the IDH status, participants with anaplastic gliomas without IDH mutation showed significantly higher CD133 expression than those with IDH mutation (0.74 ± 1.3 and 0.12 ± 0.19, respectively; P = .025; Table 1 and Fig. 3A). The mean percentage of cells with CD133 expression in the immunohistochemical assay was 5.8% ± 6.9%. When this parameter was combined with the results of the IDH status, participants with anaplastic gliomas without IDH mutation showed significantly higher CD133 expression than those with IDH mutation (11% ± 8.7% and 3.0% ± 4.4%, respectively; P = .0014; Table 1 and Fig. 3B).
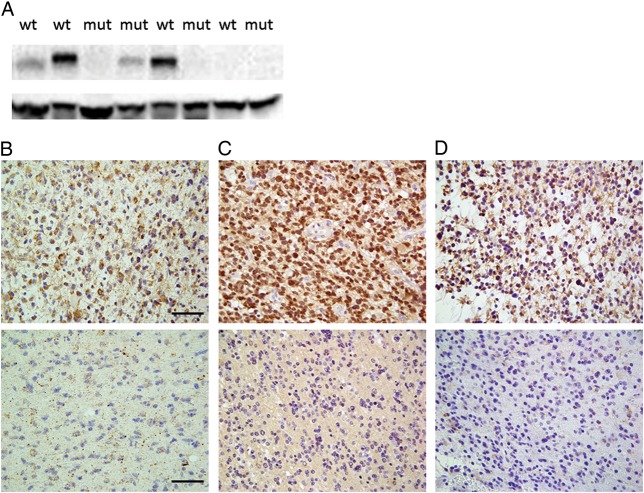
Fig. 2.
(A) Representative Western blots. The upper and lower bands show CD133 and β-actin expression (130 and 47 kDa, respectively). (B–D) Representative image of anaplastic glioma tissue sections immunohistochemically stained with an anti-CD133 antibody (B), anti-SOX2 antibody (C), or anti-NESTIN antibody (D). Positively stained cells are shown in brown. The upper panels illustrate a case with positively stained cells, and the lower panels illustrate a case with few positive cells. Scale bar, 40 μm. Abbreviations: mut, mutant IDH; wt, wild-type IDH.
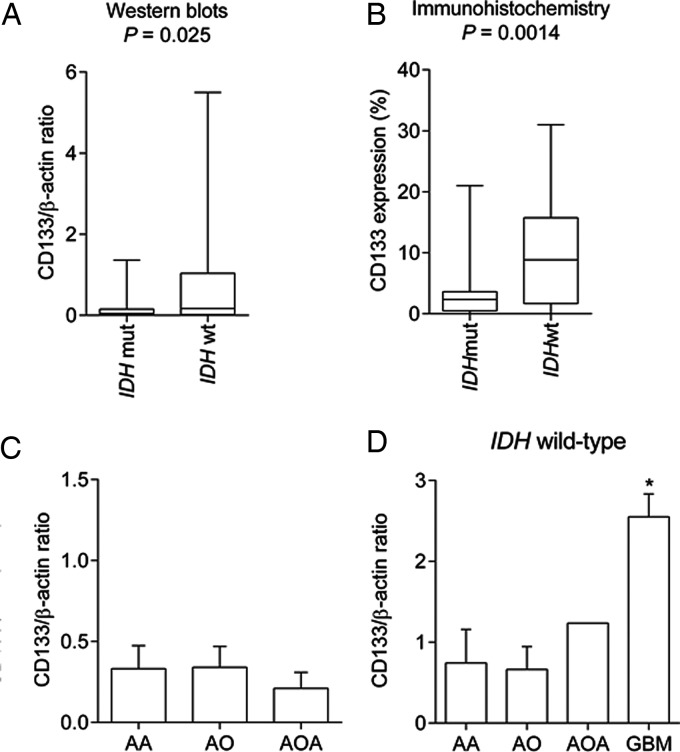
Fig. 3.
(A and B) The difference in CD133 expression between anaplastic gliomas with and without IDH mutation. (A) The CD133/β-actin ratio analyzed by Western blotting was higher in patients with anaplastic gliomas without IDH mutation (IDH wt) than patients with IDH mutation (IDH mut, P = .025). (B) CD133 expression analyzed by immunohistochemistry was higher in patients with anaplastic gliomas without IDH mutation than patients with IDH mutation (P = .0014). (C) The difference in CD133 expression among different histological types of anaplastic gliomas. The CD133/β-actin ratio was compared among patients with anaplastic astrocytoma (AA; n = 40), anaplastic oligodendroglioma (AO; n = 34), and anaplastic oligoastrocytoma (AOA; n = 12); however, no differences were observed. (D) The CD133/β-actin ratio was compared among patients with AA (n = 13), AO (n = 15), and AOA (n = 1) without IDH mutation and glioblastomas without IDH mutation (n = 109 ( data modified from our previous report21). Glioblastomas showed significantly higher CD133 expression (P < .0001).
We assessed other stem cell markers for gliomas in addition to CD133. SOX2 expression was higher in participants without IDH mutation than in those with IDH mutation (10.5% ± 15.3% and 19.4% ± 39.6% respectively; P = .020; Table 1), but NESTIN expression did not correlate with the IDH status (P = .50; Table 1). Thus, anaplastic gliomas without IDH mutation showed high CD133 expression, which was confirmed by 2 methods (Western blotting and immunohistochemistry), and high expression of stem cell markers, which was confirmed using 2 markers (CD133 and SOX2).
Next, we analyzed the differences in CD133/β-actin ratio in relation to several factors. As shown in Table 2, the recurrence pattern and molecular alterations were not associated with the CD133/β-actin ratio, whereas tumors without Gd enhancement exhibited a higher CD133/β-actin ratio than those with Gd enhancement (P = .0082).
Table 2.
Summary of patients characteristics based on CD133 expression
| Clinical Factor | Total | CD133/β-actin Ratio | P |
|---|---|---|---|
| n = 86 | n = 58 (67%) | ||
| Radiological pattern, diffuse: focal | 26:60 | 0.30 ± 0.74:0.33 ± 0.82 | .66a |
| Gd enhancement, yes: no | 63:23 | 0.22 ± 0.58:0.59 ± 1.2 | .0082a |
| Recurrence pattern, local: distant | 25:15 | 0.36 ± 0.76:0.77 ± 1.4 | .46a |
| Recurrence area, infield: outfield | 30:10 | 0.28 ± 0.54:0.43 ± 0.54 | .84a |
| Ki-67 labeling index, <15%: ≥15% | 35:51 | 0.14 ± 0.24:0.45 ± 1.0 | .48a |
| MGMT methylation | 62:24 | 0.16 ± 0.29:0.73 ± 1.4 | .14a |
| 1p19q, loss: retain | 24:62 | 0.08 ± 0.09:0.42 ± 0.91 | .072a |
| TP53, mutated: wild-type | 37:49 | 0.46 ± 1.0:0.22 ± 0.55 | .18a |
aMann-Whitney test.
CD133 Expression Based on Histological Types
We compared CD133 expression on the basis of the histological types. In anaplastic gliomas, the CD133/β-actin ratio did not differ among histological types even when IDH status was considered (Fig. 3C and D). In addition, we analyzed the CD133/β-actin ratio of glioblastoma using data from our previous report.21 This ratio was found to be 2.6 ± 3.0, which was significantly greater than that in other anaplastic gliomas without IDH mutation (P < .0001, Fig. 3D).
Outcome
The cutoff value for distinguishing high and low CD133 expression varied among studies.20,24,33,34 In our study, ORs were examined to determine the optimal cutoff value for patients with anaplastic gliomas. Among the analyzed cutoff values from 0.1 to 0.5, the OR of CD133/β-actin ( ≥0.5 or <0.5) was the highest (OR, 4.2; 95% CI,1.8–9.6; P = .00073). Therefore, 0.5 was selected as the cutoff value for the CD133/β-actin ratio. In univariate analysis, participants with high CD133 expression (CD133/β-actin ratio ≥0.5) presented significantly shorter OS, TTD, and TTL than those with low CD133 expression (CD133/β-actin ratio <0.5; P = .0006, .0009, and .022 respectively; Table 3). Of 10 participants with high CD133 expression, 9 had a tumor with wild-type IDH.
Table 3.
Clinical and genetic parameters affecting time to distant recurrence, time to local recurrence, and overall survival in anaplastic glioma
| Parameters | Patients (n = 86) | TTD |
TTL |
OS |
|||
|---|---|---|---|---|---|---|---|
| Median Months | P* | Median Months | P* | Median Months | P* | ||
| Age at diagnosis | |||||||
| <60 years | 70 | NR | .75 | 187 | .15 | NR | .058 |
| ≥60 years | 16 | NR | 51 | 69 | |||
| Preoperative KPS | |||||||
| ≥80% | 69 | NR | .43 | 187 | .55 | NR | .25 |
| <80% | 17 | NR | NR | 87 | |||
| Surgery | |||||||
| Total resection | 38 | NR | .14 | NR | .13 | NR | .014 |
| Absence of total resection | 48 | NR | 187 | 72 | |||
| Radiological appearance | |||||||
| Diffuse | 26 | NR | .57 | 52 | .017 | 50 | .0035 |
| Focal | 60 | NR | 187 | NR | |||
| Gd enhancement | |||||||
| Yes | 63 | NR | .57 | 187 | .66 | 136 | .75 |
| No | 23 | NR | NR | NR | |||
| Ki-67 labeling index | |||||||
| <15% | 35 | NR | .0028 | NR | .47 | 136 | .16 |
| ≥15% | 45 | NR | 187 | 76 | |||
| CD133 expression | |||||||
| Low | 76 | NR | .0009 | 187 | .022 | NR | .0006 |
| High | 10 | 23 | 32 | 29 | |||
| IDH1 | |||||||
| Mutated | 58 | NR | <.0001 | 187 | .0013 | NR | <.0001 |
| Wild-type | 28 | 23 | 22 | 22 | |||
| MGMT | |||||||
| Methylated | 62 | NR | .0011 | 187 | .31 | NR | .033 |
| Unmethylated | 24 | NR | NR | 44 | |||
| 1p19q loss | |||||||
| No | 62 | NR | .24 | 95 | .0026 | 69 | .0088 |
| Yes | 24 | NR | 187 | NR | |||
| TP53 mutation | |||||||
| No | 49 | NR | .83 | 187 | .18 | NR | .35 |
| Yes | 37 | NR | NR | 72 | |||
Abbreviations: KPS, Karnofsky performance status; NR, not reached; OS, overall survival; TTD, time to distant recurrence; TTL,time to local recurrence.
*Log-rank test.
Next, we assessed OS, TTD, and TTL in relation to other parameters. In univariate analysis, factors associated with prolonged OS were low CD133 expression (P = .0006), total resection (P = .014), IDH mutation (P < .0001), 1p19q loss (P = .0088), MGMT methylation (P = .033), and focal radiological appearance (P = .0035; Table 1). Factors associated with early TTD were high CD133 expression (P = .0009), Ki-67 labeling index ≥15% (P = .0028), IDH wild-type (P < .0001), and unmethylated MGMT (P = .0011; Table 1). The factors associated with early TTL were high CD133 expression (P = .022), wild-type IDH (P = .0013), retained 1p19q (P = .0026), and diffuse radiological appearance (P = .017; Table 1). In multivariate analysis for OS, independent poor prognostic factors were wild-type IDH (HR, 8.7; 95% CI, 3.1–24.0; P = .00003), absence of total resection (HR, 2.5; 95% CI, 1.0–6.1; P = .040), unmethylated MGMT (HR, 3.0; 95% CI, 1.2–7.4; P = .023), and retained 1p19q (HR, 3.2; 95% CI, 1.2–8.9; P = .026; data not shown).
In multivariate analysis for TTD considering competing risks, wild-type IDH (HR 6.6; 95% CI, 1.3–34.7; P = .025) and Ki-67 labeling index ≥15% (HR, 8.6; 95% CI, 1.3–58.7; P = .029) were independent poor prognostic factors (Table 3). In multivariate analysis for TTL considering competing risks, only retained 1p19q (HR, 3.9; 95% CI, 1.3–11.7; P = .016) remained statistically significant (Table 4).
Table 4.
Multivariate analysis of independent prognostic factors associated with time to distance recurrence and time to local recurrence. Result of competing risk analyses.
| Parameters | TTD |
TTL |
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Radiological appearance | ||||||
| Diffuse vs focal | N/A | 2.1 | 0.96–4.7 | .064 | ||
| Ki67 labeling index | ||||||
| ≥15% vs <15% | 8.6 | 1.3–58.7 | .029 | N/A | ||
| CD133 expression | ||||||
| High vs low | 1.0 | 0.30–3.6 | .96 | 1.4 | 0.49–4.3 | .51 |
| IDH | ||||||
| Wild-type vs mutated | 6.6 | 1.3–34.7 | .025 | 0.89 | 0.35–2.2 | .80 |
| MGMT | ||||||
| Unmethylated vs methylated | 1.4 | 0.36–5.3 | .64 | N/A | ||
| 1p19q | ||||||
| Retain vs loss | N/A | 3.9 | 1.3–11.7 | .016 | ||
Abbreviations: CI, confidence interval; HR, hazard ratio; N/A: not applicable; TTD, time to distant recurrence; TTL,time to local recurrence.
Discussion
We analyzed the recurrence pattern and molecular alterations including stem cell markers of anaplastic gliomas. The main pattern of initial recurrence in anaplastic gliomas was local recurrence (25 of 40 participants; 63%); however, distant recurrence was observed in 15 participants (37%). A previous report also showed that distant recurrence was frequently observed (23 of 38 patients with high-grade gliomas),35 which was in line with our results. Of 15 patients with distant recurrence, 12 had anaplastic gliomas with wild-type IDH. Thus, distant recurrence should be considered in the clinical course of patients who have anaplastic gliomas with wild-type IDH. Brandes et al. reported that the recurrence pattern of glioblastomas, whether inside or outside the radiation field, correlated strongly with MGMT methylation status.36 Accordingly, we also examined this correlation, but distant recurrence of anaplastic gliomas without IDH mutation did not correlate with either radiation field status or MGMT methylation status. We next assessed the time to recurrence. In univariate analysis, wild-type IDH is associated with early TTD; in multivariate analysis, however, wild-type IDH is an independent poor prognostic factor for TTD. These results suggest that anaplastic gliomas without IDH mutation carry a significant risk of distant recurrence, which corresponds to an aggressive phenotype and results in poor prognosis.
At present, the diagnosis of anaplastic gliomas does not always correspond to its clinical outcome because of considerable interobserver variation37 and IDH status. Hartmann et al. reported that patients with AA without IDH mutation exhibited better survival than those with glioblastomas without IDH mutation but had poorer survival than patients with AA and IDH mutation and patients with glioblastomas and IDH mutation.13 Thus, they proposed that aggressive treatment corresponding to the current standard of care for glioblastomas may be appropriate for patients with AA without IDH mutation, whereas less aggressive treatment may be suitable for patients with primary glioblastomas and IDH mutation. Nevertheless, evidence supporting this concept remains limited. In line with previous reports,20,23,24 our results also demonstrated the presence of CD133 expression in anaplastic gliomas. Of note, we showed that its expression is remarkably evident in tumors without IDH mutation. Using another stem cell marker, SOX2, we also confirmed that tumors without IDH mutation are likely to have high SOX2 expression. Thus, patients with anaplastic gliomas without IDH mutation express CD133 and SOX2, as do patients with glioblastomas,20,21,25 indicating that anaplastic gliomas without IDH mutation may share similar malignant characteristics (ie, glioma stem cell resistance to radiochemotherapy).38,39 In terms of NESTIN expression, we did not observe differences between tumors with and without IDH mutation.
We analyzed the CD133/β-actin ratio in relation to histological types. A previous report showed that CD133 expression is a prognostic factor in patients with high-grade oligodendroglioal tumors.40 Thus, we hypothesized that patients with different histological types may exhibit different CD133 expression. Nonetheless, CD133 expression did not differ by histological type, even considering the IDH status. Using data from our previous reports,21 we compared CD133 expression between anaplastic gliomas without IDH mutation and glioblastomas without IDH mutation. CD133 expression of glioblastomas was significantly higher than that of anaplastic gliomas without an IDH mutation (Fig. 3D). This result may support the concept that anaplastic gliomas without IDH mutation share some malignant characteristics of glioblastoma, but these 2 tumor types differ in malignancy of glioma stem cells.
Next, we assessed the correlation among molecular alterations including IDH status, CD133 expression, and radiographical appearance. Diffuse appearance was not associated with IDH status and CD133 expression. Gd enhancement was not associated with IDH status but was associated with low CD133 expression. An unknown mechanism may be responsible for these correlations, and further studies are needed. The status of 1p19q, TP53, MGMT, and Ki67 labeling index did not correlate with CD133 expression.
There are some limitations to the study. First, the number of participants was relatively small, and not all participants received the same treatment since this was a retrospective study. Thus, our findings need to be confirmed prospectively with a larger number of participants. Second, there are no universal methods for analysis of stem cell markers. Western blotting,17,21,23 PCR,17,26,27,34,41 immunohistochemistry,17,18,20,26,27,42 and flow cytometry42,43 are often used to assess CD133, SOX2, and NESTIN expression. To overcome this limitation, we assessed expression of 3 stem cell markers (CD133, SOX2, and NESTIN) and used 2 methods (Western blotting and immunohistochemistry) in this study.
For the first time, we demonstrate that anaplastic gliomas without IDH mutation carry a risk of distant recurrence and are likely to have high CD133 expression. Thus, anaplastic gliomas without IDH mutation may differ in clinical characteristics from anaplastic gliomas with IDH mutation. Furthermore, these findings suggest that anaplastic gliomas without IDH mutation may originate from glioma stem cells or have malignant characteristics similar to those of glioblastomas. Anaplastic gliomas without IDH mutation have poor prognosis, but the histological features and the extent of CD133 expression of anaplastic gliomas and glioblastomas are different; thus, these tumor types should not be considered identical. Collectively, because of their aggressive characteristics, anaplastic gliomas without IDH mutation may require aggressive treatment and should be considered separately from anaplastic gliomas with IDH mutation and from glioblastoma in future clinical trials. To confirm our findings, larger studies of clinical and molecular features of anaplastic gliomas without IDH mutation are needed.
Supplementary material
Funding
This work was supported in part by Grants-in-Aid for Cancer Research from the Ministry of Health and Welfare in Japan to T. T.
Supplementary Material
Acknowledgments
We would like to thank M. Fue for assistance in extracting protein and Western blotting and Enago (www.enago.jp) for the English language review.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balss J, Meyer J, Mueller W, et al. Analysis of the IDH1 codon 132 mutation in brain tumors. Acta Neuropathol. 2008;116(6):597–602. doi: 10.1007/s00401-008-0455-2. [DOI] [PubMed] [Google Scholar]
- 3.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe T, Nobusawa S, Kleihues P, et al. IDH1 mutations are early events in the development of astrocytomas and oligodendrogliomas. Am J Pathol. 2009;174(4):1149–1153. doi: 10.2353/ajpath.2009.080958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan H, Bigner DD, Velculescu V, et al. Mutant metabolic enzymes are at the origin of gliomas. Cancer Res. 2009;69(24):9157–9159. doi: 10.1158/0008-5472.CAN-09-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ohgaki H, Kleihues P. Genetic alterations and signaling pathways in the evolution of gliomas. Cancer Sci. 2009;100(12):2235–2241. doi: 10.1111/j.1349-7006.2009.01308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11(4):341–347. doi: 10.1215/15228517-2009-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukasa A, Takayanagi S, Saito K, et al. Significance of IDH mutations varies with tumor histology, grade, and genetics in Japanese glioma patients. Cancer Sci. 2012;103(3):587–592. doi: 10.1111/j.1349-7006.2011.02175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shibahara I, Sonoda Y, Kanamori M, et al. IDH1/2 gene status defines the prognosis and molecular profiles in patients with grade III gliomas. Int J Clin Oncol. 2012;17(6):551–561. doi: 10.1007/s10147-011-0323-2. [DOI] [PubMed] [Google Scholar]
- 11.van den Bent MJ, Dubbink HJ, Marie Y, et al. IDH1 and IDH2 mutations are prognostic but not predictive for outcome in anaplastic oligodendroglial tumors: a report of the European Organization for Research and Treatment of Cancer Brain Tumor Group. Clin Cancer Res. 2010;16(5):1597–1604. doi: 10.1158/1078-0432.CCR-09-2902. [DOI] [PubMed] [Google Scholar]
- 12.Shibahara I, Sonoda Y, Kanamori M, et al. New insights into glioma classification based on isocitrate dehydrogenase 1 and 2 gene status. Brain Tumor Pathol. 2011;28(3):203–208. doi: 10.1007/s10014-011-0050-4. [DOI] [PubMed] [Google Scholar]
- 13.Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120(6):707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 14.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63(18):5821–5828. [PubMed] [Google Scholar]
- 15.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432(7015):396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 16.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414(6859):105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz M, Temme A, Senner V, et al. Identification of SOX2 as a novel glioma-associated antigen and potential target for T cell-based immunotherapy. Br J Cancer. 2007;96(8):1293–1301. doi: 10.1038/sj.bjc.6603696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arai H, Ikota H, Sugawara K, et al. Nestin expression in brain tumors: its utility for pathological diagnosis and correlation with the prognosis of high-grade gliomas. Brain Tumor Pathol. 2012;29(3):160–167. doi: 10.1007/s10014-012-0081-5. [DOI] [PubMed] [Google Scholar]
- 19.Yan X, Ma L, Yi D, et al. A CD133-related gene expression signature identifies an aggressive glioblastoma subtype with excessive mutations. Proc NatlAcad Sci USA. 2011;108(4):1591–1596. doi: 10.1073/pnas.1018696108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeppernick F, Ahmadi R, Campos B, et al. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin Cancer Res. 2008;14(1):123–129. doi: 10.1158/1078-0432.CCR-07-0932. [DOI] [PubMed] [Google Scholar]
- 21.Shibahara I, Sonoda Y, Saito R, et al. The expression status of CD133 is associated with the pattern and timing of primary glioblastoma recurrence. Neuro Oncol. 2013;15(9):1151–1159. doi: 10.1093/neuonc/not066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato A, Sakurada K, Kumabe T, et al. Association of stem cell marker CD133 expression with dissemination of glioblastomas. Neurosurg Rev. 2010;33(2):175–183. doi: 10.1007/s10143-010-0239-8. [DOI] [PubMed] [Google Scholar]
- 23.Thon N, Damianoff K, Hegermann J, et al. Presence of pluripotent CD133+ cells correlates with malignancy of gliomas. Mol Cell Neurosci. 2010;43(1):51–59. doi: 10.1016/j.mcn.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 24.Zhang M, Song T, Yang L, et al. Nestin and CD133: valuable stem cell-specific markers for determining clinical outcome of glioma patients. J Exp Clin Cancer Res. 2008;27:85. doi: 10.1186/1756-9966-27-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Annovazzi L, Mellai M, Caldera V, et al. SOX2 expression and amplification in gliomas and glioma cell lines. Cancer Genomics & Proteomics. 2011;8(3):139–147. [PubMed] [Google Scholar]
- 26.Galatro TF, Uno M, Oba-Shinjo SM, et al. Differential expression of ID4 and its association with TP53 mutation, SOX2, SOX4 and OCT-4 expression levels. PloS One. 2013;8(4):e61605. doi: 10.1371/journal.pone.0061605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strojnik T, Rosland GV, Sakariassen PO, et al. Neural stem cell markers, nestin and musashi proteins, in the progression of human glioma: correlation of nestin with prognosis of patient survival. Surg Neurol. 2007;68(2):133–143. doi: 10.1016/j.surneu.2006.10.050. [DOI] [PubMed] [Google Scholar]
- 28.Pope WB, Xia Q, Paton VE, et al. Patterns of progression in patients with recurrent glioblastoma treated with bevacizumab. Neurology. 2011;76(5):432–437. doi: 10.1212/WNL.0b013e31820a0a8a. [DOI] [PubMed] [Google Scholar]
- 29.Chamberlain MC. Radiographic patterns of relapse in glioblastoma. J Neurooncol. 2011;101(2):319–323. doi: 10.1007/s11060-010-0251-4. [DOI] [PubMed] [Google Scholar]
- 30.Sonoda Y, Kumabe T, Watanabe M, et al. Long-term survivors of glioblastoma: clinical features and molecular analysis. Acta Neurochir (Wien) 2009;151(11):1349–1358. doi: 10.1007/s00701-009-0387-1. [DOI] [PubMed] [Google Scholar]
- 31.Sonoda Y, Kumabe T, Nakamura T, et al. Analysis of IDH1 and IDH2 mutations in Japanese glioma patients. Cancer Sci. 2009;100(10):1996–1998. doi: 10.1111/j.1349-7006.2009.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sonoda Y, Yokosawa M, Saito R, et al. O (6)-Methylguanine DNA methyltransferase determined by promoter hypermethylation and immunohistochemical expression is correlated with progression-free survival in patients with glioblastoma. Int J Clin Oncol. 2010;15(4):352–358. doi: 10.1007/s10147-010-0065-6. [DOI] [PubMed] [Google Scholar]
- 33.Joo KM, Kim SY, Jin X, et al. Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas. Lab Invest. 2008;88(8):808–815. doi: 10.1038/labinvest.2008.57. [DOI] [PubMed] [Google Scholar]
- 34.Metellus P, Nanni-Metellus I, Delfino C, et al. Prognostic impact of CD133 mRNA expression in 48 glioblastoma patients treated with concomitant radiochemotherapy: a prospective patient cohort at a single institution. Ann Surg Oncol. 2011;18(10):2937–2945. doi: 10.1245/s10434-011-1703-6. [DOI] [PubMed] [Google Scholar]
- 35.Zuniga RM, Torcuator R, Jain R, et al. Efficacy, safety and patterns of response and recurrence in patients with recurrent high-grade gliomas treated with bevacizumab plus irinotecan. J Neurooncol. 2009;91(3):329–336. doi: 10.1007/s11060-008-9718-y. [DOI] [PubMed] [Google Scholar]
- 36.Brandes AA, Tosoni A, Franceschi E, et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation With MGMT promoter methylation status. J Clin Oncol. 2009;27(8):1275–1279. doi: 10.1200/JCO.2008.19.4969. [DOI] [PubMed] [Google Scholar]
- 37.van den Bent MJ. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: a clinician's perspective. Acta Neuropathol. 2010;120(3):297–304. doi: 10.1007/s00401-010-0725-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 39.Murat A, Migliavacca E, Gorlia T, et al. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 2008;26(18):3015–3024. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 40.Beier D, Wischhusen J, Dietmaier W, et al. CD133 expression and cancer stem cells predict prognosis in high-grade oligodendroglial tumors. Brain Pathol. 2008;18(3):370–377. doi: 10.1111/j.1750-3639.2008.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshimoto K, Ma X, Guan Y, et al. Expression of stem cell marker and receptor kinase genes in glioblastoma tissue quantified by real-time RT-PCR. Brain Tumor Pathol. 2011;28(4):291–296. doi: 10.1007/s10014-011-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pallini R, Ricci-Vitiani L, Montano N, et al. Expression of the stem cell marker CD133 in recurrent glioblastoma and its value for prognosis. Cancer. 2011;117(1):162–174. doi: 10.1002/cncr.25581. [DOI] [PubMed] [Google Scholar]
- 43.Beier D, Hau P, Proescholdt M, et al. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 2007;67(9):4010–4015. doi: 10.1158/0008-5472.CAN-06-4180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.