Abstract
Background
According to the Response Assessment in Neuro-Oncology criteria, new enhancement within the radiation field on contrast enhanced T1-weighted images within 12 weeks after completion of radiotherapy should not qualify for progressive disease, since up to 50% of these cases may be pseudoprogression (PsP). To validate this concept, we assessed incidence and overall survival (OS) of patients with suspected and confirmed PsP dependent on different time intervals and definitions of PsP.
Methods
Patients with newly diagnosed glioblastoma and an enhancement increase of at least 25% after completion of standard radiochemotherapy at month 1, 4, 7, or 10 were eligible. Based on the development of the enhancement in follow-up examinations, patients were categorized as either PsP (subgrouped as complete resolution/decrease >50% and decrease <50%/stable) or true progression.
Results
Out of 548 patients, 79 fulfilled the inclusion criteria. Of these 79 patients, 9 (11.4%) showed PsP (6/45 patients at 1 month, 2/17 at 4 months, 1/9 at 7 months, and 0/8 at 10 months). Complete resolution of the enhancement was found in 1, decrease >50% in 3, decrease <50% in 2, and stable enhancement in 3 patients with PsP. Patients with PsP showed a significantly longer OS (P < .012). No difference in OS was found among PsP subgroups.
Conclusions
This series challenges the current concept of PsP. Even though we could confirm a prolonged OS of patients with PsP, the incidence of PsP was lower than reported previously and extended beyond 12 weeks.
Keywords: glioblastoma, pseudoprogression
One of the major changes in the Response Assessment in Neuro-Oncology (RANO) criteria1 is the recognition of a radiographic phenomenon coined pseudoprogression (PsP). PsP refers to new contrast enhancement following radiochemotherapy within the radiation field that eventually subsides without any change in therapy (Fig. 1).1 It is reported to occur more frequently under radiotherapy with temozolomide.2
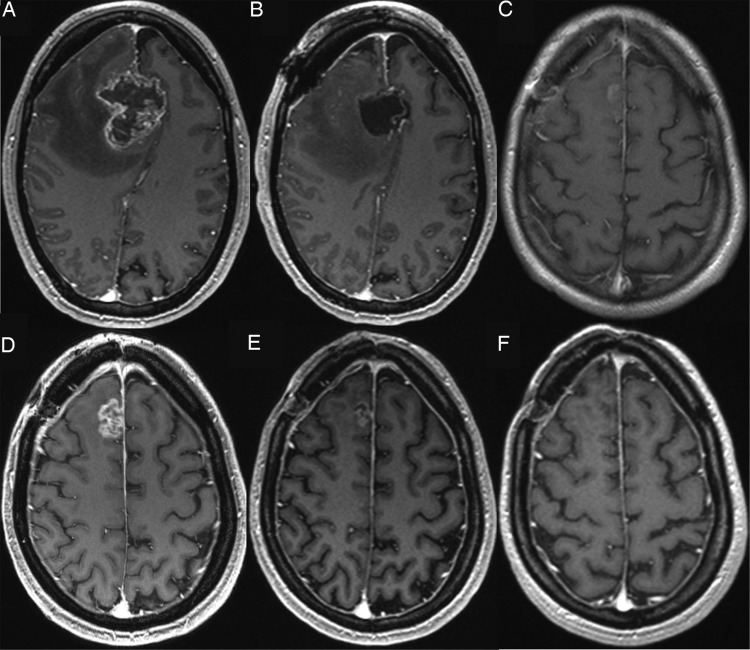
Fig. 1.
A 39-year-old female patient with glioblastoma in the right frontal lobe. Contrast enhanced T1-weighted MRI (A) before and (B) after surgery. (C) First f/u 1 month after completion of radiotherapy shows only minimal enhancement. (D) Enhancement increase of at least 25% appears in the second f/u 4 months after completion of radiochemotherapy. After (E) 7 months the enhancement was rarely visible and disappeared totally after (F) 10 months.
Even with advanced imaging techniques, it is currently not possible to differentiate true progression and PsP reliably.3–10 Hence, final diagnosis can only be achieved by histopathological verification or further follow-up examination (f/u). To address this issue, the RANO criteria suggest that “within the first 12 weeks of completion of radiotherapy, progression can only be determined if the majority of the new enhancement is outside of the radiation field or if there is pathologic confirmation of progressive disease.”1
Although PsP has been reported in up to 50%,2,11 the inclusion of PsP in the RANO criteria has not been based on large patient series with clearly defined imaging and read-out criteria.8,12 Furthermore, there is no consensus on whether the term “PsP” should also include cases of stable enhancement in the subsequent f/u or should be exclusively used for a decrease or a complete resolution of the initial enhancement.
The aim of this study was to determine the incidence of PsP at different time points and different degrees of enhancement decrease in a large patient series. Additionally, overall survival (OS) of patients with true progression and PsP was compared among subgroups. Finally it was evaluated whether changes on T2-weighted images can differentiate true progression and PsP.
Materials and Methods
Patient Selection Criteria
This retrospective study was approved by the local ethical committee of the University of Heidelberg. All patients were treated at the Medical Center in Heidelberg and had consented to MRI and therapy according to German regulations. Five hundred forty-eight subsequent patients treated for newly diagnosed glioblastoma between January 1, 2007 and August 31, 2012 were eligible for this study. Only patients treated with standard radiochemotherapy according to Stupp and colleagues13 aged 18 years or older with a postoperative baseline scan within 72 h after surgery and regular MRI scans done until enhancement increase on T1-weighted MR images were included. Patients who received additional antiangiogenic medication were excluded.
To qualify for true progression or PsP, patients had to present an enhancement increase of at least 25% of an original lesion with ≥10 mm of perpendicular diameters or a new nodular component ≥10 mm within the radiation field in the first, second, third, or fourth f/u compared with the baseline examination. Patients who did not present an enhancement increase within 1 year after completion of radiotherapy and patients who presented an enhancement increase of <25% or an enhancement outside the radiation field were excluded. Inclusion and exclusion criteria are summarized in Table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria |
|
| Exclusion criteria |
|
| Included patients | Total: 79 patients with enhancement increase of at least 25% in the first, second, third, or fourth postradiation scan |
*Numbers in parentheses are excluded patients.
Finally, corticosteroid dosage at the time of the f/u with 25% enhancement increase was assessed by chart review for each included patient.
Imaging
MRI was performed on a 3-Tesla scanner (Trio and Verio, Siemens) or a 1.5-Tesla scanner (Symphony, Siemens). The standard MRI protocol included an axial T1-weighted sequence (repetition time [TR], 400 ms; echo time [TE], 15 ms; section thickness, 5 mm; field of view [FOV], 230 mm) or alternatively, a T1-weighted 3D magnetization-prepared rapid acquisition with gradient echo sequence (TR, 1740 ms; TE, 3.45 ms; slice thickness, 1.0 mm; FOV, 250 mm) before and after application of contrast agent, a T2-weighted sequence (TR, 4890 ms; TE, 85 ms; slice thickness, 5 mm; FOV, 230 mm), or a fluid attenuated inversion recovery (FLAIR) sequence (TR, 8500 ms; TE, 85 ms; inversion time, 2400 ms; slice thickness, 5 mm; FOV, 230 mm).
Categorization as True Progression or Pseudoprogression
All included patients were categorized as either true progression or PsP on f/u MRI. Patients were categorized as true progression if they presented a further increase of the initial 25% enhancement increase within the next 2 f/u's or if the enhancement was histologically confirmed as tumor progression. In fatal outcomes within 6 months after the initial enhancement increase due to tumor-related deterioration, patients were categorized as true progression.
Patients were classified as PsP if the initial enhancement increase did not increase further within the next 2 f/u's, if the enhancement decreased within the next 2 f/u's, or if histological assessment did not reveal any tumor cells. Enhancement decrease was further classified as decrease less than 50% (PsP < 50%) or more than 50% (PsP > 50%) or as complete resolution of enhancement compared with the initial enhancement increase.
Furthermore, T2-weighted or FLAIR images at baseline and at the f/u that displayed a 25% enhancement increase were assessed. The development of the T2 signal was categorized as T2 increase >25%, stable T2 signal, or T2 signal decrease.
All MRI scans were assessed independently by 2 neuroradiologists (A.R. and P.K.), who were blinded to all patient data. Discrepancies were resolved by consensus reading. According to RANO criteria, the enhancement was quantified on contrast enhanced T1-weighted images by the sum of the products of perpendicular diameters of enhancing lesions and compared with the baseline image. Changes of hyperintensities on T2 or FLAIR images were assessed accordingly.
Statistical Analysis
Incidences of PsP in the first, second, third, and fourth f/u subgroups were compared using the χ2 test. Overall survival was measured from date of baseline scan to death, and the log-rank test was employed to compare PsP versus true progression. Patients who were alive at last evaluation (August 1, 2013) were censored. A multivariate Cox proportional hazards regression model was used for adjustment of median age and extent of tumor resection (gross total vs subtotal or biopsy). All statistical tests were performed using SPSS 21.0. P < .05 was deemed statistically significant. All described results are reported as medians with ranges or 95% CIs for continuous variables.
Results
In total, 79 of 548 patients fulfilled the inclusion criteria for this study.
In a first step, patients were categorized into 4 different groups depending on the f/u in which the initial enhancement increase of at least 25% was diagnosed: 45 patients were assigned to the first, 17 to the second, 9 to the third, and 8 to the fourth group. In a second step, patients within each group were categorized based on the further development of contrast enhancement into true progression or PsP, subgrouped as stable enhancement, PsP < 50%, PsP > 50%, or total resolution of the enhancement. Average scan date after initial diagnosis was assessed for each group: 34.0 ± 18.0 days for the first group, 113.3 ± 15.2 days for the second group, 187.8 ± 23.5 days for the third group, and 305.0 ± 53.6 days for the fourth group.
Patient distribution among the different subgroups and average scan dates after initial diagnosis are summarized in Table 2.
Table 2.
Categorization of the patients who displayed a new enhancement of ≥25% after the first, second, third, or fourth postradiation scan as either true progression or PsP
| Scan | True Progression | Pseudoprogression |
Total | |||
|---|---|---|---|---|---|---|
| Stable | Decrease <50% of Enhancement | Decrease >50% of Enhancement | Complete Resolution of Enhancement | |||
| First postradiation scan | 39 = 86.67% | 3 = 6.67% | 1 = 2.22% | 2 = 4.44% | – | 45 |
| Average scan date after initial diagnosis 34.0 ± 18.0 d, median 28 d | ||||||
| Second postradiation scan | 15 = 88.24% | – | 1 = 5.88.3% | – | 1 = 5.88% | 17 |
| Average scan date after initial diagnosis 113.3 ± 15.2 d, median 111.5 d | ||||||
| Third postradiation scan | 8 = 88.89% | – | – | 1 = 11.11% | – | 9 |
| Average scan date after initial diagnosis 187.8 ± 23.5 d, median 193 d | ||||||
| Fourth postradiation scan | 8 = 100% | – | – | – | – | 8 |
| Average scan date after initial diagnosis 305.0 ± 53.6 d, median 302.5 d | ||||||
| Total | 70 = 88.61% | 2 = 2.53% | 4 = 5.06% | 2 = 2.53% | 1 = 1.27% | 79 |
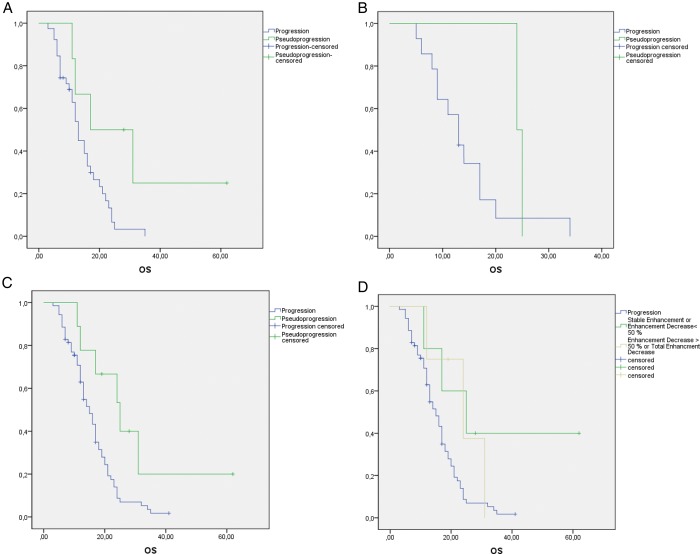
Median OS was calculated for PsP and true progression in each group. Subsequently the log-rank test was applied for the first group and cumulatively on all groups together. The isolated application of the log-rank test on the second, third, and fourth groups was precluded due to the small number of PsP cases in these groups. In the first group, median OS was longer in patients with PsP (29.9 [12.7–47.0] mo) than in patients with true progression (14.3 [11.9–16.8] mo) (log-rank test, P = .03). Taking all 4 groups together, we found a difference between patients with true progression (median OS of 15.8 [13.8–17.7] mo) and patients with PsP (median OS of 29.6 [16.5–42.6] mo) (log-rank test, P = .012).
To assess whether the amount of enhancement decrease in the PsP group had an influence on OS, we compared the OS of the patients who presented stable PsP or PsP < 50% with OS of patients with total resolution of the enhancement or PsP > 50%. For the 5 patients who presented stable PsP or PsP < 50%, median OS was 35.4 months (95% CI, 16.0–54.8 mo) and log rank revealed a significant difference compared with patients with true progression (P = .029). In contrast, median OS of the 4 patients with PsP who presented a total PsP or PsP > 50% was 23.6 (14.4–32.8) months and not different from the median OS of patients with true progression (log-rank test, P = .16). OS did not differ between the 2 PsP groups (log-rank test, P = .7) (Fig. 2D). Finally, the χ2 test did not reveal a difference among incidences of PsP in the first, second, or third f/u.
Fig. 2.
Kaplan–Meier curves of patients with a new enhancement after (A) the first f/u, (B) the second f/u, and (C) cumulatively after the first, second, third, and fourth f/u's. (D) Patients with PsP are subdivided into those with an enhancement decrease of >50% or <50% in the f/u after the initial enhancement increase was diagnosed.
A multivariate Cox proportional hazards regression model for adjustment of median age (≤58 vs >58 y) and extent of tumor resection (gross total resection vs biopsy/subtotal resection) was performed on the first group and cumulatively on all groups. The low number of PsP cases impeded individual analysis of the second and third groups. In the first group, adjustment for the above-mentioned clinical confounders yielded a hazard ratio of 3.0 (1.0–8.8) (P < .044), while the cumulative adjustment for all 4 groups was 2.9 (1.2–6.8) (P < .014). Results of the log-rank tests and of the multivariate Cox regression are summarized in Fig. 2 and in Table 3.
Table 3.
Univariate survival analysis and multivariate Cox regression analysis for type of surgery and age at initial diagnosis for different f/u groups
| Postradiation Scan | Parameter | Number of Patients, Died/Total | Univariate Survival Analysis |
Multivariate Cox Regression Analysis |
|||
|---|---|---|---|---|---|---|---|
| P, Log-Rank | Median, mo (95% CI) | Hazard Ratio (95% CI) | P | ||||
| First f/u* | Age | ≤ Median age | 17/20 | .585 | 16.6 (12.9–20.4) | 1.2 (0.6–2.3) | .65 |
| > Median age | 21/25 | 16.4 (10.8–22.0) | |||||
| Surgery | Gross total resection | 21/23 | .952 | 15.7 (11.7–19.6) | 1.1 (0.6–2.2) | .76 | |
| Subtotal resection/biopsy | 17/22 | 17.0 (11.9–22.0) | |||||
| Progression | PsP | 2/6 | .03 | 29.9 (12.7–47.0) | 3.0 (1.0–8.8) | .044 | |
| True progression | 40/45 | 14.3 (11.9–16.8) | |||||
| Second f/u | Progression | PsP | 2/2 | NA | 24.5 (23.5–25.5) | NA | NA |
| True progression | 14/15 | 14.0 (9.8–18.3) | |||||
| Third f/u | Progression | PsP | 0/1 | NA | 19.0 | NA | NA |
| True progression | 6/8 | 20.1 (13.4–26.8) | |||||
| Fourth f/u | Progression | PsP | 0 | NA | NA | NA | NA |
| True progression | /78 | 21.3 (17.0–25.6) | |||||
| First, second, third, and fourth f/u's cumulatively | Age | ≤ Median age | 34/41 | .212 | 18.3 (15.5–21.1) | 1.5 (0.9–2.5) | .102 |
| > Median age | 33/38 | 16.1 (12.4–19.9) | |||||
| Surgery | Gross total resection | 30/36 | .52 | 19.1 (14.4–23.8) | 1.4 (0.8–2.3) | .19 | |
| Subtotal resection/biopsy | 37/43 | 16.2 (13.7–18.9) | |||||
| Progression | PsP | 6/9 | .012 | 29.6 (16.5–42.6) | 2.9 (1.2–6.8) | .014 | |
| True progression | 61/70 | 15.8 (13.8–17.7) | |||||
Abbreviation: NA, not applicable.
P-values below .05 are boldfaced.
No difference was detected between T2-signal development and appearance of true progression or PsP (Table 4). Finally, no difference was detected for the percentage of patients who received steroid therapy at the initial enhancement-presenting f/u between the true progression group (15/70 patients, 21.4%) and the PsP group (2/9 patients, 22.2%).
Table 4.
Development of T2 signal in patients with 25% enhancement increase
| Development of T2 Signal at Time Point of 25% Enhancement Increase on T1 |
No Sufficient T2 at Baseline | Total | |||
|---|---|---|---|---|---|
| Increase >25% | Stable | Decrease | |||
| True progression | 46 (73.0%) | 8 (12.7%) | 9 (14.3%) | 7 | 70 |
| Pseudoprogression | 6 (75%) | 1 (12.5%) | 1 (12.5%) | 1 | 9 |
| Total | 52 | 9 | 10 | 8 | 79 |
Percent values refer to the included scans: 63 for true progression and 8 for PsP. No significant difference could be detected between T2-signal development and appearance of true progression or PsP.
Discussion
The definition of the RANO criteria to consider an increased enhancement within the first 12 weeks after completion of radiochemotherapy as potential PsP is based on the assumption that PsP is a radiographic phenomenon associated with prolonged OS14 and occurs with an incidence of up to 50%,11,15 mainly within the above-mentioned period of time.1
Our data confirm a prolonged OS in patients presenting with PsP. However, we could neither confirm the previously reported high incidence of PsP nor the narrow time-dependent definition suggested by the RANO criteria.
Incidence of Pseudoprogression
A wide variation in the incidence of PsP of between 12%16 and 64%17 has been reported in published studies that often contain small numbers of cases.2,8,18–20 In this context, it is important to note that our study refers to the incidence of PsP as only the incidence of all patients with a new enhancement on f/u and not to the cohort of all glioblastoma patients, since the latter would result in even lower incidence rates.
In a large study on PsP, the incidence of PsP was assessed within 4 weeks after completion of radiochemotherapy, and PsP was diagnosed in 32 of 50 patients.14 In contrast, we found PsP in 6 of 45 patients after 4 weeks (first f/u).
Since our study is based on the widest possible definition of PsP (including cases with stable enhancement at f/u after a suspected PsP), the amount of subsequent enhancement decrease does not explain the discrepancy in the incidences reported. The categorization of patients with stable PsP or PsP < 50% as true progression would have resulted in an even lower incidence of PsP in our study.
In contrast, the initial amount of enhancement increase required to classify a new enhancement as potential PsP plays a pivotal role. Some studies have required only a “lesion growth.”14 From a clinical point of view, this definition is questionable because the nonobservance of PsP would contribute to false clinical decisions only if the “pseudo-enhancement” increase reached 25%. Any increase below this cutoff value would not require any change in therapy, since it would be evaluated at least as stable disease.
An approach that defines any enhancement increase as potential PsP is furthermore likely to overestimate the incidence of PsP. This especially holds true if the subsequent enhancement decrease is assessed according to Macdonald/RANO criteria including stable disease. According to this approach, a minimal enhancement increase (eg, 5%) and a subsequent clear increase (eg, 15%) would qualify for PsP because the f/u does not fulfill the 25% criterion and hence has to be categorized as stable disease.
In our view, the unequivocal identification of a 25% initial enhancement increase is the main reason for the divergent incidences of PsP reported in our study and former studies. The response evaluation is highly subjective, and in case of uncertainty the assumption of an initial 25% enhancement increase should be made with caution: while an initial slight enhancement increase may appear and subsequently disappear frequently after radiochemotherapy, our study demonstrated that an unequivocal enhancement increase of at least 25% disappears very rarely in the subsequent f/u.
Furthermore, different MRI parameters at different institutions as well as the combined use of MRI and CT scans for f/u assessment might have contributed to the divergent incidences reported in prior studies. For example, Taal et al20 found in a study that used the 25% enhancement increase criterion in 36 patients with mixed CT and MRI baseline scans an incidence of PsP of 50%. Even though the subgroup analysis did not reveal any significant difference for the PsP incidence within the subgroups, this experimental setting might be a gateway to a read-out bias.
In a recently published large prospective study, the frequency of confirmed PsP was 9.3% in the standard arm, radiochemotherapy with temozolomide, supporting our findings rather than the high levels reported in the past.21
Pseudoprogression Beyond 12 Weeks
Furthermore, our study could not confirm the assumption that PsP is most prevalent within 12 weeks after completion of radiotherapy.
Systematic studies of larger patient cohorts about the incidence of PsP within different time spans do not exist. Recent studies investigated time spans from 4 weeks14,20 to 6 months.16 As mentioned above, the hitherto largest study on PsP focused on the 4-week criterion.14 In contrast, some studies emphasize the potential of late and prolonged PsP. Chaski et al16 found in a cohort of 25 patients with newly increased enhancement 3 patients with PsP 2, 4, and 6 months after completion of radiochemotherapy. They did not identify any patient with PsP immediately after completion of radiochemotherapy and concluded that PsP will occur mainly as delayed focal enhancement during the 6 months of maintenance therapy with temozolomide.16 Furthermore, Stuplich et al22 recently reported the occurrence of late and prolonged PsP in a small case series with 8 patients. Three of these 8 patients presented PsP at 10, 19, and 39 weeks after completion of radiotherapy and subsequent treatment with lomustine and temozolomide.22
In our study, we did not find a significant difference between the incidence of PsP 1 month, 4 months, or 7 months after completion of radiochemotherapy. These results should be interpreted cautiously, since there were in total only 9 patients with PsP in these 3 groups. It also has to be mentioned that we could not identify any patient with PsP among the 8 patients who presented an enhancement increase in the fourth f/u.
Taking together the low frequency of PsP within 12 weeks after radiochemotherapy, the unequivocal evidence of 3 cases of PsP beyond the 12-week criterion, and the comparable incidences of PsP at first, second, and third f/u's, our study challenges the introduced arbitrary cutoff definition of 12 weeks.
Required Enhancement Decrease for Definition of Pseudoprogression
Generally, there is no consensus about whether PsP should be exclusively limited to the complete or significant disappearance of an initially newly diagnosed enhancement or should include also cases of enhancement stabilization. The RANO criteria state that PsP should be suggested for an increased enhancement that “eventually subsides.”1 However, the vast majority of studies also include patients who present a stabilization of the enhancement.
This stands in contrast to the published MRIs frequently presenting an almost complete or a complete disappearance of contrast enhancement (Fig. 1).
Since we could not find a statistically significantly different OS between the PsP subgroups with an enhancement decrease larger or smaller than 50%, we suggest including patients with stable enhancement in the definition of PsP.
The low frequency of a complete enhancement disappearance in our study (found in only 1 patient) further supports the hypothesis that the histopathological basis of PsP is not always a transiently increased permeability of the tumor vasculature. The stable enhancement may rather be caused by a permanently disrupted blood–brain barrier or by confounding effects of recurrent tumor portions. The latter reason may potentially present the common scenario of a mixture of PsP and recurrent glioma.23,24
Pseudoprogression Under RANO Criteria
The way PsP is defined in the RANO criteria has important implications for daily clinical decision making as well as for the management of clinical trials in the event that a patient presenting true progression is mistakenly diagnosed with PsP. Using the RANO criteria, these patients will only (correctly) be diagnosed with true progression in a subsequent f/u. As highlighted by Pope and Hessel,25 this delay of the correct diagnosis to the next f/u may exclude the fastest recurring tumors from necessary therapy changes. Moreover, since the RANO criteria propose that patients with suspected PsP should be excluded from trials for recurrent glioma (and most trials follow this recommendation), this group of patients with very aggressive tumors who probably most urgently require new therapies have a delayed enrollment into clinical trials. Therefore, the risks of a premature termination of a sufficient therapy in case of PsP on the one hand and the above-mentioned risks of a delayed therapy change in case of true progression on the other hand have to be weighed against each other.25 In this respect, the results of our study challenge the current definition of PsP by the RANO criteria.
According to the results of the present study, 86.7% of all patients with a new enhancement at the first f/u after radiotherapy would erroneously be diagnosed with PsP. Even though we found significantly prolonged OS in patients with PsP, it is questionable whether this low incidence can truly justify the delay in necessary therapy changes in the majority of glioblastoma patients. Based on our findings, we would rather suggest not excluding patients from trials for recurrent gliomas who present an unequivocal enhancement increase of at least 25% within 12 weeks after completion of chemoradiotherapy. Beyond this, our study underlines the necessity of a close imaging observation of patients who were diagnosed with potential PsP, for example—as suggested by the RANO criteria—at 4-week intervals.
Finally, there are still limitations that should be considered in the current study. First of all, the number of included patients with PsP is relatively small (9 patients), which makes especially the survival analysis prone to bias. Furthermore, we did not have histopathological confirmation of suspected true progression and PsP. This confirmation would be especially relevant for the differentiation of PsP and radiation necrosis. It has been reported that radiation necrosis26 might be a more severe local tissue reaction than PsP that potentially requires surgical debulking in clinical symptomatic cases.27 These cases might progress and mimic tumor progression if left untreated and hence lead to an underestimation of the PsP incidence. However, the majority of prior studies reporting on the incidence of PsP did not have histopathological confirmation either,14,20 which makes the missing histopathological confirmation a general limitation that cannot explain the differences in the reported incidences of PsP.
A further limitation of our study is its retrospective design. We therefore suggest evaluating the incidence of PsP at different time points in a large collaborative multicenter effort. Finally, a reliable differentiation of PsP and true progression with other MR techniques would be the most preferable solution. Since we have shown in the current study that the sole development of the T2 signal does not differentiate both entities, advanced imaging and postprocessing techniques should be investigated to differentiate PsP and true progression reliably.
Conclusion
Even though we could prove a prolonged OS for patients with PsP, the results of this retrospective study challenge the current management of PsP. Under the current guidelines for PsP, 86.7% of the patients in this study were initially erroneously diagnosed with PsP. Furthermore, no difference between the incidence of PSP at months 1, 4, and 7 could be detected, questioning the proposed 3-month cutoff value.
Funding
B.W. is a scholar of the National Center for Tumor Diseases Heidelberg School of Oncology Postdoc Program. This research was supported by a grant from Guerbet and a grant from the Intramurales Förderprogramm of the German Cancer Research Center (DKFZ).
Conflict of interest statement. W.W. is on advisory boards and a steering committee and has received honoraria for lectures and research funding from Merck, Sharp & Dohme and Roche, along with Apogenix, Eli Lilly, and Boehringer Ingelheim. No other author conflicts are declared.
References
- 1.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. doi: 10.1200/JCO.2009.26.3541. [DOI] [PubMed] [Google Scholar]
- 2.Gerstner ER, McNamara MB, Norden AD, et al. Effect of adding temozolomide to radiation therapy on the incidence of pseudo-progression. J Neurooncol. 2009;94(1):97–101. doi: 10.1007/s11060-009-9809-4. [DOI] [PubMed] [Google Scholar]
- 3.Lee WJ, Choi SH, Park CK, et al. Diffusion-weighted MR imaging for the differentiation of true progression from pseudoprogression following concomitant radiotherapy with temozolomide in patients with newly diagnosed high-grade gliomas. Acad Radiol. 2012;19(11):1353–1361. doi: 10.1016/j.acra.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Sundgren PC, Fan X, Weybright P, et al. Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging. 2006;24(9):1131–1142. doi: 10.1016/j.mri.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Sundgren PC. MR spectroscopy in radiation injury. AJNR Am J Neuroradiol. 2009;30(8):1469–1476. doi: 10.3174/ajnr.A1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gahramanov S, Raslan AM, Muldoon LL, et al. Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int J Radiat Oncol Biol Phys. 2011;79(2):514–523. doi: 10.1016/j.ijrobp.2009.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suh CH, Kim HS, Choi YJ, et al. Prediction of pseudoprogression in patients with glioblastomas using the initial and final area under the curves ratio derived from dynamic contrast-enhanced T1-weighted perfusion MR imaging. AJNR Am J Neuroradiol. 2013;34(12):2278–2286. doi: 10.3174/ajnr.A3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hygino da Cruz LC, Jr, Rodriguez I, Domingues RC, et al. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32(11):1978–1985. doi: 10.3174/ajnr.A2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah AH, Snelling B, Bregy A, et al. Discriminating radiation necrosis from tumor progression in gliomas: a systematic review. What is the best imaging modality? J Neurooncol. 2013;112(2):141–152. doi: 10.1007/s11060-013-1059-9. [DOI] [PubMed] [Google Scholar]
- 10.Verma N, Cowperthwaite MC, Burnett MG, et al. Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol. 2013;15(5):515–534. doi: 10.1093/neuonc/nos307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 12.Tran DK, Jensen RL. Treatment-related brain tumor imaging changes: so-called “pseudoprogression” vs. tumor progression: review and future research opportunities. Surg Neurol Int. 2013;4(Suppl 3):S129–S135. doi: 10.4103/2152-7806.110661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 14.Brandes AA, Franceschi E, Tosoni A, et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–2197. doi: 10.1200/JCO.2007.14.8163. [DOI] [PubMed] [Google Scholar]
- 15.Brandsma D, Stalpers L, Taal W, et al. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–461. doi: 10.1016/S1470-2045(08)70125-6. [DOI] [PubMed] [Google Scholar]
- 16.Chaskis C, Neyns B, Michotte A, et al. Pseudoprogression after radiotherapy with concurrent temozolomide for high-grade glioma: clinical observations and working recommendations. Surg Neurol. 2009;72(4):423–428. doi: 10.1016/j.surneu.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 17.Brandes AA, Tosoni A, Franceschi E, et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation with MGMT promoter methylation status. J Clin Oncol. 2009;27(8):1275–1279. doi: 10.1200/JCO.2008.19.4969. [DOI] [PubMed] [Google Scholar]
- 18.Mangla R, Singh G, Ziegelitz D, et al. Changes in relative cerebral blood volume 1 month after radiation-temozolomide therapy can help predict overall survival in patients with glioblastoma. Radiology. 2010;256(2):575–584. doi: 10.1148/radiol.10091440. [DOI] [PubMed] [Google Scholar]
- 19.Sanghera P, Perry J, Sahgal A, et al. Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can J Neurol Sci. 2010;37(1):36–42. doi: 10.1017/s0317167100009628. [DOI] [PubMed] [Google Scholar]
- 20.Taal W, Brandsma D, de Bruin HG, et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113(2):405–410. doi: 10.1002/cncr.23562. [DOI] [PubMed] [Google Scholar]
- 21.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 22.Stuplich M, Hadizadeh DR, Kuchelmeister K, et al. Late and prolonged pseudoprogression in glioblastoma after treatment with lomustine and temozolomide. J Clin Oncol. 2012;30(21):e180–e183. doi: 10.1200/JCO.2011.40.9565. [DOI] [PubMed] [Google Scholar]
- 23.Fink J, Born D, Chamberlain MC. Pseudoprogression: relevance with respect to treatment of high-grade gliomas. Curr Treat Options Oncol. 2011;12(3):240–252. doi: 10.1007/s11864-011-0157-1. [DOI] [PubMed] [Google Scholar]
- 24.Perry A, Schmidt RE. Cancer therapy-associated CNS neuropathology: an update and review of the literature. Acta Neuropathol. 2006;111(3):197–212. doi: 10.1007/s00401-005-0023-y. [DOI] [PubMed] [Google Scholar]
- 25.Pope WB, Hessel C. Response assessment in neuro-oncology criteria: implementation challenges in multicenter neuro-oncology trials. AJNR Am J Neuroradiol. 2011;32(5):794–797. doi: 10.3174/ajnr.A2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamberlain MC, Glantz MJ, Chalmers L, et al. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82(1):81–83. doi: 10.1007/s11060-006-9241-y. [DOI] [PubMed] [Google Scholar]
- 27.Brandsma D, van den Bent MJ. Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol. 2009;22(6):633–638. doi: 10.1097/WCO.0b013e328332363e. [DOI] [PubMed] [Google Scholar]