Abstract
Background
CD44 is a molecular marker associated with molecular subtype and treatment resistance in glioma. More effective therapies will result from approaches aimed at targeting the CD44-high gliomas.
Methods
Protein tyrosine kinase 7 (PTK7) mRNA expression was analyzed based on The Cancer Genome Atlas glioblastoma dataset. PTK7 expression was depleted through lentivirus-mediated short hairpin RNA knockdown. Terminal deoxynucleotidyl transferase dUTP nick-end labeling was used to evaluate cell apoptosis following PTK7 knockdown. Gene expression analysis was performed on Affymetrix microarray. A nude mice orthotopic tumor model was used to evaluate the in vivo effect of PTK7 depletion.
Results
PTK7 is highly expressed in CD44-high glioblastoma and predicts unfavorable prognosis. PTK7 knockdown attenuated cell proliferation, impaired tumorigenic potential, and induced apoptosis in CD44-high glioma cell lines. Gene expression analysis identified inhibitor of DNA Binding 1 (Id1) gene as a potential downstream effector for PTK7. Overexpression of Id1 mostly restored the cell proliferation and colony formation attenuated by PTK7 depletion. PTK7 enhanced anchorage-independent growth in normal human astrocytes, which was attenuated by Id1 knockdown. Furthermore, PTK7 regulated Id1 expression through modulating TGF-β/Smad signaling, while pharmacological inhibition on TGF-β/Smad signaling or PTK7/Id1 depletion attenuated TGF-β–stimulated cell proliferation. PTK7 depletion consistently reduced Id1 expression, suppressed tumor growth, and induced apoptosis in a murine orthotopic tumor model, which could be translated into prolonged survival in tumor-bearing mice.
Conclusions
PTK7 regulates Id1 expression in CD44-high glioma cell lines. Targeting PTK7 could be an effective strategy for treating glioma with high CD44 expression.
Keywords: CD44, cell proliferation, glioma, PTK7, tumorigenesis
Despite progress in studying the molecular aspects of malignant gliomas, the prognosis of these brain tumors, especially glioblastoma multiforme (GBM), continues to be dismal.1 The biological characteristics of GBM are exemplified by prominent proliferation, active invasiveness, and rich angiogenesis. The Cancer Genome Atlas (TCGA) GBM study has unveiled several highly deregulated signaling pathways: p53/MDM2/ARF, Rb/CDK4/CDK6, and receptor tyrosine kinase (RTK)/Ras/phosphoinositide 3-kinase (PI3K) signaling in GBM.2 Studies of these signaling pathways have greatly increased our understanding of the biology and clinical behavior of GBM. An integrated view of aberrant signal transduction will provide a more useful approach for designing novel therapies for this devastating disease.
Protein tyrosine kinase 7 (PTK7) is an evolutionarily conserved receptor tyrosine kinase-like molecule with functions in various biological processes ranging from embryonic morphogenesis to epidermal wound repair.3,4 After initial identification in colon carcinoma cells, PTK7 was later shown to be required for embryonic morphogenesis ranging from axon guidance in Drosophila to the regulation of gastrulation, neural tube closure, neural crest migration, epithelial-to-mesenchymal transitions, and cardiac morphogenesis in vertebrates.5–7 Mice expressing a truncated form of PTK7 protein die perinatally, with evidence of a defect in neural tube closure and stereociliary bundle orientation.6 These findings implicate PTK7 as a regulator of planar cell polarity (PCP). It has been shown that PTK7 recruits RACK1, which affects Dsh recruitment by interaction with PKCδ1.8,9 Interaction between PTK7 and Dsh at the plasma membrane activates noncanonical Wnt signaling, which then directs PCP.10 PTK7 also interacts with β-catenin, enhancing β-catenin–dependent transcriptional events. These studies suggest that PTK7 plays a role in the Wnt and the PCP signaling pathway.11
PTK7 expression is elevated in multiple cancer types including colon cancer, gastric cancer, breast cancer, and acute myeloid leukemia and is associated with poor drug response, increased metastatic potential, and poor patient survival.5,12–15 Although the role of PTK7 in different cancers has not been studied comprehensively, PTK7 is likely to have a general role in promoting tumors. Expression of PTK7 in leukemia cells enhances cell migration and survival.12 In addition, PTK7 knockdown has been shown to inhibit proliferation and invasion of liposarcoma cells16 as well as proliferation and antiapoptotic activity of colon cancer cells.17 Moreover, treatment with the entire extracellular domain of PTK7 (soluble PTK7), acting as a decoy receptor or knockdown of PTK7, prevented vascular endothelial growth factor-induced migration, invasion, tube-formation of human umbilical vein endothelial cells, and angiogenesis in vivo.18 These data demonstrate that PTK7 is a versatile coreceptor for cancer-related signaling and supports a role of PTK7 as a molecular switch between signaling pathways. Although progress has been made in placing PTK7 in the cellular signaling network, many questions still remain; the answers may help us gain a better understanding of gene function in tumor development.
CD44 is a transmembrane glycoprotein expressed in mesenchyma-like glioma subtype and serves as a surface receptor for components of the extracellular matrix such as hyaluronic acid.19 CD44 plays a critical role in efficient cell detachment from the hyaluronic acid substrate and promotes glioma cell migration.20,21 CD44 expression levels correlated with the histopathological grade of gliomas.22 Recently, CD44 has been extensively used as a surface marker for isolating cancer stem cells from breast, prostate, pancreas, and colorectal cancers and glioma.23–25 In this study, we found that PTK7 is highly expressed in CD44-high GBM tissues and human glioma cell lines. Furthermore, PTK7 depletion suppressed cell proliferation, impaired tumorigenic potential, and induced apoptosis in CD44-high glioma cell lines. These results suggested that targeting PTK7 could be an effective strategy for combating CD44-high gliomas.
Materials and Methods
Reagents and Cell Lines
Antibodies to phosphorylated Smad2(Ser465/467)/Smad2, Smad3(Ser423/425)/Smad3, pSmad2/3, and CD44 were purchased from Cell Signaling Technology. Antibodies to PTK7, β-Actin, and Id1 were from Santa Cruz Biotechnology. Horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse antibodies were purchased from Bio-Rad. DMEM/F12, antibiotics, B27, EGF, and bFGF were all purchased from Invitrogen. TGF-βR Inhibitor SB 431542, bromodeoxyuridine (BrdU), and TGF-β1 were obtained from Sigma Chemical Company. Human glioma cell lines U87, U251, U373, LN18, A172, T98G, and LN229 were obtained from American Type Culture Collection and cultured according to the manufacturer's protocol. Human glioma cell lines D54, U343, and SF295 were kindly provided by the Type Culture Collection of the Chinese Academy of Sciences, Shanghai. Normal human astrocytes (NHAs) were purchased from Life Technologies and cultured in GIBCO astrocyte medium.
Tumors Specimens and Glioma-initiating Cells Culture
Nine primary GBM specimens were obtained freshly from the operating room following protocols approved by the research ethics committee in Central South University with informed consent having been obtained from all participants. GIC lines GIC-2, GIC-4, GIC-5, GIC-6, GIC-7, and GIC-8 (derived from GBM02, 04, 05, 06, 07, 08) were isolated and subsequently cultured in stem cell medium as reported by Lee et al.26
Lentiviral Construction and Transduction
Lentiviruses encoding short hairpin RNAs (shRNAs) silencing PTK7 and control scramble were purchased from Open Biosystems. shRNA sequence-silencing Id1 was reported previously.27 OmicsLink lentiviral ORF expression clones for PTK7 or Id1 gene were constructed by Genecopoeia Inc. Lentiviral particles were produced by transient cotransfection of packaging plasmids into the Lenti-Pac 293Ta cell line. Produced lentiviruses were concentrated by using Centricon Plus-20 centrifugal filter device (Millipore).
Tumorsphere Formation Assay
Tumorsphere formation assay of glioma-initiating cells (GICs) was performed as described previously.25
Soft Agar Assay
Anchorage-independent growth of glioma cells was tested as described previously.28 The 2 mL culture medium with 0.5% agar was first plated into each well of a 6 cm culture dish. After the agar solidified, each well received another 2 mL of 0.35% agar in culture medium containing 1 × 104 cells. After 4 weeks, colonies were fixed and stained with 0.1% crystal violet. The number of colonies was determined microscopically by manually counting from triplicate wells for each cell line.
Western Blot Analysis
Cell lysates were prepared with cell lysis buffer containing 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), and protease inhibitor cocktail. After standard SDS-PAGE and Western blotting procedures, proteins were visualized using the ECL system (Amersham Biosciences). Antibodies against PTK7, Id1, p-Smad, Smad, and CD44 were used at a 1:1000 dilution. Anti-β-Actin was used at a 1:5000 dilution.
Immunofluorescence Staining
Cells were cultured on glass coverslips precoated with polylysine in 6-well plates, rinsed 3 times with phosphate-buffered saline, fixed with 3.7% paraformaldehyde for 15 minutes, and blocked with 5% normal goat serum for 1 hour. The cells were immunostained by using primary antibodies specific to various antigens (CD44, PTK7). Images were taken under a Zeiss axiocam fluorescence microscope using AxioVision software (Zeiss). BrdU incorporation assay was performed as described previously.29
Terminal Deoxynucleotidyl Transferase Dutp Nick-end Labeling Assay
The level of apoptosis in glioma cells was assessed using the terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) method. For the PTK7/Id1 knockdown experiment, apoptotic cells were evaluated 5 days after shRNA lentivirus infection. The percentage of TUNEL-labeled cells in each section was determined at a magnification of 400 by counting 500 cells in a randomly selected field.
Cell Proliferation Assay
To determine the effect of PTK7 or Id1 knockdown on cell proliferation, cells were infected with scramble or shRNA-targeting lentivirus for 48 hours, dissociated into single cell, plated onto 6-well plates (1 × 104 cells/well, and incubated for 5 days. The cultures were trypsinized, and the number of viable cells in each group was counted with a hemocytometer using 0.2% trypan blue exclusion.
Immunohistochemistry
Immunohistochemical staining was performed as described previously.29 Briefly, frozen tissue sections were fixed with 4% paraformaldehyde, incubated with primary antibodies overnight at 4°C, and then incubated for 30 minutes each with horseradish peroxidase-labeled secondary antibody. Antigen-antibody reactions were detected by exposure to 3,3-diaminobenzidine and hydrogen peroxide chromogen substrate (DAKO). Slides were counterstained with hematoxylin and mounted. The negative controls were incubated with nonimmune mouse IgG. The immunostained slides were examined under the light microscope and scored in a double-blind manner by a pathologist and laboratory researcher.
Animal Studies
Immunocompromised nude mice were obtained from the breeding facility at the animal center of Central South University. All animal studies were performed in accordance with institutional ethical guidelines for experimental animal care. For subcutaneous xenograft study, 2 × 106 LN18 cells were inoculated into the flank of nude mice (n = 5). In order to determine tumor volume by external caliper, the greatest longitudinal diameter (a) and the greatest transverse diameter (b) were determined. Tumor volume based on caliper measurements was calculated by the modified ellipsoidal formula: tumor volume (mm3) = a × b2/2.
For survival analysis, 2 × 105 LN18 cells were injected stereotactically into 4-week-old nude mice cortex, following administration of general anesthesia. The injection coordinates were 3 mm to the left of the midline, 2 mm anterior to the lambdoid suture, and 3 mm deep. The incision was closed with wound clips and removed 4 days after inoculation. Animals that died, lost weight, or developed neurological deficits within 24 hours of cell injection were excluded. The animals were monitored daily until signs of neurological deficit developed, at which time they were euthanized and their brains removed.
For histopathological analysis, the mouse brain xenografts embedded in optimum cutting temperature were stored in liquid nitrogen overnight, and then sectioned at 5 µm thickness on a MicromHM200 cryotome (Eryostar). Hematoxylin and eosin (H&E) stained sections were evaluated for evidence of tumor.
The Cancer Genome Atlas Data Analysis
Array comparative genomic hybridization, mRNA, and gene mutation data from GBM patients were downloaded from the TCGA project data portal (http://cancergenome.nih.gov/dataportal). Details on the data processing and platforms are in the publication describing the GBM data analysis.30
Statistical Analysis
Statistical evaluations were carried out using SPSS 10.0 software (IBM). Error bars throughout the figures indicate standard deviation. The Student' t test was used to compare means of 2 groups. One-way ANOVA was used to compare means of 3 or more groups. Kaplan-Meier curves of overall survival were drawn, and survival in the groups was compared using the log-rank test. For all tests, the level of statistical significance was set at P < .05. All statistical tests were 2 sided.
Results
PTK7 Is Highly Expressed in CD44-high Glioma
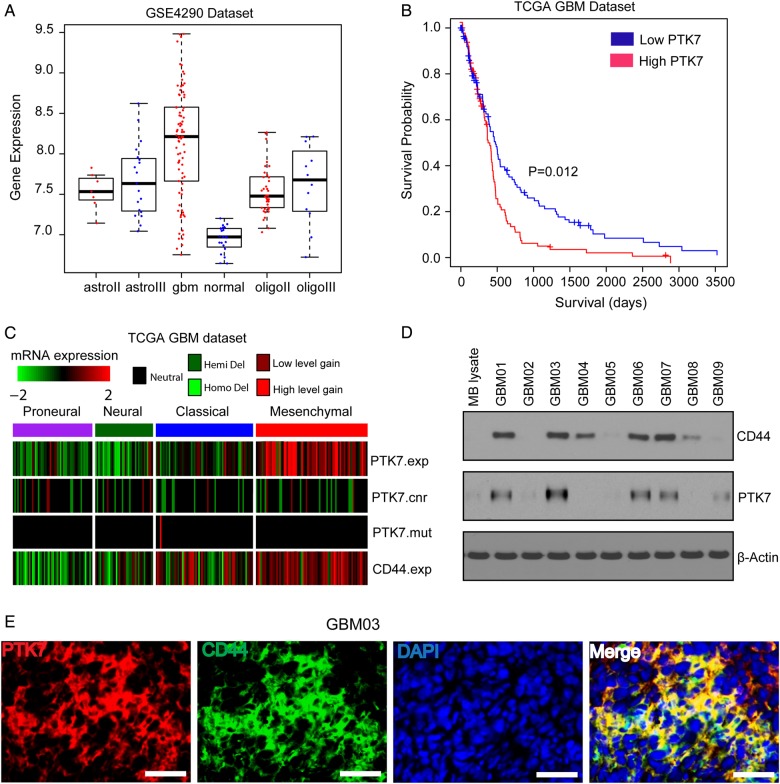
Analysis on the GSE4290 dataset showed that PTK7 mRNA was highly expressed in glioma tissues, as compared with nontumor brain tissues (P < .05) (Fig. 1A). Highest PTK7 mRNA expression was observed in GBMs, as compared with nontumor brain tissues or lower grade gliomas (P < .05) (Fig. 1A). Survival analysis indicated that high PTK7 expression in TCGA GBM tissues predicted unfavorable survival outcome, as compared with those with low PTK7 expression (log-rank survival analysis; P = .012) (Fig. 1B). Furthermore, TCGA GBM profiling revealed that higher PTK7 expression was consistent with higher CD44 expression in the mesenchymal molecular subclass (Fig. 1C). CD44 mRNA expression was significantly correlated with PTK7 expression in TCGA GBM (Pearson correlation r = 0.423; P < .001). Western blotting analysis confirmed that PTK7 expression was higher in primary GBM tissues expressing CD44 but not in normal mouse brain tissues (Fig. 1D). PTK7 immunoreactivity was also seen in CD44-positive glioma cells in the GBM03 tumor section (Fig. 1E). Therefore, PTK7 might exert an important function in CD44-high gliomas.
Fig. 1.
PTK7 is highly expressed in CD44-high GBMs and predicts poor prognosis. (A) PTK7 mRNA expression in nontumor brain tissues and gliomas (astrocytoma grade II, III; GBM; oligodendrocytoma grade II, III) based on GSE4290 dataset (http://www.ncbi.nlm.nih.gov/sites/GDSbrowser?acc=GDS1962). (B) Survival analysis on PTK7 expression in TCGA GBM dataset. (C) PTK7 is highly expressed in TCGA mesenchymal GBM subclass. (D) PTK7 and CD44 protein expression in mouse brain and human GBM tissue lysates. (E) Immunostaining on PTK7 and CD44 in GBM03 frozen tissue section. Bars: 100 μm.
Targeting PTK7 Attenuates Glioma Cell Proliferation and Impairs Tumorigenic Potential in CD44-High Glioma Cell Lines
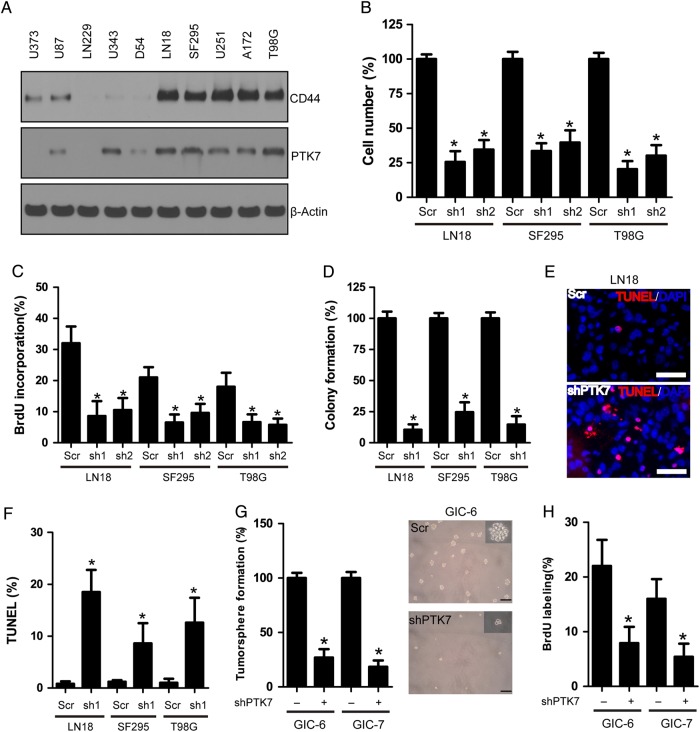
PTK7 was differentially expressed in a panel of human GBM cell lines, consistent with CD44 protein levels (Fig. 2A). To interrogate the role of PTK7 in glioma cells, we depleted endogenous PTK7 expression by lentivirus-expressing shRNA specific to PTK7. The 2 shRNAs efficiently reduced PTK7 expression in glioma cell lines, as compared with the control or scramble shRNA group (Supplementary, Fig. S1). Consequently, PTK7 knockdown significantly suppressed cell proliferation in the LN18, SF29, and T98G cell lines, accompanied with reduced BrdU incorporation (P < .05) (Fig. 2B and C). Two PTK7 shRNAs exhibited similar effects; hereafter, we used sh-1 shRNA for PTK7 knockdown. PTK7 depletion also attenuated the tumorigenic potential of glioma cells; the soft agar colony formation in PTK7 knockdown groups were significantly decreased compared with scramble groups (P < .05) (Fig. 2D). Furthermore, TUNEL assay showed that PTK7 knockdown markedly induced apoptosis in 3 glioma cell lines, as compared with scramble control (Fig. 2E and F) (P < .01). GICs have been identified in GBM and are thought to be responsible for tumor initiation, uncontrollable growth, and recurrence. We established 6 GIC lines from fresh GBM specimens. These GIC lines were grown as tumorspheric in vitro and exhibited high tumorigenicity in vivo (Supplementary, Fig. S2B and S2C). Similar to the glioma cell lines, PTK7 was also highly expressed in CD44-high GIC lines (Supplementary, Fig. S2A). PTK7 depletion attenuated tumorsphere formation and proliferative BrdU incorporation in CD44-high GIC lines (Fig. 2G and H). PTK7 shRNA did not affect cell proliferation in glioma cell lines D54 and LN229 with low endogenous PTK7 expression (Supplementary, Fig. S3). These results suggested that PTK7 might play a crucial role in CD44-high glioma.
Fig. 2.
(A) Expression of PTK7 and CD44 in human GBM cell lines. (B) PTK7 knockdown attenuated glioma cell proliferation in LN18, SF295, and T98G cells. *P < .05, as compared with scramble (Scr) control. (C) PTK7 knockdown reduced BrdU incorporation in LN18, SF295, and T98G cells. *P < .05, as compared with scramble (Scr) control. (D) PTK7 knockdown impaired soft agar colony formation in LN18, SF295, and T98G cells. *P < .05, as compared with scramble (Scr) control. (E and F) PTK7 knockdown induced apoptosis in LN18, SF295, and T98G cells. Bars: 50 μm. *P < .05, as compared with scramble (Scr) control. (G) PTK7 knockdown decreased tumorsphere formation in glioma initiating cell lines GIC-7 and GIC-7. *P < .05, as compared with scramble (Scr) control. (H) PTK7 knockdown reduced BrdU incorporation in glioma initiating cell lines GIC-7 and GIC-7. *P < .05, as compared with scramble (Scr) control.
Id1 Mediates PTK7 Signals to Promote Glioma Cell Proliferation and Tumorigenesis
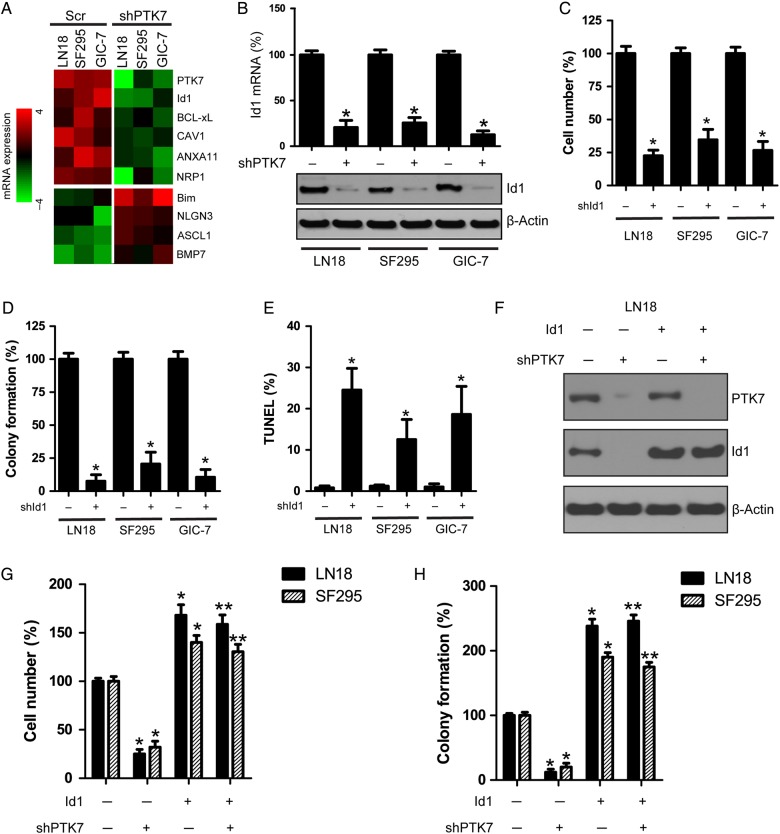
In an effort to further explore the PTK7-regulated signaling pathway in glioma cells, we compared the gene expression profiles following PTK7 knockdown in 3 glioma cell lines (LN18, SF295, GIC-7). We found that several genes (eg, Id1, Bcl-XL, CAV1, ANXA11, and NRP1) were downregulated, while some genes (Bim, NLGN3, ASCL1, BMP7) were upregulated, as compared with the scramble control (P < .05) (Fig. 3A). Id1 is a critical transcriptional factor closely linked to tumorigenesis as well as cell proliferation and survival. Id1 protein promotes cell proliferation and cell cycle progression through activation of growth-promoting pathways in certain cancer cells. Herein, we showed that PTK7 depletion decreased Id1 mRNA and protein expression as compared with scramble control in the LN18, SF295, and GIC-7 glioma cell lines (Fig. 3B). Moreover, Id1 knockdown suppressed the cell proliferation and colony formation and induced apoptosis in the LN18, SF295, and GIC-7 glioma cell lines (Fig. 3C–E). However, knockdown of Bcl-XL, CAV1, ANXA11, and NRP1 did not affect cell proliferation in LN18 cells (Supplementary, Fig. S4). In order to confirm the function of Id1 in PTK7 signaling, we constitutively expressed Id1 in PTK7-depleted LN18 and SF295 glioma cells (Fig. 3F). We showed that Id1 overexpression restored the cell proliferation and soft agar colony formation attenuated by PTK7 depletion (Fig. 3G and H). Therefore, PTK7 might regulate Id1-mediated cell proliferation and tumorigenesis in CD44-high glioma cell lines.
Fig. 3.
Id1 is a potential downstream effector of PTK7 signaling. (A) PTK7 knockdown altered gene expression in LN18, SF295 and GIC-7 glioma cells. (B) PTK7 depletion attenuated Id1 mRNA and protein expression in LN18, SF295 and GIC-7 glioma cells. *P < .05, as compared with scramble (Scr) control. (C) Id1 knockdown attenuated glioma cell proliferation in LN18, SF295, and GIC-7 glioma cells. *P < .05, as compared with scramble (Scr) control. (D) Id1 knockdown impaired soft agar colony formation in LN18, SF295, and GIC-7 glioma cells. *P < .05, as compared with scramble (Scr) control. (E) Id1 knockdown induced apoptosis in LN18, SF295, and GIC-7 glioma cells. *P < .05, as compared with scramble (Scr) control. (F–H) Overexpression of Id1 rescued cell proliferation and colony formation attenuated by PTK7 depletion. *P < .05, as compared with scramble (Scr) control. **P < .05, as compared with shPTK7 group.
PTK7 Upregulates Id1 to Induce Anchorage-independent Growth in Normal Human Astrocytes
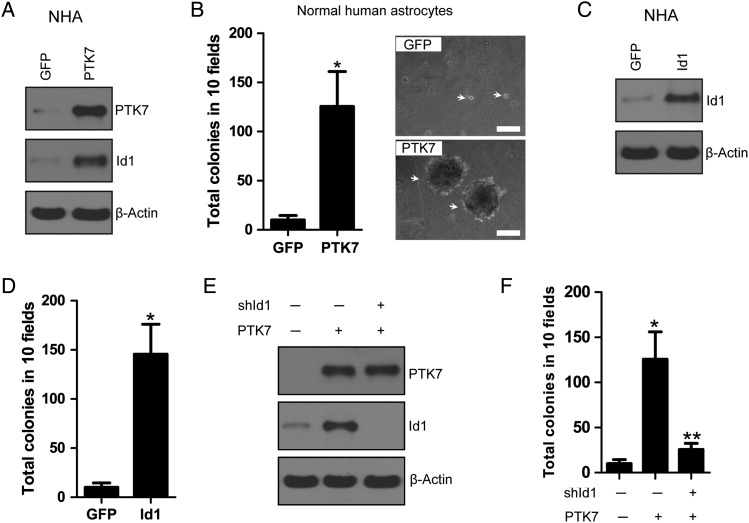
The differential expression of PTK7 expression in gliomas and normal brain tissues sparked our interest into looking at the potential role of PTK7 in gliomagenesis. Anchorage-independent growth is a crucial event in tumor initiation and progression.31 However, the oncogenic potential of PTK7 as a single gene in nontransformed cells remained undetermined. We overexpressed PTK7 in NHAs and found that PTK7 overexpression enhanced Id1 expression in NHAs as compared with GFP control (Fig. 4A). As noted, the parental NHA cells did not form colonies in soft agar, while overexpression of PTK7 in NHAs was able to grow and form colonies in soft agar (P < .05), suggesting that PTK7 might exert a crucial function in promoting anchorage-independent growth (Fig. 4B). We overexpressed Id1 in NHA and found that Id1 overexpression also promoted anchorage-independent growth in NHAs (Fig. 4C and D). Further, we showed that the effect of PTK7 on NHAs might be mediated by Id1 because Id1 knockdown attenuated PTK7 effect on anchorage-independent growth in NHAs (Fig. 4E and F). These results suggested that PTK7 regulates Id1-mediated anchorage-independent growth in NHAs.
Fig. 4.
PTK7 up-regulates Id1 to induce anchorage-independent growth in normal human astrocytes. (A) Overexpression of PTK7 upregulated Id1 expression in normal human astrocytes. (B) PTK7 promoted anchorage-independent growth in normal human astrocytes. *P < .05, as compared with GFP control. Bars: 100 μm. (C and D) Overexpression of Id1 promoted anchorage-independent growth in normal human astrocytes. *P < .05, as compared with GFP control. (E and F) Knockdown of Id1 attenuated PTK7-induced anchorage-independent growth in normal human astrocytes. *P < .05, as compared with GFP control. **P < .05, as compared with PTK7-expressing group.
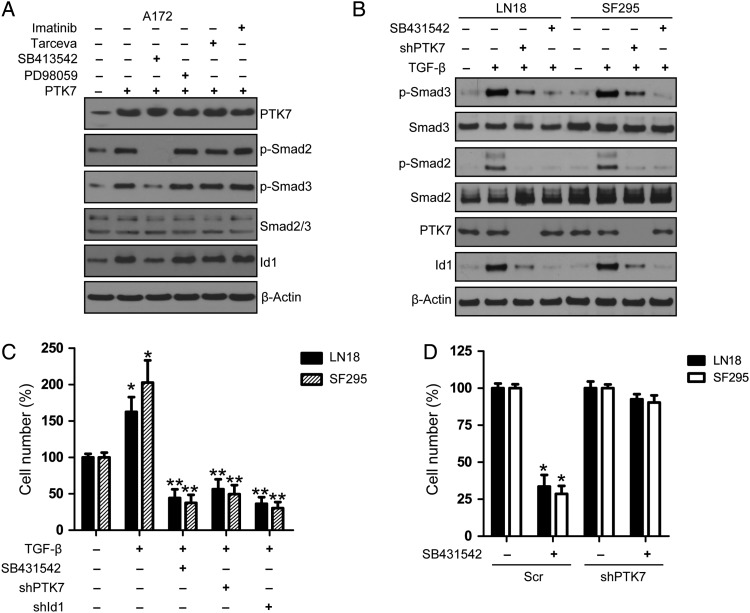
PTK7 Regulates Id1 Expression Through Modulating TGF-β/Smad Signaling Pathway
TGF-β signaling promotes cell proliferation and tumorigenesis in CD44-high GICs through upregulation of Id1 levels, therefore enhancing the capacity of cells to initiate tumors.25 Similarly, we showed that TGF-β/Smad signaling induced Id1 expression in LN18 and SF295 glioma cells (Supplementary, Fig. S5). Overexpression of PTK7 enhanced TGF-β/Smad signaling and upregulated Id1 expression in A172 glioma cells, which was blocked by TGF-β signaling inhibitor SB413542 (Fig. 5A). Similar to TGF-β signaling inhibitor, PTK7 depletion attenuated TGF-β/Smad signaling and reduced Id1 expression in LN18 and SF295 cells (Fig. 5B). Cell proliferation analysis showed that TGF-β promoted glioma cell proliferation in LN18 and SF295 cells, which was attenuated by TGF-β signaling inhibitor SB413542 or PTK7/Id1 knockdown (Fig. 5C). Although c-Myc was shown to regulate Id1 expression in breast cancer cells, we did not observe any reduction of Id1 expression following knockdown of c-Myc in the LN18 and T98G cell lines (Supplementary, Fig. S6A and S6B). In order to confirm that PTK7's effect on glioma cell proliferation is mediated by TGFβ/Smad signaling, we treated PTK7-expressing or PTK7-depleted glioma cells (LN18, SF295) with TGFβ receptor inhibitor SB431542. (The number of viable cells in PTK7-expressing or PTK7-depleted cells without SB431542 treatment was regarded as 100%.) We showed that SB431542 markedly inhibited cell proliferation in PTK7-expressing LN18 and SF295 cells. In contrast, we only observed a mild inhibitory effect of SB431542 on cell proliferation in PTK7-depleted isogenic cells (Fig. 5D). These results suggest that PTK7 might regulate glioma cell proliferation via modulation of TGFβ/Smad/Id1 signaling.
Fig. 5.
PTK7 regulates Id1 expression via TGF-β/Smad pathway. (A) TGF-β receptor inhibition attenuated PTK7-induced Id1 expression in A172 glioma cells. After PTK7 transfection, A172 cells were incubated with various inhibitors for PDGFRα (imatinib, 5 μM), Tarceva (EGFR, 5 μM), TGF-β receptor inhibitor (SB413542, 10 μM), MEK/ERK (PD98059, 10 μM) for 24 hours. (B) PTK7 regulates Id1 expression via TGF-β/Smad pathway. After serum starvation for 36 hours, glioma cells (Scr or shPTK7) were treated with TGF-β (10 ng/mL) for 24 hours. SB413542 was used as a positive control for the blockade of TGF-β signaling. (C) PTK7/Id1 knockdown attenuated TGF-β-induced cell proliferation in glioma cell lines LN18 and SF295. *P < .05, as compared with no-TGF-β control. **P < .05, as compared with TGF-β-stimulated group. (D) SB431542 effects on PTK7-expressing or PTK7-depleted glioma cells. The number of viable cells in PTK7-expressing or PTK7-depleted cells without SB431542 treatment was regarded as 100%. *P < .05, as compared with no drug control in PTK7-expressing glioma cells.
Targeting PTK7 Attenuates in Vivo Tumor Growth and Prolongs Tumor-bearing Mice Survival
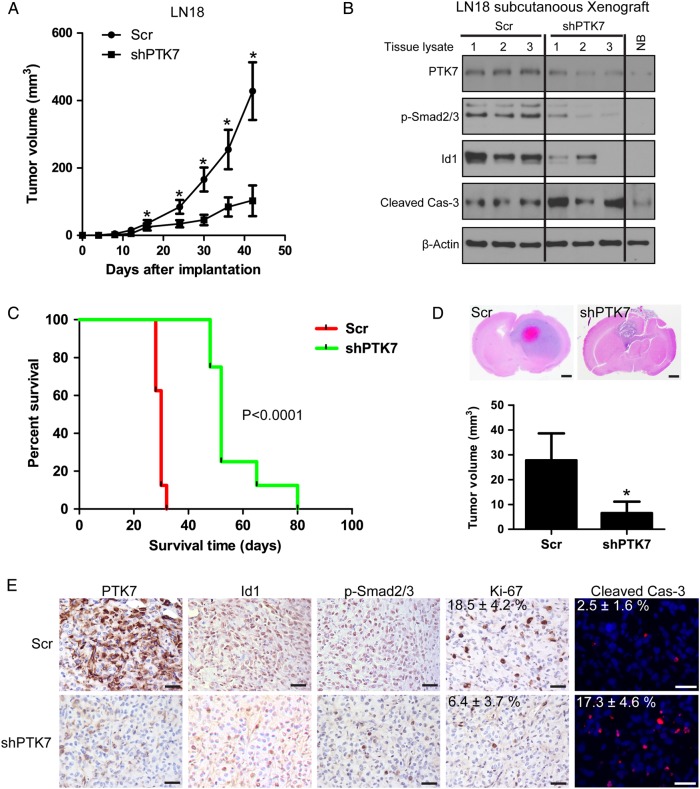
The in vitro findings that targeting PTK7 suppressed cell proliferation, impaired tumorigenesis, and induced apoptosis in CD44-high glioma cell lines were carried over to observations with in vivo studies. The effect of PTK7 depletion was examined in mice with LN18 glioblastoma subcutaneous xenografts. The group of mice implanted with LN18/Scr glioma cells developed tumors more rapidly than those with LN18/shPTK7 cells. The tumor volume in shPTK7 groups was significantly smaller than the Scr group (P < .05) (Fig. 6A). Western blotting analysis showed that PTK7 depletion attenuated p-Smad2/3 and Id1 expression and induced caspase-3 activation in xenograft lysates (Fig. 6B).
Fig. 6.
Targeting PTK7 attenuates in vivo tumor growth and prolongs tumor-bearing mice survival. (A) PTK7 depletion attenuated subcutaneous tumor growth. *P < .05, as compared with scramble (Scr) control. (B) Western blotting analysis on tumor lysates from subcutaneous tumor with PTK7 depletion at day 30 after implantation. (NB, normal mouse brain lysate). (C) Targeting PTK7 prolongs tumor-bearing mice survival in an orthotopic xenograft model. (D) PTK7 depletion attenuates in vivo tumor growth in an orthotopic xenograft model. *P < .05, as compared with scramble (Scr) control. Bars: 1 mm. (E) Immunostaining of the intracranial tumors at day 25 after implantation. The tissue sections were incubated with antibodies against indicated antibodies (PTK7, p-Smad2/3, Id1, Ki-67). Diaminobenzidine was used as a chromogen, followed by counterstaining with hematoxylin. Incidence of apoptosis was determined with TUNEL staining. Bar, 50 μm. Ki-67 and TUNEL, Scr versus shPTK7; P < .05.
We next evaluated the effect of PTK7 depletion in the intracranial xenograft model. The animals were intracranially inoculated with LN18 glioma cells with or without PTK7 knockdown (n = 13). The primary endpoint was to evaluate animal survival (n = 8). In addition, we also examined the in vivo effect of PTK7 depletion on the expression of PTK7-related signaling molecules (n = 5). The median duration of survival of the LN18/Scr group was 30 days. The median duration of survival for animals in the LN18/shPTK7 group was extended to 52 days (Scr vs shPTK7, P < .0001) (Fig. 6C). Next, we investigated whether Id1 was reduced by the effect of PTK7 knockdown in vivo. For this purpose, tumor-bearing mice from each group (n = 5) were euthanized 21 days after the treatment started. H&E staining on mice brain sections revealed apparent intratumoral necrosis and larger tumor bulk in the LN18/Scr group, as compared with the LN18/shPTK7 group (Fig. 6D). Xenografts in the LN18/shPTK7 group showed decreased PTK7, p-Smad2/3, Ki-67, and Id1 expression in the tumors, as compared with the LN18/Scr control group (Fig. 6E). TUNEL analyses identified an elevated percentage of apoptotic cells in the PTK7-depleted tumors (Fig. 6E). Therefore, PTK7 might serve as a potential therapeutic target for combatting CD44-high glioma.
Discussion
While GBM has a poor prognosis overall, molecular subsets of tumors exist with varying outcomes to initial treatment. CD44 is a cell-surface marker associated with tumor progression and treatment resistance in glioma.19,22 Heidi et al showed that CD44 is a typical biomarker for mesenchymal subtype glioma.32 Recently, Anido et al revealed the crucial role of CD44-high glioma stem cells in tumor initiation and progression.25 In this study, we found that PTK7 is a novel molecule that is highly expressed in CD44-high GBM and human glioma cell lines. Knockdown of PTK7 expression suppressed glioma cell proliferation, impaired tumorigenic potential, and induced apoptosis. We also observed high PTK7 expression in CD44-high GIC lines. PTK7 depletion attenuated the tumorsphere formation and growth of these GICs. These findings suggested that PTK7 might be a potential regulator and potential target in CD44-high glioma.
DNA-binding protein inhibitor (Id1) is an important basic helix-loop-helix transcriptional factor regulating the stem cell maintenance of embryonic stem cells and B1 type adult neural stem cells.33,34 Soroceanu et al showed that Id1 expression levels correlated positively with glioma cell invasiveness in culture and with histopathological grades in patient biopsies.35 Genetic knockdown of Id1 leads to a significant increase in survival in an orthotopic model of human GBM, suggesting that Id1 represents a novel and promising strategy for improving the therapy and outcome of GBM patients.35 Anido et al also identified a cell population enriched for glioma stem cells that expressed high levels of CD44 and Id1, suggesting the potential role of Id1 in CD44-high glioma.25 Our findings showed that Id1 is a downstream molecule regulated by PTK7. PTK7 knockdown reduced Id1 expression in CD44-high glioma cell lines, while overexpression of Id1 restored the cell proliferation and tumorigenic potential attenuated by PTK7 knockdown. We also showed that PTK7 enhanced anchorage-independent growth in normal human astrocytes, which was attenuated by Id1 knockdown. These results suggested that Id1 might be an important mediator for PTK7-regulated glioma cell proliferation, tumorigenesis, and cell transformation.
Tumor cells often use TGF-β signaling to increase EMT, invasion, and metastasis.36,37 Although TGF-β signaling suppresses proliferation of certain carcinoma cells and is well known to be a tumor suppressor, it promotes proliferation of tumors of nonepithelial origin, including glioma and osteosarcoma.38,39 In addition to induction of proliferation, the TGF-β pathway has also been implicated in invasion, tumor growth, and intratumoral angiogenesis of glioma.40 These multiple roles of TGF-β in glioma progression have promoted the development of therapeutic agents based on the inhibition of the TGF-β pathway.41 The inhibition of the TGF-β pathway impaired the maintenance of CD44-high glioma stem cell population through repression of Id1 expression, therefore inhibiting the capacity of cells to initiate tumors.25 Swarbrick et al showed that c-Myc regulates Id1 expression in breast cancer cells.42 However, c-Myc effects is often antagonized by TGF-β/Smad signaling, which suggests that TGF-β/Smad or c-Myc might differentially regulate Id1 expression in different cell systems or tissue types.43,44 In this study, we showed that PTK7 regulates Id1 expression by modulating the TGF-β pathway in CD44-high glioma cell lines. We also showed that knockdown of c-Myc did not affect Id1 expression in our glioma cell models. These results suggested that PTK7 might regulate Id1 expression through modulating TGF-β/Smad signaling rather than c-Myc in CD44-high glioma cells. Further, we showed that TGF-β receptor inhibitor SB431542 markedly inhibited the cell proliferation in PTK7-expressing glioma cells, rather than in PTK7-depleted isogenic glioma cells, which might suggest that PTK7 regulates glioma cell proliferation via modulating TGFβ/Smad/Id1 signaling. Therefore, PTK7 could be a novel target for suppressing TGF-β/Id1-mediated cell proliferation in CD44-high glioma.
In the present study, the finding that PTK7 regulates the cell proliferation and tumorigenesis of CD44-high glioma cells sparked our interest in testing the effect of PTK7 depletion on in vivo glioma models. Our results indicated that PTK7 depletion suppresses glioma growth and induces apoptosis in a subcutaneous transplant glioma model. The significance of these observations (ie, the inhibition on cell proliferation and the induction of apoptosis in CD44-high glioma cell line following PTK7 knockdown) were also evaluated with an in vivo orthotopic glioma model. The benefits of PTK7 depletion were confirmed in vivo, with more of the mice in the PTK7 knockdown group surviving long term than those in the control group. Mice bearing glioma xenografts with PTK7 knockdown also demonstrated reduced p-Smad2/3, Id1, and Ki-67 positivity and induced apoptosis. These results would identify PTK7 as a novel therapeutic target that may improve the treatment of CD44-high GBM.
Supplementary Material
Funding
This work was supported by National Natural Science Foundation of China (No. 30901539).
Supplementary Material
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Cancer Genome Atlas Research Network, 231 Collaborators. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lhoumeau AC, Puppo F, Prébet T, et al. PTK7: a cell polarity receptor with multiple facets. Cell Cycle. 2011;10(8):1233–1236. doi: 10.4161/cc.10.8.15368. [DOI] [PubMed] [Google Scholar]
- 4.Caddy J, Wilanowski T, Darido C, et al. Epidermal wound repair is regulated by the planar cell polarity signaling pathway. Dev Cell. 2010;19(1):138–147. doi: 10.1016/j.devcel.2010.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mossie K, Jallal B, Alves F, et al. Colon carcinoma kinase-4 defines a new subclass of the receptor tyrosine kinase family. Oncogene. 1995;11(10):2179–2184. [PubMed] [Google Scholar]
- 6.Lu X, Borchers AG, Jolicoeur C, et al. PTK7/CCK-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430(6995):93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 7.Shin WS, Maeng YS, Jung JW, et al. Soluble PTK7 inhibits tube formation, migration, and invasion of endothelial cells and angiogenesis. Biochem Biophys Res Commun. 2008;371(4):793–798. doi: 10.1016/j.bbrc.2008.04.168. [DOI] [PubMed] [Google Scholar]
- 8.Shnitsar I, Borchers A. PTK7 recruits dsh to regulate neural crest migration. Development. 2008;135(24):4015–4024. doi: 10.1242/dev.023556. [DOI] [PubMed] [Google Scholar]
- 9.Wehner P, Shnitsar I, Urlaub H, et al. RACK1 is a novel interaction partner of PTK7 that is required for neural tube closure. Development. 2011;138(7):1321–1327. doi: 10.1242/dev.056291. [DOI] [PubMed] [Google Scholar]
- 10.Puppo F, Thomé V, Lhoumeau AC, et al. Protein tyrosine kinase 7 has a conserved role in Wnt/β-catenin canonical signalling. EMBO Rep. 2011;12(1):43–49. doi: 10.1038/embor.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes M, Naito M, Daulat A, et al. Ptk7 promotes non-canonical Wnt/PCP-mediated morphogenesis and inhibits Wnt/β-catenin-dependent cell fate decisions during vertebrate development. Development. 2013;140(8):1807–1818. doi: 10.1242/dev.090183. [DOI] [PubMed] [Google Scholar]
- 12.Prebet T, Lhoumeau AC, Arnoulet C, et al. The cell polarity PTK7 receptor acts as a modulator of the chemotherapeutic response in acute myeloid leukemia and impairs clinical outcome. Blood. 2010;116(13):2315–2323. doi: 10.1182/blood-2010-01-262352. [DOI] [PubMed] [Google Scholar]
- 13.Kapoor S. Emerging new prognostic markers of gastric tumors besides PTK7. J Surg Oncol. 2013;107(4):450. doi: 10.1002/jso.23209. [DOI] [PubMed] [Google Scholar]
- 14.Jiang G, Zhang M, Yue B, et al. PTK7: a new biomarker for immunophenotypic characterization of maturing T cells and T cell acute lymphoblastic leukemia. Leuk Res. 2012;36(11):1347–1353. doi: 10.1016/j.leukres.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gärtner S, Gunesch A, Knyazeva T, et al. PTK 7 is a transforming gene and prognostic marker for breast cancer and nodal metastasis involvement. PLoS One. 2014;9(1):e84472. doi: 10.1371/journal.pone.0084472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gobble RM, Qin LX, Brill ER, et al. Expression profiling of liposarcoma yields a multigene predictor of patient outcome and identifies genes that contribute to liposarcomagenesis. Cancer Res. 2011;71(7):2697–2705. doi: 10.1158/0008-5472.CAN-10-3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng L, Sefah K, O'Donoghue MB, et al. Silencing of PTK7 in colon cancer cells: caspase-10-dependent apoptosis via mitochondrial pathway. PLoS One. 2010;5(11):e14018. doi: 10.1371/journal.pone.0014018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee HK, Chauhan SK, Kay E, et al. Flt-1 regulates vascular endothelial cell migration via a protein tyrosine kinase-7-dependent pathway. Blood. 2011;117(21):5762–5771. doi: 10.1182/blood-2010-09-306928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merzak A, Koocheckpour S, Pilkington GJ. CD44 mediates human glioma cell adhesion and invasion in vitro. Cancer Res. 1994;54(15):3988–3992. [PubMed] [Google Scholar]
- 20.Knüpfer MM, Poppenborg H, Hotfilder M, et al. CD44 expression and hyaluronic acid binding of malignant glioma cells. Clin Exp Metastasis. 1999;17(1):71–76. doi: 10.1023/a:1026425519497. [DOI] [PubMed] [Google Scholar]
- 21.Wiranowska M, Ladd S, Moscinski LC, et al. Modulation of hyaluronan production by CD44 positive glioma cells. Int J Cancer. 2010;127(3):532–542. doi: 10.1002/ijc.25085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida T, Matsuda Y, Naito Z, et al. CD44 in human glioma correlates with histopathological grade and cell migration. Pathol Int. 2012;62(7):463–470. doi: 10.1111/j.1440-1827.2012.02823.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Xie J, Guo J, et al. Evaluation of CD44 and CD133 as cancer stem cell markers for colorectal cancer. Oncol Rep. 2012;28(4):1301–1308. doi: 10.3892/or.2012.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ponti D, Costa A, Zaffaroni N, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005;65(13):5506–5511. doi: 10.1158/0008-5472.CAN-05-0626. [DOI] [PubMed] [Google Scholar]
- 25.Anido J, Sáez-Borderías A, Gonzàlez-Juncà A, et al. TGF-β Receptor Inhibitors Target the CD44(high)/Id1(high) Glioma-Initiating Cell Population in Human Glioblastoma. Cancer Cell. 2010;18(6):655–668. doi: 10.1016/j.ccr.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9(5):391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 27.Ushio K, Hashimoto T, Kitamura N, et al. Id1 is down-regulated by hepatocyte growth factor via ERK-dependent and ERK-independent signaling pathways, leading to increased expression of p16INK4a in hepatoma cells. Mol Cancer Res. 2009;7(7):1179–1188. doi: 10.1158/1541-7786.MCR-08-0289. [DOI] [PubMed] [Google Scholar]
- 28.Jin L, Wessely O, Marcusson EG, et al. Prooncogenic factors miR-23b and miR-27b are regulated by Her2/Neu, EGF, and TNF-α in breast cancer. Cancer Res. 2013;73(9):2884–2896. doi: 10.1158/0008-5472.CAN-12-2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu J, Yang QY, Sai K, et al. TGM2 inhibition attenuates ID1 expression in CD44-high glioma-initiating cells. Neuro Oncol. 2013;15(10):1353–1365. doi: 10.1093/neuonc/not079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verhaak RG, Hoadley KA, Purdom E, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cifone MA. In vitro growth characteristics associated with benign and metastatic variants of tumor cells. Cancer Metastasis Rev. 1982;1(4):335–347. doi: 10.1007/BF00124216. [DOI] [PubMed] [Google Scholar]
- 32.Phillips HS, Kharbanda S, Chen R, et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. 2006;9(3):157–173. doi: 10.1016/j.ccr.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 33.Romero-Lanman EE, Pavlovic S, Amlani B, et al. Id1 maintains embryonic stem cell self-renewal by up-regulation of Nanog and repression of Brachyury expression. Stem Cells Dev. 2012;21(3):384–393. doi: 10.1089/scd.2011.0428. [DOI] [PubMed] [Google Scholar]
- 34.Nam HS, Benezra R. High levels of Id1 expression define B1 type adult neural stem cells. Cell Stem Cell. 2009;5(5):515–526. doi: 10.1016/j.stem.2009.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Soroceanu L, Murase R, Limbad C, et al. Id-1 is a key transcriptional regulator of glioblastoma aggressiveness and a novel therapeutic target. Cancer Res. 2013;73(5):1559–1569. doi: 10.1158/0008-5472.CAN-12-1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Padua D, Massagué J. Roles of TGFbeta in metastasis. Cell Res. 2009;19(1):89–102. doi: 10.1038/cr.2008.316. [DOI] [PubMed] [Google Scholar]
- 37.Taylor MA, Lee YH, Schiemann WP. Role of TGF-beta and the tumor microenvironment during mammary tumorigenesis. Gene Expr. 2011;15(3):117–132. doi: 10.3727/105221611x13176664479322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruna A, Darken RS, Rojo F, et al. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11(2):147–160. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 39.Matsuyama S, Iwadate M, Kondo M, et al. SB-431542 and Gleevec inhibit transforming growth factor-beta-induced proliferation of human osteosarcoma cells. Cancer Res. 2003;63(22):7791–7798. [PubMed] [Google Scholar]
- 40.Wick W, Platten M, Weller M. Glioma cell invasion: regulation of metalloproteinase activity by TGF-beta. J Neurooncol. 2001;53(2):177–185. doi: 10.1023/a:1012209518843. [DOI] [PubMed] [Google Scholar]
- 41.Joseph JV, Balasubramaniyan V, Walenkamp A, et al. TGF-β as a therapeutic target in high grade gliomas – promises and challenges. Biochem Pharmacol. 2013;85(4):478–485. doi: 10.1016/j.bcp.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 42.Swarbrick A, Akerfeldt MC, Lee CS, et al. Regulation of cyclin expression and cell cycle progression in breast epithelial cells by the helix-loop-helix protein Id1. Oncogene. 2005;24(3):381–389. doi: 10.1038/sj.onc.1208188. [DOI] [PubMed] [Google Scholar]
- 43.Frederick JP, Liberati NT, Waddell DS, et al. Transforming growth factor beta-mediated transcriptional repression of c-myc is dependent on direct binding of Smad3 to a novel repressive Smad binding element. Mol Cell Biol. 2004;24(6):2546–2559. doi: 10.1128/MCB.24.6.2546-2559.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yagi K, Furuhashi M, Aoki H, et al. c-myc is a downstream target of the Smad pathway. J Biol Chem. 2002;277(1):854–861. doi: 10.1074/jbc.M104170200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.