Abstract
Background
Various tumor characteristics have been associated with neurocognitive functioning (NCF), though the role of tumor grade has not been adequately examined.
Methods
Seventy-two patients with histologically confirmed grade IV glioma (n = 37), grade III glioma (n = 20), and grade II glioma (n = 15) in the left temporal lobe completed preoperative neuropsychological assessment. Rates of impairment and mean test performances were compared by tumor grade with follow-up analysis of the influence of other tumor- and patient-related characteristics on NCF.
Results
NCF impairment was more frequent in patients with grade IV tumor compared with patients with lower-grade tumors in verbal learning, executive functioning, as well as language abilities. Mean performances significantly differed by tumor grade on measures of verbal learning, processing speed, executive functioning, and language, with the grade IV group exhibiting worse performances than patients with lower-grade tumors. Group differences in mean performances remained significant when controlling for T1-weighted and fluid attenuated inversion recovery MRI-based lesion volume. Performances did not differ by seizure status or antiepileptic and steroid use.
Conclusions
Compared with patients with grade II or III left temporal lobe glioma, patients with grade IV tumors exhibit greater difficulty with verbal learning, processing speed, executive functioning, and language. Differences in NCF associated with glioma grade were independent of lesion volume, seizure status, and antiepileptic or steroid use, lending support to the concept of “lesion momentum” as a primary contributor to deficits in NCF of newly diagnosed patients prior to surgery.
Keywords: brain tumor, cognition, glioma, histopathology, neuropsychology
Glioma comprises the vast majority of all malignant primary brain tumors.1 These tumors often disrupt cerebral function through direct mechanisms such as destruction of brain tissue, as well as more indirect means, including compression, displacement, and ischemia.2 As a result, most patients exhibit some impairment of neurocognitive function (NCF), even early in the disease course.3,4 However, when compared with patients with acute lesions related to stroke or trauma, individuals with brain tumors often show better preservation of NCF.5 One explanation for this has been greater compensatory neuroplasticity in brain tumor patients as a response to the more insidiously developing neuropathology characteristic of cerebral tumors.6 It follows that fast-growing high-grade glioma may cause more profound NCF dysfunction than slower-growing lower-grade tumors, as greater cerebral reorganization may occur when neuronal damage happens at a slower rate. The difference in speed of tumor growth associated with varying histopathologies has been referred to as “tumor momentum.”7
In one of the very few studies directly investigating relationships between tumor grade and NCF, Hom and Reitan8 studied 92 patients with an even distribution of tumors across the 4 grades. Their study found greater NCF deficits in patients with grade III or IV tumors compared with those with more slowly growing grade I or II tumors. While informative, the investigation failed to account for important patient- and disease-specific factors that may also impact NCF in this population.9 These factors include demographic variables such as age, as well as tumor characteristics, including lesion size (ie, tumor volume). Specifically, the median age of the patients in the grade III or IV group was significantly older than patients with grade I or II tumors, and neither demographic adjustments nor statistical controls were performed to account for the age difference when analyzing tests of NCF. As such, aging effects may have contributed to the observed differences in NCF between groups. Additionally, without any information about lesion volume it is unknown whether patients with higher-grade tumors had larger lesion volume than patients with lower-grade tumors or vice versa.
Kayl and Meyers9 attempted to address some of the shortcomings of the Hom and Reitan study. They investigated NCF in 24 patients with glioblastoma (grade IV) and 24 patients with anaplastic astrocytoma (grade III) shortly after surgical resection. Patients were matched on demographic characteristics, including age, education, and gender, as well as tumor location. While NCF performances were significantly better in grade III patients, group differences did not remain significant after controlling for preoperative tumor volume. However, the Kayl and Meyers sample completed NCF testing after surgery, while the imaging variables were obtained preoperatively.
In a more recent study, Miotto and colleagues2 evaluated NCF in 27 glioma patients prior to surgical intervention. Their results showed that high-grade glioma patients exhibited greater impairment than low-grade patients, particularly within the executive functioning and memory domains. However, the study was limited by many of the problems noted with prior studies.8,9 Specifically, important clinical characteristics were not included in analyses (eg, lesion volume, seizure status), tumors were distributed throughout different brain regions across the 2 groups, and the sample size was very small in the high-grade group (n = 8). Additionally, the authors did not describe the composition of the groups. As such, the proportion of grade IV to grade III tumors in the high-grade group and the proportion of grade II to grade I tumors in the low-grade group are unclear.
In light of the limitations noted with the existing research, the nature of relationships between tumor grade and NCF remains largely unsettled, and focused investigations with fewer potential confounds are needed. The present study investigated NCF in newly diagnosed, treatment-naïve patients with glioma restricted to the left temporal lobe (LTL) while accounting for relevant patient and tumor characteristics where possible.
Materials and Methods
Participants
Adult patients with glioma involving the LTL who had presurgical neuropsychological assessment were identified in The University of Texas M.D. Anderson Cancer Center (MDACC) neuropsychology and neurosurgery databases. Patients with a history of open surgical resection, radiotherapy, or any type of chemotherapy prior to completion of NCF testing were excluded. Those with history of other neurological disease were also excluded, with the exception of seizure disorder secondary to tumor. A single neurosurgeon (M.Z.) reviewed MRI scans of the identified patients. Cases were considered for inclusion if they had unifocal LTL tumors, as indicated on T1-weighted, gadolinium-enhanced T1-weighted, or fluid attenuated inversion recovery (FLAIR) sequences. Patients were excluded if tumors extended beyond the bounds of the LTL on T1-weighted imaging sequences. Seventy-two patients were identified who underwent detailed presurgical neuropsychological evaluation between 2001 and 2010. Specialist neuropathologists at MDACC made the histopathological diagnoses. The resulting sample comprised 37 patients with grade IV tumors, 20 patients with grade III tumors, and 15 patients with grade II tumors. The MDACC institutional review board approved the retrospective chart review for this study.
Temporal Lobe Segmentation
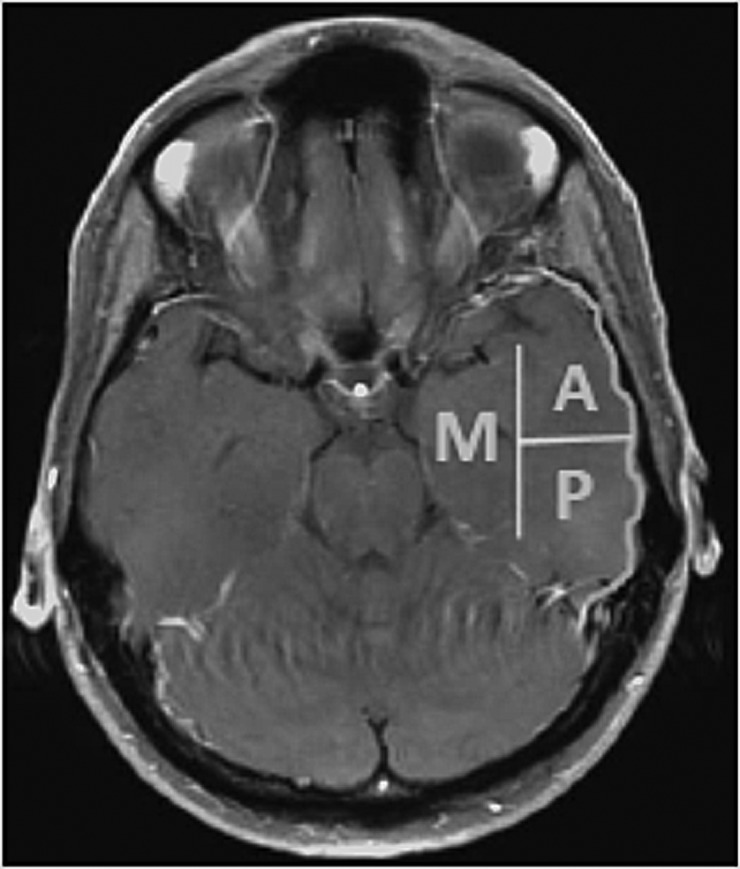
Three distinct temporal lobe areas were defined to describe tumor location, including the lateral anterior, lateral posterior, and medial regions (see Fig. 1). The anterior region comprised the temporal pole in addition to the area lateral to the temporal horn of the ventricle extending ∼30–35 mm posteriorly from the pole. The posterior region consisted of the lateral region extending posteriorly from that point, not to exceed 99 mm from the temporal pole. The medial region was designated as the area medial to the temporal horn of the lateral ventricle, including the hippocampal formation and parahippocampal gyrus. Tumors were described as localized in a region based on their extension on T1-weighted or gadolinium-enhanced T1-weighted MRI. The tumor boundary was defined as the entire occupied space on T1-weighted images or the enhancing area on gadolinium-enhanced T1-weighted images. For cases in which the temporal horn was compressed, estimated locations were obtained using the horn of the tumor-free right temporal lobe as a reference point. A fourth group, multiregion, included tumors with extension into 2 or more regions. More precise segmentation was attempted but was not useful due to sample size restrictions.
Fig. 1.
Left temporal lobe segmentation. A, lateral anterior; P, lateral posterior; M, medial.
Lesion Size
Volumetric analysis was performed on MRI scans with MedVision 1.41 software, as previously described.10 T1-weighted volume was defined as the greater of the hypointense region on T1-weighted MRI, or the hyperintense area on gadolinium-enhanced T1-weighted MRI. FLAIR volume was defined as the area of hyperintensity identified on the FLAIR MRI sequence.
Neurocognitive Assessment
NCF testing was conducted by a neuropsychologist or a trained neuropsychology staff member (ie, a psychometrist or neuropsychology fellow) supervised by a neuropsychologist, as part of a comprehensive presurgical evaluation for clinical purposes. Table 1 lists the neuropsychological tests by domain that were routinely included in the clinical test battery. The number of patients administered a given NCF measure differed by instrument, as the patient evaluations utilized a flexible battery and were performed for clinical purposes. Sample sizes are described by measure in the table and figure accompanying the results. Approximately half of the total sample did not have data for the Hopkins Verbal Learning Test–Revised (HVLT-R) Delayed Recall (DR) and HVLT-R Recognition (Recog) variables, as clinic practices initially utilized an earlier version of the HVLT that did not include the delayed memory trials. Nonetheless, HVLT-R Total Recall (TR) trials are identical between versions, and HVLT-R normative data were used for all HVLT variables.
Table 1.
Neurocognitive tests grouped by principal domain
| Measure | Abbreviations | Norms |
|---|---|---|
| Attention | ||
| WAIS-R/III Digit Span | Digit Span | Wechsler, 1981; Wechsler, 1997 |
| Learning and Memory | ||
| HVLT-R Total Recall | HVLT-R TR | Benedict, Schretlen, Groninger, & Brandt, 1998 |
| HVLT-R Delayed Recall | HVLT-R DR | Benedict, Schretlen, Groninger, & Brandt, 1998 |
| HVLT-R Recognition Discrimination | HVLT-R Recog | Benedict, Schretlen, Groninger, & Brandt, 1998 |
| Processing Speed | ||
| WAIS-R/III Digit Symbol | Digit Symbol | Wechsler, 1981; Wechsler, 1997 |
| Trail Making Test Part A | TMTA | Tombaugh, 2004 |
| Executive Function | ||
| Trail Making Test Part B | TMTB | Tombaugh, 2004 |
| WAIS-R/III Similarities | Similarities | Wechsler, 1981; Wechsler, 1997 |
| MAE Controlled Oral Word Association | COWA | Ruff, Light, Parker, & Levin, 1996 |
| Language | ||
| MAE Token Test | Token | Benton, Hamsher, & Sivan, 2000 |
| MAE Visual Naming Test or Boston Naming Test | Naming | Benton, Hamsher, & Sivan, 2000; Heaton, Miller, Taylor, & Grant, 2004 |
| Visuospatial Function | ||
| WAIS-R/III Block Design | Block Design | Wechsler, 1981; Wechsler, 1997 |
| Motor Function | ||
| Grip Strength Difference | Grip | Heaton, Miller, Taylor, & Grant, 2004 |
| Grooved Pegboard Difference | Peg | Trites, 1977 |
| Clinical Trial Battery Composite | CTB Comp | Mean of z-scores from the HVLT-R, COWA, and TMT |
Abbreviations: WAIS-R, Wechsler Adult Intelligence Scale–Revised; WAIS-III, Wechsler Adult Intelligence Scale–Third Edition; MAE, Multilingual Aphasia Examination.
NCF test scores were standardized using published normative data,11–18 all of which were stratified by patient age, in addition to gender, handedness, and level of education when appropriate, and converted into z-scores (mean = 0, SD = 1). Per convention, performance on an individual NCF test that fell at or below a z-score of −1.5 was considered impaired. Grip Strength Difference and Grooved Pegboard Difference scores were calculated as the z-score of the right hand minus the z-score of the left hand. Motor performances were considered impaired if the right hand was at least 1.5 standard deviations less than the left. Additionally, a derived composite was calculated, referred to as the Clinical Trial Battery Composite (CTB Comp). The CTB Comp variable is the mean of the z-scores for the Controlled Oral Word Association (COWA), Trail-Making Test (TMT)A, TMTB, HVLT-R TR, HVLT-R DR, and HVLT-R Recog.19,20 CTB Comp z-scores that fell at or below −0.70 were considered impaired, based on the results of prior receiver operating characteristic analyses determining optimal classification of clinician-determined impairment ratings (unpublished data). Information concerning seizure status was obtained at the same clinic visit as NCF testing or from review of available medical records.
Statistical Analysis
Descriptive statistics for demographic and clinical variables were calculated as means and standard deviations or frequencies and percentages where appropriate. One-way analysis of variance (ANOVA) and Fisher's exact tests were used to compare differences in demographic and clinical characteristics among tumor grade groups. Rates of impairment were compared among grades II, III, and IV groups with Fisher's exact tests. Mean NCF test z-scores were compared across groups with 1-way ANOVA. Associations between lesion volume (T1-weighted and FLAIR) and NCF tests were determined with Spearman's rank-order correlations (ρ) for each of the 3 groups. Follow-up analyses of NCF test performances by tumor grade were performed with 1-way analysis of covariance (ANCOVA), simultaneously controlling for T1 and FLAIR lesion volume. Given the potential impact of seizures and antiepileptic or steroid use on NCF, test performances were analyzed by tumor grade and each of these clinical characteristics with 2-way ANOVA. For all significant ANOVAs, Tukey's honestly significant difference or Games–Howell tests were used for post hoc pairwise comparisons depending on the results of Levene's tests for homogeneity of variance. All statistical analyses were performed with SPSS 21.0 (IBM).21 Two-sided tests were used with a significance level of P ≤ .05.
Results
Demographic and Clinical Characteristics
Sample sociodemographic and clinical characteristics are presented by tumor grade in Table 2. Sex, ethnicity, education, and handedness did not significantly differ among groups. Age significantly differed, F(2, 69) = 15.90, P < .001, with post hoc analyses revealing that patients with grade II glioma were significantly younger than patients with grade III (P = .004) and grade IV (P < .000) glioma. Lesion location and volume on T1-weighted and FLAIR MRI did not significantly differ among groups, though a trend was observed in which patients with grade IV glioma exhibited larger lesions than the lower-grade groups. Seizure history also significantly differed among groups (P = .002, Fisher's exact test), with patients with grade IV glioma exhibiting the lowest rate of seizure disorder. Rates of antiepileptic and steroid use were similar across groups.
Table 2.
Demographic and clinical characteristics
| Glioma Grade |
||||
|---|---|---|---|---|
| II (n = 15) | III (n = 20) | IV (n = 37) | Pa | |
| Age, y | ||||
| Mean (SD) | 37.6 (13.4) | 51.1 (14.0) | 57.9 (9.6) | <.001* |
| Median | 35 | 49 | 60 | |
| Range | 18–66 | 20–78 | 31–71 | |
| Male, % | 46.7 | 55.0 | 59.5 | .703 |
| White, % | 86.7 | 95.0 | 83.8 | .713 |
| Right hand dominant, % | 80.0 | 85.0 | 89.2 | .672 |
| Education, y | ||||
| Mean (SD) | 13.8 (2.5) | 14.3 (2.5) | 15.1 (2.6) | .243 |
| Median | 14 | 16 | 16 | |
| Range | 8–18 | 7–18 | 11–20 | |
| Histology, % | ||||
| Glioblastoma | – | – | 97.3 | – |
| Oligodendroglioma | 40.0 | 30.0 | – | |
| Astrocytoma | 20.0 | 60.0 | – | |
| Mixed glioma | 13.3 | 5.0 | – | |
| Other | 26.7 | 5.0 | 2.7 | |
| Temporal Lobe Region, % | ||||
| Anterior | 13.3 | 25.0 | 16.2 | .660 |
| Posterior | 33.3 | 20.0 | 35.1 | |
| Medial | 46.7 | 45.0 | 29.7 | |
| Multi | 7.7 | 10.0 | 18.9 | |
| MRI Volume, mm3 | ||||
| T1-weighted, Mean (SD)b | 26.6 (22.3) | 16.5 (15.1) | 29.0 (29.8) | .201 |
| FLAIR, mean (SD)c | 36.1 (34.6) | 34.9 (34.1) | 59.8 (50.2) | .068 |
| Seizure history, % yes | 53.3 | 70.0 | 24.3 | .002* |
| Antiepileptic Drug,d % yes | 50.0 | 64.3 | 67.6 | .647 |
| Steroid,e % yes | 44.4 | 42.9 | 64.7 | .294 |
aContinuous variables compared with ANOVA; categorical variables compared with Fisher's exact test.
bII, n = 14.
cIV, n = 35.
dII, n = 10; III, n = 14.
eII, n = 9; III, n = 14; IV, n = 34.
*Significant difference, P ≤ .05: age (grade II < grade III & grade II < grade IV).
Neurocognitive Performances
Impairment was common in the overall sample, with 74% of patients exhibiting impaired performances on one or more measures. Impairment on at least one measure was identified in 60% of patients with grade II, 70% with grade III, and 81% with grade IV glioma, though the difference among groups was not statistically significant (P = .274, Fisher's exact test). Impairment rates on 2 or more measures (grade II, 27%; grade III, 20%; grade IV, 27%) and 3 or more measures (grade II, 13%; grade III, 15%; grade IV, 24%) were similar among groups (P > .05, Fisher's exact tests).
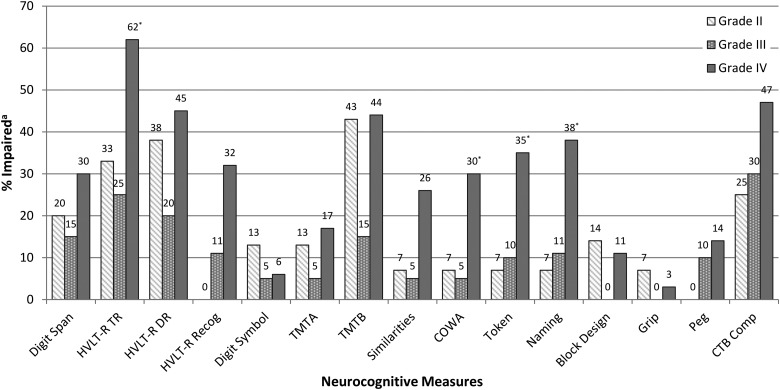
Rates of NCF impairment on individual tests are shown in Fig. 2. Patients with grade II glioma were most frequently impaired on indices of executive functioning (TMTB, 43%), learning and memory (HVLT-R TR, 33%; DR, 38%), auditory attention (Digit Span, 20%), and the composite (CTB Comp, 25%). Rates of impairment on all other measures fell below 15%. Patients with grade III glioma were most often impaired on measures of learning and memory (HVLT-R TR, 25%; DR, 20%), executive functioning (TMTB, 15%), auditory attention (Digit Span, 15%), and the composite (CTB Comp, 30%). Impairment rates were <15% on other tests for the grade III group. Patients with grade IV glioma were most commonly impaired on measures of learning and memory (HVLT-R TR, 62%; DR, 45%; Recog, 32%), executive functioning (TMTB, 44%; COWA, 30%; Similarities, 26%), expressive and receptive language (Naming, 38%; Token, 35%), auditory attention (Digit Span, 30%), processing speed (TMTA, 17%), and CTB Comp (47%). Rates of impairment were <15% on other tests for the grade IV group. Impairment frequency significantly differed across groups, as patients with grade IV glioma showed the greatest impairment frequency on measures of verbal learning (HVLT-R TR: P = .017, Fisher's exact test), executive functioning (COWA: P = .036, Fisher's exact test), receptive language (Token: P = .029, Fisher's exact test), and object naming (Naming: P = .022, Fisher's exact test).
Fig. 2.
Neurocognitive impairment by glioma grade. aImpairment defined as a z-score ≤ −1.5 for all individual measures. Impairment defined as a z-score ≤ −0.70 for CTB Comp. *Significant difference between groups, P ≤ .05; Fisher's exact tests. Sample sizes by group: Digit Span (II = 15; III = 20; IV = 37), HVLT-R TR (II = 15; III = 20; IV = 37), HVLT-R DR (II = 8; III = 10; IV = 20), HVLT-R Recog (II = 8; III = 10; IV = 19), Digit Symbol (II = 15; III = 20; IV = 36), TMTA (II = 15; III = 20; IV = 36), TMTB (II = 14; III = 20; IV = 34), Similarities (II = 14; III = 20; IV = 35), COWA (II = 15; III = 20; IV = 37), Token (II = 15; III = 20; IV = 37), Naming (II = 15; III = 19; IV = 37), Block Design (II = 14; III = 20; IV = 37), Grip (II = 15; III = 19; IV = 35), Peg (II = 15; III = 20; IV = 36), CTB Comp (II = 8; III = 10; IV = 19).
NCF test performances by glioma grade are described in Table 3. Performances significantly differed across groups on measures of verbal learning, HVLT-R TR: F(2, 69) = 6.91, P = .002; processing speed, Digit Symbol: F(2, 68) = 7.45, P = .001; executive functioning, COWA: F(2, 69) = 6.76, P = .002; auditory comprehension, Token: F(2, 69) = 6.44, P = 0.010; and object naming, Naming: F(2, 68) = 4.63, P = .013. Post hoc tests revealed that patients with grade IV glioma performed significantly worse than patients with grade III glioma on HVLT-R TR (P = .008), Digit Symbol (P = .001), COWA (P = .004), Token (P = .030), and Naming (P = .014). Patients with grade IV glioma also performed significantly below patients with grade II glioma on HVLT-R TR (P = .012), COWA (P = .038), and Token (P = .038). Patients with grade II glioma were significantly lower than patients with grade III glioma on Digit Symbol (P = .030), though all other performances were similar between patients with grades II and III glioma.
Table 3.
Neurocognitive performances by glioma grade
| Domain and Test | Glioma Grade |
Pa | |||||
|---|---|---|---|---|---|---|---|
| II |
III |
IV |
|||||
| N | z-Score Mean (SD) | N | z-Score Mean (SD) | N | z-Score Mean (SD) | ||
| Attention | |||||||
| Digit Span | 15 | −0.44 (0.66) | 20 | −0.47 (0.63) | 37 | −0.75 (0.78) | .236 |
| Learning and Memory | |||||||
| HVLT-R TR | 15 | −0.75 (1.32) | 20 | −0.81 (0.99) | 37 | −2.11 (1.79) | .002* |
| HVLT-R DR | 8 | −0.98 (1.83) | 10 | −0.88 (1.14) | 20 | −1.45 (2.19) | .688 |
| HVLT-R Recog | 8 | −0.25 (0.55) | 10 | −0.29 (1.27) | 19 | −1.27 (2.12) | .214 |
| Processing Speed | |||||||
| Digit Symbol | 15 | −0.16 (1.07) | 20 | 0.58 (0.90) | 36 | −0.30 (0.67) | .001* |
| TMTA | 15 | −0.36 (2.05) | 20 | −0.10 (1.05) | 36 | −0.49 (2.72) | .816 |
| Executive Function | |||||||
| TMTB | 14 | −1.53 (2.48) | 20 | −0.49 (2.53) | 34 | −1.88 (2.83) | .186 |
| Similarities | 14 | −0.24 (0.84) | 20 | −0.08 (0.63) | 35 | −0.46 (0.95) | .286 |
| COWA | 15 | −0.36 (0.85) | 20 | −0.19 (1.10) | 37 | −1.16 (1.08) | .002* |
| Language | |||||||
| Token | 15 | 0.16 (0.89) | 20 | 0.11 (0.87) | 37 | −0.72 (1.33) | .010* |
| Naming | 15 | −0.62 (0.93) | 19 | −0.31 (1.09) | 37 | −1.25 (1.25) | .013* |
| Visuospatial Function | |||||||
| Block Design | 14 | −0.36 (0.96) | 20 | 0.32 (0.83) | 37 | −0.17 (0.93) | .072 |
| Motor Function | |||||||
| Grip | 15 | 0.06 (1.12) | 19 | 0.07 (0.84) | 35 | −0.45 (0.82) | .063 |
| Peg | 15 | 0.27 (0.75) | 20 | 0.22 (1.35) | 36 | −0.43 (1.23) | .069 |
| CTB Comp | 8 | −0.38 (0.71) | 10 | −0.56 (0.94) | 19 | −0.97 (1.17) | .384 |
aOne-way ANOVA used for group comparisons. Post hoc comparisons performed with Tukey's honestly significant difference or Games–Howell tests.
*ANOVA significant, P ≤ .05; post hoc comparisons: HVLT-R TR (grade IV < III & grade IV < II), Digit Symbol (grade IV < grade III & grade II < grade III), COWA (grade IV < grade III & grade IV < grade II), Token (grade IV < grade III & grade IV < grade II), Naming (grade IV < grade III).
Clinical Characteristics and Neurocognitive Function
Performances did not significantly differ by seizure status and antiepileptic or steroid use, and there were no significant interaction effects between these variables and tumor grade. For patients with grade II glioma, Peg performance was significantly associated with both T1-weighted, ρ(12) = −0.56, P = .039, and FLAIR, ρ(13) = −0.60, P = .018, MRI volume. For patients with grade IV glioma, Peg performance was significantly associated with FLAIR MRI volume, ρ(32) = −0.36, P = .037. T1-weighted and FLAIR volumes were not significantly associated with any NCF test for patients with grade III glioma. Post hoc sensitivity analyses of significant differences in mean NCF test performances among glioma grade groups were performed with ANCOVA controlling for both T1-weighted and FLAIR MRI volumes. All significant group differences remained significant when controlling for lesion volume.
Discussion
To our knowledge, the present study represents the first characterization of relationships between tumor grade and NCF in treatment-naïve patients with glioma restricted to the LTL. Generally consistent with prior studies of NCF and glioma,3,4 impairment was common in the overall sample, with 74% of all patients exhibiting impairment on at least one measure. However, the rates of impairment on individual tests significantly differed across the glioma grade groups. Specifically, patients with grades II and III glioma tended to exhibit greatest frequency of impairment on measures of verbal learning and memory, auditory attention, and executive function. While patients with grade IV glioma also showed frequent impairment in these domains, they exhibited a broader range of cognitive dysfunction, including expressive and receptive language problems and processing speed, and nearly half of the group fell in the impaired range on the composite measure. Additionally, in comparison with patients with grades II and III glioma, those with grade IV glioma were significantly more frequently impaired on tests of verbal learning, executive functioning, and language.
A similar pattern was noted when comparing mean NCF performances across the 3 tumor grade groups. Specifically, patients with grade IV glioma exhibited the lowest mean scores across all measures, with significantly worse performances than patients with grade II or III glioma on measures of verbal learning, expressive and receptive language, and executive function. The grade IV group also demonstrated significantly more dysnomia and slower processing speed than patients with grade III glioma. Accordingly, while significant NCF difficulties appear common in all LTL glioma patients, those with grade IV tumors exhibit more frequent and severe impairment than patients with lower-grade tumor across most domains.
These findings are consistent with prior studies reporting greater NCF deficits in patients with higher- versus lower-grade tumors.2,8,9 However, unlike the Kayl and Meyers study,9 differences among groups remained significant even when controlling for lesion size as measured by T1-weighted and FLAIR MRI sequences. This lends support to the contention that factors other than lesion size, such as tumor momentum, play a role in the development of tumor-related NCF impairment. Slower-growing tumors may allow for greater neuroplasticity, as the brain has more time to reorganize.5 This may be reflected in the finding of less frequent language impairment in the grade II and grade III groups, which is a domain commonly impacted by damage to LTL structures.22–27 Specifically, the slower-growing tumors could allow greater time for migration of such functions to nearby structures. Such functional reorganization has been documented in temporal lobe epilepsy patients,28–31 though further investigation is needed to better understand the neuroplastic processes in patients with glioma. Functional neuroimaging techniques32,33 and intraoperative brain mapping of NCF34 may be particularly useful in addressing such questions.
Although the hypothesized influence of tumor momentum on NCF is supported by the differences between the grade IV and grades II and III groups, a similar trend was not observed between the grade III and grade II groups. Like grade IV tumors, grade III gliomas are also considered high grade with infiltrative and rapid growth. Accordingly, it was expected that patients with grade III glioma would exhibit worse NCF than those with grade II glioma. However, the two groups performed similarly across most measures, with patients with grade II glioma actually exhibiting significantly lower performance than those with grade III glioma on a measure of processing speed. While somewhat surprising, this may reflect variability related to the small sample sizes of the grades II and III groups, as group means and impairment rates may be more influenced by individual performances falling at the extremes of the distribution. Similarly, histopathological heterogeneity within each group, as well as potential referral bias, may impact the findings, as the samples do not represent a consecutive series of all diagnosed patients. Further, tumor grade alone may not be the best proxy for tumor momentum, as growth rate is known to vary within the grade classes according to molecular profile35 and even by symptomatic versus incidental presentation.36 Additionally, sample size restrictions prohibited a more fine-grained segmentation of tumor location, precluding inferential analyses of differences in NCF by LTL region involved. Further studies should aim to clarify such questions through replication with a prospective design, including a larger sample and measures sampling from a broader variety of nonverbal functions.
Few patients had data regarding delayed memory on the HVLT-R. As such, the lack of differences in delayed verbal memory across groups may relate to the limited sample size, particularly in light of the sizable difference noted in verbal learning performances between the grade IV and lower groups. Despite the sample limitations, the tumor grade groups were of similar if not favorable size in comparison with existing studies. The present study also has greater regional specificity than any other investigation of glioma grade and NCF to date, as all patients had tumors restricted to the LTL, with similar distribution of tumors throughout the LTL across tumor grade groups. Another strength of the study is the strict control of other patient and tumor characteristics. Whereas the Hom and Reitan8 study did not account for age differences, NCF performances were demographically adjusted for these analyses, which is important given the fact that low-grade tumors tend to present at an earlier age.37 Similarly, other potential confounds such as seizure status and medication use were considered. Consistent with known rates of seizure disorder in patients with glioma, seizures were least frequent for patients with glioblastoma multiforme, but NCF performances did not differ by seizure status or use of antiepileptic drugs. Additionally, the impact of tumor grade on NCF was independent of steroid use and lesion volume in sensitivity analyses, providing further support for the hypothesized role of lesion momentum in the development of NCF impairment in LTL glioma patients.
In sum, patients with glioma involving the LTL frequently present with NCF difficulties, particularly on measures of learning and memory, attention, and executive functioning, regardless of tumor grade. As such, assessment of NCF may be helpful in the treatment planning and monitoring of quality of life for all LTL glioma patients, even those with low-grade tumors. Interestingly, the findings also demonstrate that grade IV gliomas disrupt memory, language, processing speed, and executive functions to a greater extent than lower-grade tumors, suggesting more disruption of distributed brain networks in these patients. While prior research suggested that differences in NCF across tumor grades may be attributable to variation in lesion volume, this contention was not supported by follow-up analyses. In the present study, after accounting for confounding patient- and tumor-related characteristics, differences in NCF across LTL glioma tumor grades appeared to be significantly and independently influenced by tumor momentum, insofar as higher-grade tumors are likely to exhibit more rapid tumor growth. In other words, the greater lesion momentum of faster-growing tumors may prevent neuroplasticity and, as a consequence, result in greater NCF impairment.
Funding
This work was supported by the National Institute of Nursing Research of the National Institutes of Health under award no. R01NR014195 (J.S. Wefel). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
This work was previously presented in poster form at the Cancer Survivorship Research Symposium, The University of Texas M.D. Anderson Cancer Center, Houston, Texas on January 30, 2014.
Conflict of interest statement. The authors report no conflicts of interest.
References
- 1.Schwartzbaum JA, Fisher JL, Aldape KD, et al. Epidemiology and molecular pathology of glioma. Nat Clin Pract Neurol. 2006;2(9):494–503. doi: 10.1038/ncpneuro0289. [DOI] [PubMed] [Google Scholar]
- 2.Miotto EC, Junior AS, Silva CC, et al. Cognitive impairments in patients with low grade gliomas and high grade gliomas. Arq Neuro-Psiquiatr. 2011;69(4):596–601. doi: 10.1590/s0004-282x2011000500005. [DOI] [PubMed] [Google Scholar]
- 3.Tucha O, Smely C, Preier M, et al. Cognitive deficits before treatment among patients with brain tumors. Neurosurgery. 2000;47(2):324–333. doi: 10.1097/00006123-200008000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Taphoorn MJB, Klein M. Cognitive deficits in adult patients with brain tumors. Lancet Neurol. 2004;3(3):159–168. doi: 10.1016/S1474-4422(04)00680-5. [DOI] [PubMed] [Google Scholar]
- 5.Desmurget M, Bonnetblanc F, Duffau H. Contrasting acute and slow-growing lesions: a new door to brain plasticity. Brain. 2007;130(Pt 4):898–914. doi: 10.1093/brain/awl300. [DOI] [PubMed] [Google Scholar]
- 6.Heimans JJ, Reijneveld JC. Factors affecting the cerebral network in brain tumor patients. J Neurooncol. 2012;108(2):231–237. doi: 10.1007/s11060-012-0814-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ris MD, Noll RB. Long-term neurobehavioral outcome in pediatric brain-tumor patients: review and methodological critique. J Clin Exp Neuropsychol. 1994;16(1):21–42. doi: 10.1080/01688639408402615. [DOI] [PubMed] [Google Scholar]
- 8.Hom J, Reitan RM. Neuropsychological correlates of rapidly vs. slowly growing intrinsic cerebral neoplasms. J Clin Neuropsychol. 1984;6(3):309–324. doi: 10.1080/01688638408401221. [DOI] [PubMed] [Google Scholar]
- 9.Kayl AE, Meyers CA. Does tumor histology influence cognitive function? Neuro Oncol. 2003;5(4):255–260. doi: 10.1215/S1152851703000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi WM, Wildrick DM, Sawaya R. Volumetric measurement of brain tumors from MR imaging. J Neurooncol. 1998;37(1):87–93. doi: 10.1023/a:1005944724470. [DOI] [PubMed] [Google Scholar]
- 11.Wechsler D. Wechsler Adult Intelligence Scale–Revised. San Antonio: The Psychological Corporation; 1981. [Google Scholar]
- 12.Wechsler D. Wechsler Adult Intelligence Scale–III. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- 13.Benedict RHB, Schretlen D, Groninger L, et al. Hopkins Verbal Learning Test Revised: normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12(1):43–55. [Google Scholar]
- 14.Tombaugh TN. Trail Making Test A and B: normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 15.Ruff RM, Light RH, Parker SB, et al. Benton Controlled Oral Word Association Test: reliability and updated norms. Arch Clin Neuropsychol. 1996;11(4):329–338. [PubMed] [Google Scholar]
- 16.Benton A, Hamsher K, Sivan A. Multilingual Aphasia Examination Manual. Iowa City, IA: AJA Associates Inc.; 2000. [Google Scholar]
- 17.Heaton RK, Miller SW, Taylor MJ, et al. Revised Comprehensive Norm for an Expanded Halstead-Reitan Battery: Demographically Adjusted Neuropsychological Norms for African American and Caucasian Adults. Lutz, FL: Psychological Assessment Resources, Inc.; 2004. [Google Scholar]
- 18.Trites, Ronald L. Neuropsychological Test Manual. Ottawa, Ontario, Canada: Royal Ottawa Hospital; 1977. [Google Scholar]
- 19.Armstrong TR, Wefel JS, Wang M, et al. Net clinical benefit analysis of Radiation Therapy Oncology Group 0525: a phase III trial comparing conventional adjuvant temozolomide with dose-intensive temozolomide in patients with newly diagnosed glioblastoma. J Clin Oncol. 2013;31(32):4076–4084. doi: 10.1200/JCO.2013.49.6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Version 21.0. Armonk, NY: IBM Corp; [Google Scholar]
- 22.Lee TM, Yip JT, Jones-Gotman M. Memory deficits after resection from left or right anterior temporal lobe in humans: a meta-analytic review. Epilepsia. 2002;43(3):283–291. doi: 10.1046/j.1528-1157.2002.09901.x. [DOI] [PubMed] [Google Scholar]
- 23.Bell B, Lin JJ, Seidenberg M, et al. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nature Rev Neruol. 2011;7(3):154–164. doi: 10.1038/nrneurol.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martins IP, Caeiro L, Ferro JM. Cognitive and behavioral disorders according to stroke site and side. In: Godefroy O, editor. The Behavioral and Cognitive Neurology of Stroke. 2nd ed. New York: Cambridge; 2013. [Google Scholar]
- 25.Feng HM, Kuo SC, Chen CY, et al. Rapidly progressive anomic aphasia: a rare presentation of temporal lobe tumor. J Neuropsychiatry Clin Neurosci. 2013;25(2):E18–E19. doi: 10.1176/appi.neuropsych.12030067. [DOI] [PubMed] [Google Scholar]
- 26.Thiel A, Hartmann A, Rubi-Fessen I, et al. Effects of noninvasive brain stimulation on language networks and recovery in early poststroke aphasia. Stroke. 2013;44(8):2240–2246. doi: 10.1161/STROKEAHA.111.000574. [DOI] [PubMed] [Google Scholar]
- 27.Griffiths JD, Marslen-Wilson WD, Stamatakis EA, et al. Functional organization of the neural language system: dorsal and ventral pathways are critical for syntax. Cereb Cortex. 2013;23(1):139–147. doi: 10.1093/cercor/bhr386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tracy JI, Lippincott-Stamos C, Osipowicz K, et al. Epilepsy and cognitive plasticity. In: Armstrong CL, Morrow L, editors. Handbook of Medical Neuropsychology. New York: Springer; 2010. pp. 3–16. [Google Scholar]
- 29.Naegele J. Epilepsy and the plastic mind. Epiepsy Curr. 2009;9(6):166–169. doi: 10.1111/j.1535-7511.2009.01331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kücker S, Töllner K, Piechotta M, et al. Kindling as a model of temporal lobe epilepsy induces bilateral changes in spontaneous striatal activity. Neurobiol Disease. 2010;37(3):661–672. doi: 10.1016/j.nbd.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Doucet GE, Skidmore C, Evans J, et al. Temporal lobe epilepsy and surgery selectively alter the dorsal, not the ventral, default-mode network. Front Neurol. 2014;5:23. doi: 10.3389/fneur.2014.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohammadi B, Kollewe K, Samii A, et al. Functional neuroimaging at different disease stages reveals distinct phases of neuroplastic changes in amyotrophic lateral sclerosis. Hum Brain Mapp. 2011;32(5):750–758. doi: 10.1002/hbm.21064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcotte K, Perlbarg V, Marrelec G, et al. Default-mode network functional connectivity in aphasia: therapy-induced neuroplasticity. Brain Lang. 2013;124(1):45–55. doi: 10.1016/j.bandl.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 34.Duffau H. The huge plastic potential of adult brain and the role of connectomics: new insights provided by serial mappings in glioma surgery. Cortex. 2014;58:325–337. doi: 10.1016/j.cortex.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 35.Banaem HY, Ahmadian A, Saberi H, et al. Brain tumor modeling: glioma growth and interaction with chemotherapy. 2011 International Conference on Graphic and Image Processing (pp. 82851M-82851M); International Society for Optics and Photonics; 2011, October. [Google Scholar]
- 36.Torres-Reveron J, Piepmeier JM, Becker KP. Diffuse Low-Grade Gliomas in Adults. London: Springer; 2013. Clinical presentation in diffuse low-grade gliomas; pp. 179–188. [Google Scholar]
- 37.Porter KR, McCarthy BJ, Freels S, et al. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol. 2010;12(6):520–527. doi: 10.1093/neuonc/nop066. [DOI] [PMC free article] [PubMed] [Google Scholar]