Abstract
Background
Spinal myxopapillary ependymomas (MPEs) are slowly growing ependymal gliomas with preferential manifestation in young adults. The aim of this study was to assess the outcome of patients with MPE treated with surgery, radiotherapy (RT), and/or chemotherapy.
Methods
The medical records of 183 MPE patients (male: 59%) treated at the MD Anderson Cancer Center and 11 institutions from the Rare Cancer Network were retrospectively reviewed. Mean patient' age at diagnosis was 35.5 ± 15.8 years. Ninety-seven (53.0%) patients underwent surgery without RT, and 86 (47.0%) were treated with surgery and/or RT. Median RT dose was 50.4 Gy. Median follow-up was 83.9 months.
Results
Fifteen (8.2%) patients died, 7 of unrelated cause. The estimated 10-year overall survival was 92.4% (95% CI: 87.7–97.1). Treatment failure was observed in 58 (31.7%) patients. Local failure, distant spinal relapse, and brain failure were observed in 49 (26.8%), 17 (9.3%), and 11 (6.0%) patients, respectively. The estimated 10-year progression-free survival was 61.2% (95% CI: 52.8–69.6). Age (<36 vs ≥36 y), treatment modality (surgery alone vs surgery and RT), and extent of surgery were prognostic factors for local control and progression-free survival on univariate and multivariate analysis.
Conclusions
In this series, treatment failure of MPE occurred in approximately one third of patients. The observed recurrence pattern of primary spinal MPE was mainly local, but a substantial number of patients failed nonlocally. Younger patients and those not treated initially with adjuvant RT or not undergoing gross total resection were significantly more likely to present with tumor recurrence/progression.
Keywords: myxopapillary ependymoma, radiotherapy, spinal failures, spinal tumors
Myxopapillary ependymoma (MPE) is classified as a World Health Organization (WHO) grade I glioma, located almost exclusively in the region of the conus medullaris, cauda equina, and filum terminale of the spinal cord. Less than 5% of MPEs occur in sites outside the lumbar thecal sac. This tumor, with an incidence of 0.05–0.08 per 100 000 persons per year,1 is characterized by a papillary arrangement of cuboidal tumor cells around a vascular stromal core that undergoes mucinous degeneration. MPE arises from the ependymal lining of the spinal canal and can be infrequently observed in the brain parenchyma2 or lateral ventricle.3 Anaplastic variants are virtually unknown.1 Young adults (18–29 y) are usually affected, and ∼8%–20% of all MPEs occur in the pediatric population.4–7 It is believed to be a slowly growing ependymal glioma, thus with a favorable prognosis, which can, however, occasionally exhibit elevated mouse intestinal bacteria 1 labeling indices.8 Seeding of the subarachnoid space8 and extraneural metastasis9,10 can take place, and the outcome of MPE is thus not universally favorable for all MPE patients. Patient' age, extent of surgery, and tumor size have been identified as major prognosticators for this tumor entity in 2 large retrospective studies.11,12 Notwithstanding the favorable outcome assumed in the neurosurgical cognoscenti, we assessed the outcome and pattern of failures of spinal MPE using the 2 largest databases published. These 2 identical datasets were merged, updated, and expanded with new MPE cases to address more fully the prognostic factors of this rare tumor entity with a debatable good prognosis.
Patients and Methods
The Rare Cancer Network (RCN; http://www.rarecancer.net) is a multi-institutional cooperative group created to initiate large retrospective studies of rare cancers to improve our knowledge of their treatment outcomes and prognostic factors. The computerized database, based at Geneva University Hospital, which initiated the first RCN analysis of the primary spinal MPE cohort,12 was purposely identical to the one created previously at the MD Anderson Cancer Center (MDACC).11 The present study strategy was to merge the 2 databases in order to update the follow-up period of patients and add new patients managed at the MDACC or one of the RCN institutions. The institutional databases were queried and 183 patients with spinal MPE were identified during a 44-year period (January 1968–March 2012). The inclusion criteria were histology-proven MPE (WHO grade I; International Classification of Diseases for Oncology M-9394/1), with only primary spinal localization. Mixed tumors (with a minor WHO grade II component) were also included. In this study, all investigators obtained their own institutional review board approvals for patients' data collection. The updated data were resubmitted electronically to the principal investigator (D.C.W.) at Geneva University Hospital. Follow-up data of varying duration were available for all patients in this study. The median follow-up period of the surviving patients was 60.0 months (range, 0.2–316.6).
Patient Characteristics
Patient characteristics are detailed in Table 1. The majority (>60%) of patients presented with MPE localized to the lumbosacral spine or cauda equina (Table 1). All but 4 (2.2%) patients had nonmetastatic disease at diagnosis. Two patients presented with supratentorial brain and spinal metastases and another 2 patients presented with spinal metastases at diagnosis. One (0.5%) patient presented with a synchronous temporoparietal oligodendroglioma. Seven (3.8%) patients had MPE mixed with WHO grade II ependymoma.
Table 1.
Patient characteristics and treatment details
| Variable | Number (%) |
|---|---|
| Number of patients | 183 (100) |
| Age, y | |
| Median | 35.5 |
| SD | ±15.8 |
| Gender | |
| Female/male | 77/108 |
| Histology | |
| Grade I only (MPE) | 176 (96.2) |
| Grades I and II | 7 (3.8) |
| Symptoms | |
| Low-back pain | 155 (84.7) |
| Extremity numbness | 85 (46.4) |
| Extremity weakness | 75 (41.0) |
| Urinary dysfunction | 54 (29.5) |
| Abnormal gait | 37 (20.2) |
| 85 (46.4) | |
| Tumor location | |
| Cervical only | 1 (0.5) |
| Cervicothoracic | 2 (1.1) |
| Thoracic only | 1 (0.5) |
| Thoracolumbar | 58 (31.7) |
| Lumbosacral/filum terminale | 121 (66.2) |
| Spinal dissemination at initial presentation | |
| Yes | 4 (2.2) |
| No | 179 (97.8) |
| Primary treatment | |
| Surgery alone | 96 (52.6) |
| Surgery and radiotherapy | 85 (46.4) |
| Surgery and chemotherapy | 1 (0.5) |
| RT alone | 1 (0.5) |
| Extent of surgery | |
| Gross total resection | 99 (54.1) |
| Subtotal resection | 73 (39.9) |
| Biopsy only | 6 (3.3) |
| Unknown | 5 (2.7) |
No central histopathologic review by an expert pathologist was performed. The most common presenting symptom was low-back pain (Table 1). Neurological function was evaluated by the Frankel classification system (ranging from normal motor function [grade E] to complete neurological injury [grade A]).13 Grading was performed before surgery and during last follow-up visits. Complete motor or sensory loss of function was rare (3.4%; Table 1), but numbness of the extremities was frequent (47.8%; Table 1). Bladder sphincter dysfunction occurred in ∼30% of patients (Table 1). Spinal lesions were identified with MRI in a majority of patients (n = 150). MRI was interpreted as abnormal in all cases. Noteworthy, no central radiological review was performed for this study. Other imaging modalities were CT scan (n = 35) and myelography (n = 31). All myelographies were interpreted as abnormal, but 2/35 CT scans were considered normal at the time of diagnosis. Tumor size was available for 86 (47%) patients. Largest dimension (radiology and/or operative notes) of MPE ranged from 1.0 to 200.0 mm (median, 20.0).
Therapy
Surgery was the initial treatment for all but one patient (radiotherapy [RT] alone, n = 1; Table 1). Initial treatment modality of these patients was surgery alone, with (n = 1) or without chemotherapy (n = 96), and surgery with pre- and postoperative RT in 97 (53.0%) and 85 (46.4%) patients, respectively.
The extent of surgery was determined from the surgical notes and/or postoperative imaging studies. Gross total removal (GTR) was defined as complete resection of the MPE by the surgeon or by the absence of residual tumor on postoperative CT/MRI scans. Subtotal removal was performed if the surgeon observed unresected MPE in the tumor bed or if residual tumor was identified on postoperative imaging studies. The extent of surgery is detailed in Table 1. Six patients underwent biopsy only (Table 1).
All RT patients were treated with megavoltage photon beams. The median overall treatment time of RT was 37 days (range, 17–59). Median administered dose was 50.4 Gy and ranged from 25.2 to 60.0 Gy. A median of 28 fractions (range, 14–40) of 1.0–2.3 Gy (median, 1.8) were delivered. The RT volume was usually the primary tumor plus one vertebral body above and below the vertebral level affected by the MPE. This treatment technique was described as focal RT (n = 45 patients). Thirty other patients were treated with focal RT with unspecified margins. Craniospinal irradiation with and without a focal boost was delivered to 3 and 1 patient, respectively. The treatment volume was unknown for 6 patients.
Statistical analysis
Progression-free survival (PFS) and overall survival (OS) were calculated from the date of surgery using Kaplan–Meier estimates. The events were death (all causes included) for OS and progressive disease or death for PFS. Progressive disease was defined as any treatment failure occurring locally (initial spinal involvement) and/or distantly (spine and/or brain). We used a 2-tailed t-test to compare clinical or therapeutic covariates. For frequency analysis, we used Fisher's exact test for 2-way tables. Differences between groups were assessed using the log-rank test.14 The log-rank test was used to compare different survival functions according to various clinical factors (age, gender, tumor location, tumor volume, metastasis at diagnosis, and laterality of symptoms) and therapeutic factors (initial treatment modality, extent of surgery). Multivariate analysis was performed using Cox stepwise regression analysis to define the independent contribution of each prognostic factor.15 Clinical and therapeutic factors shown to be either significant or potentially relevant (P ≤ .1) in the univariate analysis were forced in the Cox model. Statistical tests were based on a 2-sided significance level, and a P-value of .05 or less was considered to be statistically significant. Statistical analysis was performed on Statistical Package for Social Sciences version 18.0.
Results
Patient Outcomes
At the time of the analysis, 15 (8.2%) patients had died, 8 (53.3%) from tumor progression. The estimated 10-year OS was 92.4% (95% CI: 87.7–97.1).
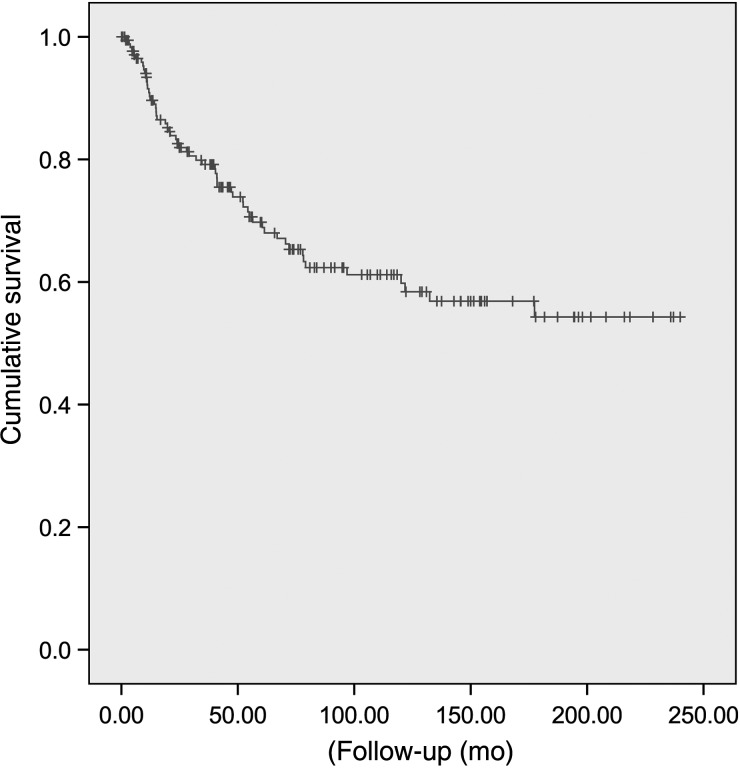
Fifty-eight (31.7%) patients experienced treatment failure. Median time to treatment failure was 26.5 months (range, 1.7–177.4). The estimated 5- and 10-year PFS rates were 69.5% (95% CI: 61.9–77.1) and 61.2% (95% CI: 52.8–69.6), respectively (Fig. 1). Local recurrence was observed in a majority (49/58; 84.5%) of patients with treatment failure. Among patients recurring locally, 37 presented with local failure only. Local and distant spinal failures were observed in 6 patients; 2 patients had combined local and brain failures. Four other patients presented with local, distant spinal, and brain concomitant failure. A substantial number of patients (n = 11; 19.0% of all tumor progression) presented with brain failures (Table 2). Brain failure only was observed in 2 patients, while in 3 patients concomitant distant spinal failure was observed. Distant spinal failure only was observed in 4 patients. No extraneural metastasis was observed. Table 2 details the pattern of tumor progression. The estimated 5- and 10-year local failure-free and brain-failure-free survivals were 73.2% (95% CI: 65.9–80.5)–66.8% (95% CI: 58.6–75.0) and 93.9% (95% CI: 90.0–97.8)–92.8% (95% CI: 88.3–97.3), respectively.
Fig. 1.
Progression-free survival for 183 MPE patients.
Table 2.
Pattern of treatment failure in 183 MPE patients
| Type of Treatment Failure | Number of Patients | %a |
|---|---|---|
| Local | 49 | 84.5 |
| Local only | 37 | 63.8 |
| Local and distant spinal | 6 | 10.3 |
| Local and brain | 2 | 3.4 |
| Local, brain, and distant spinal | 4 | 6.8 |
| Brain | 11 | 19.0 |
| Brain only | 2 | 3.4 |
| Brain and local | 2 | 3.4 |
| Brain and distant spinal | 3 | 5.2 |
| Brain, local, and distant spinal | 4 | 6.8 |
| Distant spinal | 17 | 29.3 |
| Distant spinal only | 4 | 6.8 |
| Distant spinal and local | 6 | 10.3 |
| Distant spinal and brain | 3 | 5.2 |
| Distant spinal, local, and brain | 4 | 6.8 |
aDue to the ratio approximations, percentages may not exactly add.
Of the 58 patients with treatment failure, the majority (48/58; 82.8%) underwent salvage treatment. Patients were salvaged with surgery alone (n = 14), surgery and RT (n = 13), focal RT (n = 11), surgery with RT and chemotherapy (n = 3), chemotherapy only (n = 3), extensive spinal RT (n = 2), whole brain RT with distant spinal RT (n = 1), and surgery for local recurrence with craniospinal RT for distant spinal relapse (n = 1). No salvage treatment was offered to 10 (17.2%) patients. Overall, the 5- and 10-year OS rates for recurring and nonrecurring patients were 90.3% (95% CI: 82.3–98.3)–88.1% (95% CI: 79.1–97.1) and 97.2% (95% CI: 94.1–100.0)–94.9% (95% CI: 89.6–100.0), respectively (P = .32).
Acute and Late Adverse Effects
Acute toxicity occurred in 19 (10.4%) patients. The most frequent acute toxicity was skin erythema and nausea. Grades I, II, and III adverse events of the Common Terminology Criteria for Adverse Events (CTCAE v3; http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf) were observed in 6, 11, and 1 patient, respectively. One patient presented posttreatment intestinal perforation and peritonitis (CTCAE grade 5).
Late adverse events were observed in 42 (23.0%) patients. Reported during follow-up were chronic pain (n = 8), motor paraplegia (n = 5), hypoesthesia (n = 4), urinary sphincter dysfunction (n = 2), urinary sphincter dysfunction and paraplegia (n = 2), bowel sphincter dysfunction (n = 1), scoliosis (n = 1), ataxia (n = 1), subcutaneous fibrosis (n = 1), and amenorrhea (n = 1). Late adverse events were unknown in 16 patients. Of note, no radiation-induced myelopathy was observed among patients treated with surgery + RT. The 5- and 10-year probabilities of complication-free survival were 80.5% (95% CI: 73.6–87.4) and 72.6% (95% CI: 64.0–81.2), respectively. There was a statistical trend toward significance for treatment modality and late adverse events: patients treated with surgery alone had usually less late toxicity compared with those treated with surgery + RT (43.9% vs 56.1%; P = .07). No other clinical factors (gender, P= .21; age, P = .80; tumor location, P = .84; tumor size, P = .25) or therapeutic factors (total dose, P = .86; extent of resection, P = .42) were predictive of late adverse events.
Secondary cancers were observed in 6 (3.3%) patients, 0.2–16.0 years (median, 4.2) after treatment. Three (50%) patients were treated initially for their MPE with surgery alone, and 3 other (50%) patients were treated with surgery and postoperative RT. In these, all but 1 tumor occurred in the treatment field and were thus possibly radiation induced (total, n = 2/6). All patients with secondary tumors were alive at last follow-up. No clinical factors (gender, P = .24; age, P = .44; tumor location, P = .99; tumor size, P= .99) or therapeutic factors (treatment modality, P = 1.0; total dose, P = .5; RT volume, P = .52; type of RT, P = 1.0) were predictive of secondary tumors. The survival between patients with and without secondary tumors was not significantly different: the estimated 5-year OS was 94.4% (95% CI: 90.7–98.1) and 100% for patients with and without secondary cancers, respectively (P = .51).
Prognostic Factors for OS and PFS
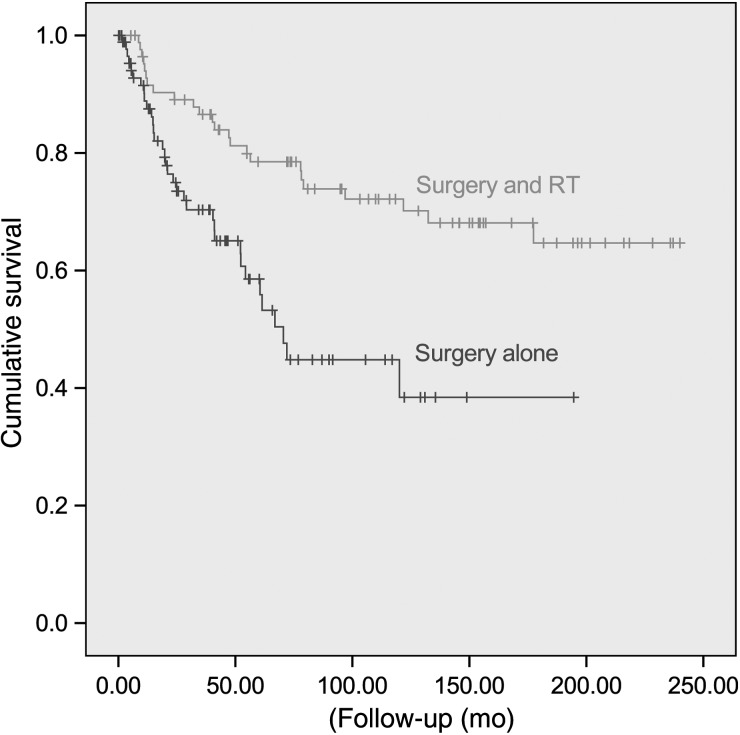
The influence on local control, PFS, and OS of both clinical and treatment variables was examined. Table 3 details the results of the univariate analysis. Local control and PFS were negatively influenced (Table 3) by younger age (<36 y) and initial surgery without RT (Fig. 2). The extent of surgery was also a significant detrimental factor for local control and PFS (Table 3). Metastasis at diagnosis negatively influenced PFS and OS (Table 3). Patients undergoing salvage therapy were significantly more controlled locally (Table 3). There were no statistically significant associations between gender, tumor location, tumor size, or laterality of symptoms on local control, PFS, or OS rates (Table 3).
Table 3.
Univariate analysis for local control, PFS, and OS
| Parameters | Cumulative Proportion of Patients With Local Tumor Control (SE) | P | Cumulative Proportion of Patients With Local and Distant Tumor Control (SE) | P | Cumulative Proportion of Surviving Patients (SE) | P |
|---|---|---|---|---|---|---|
| Age, y | <.001 | <.001 | .11 | |||
| <36 | 0.40 (0.06) | 0.30 (0.06) | 0.86 (0.07) | |||
| ≥36 | 0.84 (0.05) | 0.81 (0.05) | 0.64 (0.12) | |||
| Gender | .22 | .29 | .26 | |||
| Female | 0.68 (0.06) | 0.59 (0.07) | 0.96 (0.03) | |||
| Male | 0.56 (0.06) | 0.50 (0.06) | 0.89 (0.05) | |||
| Tumor location | .66 | .58 | .46 | |||
| Lumbosacral/filum terminale | 0.68 (0.04) | 0.53 (0.05) | 0.75 (0.07) | |||
| Cervicothoracic | 0.75 (0.21) | 0.75 (0.21) | 1.0 | |||
| Metastasis at diagnosis | .28 | .03 | .03 | |||
| No | 0.77 (0.07) | 0.54 (0.05) | 0.77 (0.07) | |||
| Yes | 0.50 (0.21) | 0.66 (0.20) | 0.50 (0.30) | |||
| Tumor size | .28 | .35 | ||||
| <20 mm | 0.59 (0.11) | 0.52 (0.11) | 1.0 | .17 | ||
| ≥20 mm | 0.80 (0.07) | 0.58 (0.12) | 0.89 (0.07) | |||
| Symptoms | .20 | .52 | .16 | |||
| Unilateral | 0.36 (0.25) | 0.30 (0.22) | 0.93 (0.04) | |||
| Bilateral | 0.74 (0.05) | 0.58 (0.07) | 0.95 (0.02) | |||
| Initial treatment modality | .006a | .005a | .99a | |||
| Surgery alone | 0.45 (0.08) | 0.38 (0.08) | 0.92 (0.03) | |||
| Surgery and standard-dose RTb | 0.71 (0.08) | 0.56 (0.08) | 0.86 (0.06) | |||
| Surgery and high-dose RTc | 0.66 (0.10) | 0.66 (0.11) | 0.71 (0.15) | |||
| Extent of surgery | .02 | .02 | .10 | |||
| GTR | 0.68 (0.06) | 0.63 (0.07) | 0.90 (0.04) | |||
| STR or biopsy | 0.55 (0.06) | 0.45 (0.06) | 0.70 (0.10) | |||
| Salvage treatment | <.001 | .86 | .87 | |||
| Yes | 0.03 (0.02) | 0.02 (0.02) | 0.74 (0.09) | |||
| No | 0.55 (0.06) | 0.11 (0.10) | 0.88 (0.10) |
Abbreviation: STR, subtotal removal.
aSurgery vs surgery + standard-/high-dose RT.
b<50.4 Gy.
c≥50.4 Gy.
Fig. 2.
Progression-free survival for patients treated with surgery alone or surgery + RT.
Multivariable Cox proportional hazards models were used to examine factors associated with local control, PFS, and OS while controlling for potential confounders. Only age, treatment modality, and extent of surgery remained independent predictors of local control and PFS (Table 4). Interestingly, the hazard ratios for local control and PFS were 0.34 and 0.28 for adjuvant low-dose and high-dose RT, respectively (Table 4). On multivariate analysis, no independent factors were associated with OS.
Table 4.
Multivariate analysis for local control (LC), PFS, and OS
| Parameters | Hazard Ratios for LC (95% CI) | P | Hazard Ratios for PFS (95% CI) | P | Hazard Ratios for OS (95% CI) | P |
|---|---|---|---|---|---|---|
| Age, y | ||||||
| <36 vs ≥36 | 0.21 (0.10–0.43) | <.001 | 0.2 (0.1–0.39) | <.001 | 2.56 (0.73–8.61) | .13 |
| Initial treatment modality | ||||||
| Surgery alone | 1.0 | <.001a | 1.0 | <.001a | 1.0 | .73 |
| Surgery + standard-dose RT | 0.34 (0.15–0.77) | 0.34 (0.16–0.71) | 0.66 (0.16–2.70) | |||
| Surgery + high-dose RT | 0.28 (0.12–0.60) | 0.28 (0.14–0.57) | 0.55 (0.11–2.61) | |||
| Extent of surgery | ||||||
| GTR vs STR or biopsy | 2.08 (1.13–3.82) | .01 | 2.14 (1.38–4.24) | .02 | 2.70 (0.76–9.52) | .11 |
| Metastasis at diagnosis | ||||||
| Yes vs no | 1.35 (0.25–15.13) | .51 | 1.51 (0.19–11.48) | .69 | 6.0 (0.62–58.38) | .12 |
Abbreviation: STR, subtotal removal.
aSurgery vs surgery + standard-/high-dose RT.
Discussion
To the best of our knowledge, the present series is the largest study published on spinal MPE and raises several key issues that should have a substantial impact on present therapeutic approaches for this tumor entity.
First, young age was a significant detrimental independent predictor for MPE patient' outcome, be it on local, distant spinal, or brain control (Table 4). The survivorship of younger patients was, however, better, although the difference was not statistically significant (Table 4). This may be due to the fact that MPE may not substantially impact the survivorship of young and healthy individuals with no comorbidities. Likewise, the survival of nonsalvaged patients in this series was not significantly different than that of salvaged patients, suggesting that MPE produces substantial impairment but does not critically reduce survival. This finding was reported by the MDACC group11 and subsequently by the RCN institutions.12 In the pediatric population, the reported MPE' behavior is all but benign. Fassett and colleagues9 reported on 26 children with MPE (mean age, 11 ± 2.9 y) treated with surgery, with and without RT.9 In those cases in which patients underwent spinal screening at diagnosis, a majority (58.3%) presented with disseminated spinal disease. A high incidence of tumor dissemination has also been observed in adult and pediatric MPE.16 The reported event-free survival was only 50% in another small MPE pediatric series, compared with 100% for spinal ependymoma.4 These data are in line with the seminal paper stemming from the Mayo group, showing that recurrences were frequently observed in younger patients.6 Conversely, age was not a prognosticator in a small series encompassing spinal ependymomas and MPEs in adult and pediatric patients (mean age, 37 y; range, 8–66), although all patients received postoperative RT, and the impact of age was not reported specifically for the MPE variant.17 In our series, three quarters of all patients with metastatic cases at presentation were younger than 36 years (mean, 16.2 ± 3.9 y; data not shown). Although spinal dissemination at diagnosis is rare in adults (Table 1), we recommend that all patients undergo brain and spinal MRI and CSF cytology in their initial workups before initiation of treatment. Moreover, the 10-year PFS in our series was ∼40% for younger patients, compared with 85% for older patients. As such, the former cohort should be treated aggressively with adjuvant RT, when complete resection has not been obtained by the surgeon. A recent series has shown that local control rates were 30% and 100% for pediatric patients treated with surgery alone and surgery with adjuvant RT, respectively.18
Second, adjuvant RT increased 10-year PFS from <40% to 70% in patients receiving this modality, compared with those not treated with postoperative radiation (Table 3). This would justify more liberal use of adjuvant RT, especially in those patients with subtotal resection and piecemeal resection, or questionable GTR in patients with spinal MPE. Immediate RT following resection is an accepted standard treatment for intracranial ependymomas, as a result of several retrospective studies showing a benefit of postoperative RT over surgery alone.19–22 Adjuvant radiation for MPE has been questioned, especially for children, as a result of its presumed benign nature (WHO grade I). It can be counter-argued (i) that complete resection of tumor is not common, occurring in approximately 1 patient out of 2 (Table 1), as observed in other series,6,11,12 and (ii) that, given time, tumor progression with spinal and/or brain dissemination will occur. MPE produces a myxoid matrix material that may render GTR chiefly challenging, particularly so in the filum terminale. As such, radiation may control these tumor cells, due to the known radiosensitivity of ependymal cells,23 and RT is consequently the mainstay in pursuing this aim. Although late recurrence was observed in this series, the median time to treatment failure was ∼2 years, suggesting that the benefit of RT may not be restricted to younger patients with longer life expectancies, who have a longer time horizon to experience tumor progression or recurrence.
Third, the role of surgery is of paramount importance for this tumor entity. GTR has been shown to be the most important prognosticator for ependymoma patients. As discussed, GTR for MPE, unlike ependymoma, with low neurological disability, can infrequently be accomplished, but the extent of resection is indeed also of paramount importance for this ependymal variant. In the Mayo study, the risk of treatment failure was decreased from 34% to 10%.6 We have also observed a significant (P = .01) decrease of treatment failure with GTR (Table 4).
Fourth, although the observed recurrence rate was substantial (Fig. 1), the survival of MPE patients was indeed good. Interestingly, salvage therapy did not improve the survivorship of recurring patients. Only a minority of patients died of MPE, suggesting that this tumor is indeed locally aggressive but does not have a major impact on the survival status of MPE patients. We did observe that patients with local failures, compared with those with distant spinal or brain failures, with or without a local component, had better survival, although the observed difference was not significant. Treatment failure may not have a major impact on survival but it may indeed have an impact on quality of life. Due to the retrospective nature of this study, this metric could not be assessed. Of note, patients with spinal or brain failures, with or without a local component, had substantially more late side effects (16.2% vs 23.8%), although the difference was not significantly relevant (P = .19; data not shown).
Finally, it has always been assumed that MPE was more prevalent in male patients. The reported male/female ratio varies from 1.4:1 to 2.5:1.11,12,24 In this larger cohort, we did observe a gender ratio at the lower published range, suggesting that this tumor is more gender neutral than previously assumed.
Concerns regarding long-term radiation-induced toxicity have been made by patients, parents, and caregivers alike. We did not observe any substantial late toxicity in patients receiving RT compared with those treated with surgery only. Secondary cancers were observed in a minority of patients, and only 2/183 (3.4%) secondary tumors in the entire cohort may have been radiation induced. The use of more conformal treatment, not limited to but including proton beam therapy25 and stereotactic fractionated RT,26 may somewhat decrease this rare but feared serious adverse effect.
We have observed an increase of local and distant control with high-dose (≥50.4 Gy) as opposed to low-dose RT on univariate analysis (Table 3). This factor was not significant in multivariate analysis, as patients with subtotal resection usually received a higher dose of RT (data not shown). Eleven ependymoma series with dose responses have been published, with 6 studies showing a beneficial effect of high-dose radiation on outcome.23 Although the data are somewhat inconclusive, there is some suggestion of a dose-response effect, and we would recommend administering 50.4 to 54.0 Gy to MPE patients.
Tailoring adjuvant treatment to high-risk patients only would be most desirable, as clinical factors, such as those identified in this study, may be too rough a prognostication tool to be clinically useful. Pathogenesis of ependymal tumors is indeed poorly understood, and molecular markers for risk-adapted patient stratification are yet to be identified. Korshunov and colleagues,27 in 39 newly diagnosed ependymomas, reported a unique gene expression pattern for spinal ependymomas that distinguished those from intracranial tumors using microarray technology. Identically, a French study using comparative genomic hybridization reported that the most frequently detected chromosomal (chr) abnormalities in spinal ependymal tumors were gain of chr 7, 9, 12, and 15.28 Losses of chr 22 were also more commonly identified in spinal tumors. The analysis of the pattern of chromosomal imbalance in this study could also distinguish between classical ependymomas and MPEs. MPE (n = 5) displayed a specific genomic signature, defined by concurrent gain of chr 5, 7, 9, 16, and 18, whereas none of the other non-MPE specimens (n = 40) presented this genetic profile. Chromosomal changes and epigenetic studies may well be necessary to optimally risk-stratify these ependymal tumors. Stephen et al,4 reporting on 19 pediatric cases (MPE, n = 8), reported recently no immunohistochemistry difference between spinal ependymomas and MPEs, with the exception of EPHB3, which was usually positive in MPE. Interestingly, this protein expression has also been reported in supratentorial ependymomas,29 which have a more aggressive clinical course compared with infratentorial tumors. Although a substantial number of studies have assessed the correlation between protein or gene expression and tumor location, grade, and tumor type,4,27–29 none have analyzed specifically the association with tumor recurrence for a given histological subtype. A large German study reported a gene profile of 27 genes that were associated with poor outcome (survival <10 y) in patients with ependymomas, but all grades I–III were analyzed together.30 Future research regarding the molecular prognostication of this rare ependymal tumor is justified in the framework of collaborative studies.
This study has potential limitations inherent in all retrospective analyses, including uncontrolled patient selection into different treatment groups. Most importantly, the absence of central pathology revision and radiological review may have included in this cohort non-MPE histologies. This is particularly relevant for the minority of patients with mixed histologies (Table 1). Not all patients underwent pretreatment MRI screening for tumor dissemination. It remains unclear whether cases with distant spinal or brain failures represent clinically latent disease that was present but undetected at the time of initial presentation or instances of seeding after treatment. Additionally, the surgical techniques have evolved during the study period, and patient' outcome, including local control and toxicity, could be somewhat improved in both groups of MPE patients in our series. We performed multivariate analyses to control for potential confounding factors in our examination of PFS and OS, but some residual confounding likely remains. Notwithstanding these legitimate reservations, this series is the largest MPE study with a long follow-up period, and the treatment strategy and techniques have been fairly uniform among centers.
In summary, although a considerable number of patients did present with treatment failure, OS of all MPE patients was good. A substantial number of patients presented with nonlocal failures (distant spinal and/or brain). Younger age, surgery alone, and non-GTR were adverse prognostic factors for local control and PFS. We recommend administering adjuvant high-dose RT (≥50.4 Gy) to patients undergoing subtotal resection or biopsy.
Funding
None.
Conflict of interest statement. None declared.
References
- 1.McLendon R, Rosenblum M, Schiffer D, et al. Myxopapillary ependymoma. In: Louis D, Ohgaki H, Wiestler O, Cavenee W, editors. WHO Classification. Lyon: WHO; 2007. pp. 72–73. [Google Scholar]
- 2.Warnick RE, Raisanen J, Adornato BT, et al. Intracranial myxopapillary ependymoma: case report. J Neurooncology. 1993;15(3):251–256. doi: 10.1007/BF01050071. [DOI] [PubMed] [Google Scholar]
- 3.Schroder R, Firsching R, Kochanek S. Hemangiopericytoma of meninges. II. General and clinical data. Zentralbl Neurochir. 1986;47(3):191–199. [PubMed] [Google Scholar]
- 4.Stephen JH, Sievert AJ, Madsen PJ, et al. Spinal cord ependymomas and myxopapillary ependymomas in the first 2 decades of life: a clinicopathological and immunohistochemical characterization of 19 cases. J Neurosurg Pediatr. 2012;9(6):646–653. doi: 10.3171/2012.2.PEDS11285. [DOI] [PubMed] [Google Scholar]
- 5.Ross DA, McKeever PE, Sandler HM, et al. Myxopapillary ependymoma. Results of nucleolar organizing region staining. Cancer. 1993;71(10):3114–3118. doi: 10.1002/1097-0142(19930515)71:10<3114::aid-cncr2820711036>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 6.Sonneland PR, Scheithauer BW, Onofrio BM. Myxopapillary ependymoma. A clinicopathologic and immunocytochemical study of 77 cases. Cancer. 1985;56(4):883–893. doi: 10.1002/1097-0142(19850815)56:4<883::aid-cncr2820560431>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Gagliardi FM, Cervoni L, Domenicucci M, et al. Ependymomas of the filum terminale in childhood: report of four cases and review of the literature. Childs Nerv Syst. 1993;9(1):3–6. doi: 10.1007/BF00301925. [DOI] [PubMed] [Google Scholar]
- 8.Burger P, Scheithauer B. Tumors of the Central Nervous System. Washington, DC: ARP Press; 2007. [Google Scholar]
- 9.Fassett DR, Pingree J, Kestle JR. The high incidence of tumor dissemination in myxopapillary ependymoma in pediatric patients. Report of five cases and review of the literature. J Neurosurg. 2005;102(1 Suppl):59–64. doi: 10.3171/ped.2005.102.1.0059. [DOI] [PubMed] [Google Scholar]
- 10.Rickert CH, Kedziora O, Gullotta F. Ependymoma of the cauda equina. Acta Neurochir (Wien). 1999;141(7):781–782. doi: 10.1007/s007010050376. [DOI] [PubMed] [Google Scholar]
- 11.Akyurek S, Chang EL, Yu TK, et al. Spinal myxopapillary ependymoma outcomes in patients treated with surgery and radiotherapy at M.D. Anderson Cancer Center. J Neurooncology. 2006;80(2):177–183. doi: 10.1007/s11060-006-9169-2. [DOI] [PubMed] [Google Scholar]
- 12.Pica A, Miller R, Villa S, et al. The results of surgery, with or without radiotherapy, for primary spinal myxopapillary ependymoma: a retrospective study from the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2009;74(4):1114–1120. doi: 10.1016/j.ijrobp.2008.09.034. [DOI] [PubMed] [Google Scholar]
- 13.Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7(3):179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 14.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox D. Regression models and life tables. J R Stat Soc. 1972;34(2):187–220. [Google Scholar]
- 16.Rezai AR, Woo HH, Lee M, et al. Disseminated ependymomas of the central nervous system. J Neurosurg. 1996;85(4):618–624. doi: 10.3171/jns.1996.85.4.0618. [DOI] [PubMed] [Google Scholar]
- 17.Waldron JN, Laperriere NJ, Jaakkimainen L, et al. Spinal cord ependymomas: a retrospective analysis of 59 cases. Int J Radiat Oncol Biol Phys. 1993;27(2):223–229. doi: 10.1016/0360-3016(93)90231-j. [DOI] [PubMed] [Google Scholar]
- 18.Agbahiwe HC, Wharam M, Batra S, et al. Management of pediatric myxopapillary ependymoma: the role of adjuvant radiation. Int J Radiat Oncol Biol Phys. 2013;85(2):421–427. doi: 10.1016/j.ijrobp.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rousseau P, Habrand JL, Sarrazin D, et al. Treatment of intracranial ependymomas of children: review of a 15-year experience. Int J Radiat Oncol Biol Phys. 1994;28(2):381–386. doi: 10.1016/0360-3016(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 20.Nazar GB, Hoffman HJ, Becker LE, et al. Infratentorial ependymomas in childhood: prognostic factors and treatment. J Neurosurg. 1990;72(3):408–417. doi: 10.3171/jns.1990.72.3.0408. [DOI] [PubMed] [Google Scholar]
- 21.Pollack IF, Gerszten PC, Martinez AJ, et al. Intracranial ependymomas of childhood: long-term outcome and prognostic factors. Neurosurgery. 1995;37(4):655–666. doi: 10.1227/00006123-199510000-00008. discussion 666–657. [DOI] [PubMed] [Google Scholar]
- 22.Merchant TE. Current management of childhood ependymoma. Oncology (Williston Park). 2002;16(5):629–642. 644; discussion 645–626, 648. [PubMed] [Google Scholar]
- 23.Taylor RE. Review of radiotherapy dose and volume for intracranial ependymoma. Pediatr Blood Cancer. 2004;42(5):457–460. doi: 10.1002/pbc.10470. [DOI] [PubMed] [Google Scholar]
- 24.Cervoni L, Celli P, Caruso R, et al. Neurinomas and ependymomas of the cauda equina. A review of the clinical characteristics. Minerva Chir. 1997;52(5):629–633. [PubMed] [Google Scholar]
- 25.MacDonald SM, Yock TI. Proton beam therapy following resection for childhood ependymoma. Childs Nerv Syst. 2010;26(3):285–291. doi: 10.1007/s00381-009-1059-4. [DOI] [PubMed] [Google Scholar]
- 26.Weber DC, Zilli T, Do HP, et al. Intensity modulated radiation therapy or stereotactic fractionated radiotherapy for infratentorial ependymoma in children: a multicentric study. J Neurooncology. 2011;102(2):295–300. doi: 10.1007/s11060-010-0318-2. [DOI] [PubMed] [Google Scholar]
- 27.Korshunov A, Neben K, Wrobel G, et al. Gene expression patterns in ependymomas correlate with tumor location, grade, and patient age. Am J Pathol. 2003;163(5):1721–1727. doi: 10.1016/S0002-9440(10)63530-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rousseau A, Idbaih A, Ducray F, et al. Specific chromosomal imbalances as detected by array CGH in ependymomas in association with tumor location, histological subtype and grade. J Neurooncology. 2010;97(3):353–364. doi: 10.1007/s11060-009-0039-6. [DOI] [PubMed] [Google Scholar]
- 29.Taylor MD, Poppleton H, Fuller C, et al. Radial glia cells are candidate stem cells of ependymoma. Cancer Cell. 2005;8(4):323–335. doi: 10.1016/j.ccr.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Lukashova-v Zangen I, Kneitz S, Monoranu CM, et al. Ependymoma gene expression profiles associated with histological subtype, proliferation, and patient survival. Acta Neuropathol. 2007;113(3):325–337. doi: 10.1007/s00401-006-0190-5. [DOI] [PubMed] [Google Scholar]