Abstract
Background
Posterior fossa syndrome (PFS) is an important complication of posterior fossa surgery in children. The pathophysiology of this condition remains unclear, but there is evidence implicating surgical injury of the proximal efferent cerebellar pathway (pECP) and the cerebellar vermis to PFS. We aimed to evaluate if diffusion abnormalities involving these structures on the final intraoperative MRI can predict the development of PFS.
Methods
Diffusion-weighted imaging from 31 posterior fossa resections were anonymized and evaluated for abnormalities involving the dentate nucleus, superior cerebellar peduncle, and the mesencephalic tegmentum forming the pECP, vermis, and middle cerebellar peduncle. The case notes were independently evaluated for evidence of PFS.
Results
The diffusion imaging in 28 cases was of optimal quality for evaluation. Diffusion abnormalities were identified in 10 cases, 7 of which involved the pECP. Retrospective evaluation revealed evidence of PFS in 6 cases. There was a significant association between abnormalities involving pECP structures (P = .001) and development of PFS. Bilateral involvement of pECP (P = .006) was a highly specific risk factor for predicting the development of PFS. Diffusion abnormality of the inferior vermis was significantly associated with PFS (P = .001) but may not represent a risk factor in isolation.
Conclusion
This study demonstrates the feasibility of identifying children at risk for developing PFS at the earliest stage post tumor resection and thus adds to the growing evidence base on its pathophysiology.
Keywords: cerebellar mutism syndrome, diffusion-weighted imaging, efferent cerebellar pathway, intraoperative MRI, posterior fossa syndrome
Posterior fossa syndrome (PFS), also referred to as cerebellar mutism syndrome (CSM), is a well-recognized complication in children following posterior fossa tumor surgery and was first described by Rekate et al in 1985.1 It is characterized by neurological problems including diminished speech, ataxia, hypotonia, cranial nerve palsies, and emotional lability.2 The incidence of PFS after posterior fossa surgery varies between 11% and 29%.3 Although the symptoms improve with time, many children can exhibit long-term neurological sequelae that impact on the patient, the carer, and the health care provider.4,5
Although surgery is the primary predisposing event, the exact pathophysiology of PFS is not fully understood. Various hypotheses have been proposed, with varying levels of evidence.3 Among various factors that have been implicated in its etiology, interruption of the dento-thalamo-cortical (DTC) pathway and in particular, damage to structures of the proximal efferent cerebellar pathway (pECP) namely the dentate nucleus (DN) the superior cerebellar peduncle (SCP) and the mesencephalic tegmentum (MT) have been well documented in literature.6–10 Surgical damage to the cerebellar vermis has also been implicated in the etiology of PFS.11,12 These abnormalities can be detected on the immediate postoperative MRI, which is usually performed 24–48 hours following surgery. Assessment of postsurgical damage can be challenging on the conventional MRI sequences, namely the T1-weighted, T2-weighted, and the fluid-attenuated inversion recovery (FLAIR) sequence due to multiple factors including mass effect of the tumor on adjacent structures and preexisting peritumoral edema. Diffusion-weighted imaging is sensitive in identifying acute postsurgical changes, but the appearances can vary depending on the timing of the scan and postoperative factors such as hemorrhage and artifacts related to intracranial air.
Since October 2009, surgical resection of intracranial tumors has been guided by intraoperative MRI (ioMRI) at our tertiary level pediatric neurosurgical center. During these procedures, the final (preclosure) ioMRI has served as the immediate postoperative MR scan if no further resection had been performed following the scan. Our postoperative MRI protocol includes diffusion-weighted imaging (DWI) or diffusion tensor imaging (DTI) depending on the needs of the patient. In our initial experience with the use of ioMRI, the DWI and DTI data have been useful for identifying surgically induced parenchymal abnormalities that include edema (cytotoxic/vasogenic) and hemorrhage.13,14
The availability of these postoperative scans performed at a uniform time period (immediately following surgery) provides an opportunity to study the association between diffusion abnormalities in children undergoing posterior fossa tumor surgery and the occurrence of PFS. In particular, these scans offer an opportunity for potentially predicting postoperative neurological deficits at the earliest possible time point and prior to any other potentially confounding postoperative events. The aim of this study was to evaluate if diffusion abnormalities on the final ioMRI could predict the development of PFS following posterior fossa tumor resections.
Materials and Methods
This was a retrospective cohort study in which all suitable cases were analyzed for evidence of diffusion abnormalities at preestablished anatomical locations, and their risk of developing PFS was subsequently evaluated. The study was based on the standard clinical imaging protocol and in this respect was exempt from consideration by a research ethics committee. The work was approved by our institutional research department.
Participants
All cases of ioMRI-guided posterior fossa brain tumor resections between October 2009 and December 2012 were included. Thirty-one ioMRI-guided resections were performed in 29 children during that period, and DWI or DTI sequences were performed in all of them during the final ioMRI. Cases with significant image distortion were excluded from the study.
Magnetic Resonance Imaging and Analysis
Our ioMRI protocol is based on the recommendations by the United Kingdom Children's Cancer and Leukaemia Group (CCLG), and either DWI or DTI sequences were performed on final intraoperative/immediate postoperative scans.13 Echo planar DWI sequences were performed with b values of 0 and 1000 mm2/sec (TR, 2750 ms; TE, 70 ms; echo train length, 59; matrix, 192 x113; FOV’ 239 mm; slice thickness/spacing, 4/4 mm). Echo planar DTI sequences were performed with b values of 0 and 800 in 16 or 32 directions (TR, 2750 ms; TE, 70 ms; echo train length, 47; matrix, 112 x110; FOV, 22 mm; slice thickness/spacing, 4/4 mm). The DTI sequence is the sequence of choice and takes up to 4.47 minutes of scan time. The DWI sequence is quicker (< 1 min) and is sometimes performed instead of DTI when there are time constraints. The echo planar sequences are prone to susceptibility artifact caused by air or metal, particularly in the intraoperative setting. Intracranial and intracavitary (within the surgical cavity) air is reduced as much as possible by irrigating the surgical field with saline. The fixation screws are usually placed away from the region of interest to minimize the influence of susceptibility artifact on image interpretation.
The diffusion-weighted images of all cases that met the inclusion criteria were anonymized and coded by one of our senior radiographers (DG). The anonymized scans, comprising b 1000/800 (DWI/DTI) maps and apparent diffusion coefficient (ADC) maps, were qualitatively evaluated by a pediatric neuroradiologist (SA) with 7 years of experience in pediatric neuroimaging, including 4 years in ioMR image interpretation. The scans were initially evaluated for image distortion caused by susceptibility artifact. The scans that were significantly distorted were excluded from the study. Significant distortion was defined as distortion involving all or most regions of interest (ie, pECP (DN, SCP, and MT), the vermis, and the middle cerebellar peduncle (MCP). Cases in which the distortion did not affect the aforementioned structures or involved only a single structure were included. The nondistorted/minimally distorted scans were assessed for evidence of abnormal diffusion in the DN, SCPs, MT, vermis, and MCP (Fig. 1). The vermian abnormalities were subdivided as abnormalities of the superior or inferior vermis based upon their location in relation to the fastigium. The imaging findings were scored as binary categorical variables (ie, presence or absence of diffusion abnormality). The signal intensity in regions of interest was assessed as increased, decreased, or similar to contralateral normal white matter (bilateral when in the midline) on the b800 and b1000 images. The ADC maps were assessed similarly in a qualitative fashion. Diffusion restriction is seen in areas of cytotoxic edema or hemorrhage in the postoperative setting (hypointense on ADC). Areas of increased diffusion (hyperintense on ADC) may be related to vasogenic edema. Conventional T1- and T2- weighted imaging was reviewed in cases with diffusion restriction in the posterior fossa to exclude hemorrhage as a cause for the diffusion abnormality. Subsequently, preoperative MRI scans were reviewed in the cases with abnormal diffusion to evaluate for the presence of similar abnormalities prior to surgery. Because the intention was to identify areas of surgical damage that could manifest as cytotoxic edema, vasogenic edema, or a combination of both in the intraoperative setting, hyperintense foci on the b800/1000 images attributed to surgical injury were considered as showing diffusion abnormality.
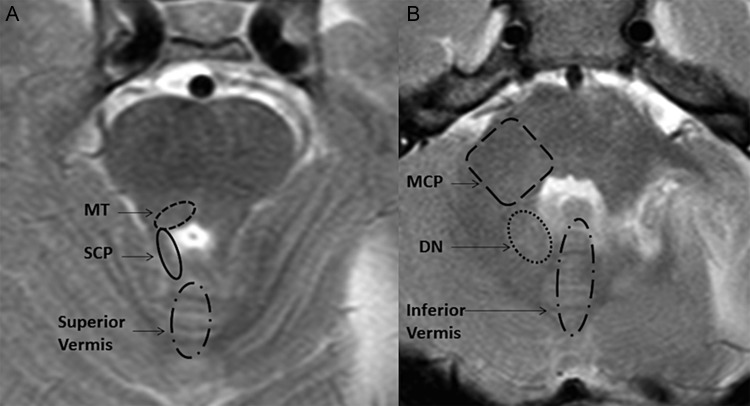
Fig. 1.
T2-weighted images of the posterior fossa at the level of the upper (A) and lower (B) pons showing the regions of interest being evaluated.
Evaluation for Posterior Fossa Syndrome
The case notes of all patients included in the study were independently reviewed retrospectively by an experienced pediatric neurologist (RK) for evidence of PFS. Since there are no agreed operational definitions of PFS, the following criteria were used (derived from existing literature and clinical experience). The inclusion criteria were persistence at day 7 after surgery of either (i) mutism or marked reduction in speech output with little attempt at nonverbal communication or (ii) disturbed behavior including irritability or lack of volitional movements. The exclusion criteria were (i) symptoms not more readily explained by another cause (eg, hydrocephalus, CSF infection, postoperative nausea, pain), (ii) symptoms resolving within 7 days after surgery, or (iii) symptoms that were present prior to surgical resection. The absence or presence of long tract signs, ataxia, and oculomotor signs were not used to include or exclude PFS.
Statistical Analysis
Patients with and without PFS were compared with respect to presence of diffusion abnormalities in one or more of the predetermined regions of interest and presence of unilateral or bilateral diffusion abnormalities. The sensitivity, specificity, and accuracy were calculated for each variable using standard formulas. Statistical analysis was performed using the Student t test for continuous variables and the chi-square or Fisher exact test for categorical variables. Analysis was performed using SPSS version 20.0, and P values <.05 were considered statistically significant.
Results
Cohort Characteristics
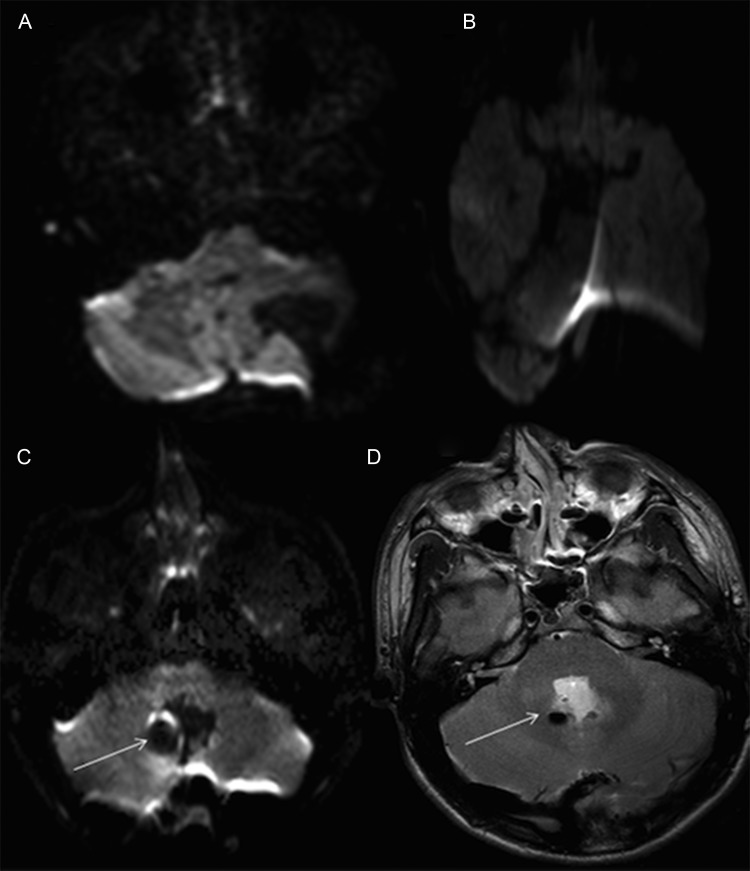
Three cases out of 31 ioMRI guided resections were excluded from the study because the images were significantly distorted. Of the remaining 28 resections (involving 26 children), 7 cases revealed partial/limited distortion and were included in the study. Figure 2 shows examples of cases with absent, partial, and significant distortion. Of the 26 children included in the study, 16 were boys. The mean age was 9.2 years (range, 1.6–17.6 years).
Fig. 2.
Diffusion-weighted b800 images demonstrating absence of distortion (A) and significant distortion (B). There is partial distortion (C) affecting the right DN (arrow) due to a focus of air shown on the T2 sequence (D).
Image Analysis
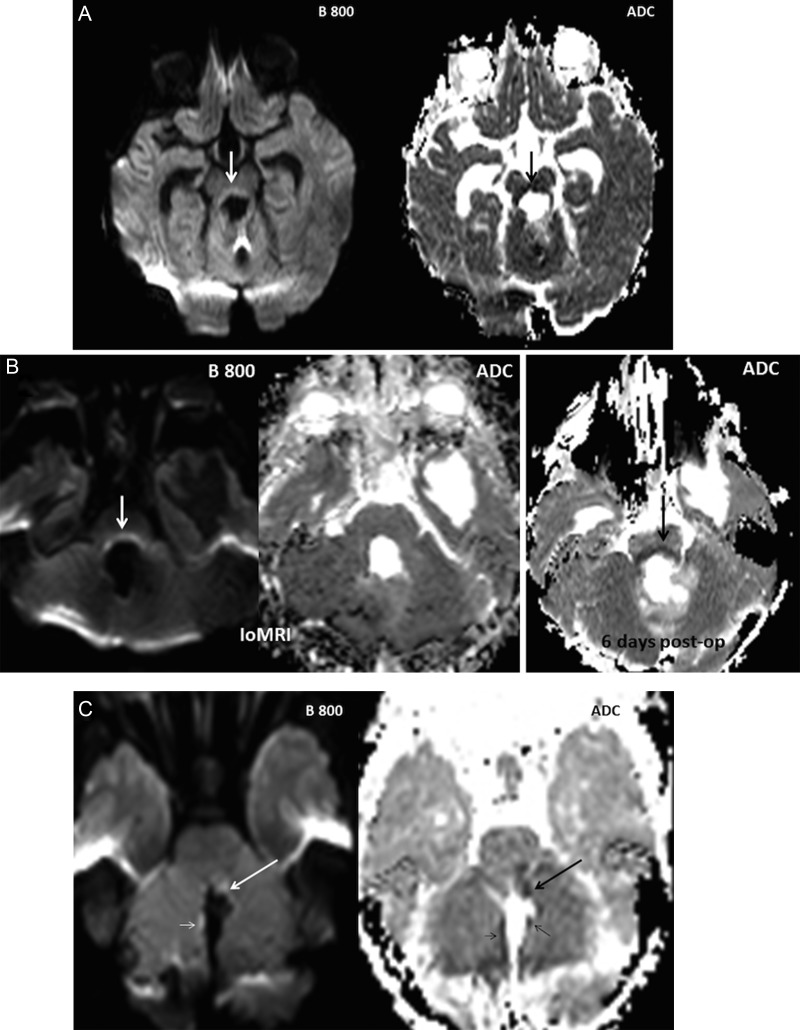
Of the 28 cases, 10 showed diffusion abnormalities in one or more regions of interest. Subsequent evaluation of the T1-/T2-weighted sequences and preoperative MRI ruled out underlying hemorrhage or preexisting abnormality. The anatomical distribution of the abnormalities is shown in Table 1. Six cases demonstrated diffusion abnormality in the pECP. Three of these 6 cases showed bilateral abnormalities in at least one region of the pECP (Fig. 3A), and one showed bilateral diffusion abnormalities affecting the SPC and the MT. The vermis was affected in 9 cases, with 7 involving the inferior vermis. The b1000/800 maps revealed increased signal intensity in all 10 cases, and 8 of them revealed hypointensity on the ADC map in keeping with cytotoxic edema. One case with bilateral diffusion abnormalities revealed both hyperintense and hypointense areas on ADC, suggesting cytotoxic edema in at least one area (MT). One case with bilateral diffusion abnormality of the SCP and MT on the b800 images showed normal signal intensity on the ADC map. A subsequent scan, performed 6 days later to guide neurological management, showed hypointensity at the same site on the ADC map in keeping with cytotoxic edema (Fig. 3B).
Table 1.
Anatomical distribution of diffusion abnormalities
| No. | Proximal Efferent Cerebellar Pathway (pECP) |
Vermis |
Middle Cerebellar Peduncle | Posterior Fossa Syndrome | |||
|---|---|---|---|---|---|---|---|
| Dentate | Supcerebellar Peduncle | Dorsal Pontine Tegmentum | Superior Vermis | Inferior Vermis | |||
| 1 | Bilateral | Bilateral | − | + | − | Yes | |
| 2 | Right | Right | Bilateral | − | + | − | Yes |
| 3 | Right | Bilateral | − | + | − | Yes | |
| 4 | Right | Right | − | + | − | Yes | |
| 5 | Left | − | − | + | No | ||
| 6 | Left | − | + | − | No | ||
| 7 | − | + | − | No | |||
| 8 | Right | − | + | − | Yes | ||
| 9 | + | − | − | No | |||
| 10 | + | − | − | No | |||
Fig. 3.
Cases illustrating various patterns of diffusion abnormalities. (A) Bilateral diffusion restriction of the MT in a 3-year-old girl who developed PFS. (B) IoMR in a 15-year-old girl who developed PFS, showing bilateral hyperintensity of the MT on the b800. The corresponding region on the ADC map appeared isointense, but a subsequent scan 6 days later showed hypointensity on ADC in keeping with restricted diffusion. (C) Diffusion restriction involving the left SCP (large arrows) and the vermis (small arrows) noted in an 11-year-old boy who did not develop PFS.
Review of the Postoperative Period
The case notes of 26 children were reviewed. Two of them had 2 separate surgical events, accounting for a total of 28 surgical events. One girl with an extensive ependymoma had 2 staged surgical procedures with a gap of 1 month. The other patient, a boy with a pilocytic astrocytoma, was reoperated 3 months following the initial surgery because of disease progression. The individual postoperative periods were assessed separately for evidence of PFS in both of these cases.
Based on the clinical features during the postoperative period, PFS was diagnosed in 6 children (21%). The 2 children who had 2 separate surgical episodes did not develop PFS in either of their postoperative periods. One child, aged 18 months, was too young to be assessed for PFS.
Predictors of Posterior Fossa Syndrome
The age at surgery did not differ significantly between children with and without PFS (P = .27).
As seen in Table 2, there was no significant association with the development of PFS when all regions of interest were considered together. When considered individually or as separate groups, there was a significant association between the development of PFS and the presence of abnormal diffusion in the pECP (SCP, DN, and MT), the vermis, and the inferior vermis. The sensitivity, specificity, positive and negative predictive values, and relative risk were similar for pECP and inferior vermis. Bilateral involvement of the pECP also had a significant association with development of PFS (P = .006) and was highly specific (100%). Unilateral diffusion abnormalities of the pECP and abnormalities of the superior vermis and MCP showed no significant association with PFS.
Table 2.
Association between the distribution of diffusion abnormality and posterior fossa syndrome
| Abnormal Diffusion | Posterior Fossa Syndrome |
|||||||
|---|---|---|---|---|---|---|---|---|
| P Value | Sensitivity | Specificity | PPV | NPV | RR | 95% CI | OR | |
| All regions of interest | .13 | 83% | 77% | 50% | 94% | 9 | 1.2–66.7 | 17 |
| pECP | .001 | 83% | 90% | 71% | 95% | 15 | 2.1–107.4 | 50 |
| pECP (unilateral) | .191 | 33% | 90% | 50% | 83% | 3 | 0.79–11.3 | 5 |
| pECP (bilateral) | .006 | 50% | 100% | 100% | 88% | 8 | 2.8–24.1 | n/a |
| Vermis | .007 | 83% | 81% | 56% | 95% | 10.6 | 1.4–77.6 | 22.5 |
| Inferior vermis | .001 | 83% | 91% | 71% | 95% | 15 | 2.1–107.5 | 50 |
| Superior vermis | >.99 | 0% | 90% | 0% | 76% | 1.3 | 1.05–1.64 | n/a |
| Middle cerebellar peduncle | >.99 | 0% | 95% | 0% | 78% | 1.2 | 1.05–1.57 | n/a |
Abbreviations: CI, confidence interval; OR, odds ratio; pECP, proximal efferent cerebellar pathway; PPV, positive predictive value; NPV, negative predictive value; RR, relative risk; n/a, not applicable.
Among the pECP group were 2 false-positive cases in which subtle foci of diffusion restriction were noted along the left SCP in a 14-year-old girl and an 11-year-old boy (Fig. 3C). Neither child developed PFS. There was one false-negative result in a 17-year-old boy who underwent resection of a medulloblastoma and developed PFS. The diffusion-weighted images were partially distorted by susceptibility artifact affecting the right DN, but the SCP and MT were not affected, and the images were therefore included in the study (Fig. 2C and D). Two children with inferior vermian abnormalities and all children with abnormalities of the superior vermis and MCP did not develop PFS.
Discussion
The pathophysiology of PFS has been a source of debate for the past 2 decades. Theories proposed on the mechanism of injury and predisposing factors include cerebral and cerebellar perfusion defects, vasospasm, transient disruption of neurotransmitter release, concomitant hydrocephalus, postoperative edema, and axonal injury related to surgical manipulation. Some of these have been refuted, and none have been proven.2,3,10,15–20 Based on neuroimaging studies, there is a greater degree of agreement that injury to the cerebello-cerebral efferent pathways can contribute to development of PFS.6–10,17,21 Our study is based on the published evidence linking injury to the pECP structures (DN, SCP, and MT) to PFS following surgery.
This study incorporated 2 important factors that have not been considered in any other study to date. All scans were performed at a relatively uniform time point following tumor resection. Having been performed immediately following resection, there is an opportunity to evaluate the postsurgical imaging appearance at its earliest time point. In contrast, conventional postoperative imaging is usually performed any time between 24 and 48 hours after surgery, and the postoperative physiological, pathological, and therapeutic factors can influence the imaging appearances. For instance, cerebral blood perfusion, which has been implicated in the pathophysiology of PFS, can vary during the postoperative period. Jabre et al reported a significant rise of cerebral perfusion in adults, averaging 18% of the preoperative value, during the first postoperative day following craniotomy. This was believed to be a normal reaction of the cerebral vasculature to surgery.22 Imaging performed immediately after surgery can therefore untether such postoperative influences on the MRI findings, and the postsurgical changes can be directly attributed to surgery.
Our study showed a significant association between diffusion abnormality involving the pECP (DN, SCP, and MT) and development of PFS. Bilateral foci of diffusion abnormality were not only significantly associated with PFS but were also a highly specific finding that could predict the development of PFS. These findings resonate with those of recent studies. Miller et al noted a positive association between bilateral damage to the DTC and PFS in which the patients with bilateral damage were 12 times more likely to develop PFS.7 Later, the same cohort of patients were analyzed by Morris et al, who reported a significant association between patients with T2 signal abnormalities in the pons (P = .029), midbrain (P = .003), and SCP (P = .030). Assessment of T2 abnormalities within the pons, midbrain, DN, and SCP demonstrated that 90% of patients with PFS showed abnormalities in 3 or more of these structures.6 A recent study evaluating the SCP in patients with PFS using DTI and tractography reported bilateral injury to the SCP in patients with PFS.23 Patay et al recently published a study showing significant association of PFS with hypertrophic olivary degeneration, which was characterized by degeneration of the inferior olivary nucleus following damage to structures including the SCP and DN. These results reinforce the association between pECP and PFS.24
The results of our study not only strengthen the association between pECP injury and PFS but also provide the earliest opportunity to identify patients at risk for developing PFS (even prior to leaving the operating theater) and to help plan appropriative rehabilitative measures that may influence their clinical outcome. It also has the potential to help identify patients for early therapeutic intervention or related clinical trials in the future.
Among the pECP-positive cases was one false-negative scan, but this was partly degraded by susceptibility artifact obscuring the right DN (Fig. 2C and D). This is a recognized limitation of ioMRI, and irrigation of the surgical cavity with saline has helped ameliorate this problem to a large extent. The 2 false-positive cases in the pECP group showed unilateral diffusion abnormalities in the left SCP. This is in keeping with other studies that showed greater risk in children with multiple and bilateral abnormalities.6–9 A recent study by Law et al evaluated the cerebello-thalamo-cerebral (C-T-C) pathways in children with CSM and control groups with and without posterior fossa tumors using DTI.21 They noted that the right cerebellar white matter within the C-T-C pathway was compromised in children with CSM relative to children without CSM and healthy children (P < .02). This is thought to be due to disruption of the C-T-C pathway connecting the right cerebellum with the areas of speech production and expressive language in the left frontal lobe. A similar observation was made by Puget et al, who noted poor cognitive outcome in children with injury to the right cerebellar hemisphere and DN. In the light of this novel finding, one could speculate that the 2 false-positive cases in our study with diffusion abnormalities in the left pECP had a lesser risk of developing PFS. All patients with diffusion abnormality in our cohort who developed PFS had right-sided changes. This is a useful predictive factor but needs to be validated with larger patient cohorts.
Our study shows significant association between vermian abnormalities, in particular the inferior vermis and PFS. The evidence for implicating vermian injury to PFS is unclear. Puget et al noted a significant association between damage of the inferior vermis and PFS (P = .004).11 Grill et al evaluated risk factors for intellectual impairment in children with posterior fossa tumors and noted that incision of the vermis had a significant association with low performance intelligence quotient (P = .02). On the other hand, Wells et al noted no association between inferior vermian incision and PFS. The association between vermian incision/injury and PFS has been challenged by other observations in which avoidance of vermis-splitting surgery (the telovelar approach) has not prevented PFS.25 PFS has occurred in non–vermis-splitting surgery.26 In our group, all patients with diffusion abnormalities of the vermis underwent vermis-splitting surgery. Two of the children with diffusion abnormality of the inferior vermis and all children with superior vermis abnormality did not develop PFS. All children with PFS and vermian abnormality also showed diffusion abnormality of the pECP. Based on these factors, we speculate that diffusion abnormality of the vermis on its own does not necessarily represent a risk factor for developing PFS.
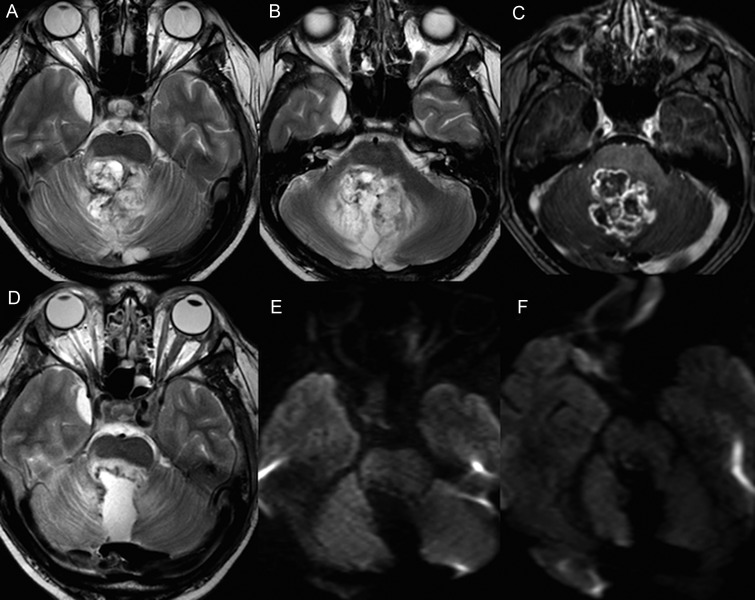
Our study also emphasizes the importance of using DWI in early assessment of patients undergoing posterior fossa surgery. This is a relatively new technique, and understandably the earlier reports and studies related to PFS have relied on imaging appearances on the conventional T2 and FLAIR sequences to identify edema involving the structures surrounding the fourth ventricle, including the pECP. Differentiating edema due to surgical damage from peritumoral edema can be challenging, particularly in large tumors where the surrounding structures are compressed and are not clearly visualized on preoperative imaging. DWI is much more sensitive in identifying acute tissue injury caused by surgery. Restricted diffusion (low ADC) is commonly observed at the operative site, indicating cytotoxic edema.27 Increased and normal ADC can also be noted in some instances, as we observed in 2 of our patients with PFS (one of them demonstrated bilateral restricted diffusion within the SCP and MT on the scan performed 6 days later). These variations of ADC are probably related to early postsurgical changes occurring at the cellular level. Hemorrhage can also result in restricted diffusion but was excluded on the conventional sequences. The utility of diffusion imaging for assessing surgical injury is best illustrated by a case involving a 15-year-old boy who underwent surgery for a diffusely infiltrative recurrent pilocytic astrocytoma (Fig 4). The tumor was noted to infiltrate the DN, SCP, and dorsal brainstem and was partially resected. Postoperative scan showed no diffusion abnormality of the pECP structures, which indicated absence of surgical damage to these structures. Despite involvement of the pECP structures on the preoperative imaging, he did not develop PFS.
Fig. 4.
Preoperative imaging in a 15-year-old boy with recurrent pilocytic astrocytoma reveals an enhancing tumor infiltrating the DN, SCP, and MT on the axial T2 (A and B) and T1-postcontrast sequence (C). T2-weighted image (D) on the final intraoperative scan shows partial resection of the tumor with minimal hemorrhage along the surgical margins. The b1000 images (E & F) show no diffusion abnormality along the pECP and vermis. The patient did not develop PFS.
One limitation of this study is the qualitative nature of assessment of diffusion abnormalities. Quantification of the diffusion abnormalities in this study poses a few challenges: (a) the structures constituting the pECP are small and do not have well-defined margins; (b) the foci of diffusion abnormality are irregular, multifocal, and often seen along the surgical margins, and their extent is difficult to quantify by conventional radiological software; and (c) diffusion restriction is usually quantified by ADC measurement. However, in this study we also wanted to identify areas of increased diffusion, which could represent vasogenic edema due to surgical damage. Accurate quantification of these subtle abnormalities will require detailed mathematical analysis and will certainly add to the growing evidence on the pathogenesis of PFS. The authors appreciate the relatively small size of the cohort studied. The study population reflects our early ioMRI experience, and we intend to validate our findings with a larger number of cases in the future. Our study is built on the existing evidence base relating PFS to the pECP structures, and the aim was to evaluate these structures in an intraoperative setting. The results have proven the utility of ioMRI for predicting the development of PFS at a very early stage post surgery. Despite the limited patient numbers, we believe our findings contribute to the increasing evidence base on the pathophysiology of PFS. Five out of the 6 children with PFS demonstrated diffusion abnormalities on ioMRI, suggesting that the injury to the affected structures occurred during surgery. This does not discount previously suggested preoperative risks/contributory factors such as tumor size,21,28 brainstem invasion,20,29,30 rostral location of the tumor, and splaying of SCP,6 but it does make a delayed postoperative insult less likely, as suggested by some studies.2,15
A limitation of all studies on PFS is the lack of an agreed operational definition with clearly stated inclusion and exclusion criteria. Even the terminology is not agreed upon, with the alternative term “cerebellar mutism” being used variably by authors to mean either the same or a similar but distinct disorder. Others have used loosely applied criteria, including the presence of ataxia, as an indicator of severity of the PFS, or even nausea and vomiting. Our study sets forth a proposed operational definition, based on clarifying the implicit definitions in previous literature and our own clinical experience. Although the classification of patients as having PFS was assigned retrospectively by case note review in this study, we note that prospective bedside diagnosis of PFS is not as straightforward as implied in other studies. In particular, early postoperative assessment is often clouded by postoperative pain, nausea, and presurgical obtundation. Thus, we used the persistence of symptoms at 7 days after surgery as a more specific indicator of PFS. In addition, the symptoms of PFS may be difficult to identify in infants and in the presence of evolving hydrocephalus.
In summary, postoperative MRI performed immediately after resection can help identify abnormalities that can predict the development of PFS. Because PFS is often an evolving and delayed phenomenon (suggesting reversibility), predicting those most at risk as soon as possible after surgery offers an early opportunity for a therapeutic window to try reducing or preventing the severity of the impending development of the syndrome.
Availability of ioMRI has facilitated early imaging of these patients in our institution. Diffusion abnormalities involving the pECP structures (DN, SCP, and MT) have a significant association with PFS. Bilateral abnormality of the aforementioned structures is a highly specific predictive factor. Early identification of children at risk will prevent delays in initiating rehabilitative care and has the potential to inform selection of patients for therapeutic interventions if available in the future.
Funding
National Institute of Health Research - Research and Capability Funding (Alder Hey).
Conflict of interest statement. None declared.
References
- 1.Rekate HL, Grubb RL, Aram DM, et al. Muteness of cerebellar origin. Arch Neurol. 1985;42(7):697–698. doi: 10.1001/archneur.1985.04060070091023. [DOI] [PubMed] [Google Scholar]
- 2.Pollack IF, Polinko P, Albright AL, et al. Mutism and pseudobulbar symptoms after resection of posterior fossa tumors in children: incidence and pathophysiology. Neurosurgery. 1995;37(5):885–893. doi: 10.1227/00006123-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Gudrunardottir T, Sehested A, Juhler M, et al. Cerebellar mutism: review of the literature. Childs Nerv Syst. 2011;27(3):355–363. doi: 10.1007/s00381-010-1328-2. [DOI] [PubMed] [Google Scholar]
- 4.Steinbok P, Cochrane DD, Perrin R, et al. Mutism after posterior fossa tumour resection in children: incomplete recovery on long-term follow-up. Pediatr Neurosurg. 2003;39(4):179–183. doi: 10.1159/000072468. [DOI] [PubMed] [Google Scholar]
- 5.Huber JF, Bradley K, Spiegler BJ, et al. Long-term effects of transient cerebellar mutism after cerebellar astrocytoma or medulloblastoma tumor resection in childhood. Childs Nerv Syst. 2006;22(2):132–138. doi: 10.1007/s00381-005-1223-4. [DOI] [PubMed] [Google Scholar]
- 6.Morris EB, Phillips NS, Laningham FH, et al. Proximal dentatothalamocortical tract involvement in posterior fossa syndrome. Brain. 2009;132(Pt 11):3087–3095. doi: 10.1093/brain/awp241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller NG, Reddick WE, Kocak M, et al. Cerebellocerebral diaschisis is the likely mechanism of postsurgical posterior fossa syndrome in pediatric patients with midline cerebellar tumors. AJNR Am J Neuroradiol. 2010;31(2):288–294. doi: 10.3174/ajnr.A1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koh S, Turkel SB, Baram TZ. Cerebellar mutism in children: report of six cases and potential mechanisms. Pediatr Neurol. 1997;16(3):218–219. doi: 10.1016/s0887-8994(97)00018-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kusano Y, Tanaka Y, Takasuna H, et al. Transient cerebellar mutism caused by bilateral damage to the dentate nuclei after the second posterior fossa surgery. Case report. J Neurosurg. 2006;104(2):329–331. doi: 10.3171/jns.2006.104.2.329. [DOI] [PubMed] [Google Scholar]
- 10.van Dongen HR, Catsman-Berrevoets CE, van Mourik M. The syndrome of ‘cerebellar' mutism and subsequent dysarthria. Neurology. 1994;44(11):2040–2046. doi: 10.1212/wnl.44.11.2040. [DOI] [PubMed] [Google Scholar]
- 11.Puget S, Boddaert N, Viguier D, et al. Injuries to inferior vermis and dentate nuclei predict poor neurological and neuropsychological outcome in children with malignant posterior fossa tumors. Cancer. 2009;115(6):1338–1347. doi: 10.1002/cncr.24150. [DOI] [PubMed] [Google Scholar]
- 12.Grill J, Viguier D, Kieffer V, et al. Critical risk factors for intellectual impairment in children with posterior fossa tumors: the role of cerebellar damage. J Neurosurg. 2004;101(2 Suppl):152–158. doi: 10.3171/ped.2004.101.2.0152. [DOI] [PubMed] [Google Scholar]
- 13.Avula S, Mallucci CL, Pizer B, et al. Intraoperative 3-Tesla MRI in the management of paediatric cranial tumours--initial experience. Pediatr Radiol. 2012;42(2):158–167. doi: 10.1007/s00247-011-2261-6. [DOI] [PubMed] [Google Scholar]
- 14.Yousaf J, Avula S, Abernethy LJ, et al. Importance of intraoperative magnetic resonance imaging for pediatric brain tumor surgery. Surg Neurol Int. 2012;3(Suppl 2):S65–S72. doi: 10.4103/2152-7806.95417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turgut M. Transient “cerebellar” mutism. Childs Nerv Syst. 1998;14(4–5):161–166. doi: 10.1007/s003810050204. [DOI] [PubMed] [Google Scholar]
- 16.Catsman-Berrevoets CE, van Breemen M, van Veelen ML, et al. Supratentorial arterial ischemic stroke following cerebellar tumor resection in two children. Pediatr Neurosurg. 2005;41(4):206–211. doi: 10.1159/000086563. [DOI] [PubMed] [Google Scholar]
- 17.Ersahin Y, Mutluer S, Cagli S, et al. Cerebellar mutism: report of seven cases and review of the literature. Neurosurgery. 1996;38(1):60–65. doi: 10.1097/00006123-199601000-00015. discussion 66. [DOI] [PubMed] [Google Scholar]
- 18.Aguiar PH, Plese JP, Ciquini O, et al. Transient mutism following a posterior fossa approach to cerebellar tumors in children: a critical review of the literature. Childs Nerv Syst. 1995;11(5):306–310. doi: 10.1007/BF00301766. [DOI] [PubMed] [Google Scholar]
- 19.Wells EM, Walsh KS, Khademian ZP, et al. The cerebellar mutism syndrome and its relation to cerebellar cognitive function and the cerebellar cognitive affective disorder. Dev Disabil Res Rev. 2008;14(3):221–228. doi: 10.1002/ddrr.25. [DOI] [PubMed] [Google Scholar]
- 20.Wells EM, Khademian ZP, Walsh KS, et al. Postoperative cerebellar mutism syndrome following treatment of medulloblastoma: neuroradiographic features and origin. J Neurosurg Pediatr. 2010;5(4):329–334. doi: 10.3171/2009.11.PEDS09131. [DOI] [PubMed] [Google Scholar]
- 21.Law N, Greenberg M, Bouffet E, et al. Clinical and neuroanatomical predictors of cerebellar mutism syndrome. Neuro Oncol. 2012;14(10):1294–1303. doi: 10.1093/neuonc/nos160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabre A, Symon L, Richards PG, et al. Mean hemispheral cerebral blood flow changes after craniotomy. Significance and prognostic value. Acta Neurochir (Wien) 1985;78(1–2):13–20. doi: 10.1007/BF01809235. [DOI] [PubMed] [Google Scholar]
- 23.Ojemann JG, Partridge SC, Poliakov AV, et al. Diffusion tensor imaging of the superior cerebellar peduncle identifies patients with posterior fossa syndrome. Childs Nerv Syst. 2013;29(11):2071–2077. doi: 10.1007/s00381-013-2205-6. [DOI] [PubMed] [Google Scholar]
- 24.Patay Z, Enterkin J, Harreld JH, et al. MR imaging evaluation of inferior olivary nuclei: comparison of postoperative subjects with and without posterior fossa syndrome. AJNR Am J of Neuroradiol. 2014;35(4):797–802. doi: 10.3174/ajnr.A3762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siffert J, Poussaint TY, Goumnerova LC, et al. Neurological dysfunction associated with postoperative cerebellar mutism. J Neurooncol. 2000;48(1):75–81. doi: 10.1023/a:1006483531811. [DOI] [PubMed] [Google Scholar]
- 26.Ersahin Y. Is splitting of the vermis responsible for cerebellar mutism? Pediatr Neurosurg. 1998;28(6):328. doi: 10.1159/000028673. [DOI] [PubMed] [Google Scholar]
- 27.Ozturk A, Oguz KK, Akalan N, et al. Evaluation of parenchymal changes at the operation site with early postoperative brain diffusion-weighted magnetic resonance imaging. Diagn Interv Radiol. 2006;12(3):115–120. [PubMed] [Google Scholar]
- 28.Gelabert-Gonzalez M, Fernandez-Villa J. Mutism after posterior fossa surgery. Review of the literature. Clin Neurol Neurosurg. 2001;103(2):111–114. doi: 10.1016/s0303-8467(01)00125-1. [DOI] [PubMed] [Google Scholar]
- 29.Robertson PL, Muraszko KM, Holmes EJ, et al. Incidence and severity of postoperative cerebellar mutism syndrome in children with medulloblastoma: a prospective study by the Children's Oncology Group. J Neurosurg. 2006;105(6 Suppl):444–451. doi: 10.3171/ped.2006.105.6.444. [DOI] [PubMed] [Google Scholar]
- 30.Doxey D, Bruce D, Sklar F, et al. Posterior fossa syndrome: identifiable risk factors and irreversible complications. Pediatr Neurosurg. 1999;31(3):131–136. doi: 10.1159/000028848. [DOI] [PubMed] [Google Scholar]