Abstract
Diffuse low-grade glioma grows, migrates along white matter tracts, and progresses to high-grade glioma. Rather than a “wait and see” policy, an aggressive attitude is now recommended, with early surgery as the first therapy. Intraoperative mapping, with maximal resection according to functional boundaries, is associated with a longer overall survival (OS) while minimizing morbidity. However, most studies have investigated the role of only one specific treatment (surgery, radiotherapy, chemotherapy) without taking a global view of managing the cumulative time while preserving quality of life (QoL) versus time to anaplastic transformation. Our aim is to switch towards a more holistic concept based upon the anticipation of a personalized and long-term multistage therapeutic approach, with online adaptation of the strategy over the years using feedback from clinical, radiological, and histomolecular monitoring. This dynamic strategy challenges the traditional approach by proposing earlier therapy, by repeating treatments, and by reversing the classical order of therapies (eg, neoadjuvant chemotherapy when maximal resection is impossible, no early radiotherapy) to improve OS and QoL. New individualized management strategies should deal with the interactions between the course of this chronic disease, reaction brain remapping, and oncofunctional modulation elicited by serial treatments. This philosophy supports a personalized, functional, and preventive neuro-oncology.
Keywords: awake surgery, diffuse low-grade gliomas, individualized management, multistage therapeutic approach, quality of life
Supratentorial WHO grade II glioma in adults (diffuse low-grade glioma [DLGG]) is a complex and heterogeneous entity that accounts for about 15% of all gliomas.1 Management of DLGG patients has been a matter of debate for many decades. This controversy is due to several issues, namely (i) what is the actual natural history of DLGG; (ii) what is the real impact of treatments on this course; and (iii) what is the functional risk of therapies?
For a long time, most authors have considered DLGG to be a stable and benign brain tumor. Therefore, the “wait and see” approach was advocated, especially because DLGG usually involves young adults (fourth decade of life) who are enjoying a “normal life” with no, or only mild, deficits on a standard neurological examination. The traditional management has been performing only a biopsy to obtain samples for neuropathological examination and then choosing between a single follow-up or a radiotherapy according to the morphological criteria defined by the WHO classification (astrocytoma vs oligodendroglioma vs mixed glioma; grade II vs III). The clinical results were usually evaluated using few parameters (ie, progression free-survival [PFS], overall survival [OS], and eventually Karnofsky performance score [KPS]).
Recent technical and conceptual advances in cognitive neurosciences, imaging, genetics, and treatments have revolutionized our knowledge of DLGG, leading to the seminal principle of personalized management.2 DLGG is not a tumor mass within the brain but rather a progressive, invasive, and chronic disease of the central nervous system. This aggressive lesion grows continuously, migrates along the white matter pathways, and inevitably progresses to a higher grade of malignancy and leads to neurological disability and ultimately to death.2 Thus, the wait and see dogma should definitely be abandoned and evolve instead toward an early therapeutic approach with the aim of delaying anaplastic transformation. Such a strategy should be adapted to the complex biological course of DLGG at the individual level.2 In the traditional literature, most studies have investigated the role of only one specific treatment (eg, impact of surgery, radiotherapy, or chemotherapy) on OS without a global view of the whole management strategy on the patient's cumulative time with preserved quality of life (QoL) versus time to malignization. Furthermore, when different therapies were nonetheless associated, a classical order of therapies (surgery followed by irradiation in incomplete resection, followed by chemotherapy at recurrence) was rigidly applied to the group of DLGGs (as it was homogeneous), generally without an attempt to tailor the sequence of treatments to the specific patient.
Here, our aim is to switch to a more holistic view based on the anticipation of a personalized, long-term multistage therapeutic approach, with online adaptation of the management over the years using feedback provided by clinical, radiological, and histomolecular monitoring at the individual level. This dynamic strategy challenges the traditional attitude with regard to different issues by proposing earlier therapy; repeating treatments, and reversing the classical order of therapies, with the ultimate goal of increasing OS and preserving (or even improving) QoL. Neuro-oncologists should tailor their management strategy during the follow-up on the basis of real-time oncological control and functional outcome. The patient's neurological and neurocognitive status can be preserved, thanks to (i) neuroplasticity mechanisms allowing compensation of the tumor growth/invasion and the effect of the different therapies taken alone and together; and (ii) the selection of the best-tolerated treatment (with similar efficacy) at the right time for the right patient. We propose a new personalized and multistage therapeutic management strategy dealing with the chronic interactions between the natural course of DLGG, the reaction brain remapping, and the oncofunctional modulation elicited by serial treatments.
New Insights Into the Natural History of DLGG: Tumor Progression and Neuroplasticity
Pathology and Genetics: Towards a Molecular classification of DLGG?
The WHO classification recognizes grade II astrocytomas, oligodendrogliomas, and oligoastrocytomas.1 However, it suffers from several limitations.3 First, it is not reproducible. The interobserver or intraobserver discordance may reach 48%. Second, some elements are too subjective with regard to grading, such as the notion of anaplasia or cell density. Third, the WHO classification does not distinguish tumoral cells from infiltrated residual brain parenchyma, and it considers the tumor as homogeneous. Nonetheless, heterogeneous foci are frequently found on a background of DLGG that corresponds to foci of increased cell density, possibly with pronounced cytonuclear atypia. Yet, the WHO classification does not recognize the existence of a continuum between grade II and grade III glioma. Thus, the term of “intermediate diffuse glioma” was introduced for cases in which the presence of these foci may lead to faster evolution toward anaplasia.3 This explains why we prefer to use the term DLGG.2
Advances in genetics have brought new insights into the comprehension of DLGG biology.4 The most frequent molecular alteration (in about 80% of DLGGs) is the IDH1/2 mutation, which occurs at a very early stage. Diffuse astrocytomas frequently carry TP53 mutations, which constitute a prognostic marker for shorter survival; gemistocytic astrocytomas carry TP53 mutations in >80% of tumors, while combined 1p/19q deletion is rare. The molecular profile of oligodendrogliomas is the combined loss of 1p/19q occurring in 70%–80% of these tumors, which is associated with longer survival, while TP53 mutations are encountered in only 5% of tumors. Most oligoastrocytomas carry either 1p/19q loss or TP53 mutations, with a tendency for these aberrations to be present in both tumor compartments. Because more than 90% of DLGGs carry at least one of these alterations, it has been suggested that a new molecular classification be developed that would complement and eventually replace histological typing. The molecular profile of DLGG based on IDH1/2 mutations, TP53 mutations, and 1p/19q loss seems to provide a more objective classification that correlates well with OS.4
Tumor Growth
There is no stable DLGG. Objective calculation of growth rate (based on at least 2 MRIs spaced by 3 months before treatment) have shown that all DLGGs had a constant growth during their premalignant phase, with a linear increase of the mean diameter (computed from the volume) of ∼ 4 mm/year.5 This growth has been observed both in symptomatic patients and in those with incidentally discovered DLGGs. Thus, the concept of PFS is meaningless in DLGG before treatment or after incomplete surgical resection because, in essence, all DLGGs are continuously growing, whereas this endpoint would be unambiguous after a total resection.2 Indeed, “total resection” is defined as an absence of signal abnormality on postoperative fluid-attenuated inversion recovery (FLAIR)-weighted MRI. In this setting, PFS is unambiguous because relapse will be defined as a recurrence of signal abnormality. Thus, the classical radiological criteria, as proposed by Macdonald or by the RANO group,6 are not appropriate for monitoring DLGG kinetics. Indeed, the RANO criteria do not recommend performing objective 3D volumetric assessment of these tumors based upon segmentation on digital imaging and communications in medicine images, in spite of the fact that DLGGs are often irregular. As a consequence, calculation of growth rate is not reliable, which could be a major problem in DLGG with slow kinetics; one might believe that the tumor is stable when it is actually a slow-growing glioma, thus preventing adaptation for optimal management.
Furthermore, there is an inverse, significant correlation between growth rates and OS in DLGG. OSs of 253, 210, 91, and 75 months correlated with growth rate < 4 mm/year, 4–8 mm/year, 8–12 mm/year and >12 mm/year, respectively.5 The growth rate data will also be useful throughout for surveillance and monitoring of response to therapies.
Migration
These tumors migrate along the white matter tracts.7 DLGG is not a well-delinated tumor mass but is rather an infiltrating chronic disease that progressively invades the central nervous system, especially the subcortical connectivity known to be critical for brain functions. Such a diffusion of glioma cells may induce neurocognitive disorders, probably due, at least partly, to a disconnection syndrome.7 Glioma migration along fibers can limit the extent of surgical resection necessary for preserving QoL.8
Anaplastic Transformation and Survival
DLGG will inevitably become malignant,2,5 and such anaplastic transformation (AT) will lead to functional deficit and ultimately to death. In European Organisation for Research and Treatment of Cancer (EORTC) randomized multicenter trials with more than 600 patients, the OS was 7.7 years in the subgroup of patients with favorable prognostic scores, whereas the OS was only 3.2 years in the subgroup of particpants with poor prognostic scores.9–11 In the EORTC trial with 314 DLGGs, OS was between 7.2 and 7.4 years.12 Recently, a study comparing early resection versus single biopsy demonstrated that the OS was 5.8 years in the biopsy group (not reached in the surgical group).13 Thus, DLGG cannot be considered to be a benign tumor.
Spontaneous Prognostic Factors
Clinically, age >40 years, presence of neurological deficits or absence of seizures at onset, and low performance status (KPS <70%) are associated with a poorer outcome.11,14 Radiologically, larger tumors, tumors crossing the midline12 (especially with a volume >10cc15), and rapid growth rate5 are adverse prognostic factors. A low cerebral blood volume correlates with longer OS, while the presence of lactates and lipids on MR spectroscopy is related to more aggressive behavior.16 Histologically, oligodendrogliomas have a better prognosis than astrocytomas, whereas oligoastrocytomas have an intermediate outcome. Among molecular markers, 1p-19q codeletion and IDH1 mutation are the most important prognostic factors.4
Functional Considerations and Cerebral Plasticity in DLGG Patients
DLGG usually involves young adults who enjoy a normal life. After an asymptomatic period that lasts several years (as demonstrated in incidental DLGG), seizures are the most common presentation. They occur in 80%–90% of patients and are intractable in 50%, especially in rolandic, mesiotemporal, and insular/paralimbic locations.17 Neurological deficits are rare in patients with DLGG, even if these tumors are frequently located within eloquent areas.18 This is due to cerebral plasticity because DLGG is a slow-growing tumor, giving many years to the brain for functional remapping.19 The recent integration of these concepts into the therapeutic strategy resulted in dramatic changes in the management of DLGG patients, with an increase of surgical indications in functional areas classically considered to be inoperable.7,8,19
Nonetheless, cognitive deficits are often observed when objective neuropsychological assessments are performed at the time of diagnosis, despite a normal social and professional life. Many DLGG patients experience disorders of executive functions, attention, concentration, working memory, or emotion.20,21 These deficits can be attributed to the tumor itself, to seizures, and to antiepileptic drug(s). Thus, a systematic assessment of higher functions and health-related QoL is now recommended before oncological treatment (i) to search subtle neuropsychological deficits; (ii) to tailor the therapeutic strategy (eg, decision of neoadjuvant chemotherapy rather than surgery first in cases of very diffuse DLGG inducing cognitive disturbances); (iii) to adapt the surgical methodology (eg, to select the optimal tasks that should be administrated during awake surgery); (iii) to have a pretherapeutic baseline allowing a comparison with the posttherapeutic evaluation; and (iv) to plan specific functional rehabilitation following surgery, which can induce a transient worsening.20,21
In summary, although it was traditionally claimed that DLGG was a benign tumor affecting patients with a normal life, in fact this disease induces functional disturbances and progresses to malignant glioma. Thus, neuro-oncologists should definitely switch from a traditional wait and watch policy to an early therapeutic strategy with the aim of delaying malignant transformation (MT) and increasing OS while preserving QoL.
The Impact of Surgical Resection in DLGG
The Traditional Literature
Despite controversies for many decades regarding the value of surgery in DLGG, recent reviews have suggested that extensive resection was correlated with a more favorable life expectancy.22 An analysis of 10 studies since 1990 showed that OS changed from 61.1 to 90.5 months with a greater extent of resection (EOR).23 Discrepancies in classical literature are related to the fact that EOR was not objectively assessed on postoperative MRI but was instead based on the sole subjectivity of the surgeons or on a single computed tomography scan. Due to the invasive feature of DLGG, the residual tumor was doubtlessly underestimated in numerous studies, resulting in erroneous conclusions about the benefit of surgery. Today, T2/FLAIR-weighted MRI is the only way to objectively calculate the postsurgical volume of residual tumor.
The Modern Literature Based on Objective Calculation of EOR on Postoperative MRI
In all recent series with objective postoperative evaluation of EOR on T2/FLAIR-MRI, more aggressive resection predicted a significant improvement in OS compared with simple debulking. When no signal abnormality was visible on control MRI (complete resection), patients had a significantly longer OS compared with patients having any residual abnormality. Moreover, even in incomplete tumor removal (especially subtotal resection with a residual volume <10 ± 5cc), patients with a greater percentage of resection had a significantly longer OS. This is due to the fact that surgery delayed histological upgrading because the volume of residual tumor serves as a predictor of AT.15 In the series by Smith et al including 216 DLGGs, after adjusting for the effects of age, KPS, tumor location, and tumor subtype, EOR remained a significant predictor of malignant PFS (P = .005) and OS (P < .001), with an 8-year OS of 98% of patients who underwent complete resection. Furthermore, the survival was significantly better with at least 90% EOR compared with <90% EOR, whereas EOR of at least 80% also remained a significant predictor of OS.24 In 156 DLGGs, Claus et al reported that patients who underwent incomplete resection had 4.9 times the risk of death compared with those who underwent total resection.25 In 222 DLGGs with a median follow-up of 4 years, Duffau et al found that 20.6% of patients with more than 10 cc of residue died, while only 8% of patients with <10 cc of residue died and no patients with complete resection died (P = .02).26 Yeh et al demonstrated that EOR and postoperative KPS showed independent prognostic significance for OS using multivariate analysis in 93 consecutive DLGGs.27 McGirt et al observed that gross-total resection versus subtotal resection was independently associated with increased OS (P = .017).28 In 191 consecutive DLGGs, the same team also showed that gross-total resection was an independent factor associated with AT (P = 0.05).29 In 130 DLGGs studied by Ahmadi et al extended surgery significantly prolonged OS.30 In a study of 314 DLGG patients, Schomas et al reported that the adverse prognostic factors for OS identified by multivariate analysis were in patients undergoing less than subtotal resection.31 The same team has also recently confirmed that gross-total resection and subtotal resection were factors associated with improved OS in a series with 852 DLGGs.32 In 190 DLGGs, Ius et al demonstrated that patients with an EOR ≥ 90% had an estimated 5-year OS of 93%, those with EOR between 70% and 89% had a 5-year OS of 84%, and those with EOR <70% had a 5-year OS of 41% (P < .001).33 Jakola et al investigated survival in population-based parallel cohorts of DLGGs from 2 hospitals with different surgical strategies. Treatment at a center that favored early surgical resection was associated with better OS (median survival, not reached) than treatment at a center that favored biopsy and watchful waiting (median survival, 5.9 years).13 Finally, the French Glioma Network (FGN) published the largest surgical series of DLGG ever reported (1097 patients), showing that EOR and postsurgical residual volume were independent prognostic factors significantly associated with longer OS.34 These results (ie, that surgical resection is an independent prognostic factor associated with increased malignant PFS [P < .001] and OS [P = .016]), have recently been confirmed in a series of 1509 DLGGs by the FGN.17 In this experience, the OS was almost 15 years (ie, about double of the OS reported in classical studies with no attempt to perform extensive resection10–12 or in series with only a biopsy13).
To sum up, early and maximal surgical resection is the first therapeutic option to consider in DLGG, as recommended by the European guidelines.35 These significant oncological results explain why a randomized trial would be unethical today.
The Concept of Supratotal Resection
Biopsy samples beyond MRI-defined abnormalities showed that conventional MRI underestimated the actual spatial extent of the DLGG because tumor cells were present around the MRI signal abnormalities (up to 20 mm). A recent series reported that a supratotal resection (ie, resection extending beyond the MRI signal abnormalities) performed in patients with a DLGG within noneloquent brain regions avoided AT (mean follow-up of 35.7 months and maximum follow-up of 135 months).36 This series was compared with a control group having only complete resection for a DLGG: AT was observed in 7 patients in the control group but not in any of the patients who underwent supratotal resection (P = .037). Furthermore, adjuvant treatment was administrated to 10 patients in the control group versus only one patient with supracomplete resection (P = .043).36
The Limited Role of Biopsy in DLGG
The indications for biopsy are very limited in DLGG. The goal of neuropathological examination is to provide the actual grade of the glioma in addition to molecular data. However, there is a high risk of sampling error. Overgrading of WHO grade I gliomas may occur in 11% of cases and undergrading of WHO grade III gliomas in 28%.37 Interestingly, maximal DLGG resection provides a more extensive amount of tumoral tissue, thus increasing the reliability of the histological grading.3 Furthermore, because the risk of biopsy is still around 2%, (ie, about the same rate as surgical resection; <2% in recent series),38,39 its indication for DLGG is essentially a contraindication for surgery. Beyond patients who do not want or who are not able to undergo resection for medical reasons, biopsy can be mainly considered for diffuse lesions, such as gliomatosis, when a subtotal resection is not a priori possible.40
Functional Considerations: The Conceptual Shift From an Image-guided Surgery to a Functional-mapping Guided Resection
The neurosurgeon should not be content with a single tumorectomy (ie, removal of the part of the glioma visible on imaging) but should instead perform the most extensive resection of the parenchyma invaded by this chronic tumoral disease, with the stipultion that this part of the brain is not crucial for cerebral functions.2,8 Intrasurgical imaging (ultrasonography, neuronavigation, intraoperative MRI) suffers from serious limitations. As mentioned, conventional T2/FLAIR MRI does not show the whole tumoral disease but only the “tip of the iceberg.” When DLGG is distant from eloquent structures, image-guided resection is by definition a non-sense, because it is possible to remove more tumoral cells while preserving QoL, i.e. to achieve a “supratotal” resection, with the impact on MT detailed above - on the condition nonetheless to not constraint the resection according to imaging.36 Consequently, performing image-guided resection may represent a loss of odds for patients with a DLGG outside the critical regions, which could be extensively removed using functional-guided resection.2,8
The aim is to continue resection until eloquent structures have been encountered, both at the cortical and subcortical levels, with no margin around these functional boundaries.38 Because of major interindividual anatomofunctional variability, anatomical landmarks are not enough. Despite recent advances in functional neuroimaging, the data provided by functional MRI and diffusion tensor imaging are not reliable. Their results may change according to the biomathematical used model, which explains their lack of reliability at the individual level.41 Above all, neuroimaging is not able to differentiate areas crucial for brain functions from regions that could be functionally compensated. Thus, there is a double risk of (i) not selecting a DLGG patient for surgery because functional activations are visible within the tumor on neuroimaging, while it was in fact possible to remove it with no permanent deficit; or (ii) inducing a permanent deficit due to a false negative.
Intraoperative electrostimulation mapping (IEM) in an awake patient is actually the more reliable method for identifying eloquent regions.38,39,41 This is a safe, inexpensive, and reproducible technique that allows the identification of crucial (nonfunctionally compensable) structures at the level of the cortex, white matter pathways, and deep gray nuclei.7,8 In the past decade, IEM has led to impressive improvement in both functional and oncological outcomes in DLGG surgery. First, it has allowed a significant increase of surgical indications for DLGG involving eloquent areas when compared with a control group of patients who underwent resection under general anesthesia with no mapping.26 A better understanding of neuroplasticity has opened the door to surgery in areas previously thought as unresectable while preserving QoL, as in Broca's area (Fig. 1), Wernicke's area, insula, or central region.7,19 Second, the rate of permanent neurological deficits is significantly lower thanks to awake IEM mapping (ie, <2%).38,39 This rate is very reproducible among the teams using IEM worldwide. A meta-analysis studying 8091 patients who underwent resection for a glioma demonstrated that the use of IEM allowed a significant reduction of late deficit, despite an increased rate of resection within eloquent areas.42 Surgical resection may also improve the QoL. In a series of 1509 DLGG patients, the FGN demonstrated that subtotal (P = .007) and total (P < .001) resections were independent predictors of total epileptic seizure control.17 Moreover, neuropsychological improvement has been shown following postoperative personalized cognitive rehabilitation, particularly concerning working memory.21 Third, the meta-analysis by De Witt Hamer et al also showed that EOR was increased, thanks to IEM.42 Fourth, a series of 281 patients confirmed that the use of functional mapping-guided resection of DLGG in presumed eloquent areas allowed both maximization of EOR and significant improvement of OS.43 Therefore, these results indicate that IEM should be universally implemented as a standard of care in glioma surgery.42
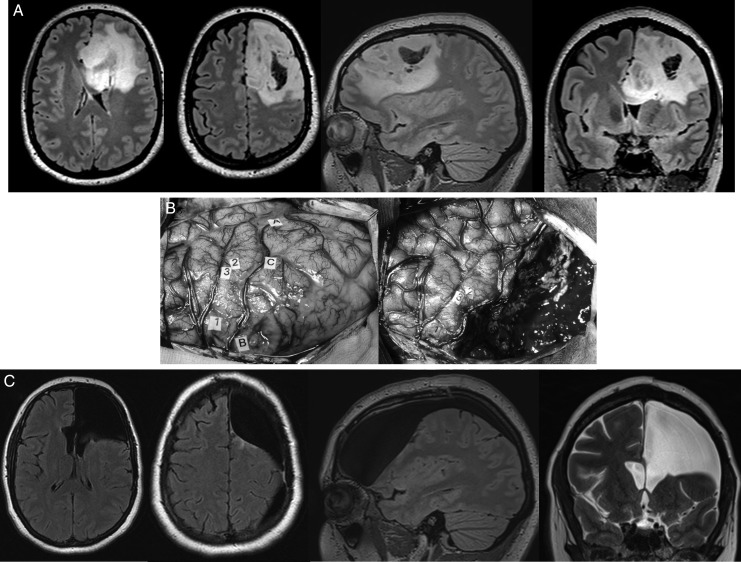
Fig. 1.
(A) Preoperative axial, sagittal, and coronal FLAIR-weighted MRI showing a left frontal DLGG incidentally discovered (headaches) in a young right-handed adult with a normal neurological examination and enjoying a normal life. The preoperative neuropsychological examination demonstrated slight attentional disorders. (B) Intraoperative photograph. Left: view before resection showing the tumor limits (letter tags) identified by ultrasonography as well as the eloquent cortical structures (number tags) detected by IEM. Right: view after glioma removal, performed according to functional boundaries and detected by IEM, both at the cortical and subcortical levels throughout the resection (no neuronagivation, no intraoperative MRI). In other words, a functional-mapped guided resection was achieved, not an image-guided resection. (C) Postoperative axial and sagittal FLAIR-weighted MRI as well as coronal T2-weighted MRI, demonstrating a complete resection of the DLGG (histologically confirmed), including its part involving the so-called Broca's area as well as within the corpus callosum. Despite an extensive left frontal lobectomy, the patient recovered and returned to a normal social and professional live (working full time) 3 months after surgery, with no symptoms. Interestingly, there was an improvement of the objective cognitive assessment performed 3 months following the resection in comparison with the presurgical evaluation, thanks to a specific cognitive rehabilitation. No antiepileptic drugs and no adjuvant oncological treatments were given.
Early and radical surgical resection has been recently proposed for incidental DLGG. A higher rate of total and supratotal resections have been achieved because the tumors were smaller. Furthermore, the rate of permanent deficit was nil, thanks to IEM awake surgery. These preliminary results support the development of preventive neurosurgery.44
Nonsurgical Therapies
Although watchful waiting should be the rule following a complete or supratotal resection for DLGG, adjuvant therapy could be considered for high-risk patients (ie, those with partial resection [with a residual volume >10 ± 5cc], rapid progression calculated on regular postoperative MRIs, or intractable postoperative intractable seizures).
Chemotherapy
The usefulness of chemotherapy (CT) for patients progressing after surgery and RT is well established. PCV (procarbazine, CCNU, and vincristine) and temozolomide (TMZ) yield similar objective response rates on MRI (45%–62%) and duration of response (10–24 months). However, a toxicity profile favors TMZ in terms of better tolerability (reduced myelotoxicity) and QoL. CT may also result in improvement of QoL, especially for patients responding radiologically and some patients with stable disease, thanks to reduction of seizures and neurological deficits.45
CT as initial treatment after surgery has also been investigated in DLGG patients with incomplete resection, persisting seizures, or rapid progression on control MRIs.46 In most cases, a response was observed by objectively calculating the growth rate on repeated MRIs with at least minor, or even partial, shrinkage of the tumor.47 The volume decrease can be delayed as long as 24–30 months and may persist once CT has been terminated, even though this is not the situation for the majority of patients. Most patients with seizures have a clinical benefit, even in the absence of a radiological change. Patients with oligodendroglial tumors are more likely to respond, but those with mixed or astrocytic tumors may respond as well. Although the response rate after CT seems to be similar regardless of the molecular profile, the duration of response is longer for patients with 1p/19q loss than for those with 1p/19q intact.48 Protracted low doses of TMZ could offer potential advantages over standard doses, especially in unmethylated tumors, but toxicity could be increased.35
Radiotherapy
Two phase III randomized trials have demonstrated no advantage for high versus low radiation doses and increased toxicity in higher doses.9,49 Regarding the timing of radiotherapy (RT), EORTC 22845 phase III randomized trial has shown that early RT had no impact on OS, despite an increase in PFS.10,12 Although RT may be beneficial for seizure control, patients who had neuropsychological follow-up at a mean of 12 years and were free of tumor progression maintained their cognitive status if not irradiated, whereas patients who received RT experienced worsening in their attentional and executive functioning as well as their information-processing speed.50 It is nonetheless important to acknowledge some limitations in this study, those being the lack of baseline testing, the inherent selection bias of patients who received radiotherapy having more aggressive disease, and the use of outdated techniques such as whole brain radiotherapy. RTOG 9802 compared RT alone versus RT + PCV, and PFS, but not OS, was improved. However, on post hoc analysis for 2-year survivors (n = 211), the addition of PCV to RT conferred a survival advantage that suggested a delayed benefit for CT. The probability of OS for an additional 5 years was 74% with RT + PCV versus 59% with RT alone (P = .02).51
Because of its potentially delayed neurotoxicity and the equivalent results in terms of OS whatever the timing of treatment (early or late), RT is being increasingly offered to high-risk patients32 with unresectable tumors (or tumor that cannot be reoperated) and in cases of progression after CT (but not as a first therapeutic option).
Proposal for Individualized Multistage Therapeutic Strategies in DLGG
The first step for managing DLGG is to investigate the tumor's behavior and its possible consequences on brain functions. It is crucial (i) to calculate the volume of the tumor and its growth rate because it has been demonstrated that both parameters are prognostic factors correlated with survival;5,34 (ii) to analyze the precise location of the glioma, both at the cortical and subcortical levels; and (iii) to perform extensive neuropsychological assessments (even in DLGG discovered incidentally) in order to adapt the intraoperative mapping, which has been demonstrated to increase the extent of resection while decreasing the morbidity.21,38,39,42 Such a baseline is essential for developing a personalized strategy because starting treatment too quickly, without this initial examination, will result in a loss of precious information (ie, not anticipating long-term optimal management at the individual level).
Even though maximal surgical resection has a significant impact on AT and OS, DLGG cannot be cured by surgery, even with supratotal resection. In the series by Yordanova et al 4 of 15 patients experienced a recurrence, even if MT did not occur, with a mean delay of about 38 months because isolated tumoral cells were still present beyond the glioma visible on MRI.36 This issue should be explained to the patient after diagnosis in order (i) to inform him/her about the fact that additional treatment(s) will be given regularly over the years; and (ii) to improve his/her compliance. Indeed, honest information is very well accepted by patients and helps build trust that will last throughout the management of this chronic disease.
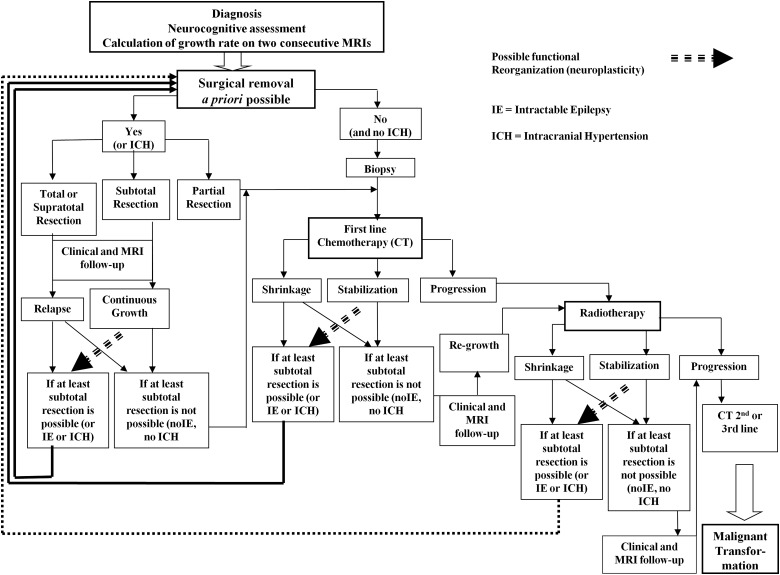
In practice, DLGG will likely recur several years after an initial maximal surgery, even after supracomplete or complete resection, and the growth rate of residual glioma after incomplete resection will be similar to its presurgical kinetics.2 Therefore, one can predict at the individual level when the tumor volume will reach 10 ± 5cc, which represents the threshold for a higher risk of AT. Consequently, a second preventive treatment can be proposed just before this threshold is reached—but not too early— in order (i) to not use future therapies prematurely; and (ii) to preserve QoL by limiting the use of too much treatment(s) (iii) while controlling the tumor by avoiding MT (Fig. 2).
Fig. 2.
Proposal of dynamic therapeutic strategy in DLGG before malignant transformation, with special emphasis on the role of early and maximal surgical resection(s) as well as multistage therapies tailored to each patient over years (modified from22).
The Multistage Surgical Approach: The Value of Reoperation(s)
The impact of multiple surgeries on DLGG has been investigated. In 40 patients reoperated for recurrent DLGG, Schmidt et al showed that gross-total resection was associated with increased time before further surgery.52 In 130 DLGGs, Ahmadi et al observed that extended resection for nonmalignant relapse prolonged the OS significantly.30 In the FGN series using multivariate analysis, subsequent surgical resection was an independent prognostic factor significantly associated with longer OS.34 Martino et al reported a consecutive series of patients who underwent a second surgery for recurrent DLGG in eloquent areas.53 A total or subtotal resection was achieved in 73.7% of patients during the reoperation, despite an involvement of functional areas. The median time between surgeries was 4.1 years, and the median follow-up from initial diagnosis was 6.6 years with no deaths during this period. Thus, the authors suggested consideration of reoperation(s) in all recurrent DLGGs. Nevertheless, due to the high rate (57%) of histologically proven AT at reoperation, it was proposed to overindicate early reintervention rather than perform late surgery after AT had already occurred.53
Such a multistage surgical approach—beginning with initial function-guided resection, followed by a period of several years, and then a second surgery with optimization of EOR while preserving QoL—is possible, thanks to brain plasticity.7,19 Cerebral remapping can be induced by (i) the first surgery itself; (ii) the postsurgical functional rehabilitation; and (iii) the slow DLGG regrowth.41 Regular neurocognitive assessments and serial functional neuroimaging can provide helpful data for predicting EOR of a second (or even third or fourth) surgery.7,21 Thus, the oncofunctional balance of multiple surgeries can be optimally found for each patient only if relationships between the DLGG course and the brain adaptation are taken into account.8
However, a significant oncological benefit of surgery was demonstrated only when the resection was (or resections were) at least subtotal. Therefore, when the glioma is very diffuse, especially with wide invasion of the cortico-subcortical structures that cannot be removed due to limitations of cerebral plastic potential7 and/or with bi-hemispheric infiltration, one can predict that surgical removal will be only partial.41 In these cases, there is no indication to perform (re-)operation except (i) in patients with intractable epilepsy because even partial resection may allow a relief of seizures, particularly within the insula or mesiotemporal structures; and (ii) in rare cases of intracranial hypertension. Otherwise, alternative treatment should be considered, without the need of biopsy.
The Place of Chemotherapy in a Dynamic Multimodal Therapeutic Strategy
CT is adapted for invasive DLGG when (re-)operation is not possible.54,55 Whatever the protocol used (PCV or TMZ), CT may diffuse into the entire brain, even in the eloquent areas, without inducing functional deficits. If one or several surgeries have already been performed, CT can be considered when the tumor regrows with a volume reaching 10 ± 5cc by involving critical structures that cannot be functionally compensated (eg, bilateral extension or invasion within subcortical connectivity). Again, the goal is to control the tumor volume, in order to delay AT while preserving QoL. TMZ is generally preferred because of fewer adverse effects. QoL does not seem to change over time while patients are receiving TMZ. Furthermore, CT has improved the QoL for patients with intractable seizures by controlling their seizures, thus leading us to give TMZ to these individuals sooner.54,55
More than 90% of patients experienced initial decrease of the mean tumor diameter.47 When CT was discontinued in the absence of tumor progression, a majority of DLGGs resumed their progressive growth within one year,47 while tumor volume decreases could be delayed longer, particularly for patients with 1p/19q loss.48
When tumor shrinkage is important, especially with regression of the glioma invasion within eloquent structures, CT may open the door to a subsequent surgery.54,55 This concept of neoadjuvant chemotherapy in neuro-oncology may be considered following previous surgical resection(s), when the DLGG relapsed with a more invasive pattern, or as the first therapeutic option at the time of diagnosis in very diffuse gliomas (“gliomatosis-like”). This strategy should not be guided by the molecular profile at the individual level. It has no negative impact on QoL and can even improve it (Fig. 3).2,54,55 Of note, prospective trials should be performed to validate this new therapeutic approach.
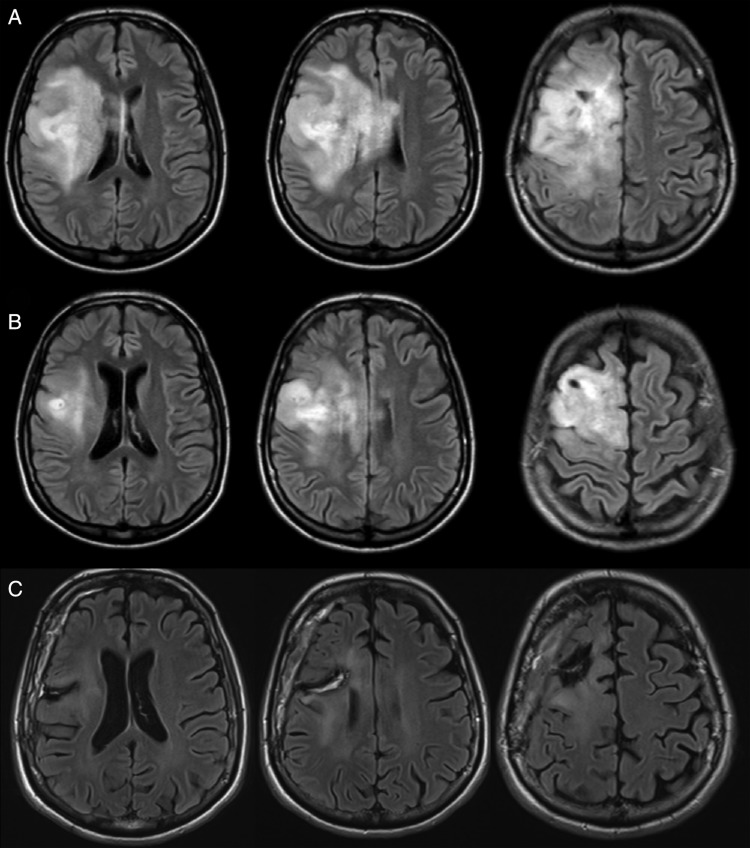
Fig. 3.
(A) Axial FLAIR-weighted MRI revealing a wide DLGG, which involved the right frontal and central areas as well as the corona radiata, in a woman who experienced intractable epilepsy. (B) After 20 cycles of neo-adjuvant chemotherapy (temozolomide) a dramatic shrinkage was observed, and awake surgery with resection guided by functional mapping was achieved. (C) Postoperative axial FLAIR-weighted MRI, demonstrating a subtotal resection in a patient who returned to a normal life with no seizures.
When CT allows only stabilization of tumor volume, without opening the window to a (re-)operation, the duration of TMZ is still a matter of debate. (PCV is stopped after a maximum of 4–6 cycles.) It is difficult to predict the behavior of DLGG after interrupting CT because distinct patterns have been described (ie, continuation of shrinkage, prolonged stabilization, or rapid regrowth).47 Several criteria for monitoring should be taken into account to solve this issue on an individual basis. First, with the aim of preserving QoL, CT should be interrupted if it is (or if it becomes) poorly tolerated. Second, radiologically, the tumor volume is one of the most important markers related to the risk of MT and OS.15,34 If the volume is >10 ± 5cc, the tendency is to give TMZ longer because the risk of MT is higher. Third, the growth rate before administration of CT should be taken into account because this paramater is correlated with OS. Therefore, CT should be administrated earlier and longer for the DLGG with a higher growth rate. Fourth, neuropathological parameters are also interesting, especially when microfoci of AT are detected within a DLGG, leading to give TMZ longer. Fifth, despite significant correlations between growth rate and 1p19q status, the decision to begin CT should not be based on molecular biology because of its poor predictive value of tumor response.47 On the other hand, significant correlations between 1p19q status and delay of relapse after interruptng TMZ have been reported (ie, tumor recurred earlier after interruption of TMZ in non-coledeted DLGG).47,48
Prospective studies are needed to optimize the management of DLGG under CT, especially to evaluate the possible benefit-to-risk ratio of new protocols with alternating periods of several months of TMZ, broken by periods of single-treatment clinical and radiological follow-up.
When to Irradiate DLGG?
One prospective randomized trial demonstrated that early RT had no impact on OS.10,12 Furthermore, another study showed that the QoL was worsened because of late cognitive decline that was induced by irradiation.50 Unlike surgery and CT, RT cannot be repeated because of potential neurotoxicity. Therefore, early RT should not be considered as a first option in strategies that aim to optimize the cumulative time with preserved QoL while preventing AT. Irradiation should be kept in reserve for progressive DLGG that has relapsed under chemotherapy and cannot be (re-)operated.
Conclusions
The current philosophy for DLGG patients is to anticipate (before neurological or even cognitive worsening) a personalized, multimodal, and long-term management strategy, with online treatments adjusted over time on the basis of regular functional feedback and radiological monitoring. This dynamic strategy challenges the traditional attitude, namely, (i) by proposing earlier therapy after the diagnosis, (ii) by repeating treatments (eg., multiple surgeries spaced by several years, periods of months of CT spaced by periods of follow-up); and (iii) by reversing the classical order of therapies (eg, neoadjuvant chemotherapy followed by surgery after tumor shrinkage, no early radiotherapy), with the ultimate goal of improving cumulative time with preserved QoL and OS. The ultimate aim is not (yet) curing this tumor but rather taking measures to delay AT as long as possible, while giving DLGG patients a real life that includes planning for their long-term future (eg, such as deciding whether to have a baby). This concept leads to individualized, functional, and prophylactic neurooncology. The next step is to envision a screening policy for silent DLGG, in order to achieve more frequent total and supratotal resections in smaller tumors.56
Funding
None.
Conflict of interest statement. None declared.
References
- 1.Louis D, Ohgaki H, Wiestler O, et al. World Health Organization Classification of Tumors of the Central Nervous System. 4th ed. Lyon: IARC; 2007. [DOI] [PubMed] [Google Scholar]
- 2.Duffau H. In: Diffuse Low Grade Glioma in Adults: Natural History, Interaction with the Brain, and New Individualized Therapeutic Strategies. Duffau H, editor. London: Springer; 2013. [Google Scholar]
- 3.Rigau V. In: Histological Classification. In Diffuse Low Grade Glioma in Adults: Natural History, Interaction with the Brain, and New Individualized Therapeutic Strategies. Duffau H, editor. London: Springer; 2013. [Google Scholar]
- 4.Kim YH, Nobusawa S, Mittelbronn M, et al. Molecular classification of low-grade diffuse gliomas. Am J Pathol. 2010;177(6):2708–2714. doi: 10.2353/ajpath.2010.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pallud J, Blonski M, Mandonnet E, et al. Velocity of tumor spontaneous expansion predicts long-term outcomes for diffuse low-grade gliomas. Neuro Oncol. 2013;15(5):595–606. doi: 10.1093/neuonc/nos331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Bent M, Wefel J, Schiff D, et al. Response assessment in neuro-oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. doi: 10.1016/S1470-2045(11)70057-2. [DOI] [PubMed] [Google Scholar]
- 7.Duffau H. The huge plastic potential of adult brain and the role of connectomics: New insights provided by serial mappings in glioma surgery. Cortex. 2013 doi: 10.1016/j.cortex.2013.08.005. pii: S0010–9452(13)00207–4. doi:10.1016/j.cortex.2013.08.005. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 8.Duffau H. The challenge to remove diffuse low grade gliomas while preserving brain functions. Acta Neurochir (Wien) 2012;154(4):569–574. doi: 10.1007/s00701-012-1275-7. [DOI] [PubMed] [Google Scholar]
- 9.Karim AB, Maat B, Hatlevoll R, et al. A randomized trial on dose-response in radiation therapy on low-grade cerebral glioma. European Organization for Research and Treatment of Cancer (EORTC) Study 22844. Inj J Radiat Oncol Biol Phys. 1996;36(3):549–556. doi: 10.1016/s0360-3016(96)00352-5. [DOI] [PubMed] [Google Scholar]
- 10.Karim AB, Afra D, Cornu P, et al. Randomized trial on the efficacy of radiotherapy for cerebral low-grade glioma in the adult: European Organization for Research and Treatment of Cancer Study 22845 with the Medical Research Council study BRO4: an interim analysis. Inj J Radiat Oncol Biol Phys. 2002;52(2):316–324. doi: 10.1016/s0360-3016(01)02692-x. [DOI] [PubMed] [Google Scholar]
- 11.Pignatti F, van den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20(8):2076–2084. doi: 10.1200/JCO.2002.08.121. [DOI] [PubMed] [Google Scholar]
- 12.van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. doi: 10.1016/S0140-6736(05)67070-5. [DOI] [PubMed] [Google Scholar]
- 13.Jakola AS, Myrmel KS, Kloster R, et al. Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA. 2012;308(18):1881–1888. doi: 10.1001/jama.2012.12807. [DOI] [PubMed] [Google Scholar]
- 14.Daniels TB, Brown PD, Felten SJ, et al. Validation of EORTC prognostic factors for adults with low-grade glioma: A report using intergroup 86–72–51. Int J Radiat Oncol Biol Phys. 2011;81(1):218–224. doi: 10.1016/j.ijrobp.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Berger MS, Deliganis AV, Dobbins J, et al. The effect of extent of resection on recurrence in patients with low-grade cerebral hemisphere gliomas. Cancer. 1994;74(6):1784–1791. doi: 10.1002/1097-0142(19940915)74:6<1784::aid-cncr2820740622>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 16.Guillevin R, Menuel C, Duffau H, et al. Proton magnetic resonance spectroscopy predicts proliferative activity in diffuse low-grade gliomas. J Neurooncol. 2008;87(2):181–187. doi: 10.1007/s11060-007-9508-y. [DOI] [PubMed] [Google Scholar]
- 17.Pallud J, Audureau E, Blonski M, et al. Epileptic seizures in diffuse low-grade gliomas in adults. Brain. 2014;137(Pt 2):449–462. doi: 10.1093/brain/awt345. [DOI] [PubMed] [Google Scholar]
- 18.Duffau H, Capelle L. Preferential brain locations of low grade gliomas. Cancer. 2004;100(12):2622–2626. doi: 10.1002/cncr.20297. [DOI] [PubMed] [Google Scholar]
- 19.Duffau H. Lessons from brain mapping in surgery for low-grade glioma: insights into associations between tumour and brain plasticity. Lancet Neurol. 2005;4(8):476–486. doi: 10.1016/S1474-4422(05)70140-X. [DOI] [PubMed] [Google Scholar]
- 20.Klein M, Duffau H, De Witt Hamer PC. Cognition and resective surgery for diffuse infiltrative glioma: an overview. J Neurooncol. 2012;108(2):309–318. doi: 10.1007/s11060-012-0811-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duffau H. Cognitive assessment in glioma patients. J Neurosurg. 2013;119(5):1348–1349. doi: 10.3171/2012.2.JNS112372. [DOI] [PubMed] [Google Scholar]
- 22.Duffau H. Surgery of low-grade gliomas: towards a “functional neurooncology”. Curr Opin Oncol. 2009;21(6):543–549. doi: 10.1097/CCO.0b013e3283305996. [DOI] [PubMed] [Google Scholar]
- 23.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurgery. 2008;62(4):753–766. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 24.Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26(8):1338–1345. doi: 10.1200/JCO.2007.13.9337. [DOI] [PubMed] [Google Scholar]
- 25.Claus EB, Horlacher A, Hsu L, et al. Survival rates in patients with low-grade glioma after intraoperative magnetic resonance image guidance. Cancer. 2005;103(6):1227–1233. doi: 10.1002/cncr.20867. [DOI] [PubMed] [Google Scholar]
- 26.Duffau H, Lopes M, Arthuis F, et al. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985–96) and with (1996–2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry. 2005;76(6):845–851. doi: 10.1136/jnnp.2004.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yeh SA, Ho JT, Lui CC, et al. Treatment outcomes and prognostic factors in patients with supratentorial low-grade gliomas. Br J Radiol. 2005;78(927):230–235. doi: 10.1259/bjr/28534346. [DOI] [PubMed] [Google Scholar]
- 28.McGirt MJ, Chaichana KL, Attenello FJ, et al. Extent of surgical resection is independently associated with survival in patients with hemispheric infiltrating low-grade gliomas. Neurosurgery. 2008;63(4):700–707. doi: 10.1227/01.NEU.0000325729.41085.73. [DOI] [PubMed] [Google Scholar]
- 29.Chaichana KL, McGirt MJ, Laterra J, et al. Recurrence and malignant degeneration after resection of adult hemispheric low-grade gliomas. J Neurosurg. 2010;112(1):10–17. doi: 10.3171/2008.10.JNS08608. [DOI] [PubMed] [Google Scholar]
- 30.Ahmadi R, Dictus C, Hartmann C, et al. Long-term outcome and survival of surgically treated supratentorial low-grade glioma in adult patients. Acta Neurochir (Wien) 2009;151(11):1359–1365. doi: 10.1007/s00701-009-0435-x. [DOI] [PubMed] [Google Scholar]
- 31.Schomas DA, Laack NN, Rao RD, et al. Intracranial low-grade gliomas in adults: 30-year experience with long-term follow-up at Mayo Clinic. Neuro Oncol. 2009;11(4):437–445. doi: 10.1215/15228517-2008-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Youland RS, Schomas DA, Brown PD, et al. Changes in presentation, treatment, and outcomes of adult low-grade gliomas over the past fifty years. Neuro Oncol. 2013;15(8):1102–1110. doi: 10.1093/neuonc/not080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ius T, Isola M, Budai R, et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients. J Neurosurg. 2012;117(6):1039–1052. doi: 10.3171/2012.8.JNS12393. [DOI] [PubMed] [Google Scholar]
- 34.Capelle L, Fontaine D, Mandonnet E, et al. Spontaneous and therapeutic prognostic factors in adult hemispheric WHO grade II gliomas: a series of 1097 cases. J Neurosurg. 2013;118(6):1157–1168. doi: 10.3171/2013.1.JNS121. [DOI] [PubMed] [Google Scholar]
- 35.Soffietti R, Baumert B, Bello L, et al. Guidelines on management of low-grade gliomas: report of an EFNS-EANO task force. Eur J Neurol. 2010;17(9):1124–1133. doi: 10.1111/j.1468-1331.2010.03151.x. [DOI] [PubMed] [Google Scholar]
- 36.Yordanova Y, Moritz-Gasser S, Duffau H. Awake surgery for WHO grade II gliomas within “noneloquent” areas in the left dominant hemisphere: toward a “supratotal” resection. J Neurosurg. 2011;115(2):232–239. doi: 10.3171/2011.3.JNS101333. [DOI] [PubMed] [Google Scholar]
- 37.Muragaki Y, Chernov M, Maruyama T, et al. Low-grade glioma on stereotactic biopsy: how often is the diagnosis accurate? Minim Invasive Neurosurg. 2008;51(5):275–279. doi: 10.1055/s-0028-1082322. [DOI] [PubMed] [Google Scholar]
- 38.Duffau H, Gatignol P, Mandonnet E, et al. Contribution of intraoperative subcortical stimulation mapping of language pathways: a consecutive series of 115 patients operated on for a WHO grade II glioma in the left dominant hemisphere. J Neurosurg. 2008;109(3):461–471. doi: 10.3171/JNS/2008/109/9/0461. [DOI] [PubMed] [Google Scholar]
- 39.Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358(1):18–27. doi: 10.1056/NEJMoa067819. [DOI] [PubMed] [Google Scholar]
- 40.Sanai N, Chang S, Berger MS. Low-grade gliomas in adults. J Neurosurg. 2011;115(5):948–965. doi: 10.3171/2011.7.JNS101238. [DOI] [PubMed] [Google Scholar]
- 41.Duffau H. In: Brain Mapping: From Neural Basis of Cognition to Surgical Applications. Duffau H., editor. New York: Springer Wien; 2011. [Google Scholar]
- 42.De Witt Hamer PC, Gil Robles S, Zwinderman A, et al. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559–2565. doi: 10.1200/JCO.2011.38.4818. [DOI] [PubMed] [Google Scholar]
- 43.Chang EF, Clark A, Smith JS, et al. Functional mapping-guided resection of low-grade gliomas in eloquent areas of the brain: improvement of long term survival. Clinical article. J Neurosurg. 2011;114(3):566–573. doi: 10.3171/2010.6.JNS091246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duffau H. Awake surgery for incidental WHO grade II gliomas involving eloquent areas. Acta Neurochir (Wien) 2012;154(4):575–584. doi: 10.1007/s00701-011-1216-x. [DOI] [PubMed] [Google Scholar]
- 45.Quinn JA, Reardon DA, Friedman AH, et al. Phase II trial of temozolomide in patients with progressive low-grade glioma. J Clin Oncol. 2003;21(4):646–651. doi: 10.1200/JCO.2003.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Hoang-Xuan K, Capelle L, Kujas M, et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22(15):3133–3138. doi: 10.1200/JCO.2004.10.169. [DOI] [PubMed] [Google Scholar]
- 47.Ricard D, Kaloshi G, Amiel-Benouaich A, et al. Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann Neurol. 2007;61(5):484–490. doi: 10.1002/ana.21125. [DOI] [PubMed] [Google Scholar]
- 48.Kaloshi G, Benouaich-Amiel A, Diakite F, et al. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;68(21):1831–1836. doi: 10.1212/01.wnl.0000262034.26310.a2. [DOI] [PubMed] [Google Scholar]
- 49.Shaw E, Arusell R, Scheithauer B, et al. Prospective randomized trial of low- versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a NCCTG/RTOG/ECOG study. J Clin Oncol. 2002;20(9):2267–2276. doi: 10.1200/JCO.2002.09.126. [DOI] [PubMed] [Google Scholar]
- 50.Douw L, Klein M, Fagel SS, et al. Cognitive and radiological effects of radiotherapy in patients with low-grade glioma: long-term follow-up. Lancet Neurol. 2009;8(9):810–818. doi: 10.1016/S1474-4422(09)70204-2. [DOI] [PubMed] [Google Scholar]
- 51.Shaw EG, Wang M, Coons SW, et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol. 2012;30(25):3065–3070. doi: 10.1200/JCO.2011.35.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmidt MH, Berger MS, Lamborn KR, et al. Repeated operations for infiltrative low-grade gliomas without intervening therapy. J Neurosurg. 2003;98(6):1165–1169. doi: 10.3171/jns.2003.98.6.1165. [DOI] [PubMed] [Google Scholar]
- 53.Martino J, Taillandier L, Moritz-Gasser S, et al. Re-operation is a safe and effective therapeutic strategy in recurrent WHO grade II gliomas within eloquent areas. Acta Neurochir (Wien) 2009;151(5):427–436. doi: 10.1007/s00701-009-0232-6. [DOI] [PubMed] [Google Scholar]
- 54.Blonski M, Taillandier L, Herbet G, et al. Combination of neoadjuvant chemotherapy followed by surgical resection as a new strategy for WHO grade II gliomas: a study of cognitive status and quality of life. J Neurooncol. 2012;106(2):353–366. doi: 10.1007/s11060-011-0670-x. [DOI] [PubMed] [Google Scholar]
- 55.Blonski M, Pallud J, Gozé C, et al. Neoadjuvant chemotherapy may optimize the extent of resection of WHO grade II gliomas: a case series of 17 patients. J Neurooncol. 2013;113(2):267–275. doi: 10.1007/s11060-013-1106-6. [DOI] [PubMed] [Google Scholar]
- 56.Mandonnet E, De Witt Hamer PC, Pallud J, et al. Silent diffuse low-grade glioma: towards screening and preventive treatment? Cancer. 2014 doi: 10.1002/cncr.28610. doi:10.1002/cncr.28610. Cancer. 2014;120(12):1758–1762. [DOI] [PubMed] [Google Scholar]