Abstract
Background
Circulating microRNAs (miRNAs) are emerging as promising biomarkers for human cancer. In the current study, we investigated the potential use of serum miRNAs as biomarkers for diagnosis and prognosis in a cohort of Chinese astrocytoma patients.
Methods
An initial screening of the circulating miRNA expression profile was performed on pooled serum samples from 10 preoperative patients and 10 healthy controls using a TaqMan low-density array. The selected serum miRNAs were then validated in 90 preoperative patients and 110 healthy controls who were randomly divided into a training set and a validation set. An additional double-blind test was performed in 50 astrocytomas and 50 controls to assess the serum miRNA-based biomarker accuracy in predicting astrocytoma. The differentially expressed miRNAs were evaluated in paired preoperative and postoperative serum samples from 73 astrocytoma patients. The correlation of the miRNA levels with survival in astrocytoma samples was estimated.
Results
Nine serum miRNAs were significantly increased in the astrocytoma patients. The biomarker composed of these 9 miRNAs had high sensitivity, specificity, and accuracy. These 9 miRNAs were markedly decreased in the serum after operation. The upregulation of miR-20a-5p, miR-106a-5p, and miR-181b-5p was associated with advanced clinical stages of astrocytoma. Kaplan-Meier survival analysis showed that the high expression of miR-19a-3p, miR-106a-5p, and miR-181b-5p was significantly associated with poor patient survival. Finally, the combined 3-miRNAs panel was an important prognostic predictor, independent of other clinicopathological factors.
Conclusions
The results indicated the potential of serum miRNAs as novel diagnostic and prognostic biomarkers for human astrocytoma.
Keywords: astrocytoma, diagnosis, prognosis, serum microRNA
Astrocytomas are the most common primary brain tumors in the central nervous system.1 The World Health Organization (WHO) established a 4-tier grading guideline for astrocytomas based on their histological and morphological features, with grade I being the least aggressive and grade IV being the most aggressive.2 Despite new biological insights and therapeutic advances, the general prognosis for astrocytoma patients remains poor, particularly for patients with grade IV astrocytomas; these patients have a median survival time of only 15 months.3 Specific, sensitive, and noninvasive biomarkers that can facilitate early diagnosis, help monitor disease progression, and assist in the prognosis of astrocytoma remain to be identified for clinical use.
MicroRNAs (miRNAs) are small, noncoding endogenous RNAs, 19–24 nucleotides in length, that modulate gene expression by base-pairing to complementary sites in target mRNAs and thereby block translation or triggering degradation of the target mRNAs.4 Increasing evidence has revealed that miRNAs are aberrantly expressed in a wide variety of human cancers and exhibit a causal role in tumorigenesis as either tumor suppressors or oncogenes.5,6 Unique patterns of altered miRNA expression provide complex fingerprints that may serve as molecular biomarkers for tumor diagnosis and predict disease-specific outcomes or the therapeutic response of patients.7 In our previous study, we established a tissue miRNA expression profile for astrocytoma risk prediction, histological classification, and assessment of prognosis.8 Blood-based biomarkers would be advantageous compared with the examination of tumor tissue due to the minimally invasive nature of the sampling, the ease of standardization in the sample analysis, and the possibility of repeated sampling. Several recent reports have suggested that circulating miRNAs are stable and detectable in serum/plasma and that the expression patterns of these miRNAs can potentially be used to identify various types of cancer and as noninvasive biomarkers. Malzkorn et al investigated the miRNA expression profile in the malignant progression of human glioma.9 Roth et al established a specific miRNA signature in the peripheral blood of grade IV astrocytoma patients.10 Wang et al found that 3 miRNAs were significantly altered in the plasma of glioma patients and were predictive for the diagnosis and prognosis of glioma.11 Ilhan-Mutlu et al reported that plasma miR-21 may be a useful biomarker associated with glioblastoma development in individual cases.12,13 Yang et al identified 7 serum miRNAs as potential noninvasive biomarkers for malignant astrocytomas by Solexa sequencing.14 Although these studies established their own miRNA signatures for astrocytoma, the results were from a small number of cases, were only specific to glioblastoma, or were not associated with prognosis. The objective of this study was to systematically and extensively investigate the potential of serum miRNAs as noninvasive biomarkers for the diagnosis and prognosis of human astrocytoma.
Materials and Methods
Study Design, Participants, and Controls
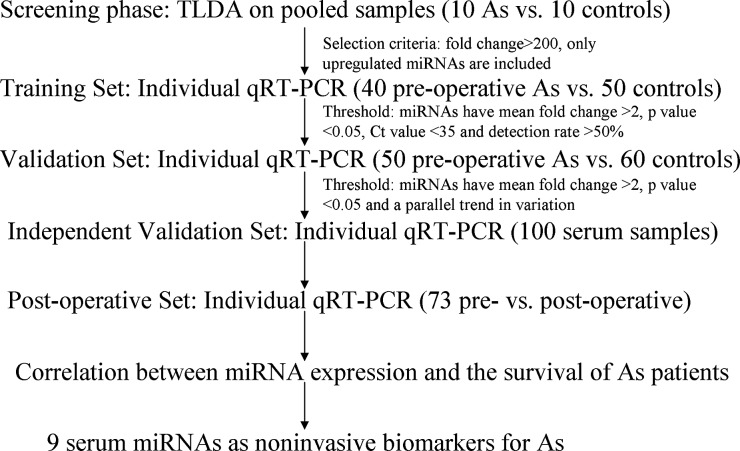
The study was approved by the Research Ethics Board of the Third Affiliated Hospital of Soochow University and the Research Ethics Committee of the Second Affiliated Hospital of Soochow University, and written informed consent was obtained from each participant. Ninety astrocytoma patients and 110 healthy controls were seen at the Third Affiliated Hospital of Soochow University between 2007 and 2013. Fifty astrocytoma patients and 50 healthy controls were seen at the Second Affiliated Hospital of Soochow University between 2012 and 2013. The demographic and clinical features of all patients and healthy controls are listed in Table 1. A multiphase case-control study was designed to identify serum miRNAs that may be potential biomarkers for astrocytoma (Fig. 1). Details of the patient enrollment procedure, blood-sample collection, and study design are available in the online supplementary methods. All samples were collected before and after surgery prior to any chemo- or radiotherapeutic treatment.
Table 1.
Summary of the clinical and pathological characteristics of astrocytoma and healthy control serum samples
| Variable | Preoperative |
P Valuec | Postoperative |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Training Set |
Validation Set |
||||||||||||
| Astrocytoma (n = 40) |
Control (n = 50) |
P Valuea | Astrocytoma (n = 50) |
Control (n = 60) |
P Valueb | Astrocytoma (n = 73) |
|||||||
| No. | % | No. | % | No. | % | No. | % | No. | % | ||||
| Average age (years) | 50.1 ± 13.1 | 49.2 ± 16.2 | 0.742d | 49.7 ± 13.8 | 49.1 ± 14.0 | 0.833 d | 0.536 d | 52.1 ± 14.3 | |||||
| Age (years) | 0.850 e | 0.848 e | 0.850 e | ||||||||||
| ≤50 | 20 | 50.0 | 26 | 52.0 | 26 | 52.0 | 29 | 48.3 | 35 | 47.9 | |||
| >50 | 20 | 50.0 | 24 | 48.0 | 24 | 48.0 | 31 | 51.7 | 38 | 52.0 | |||
| Sex | 0.981 e | 0.930 e | 0.555 e | ||||||||||
| Male | 19 | 47.5 | 25 | 50.0 | 28 | 56.0 | 32 | 53.3 | 36 | 49.3 | |||
| Female | 21 | 47.5 | 25 | 50.0 | 22 | 44.0 | 28 | 46.7 | 37 | 50.7 | |||
| WHO grade | 0.814 e | ||||||||||||
| Diffuse astrocytoma (WHO grade II) | 12 | 30.0 | 16 | 32.0 | 13 | 17.8 | |||||||
| Anaplastic astrocytoma (WHO grade III) | 16 | 40.0 | 22 | 44.0 | 34 | 46.6 | |||||||
| Glioblastoma multiforme (GBM, WHO grade IV) | 12 | 30.0 | 12 | 24.0 | 26 | 35.6 | |||||||
| Follow-up | |||||||||||||
| Alive | 21 | 52.5 | 27 | 54.0 | 0.887 e | ||||||||
| Dead | 19 | 47.5 | 23 | 46.0 | |||||||||
| Mean survival time (months) | 34.1 ± 6.8 | 28.2 ± 12.6 | |||||||||||
aAstrocytoma samples from training set versus control samples from training set.
bAstrocytoma samples from validation set versus control samples from validation set.
cAstrocytoma samples from training set versus astrocytoma samples from validation set.
dStudent t test.
e2-sided λ2 test.
Fig. 1.
A flow chart of the experimental design (As: astrocytoma).
Analysis of the Serum miRNA Profile by Taqman Low-density Array
The serum samples from 10 preoperative astrocytoma patients and 10 healthy controls were pooled in their respective groups. Total RNA was isolated from each pool of serum samples using the mirVana miRNA Isolation Kit (Applied Biosystems) according to the manufacturer's instructions. The Taqman low density array (TLDA) experiment was performed and analyzed by CapitalBio (CapitalBio Corp.) (see online supplementary methods).
Quantification of miRNAs by Quantitative Reverse Transcriptase Polymerase Chain Reaction Analysis
For the quantitative reverse-transcriptase (qRT) PCR assay, total RNA was extracted from 100 μL of serum by phenol/chloroform purification and centrifugation in isopropyl alcohol, as previously reported.15 Typically, the RNA yield was in the range of ∼50–100 ng after extracting the total RNA from 100 μL serum. The TaqMan microRNA assay was performed using a TaqMan PCR kit with the Applied Biosystems 7500 Sequence Detection System according to the manufacturer's instructions, with minor modifications as previously reported.16 All reactions, including the controls that contained no template RNA, were performed in triplicate. The expression data for the miRNAs were acquired and analyzed using the ABI PRISM 7500 Sequence Detection System and 7500 Software v2.0.1 (Applied Biosystems). The resulting threshold cycle (CT) values were determined using the fixed threshold settings. Due to the lack of a consensus on housekeeping miRNA for the qRT-PCR analysis of serum miRNA, we selected a normalization method that compared the miRNA concentration with the serum volume, as described in our previous report.17
Statistical Analysis
The data were presented as the means ± SD. Student' t test or the 2-sided χ2-test was used to compare the differences in the serum miRNA concentrations between the 2 groups. Comparisons between more than 2 groups were performed using a 1-way ANOVA, and the differences between the groups were subsequently determined by the Fisher LSD test when appropriate. A P value <.05 was considered statistically significant. For each miRNA, we constructed the receiver operating characteristic (ROC) curve and calculated the area under the ROC curve (AUC) to evaluate the predictive power of the miRNA for astrocytoma. Risk score analysis was performed to evaluate the associations between the expression levels of the serum miRNAs and astrocytoma. The risk score of each miRNA, denoted as s, was set to 1 if the expression level was greater than the upper 95% reference interval for the corresponding miRNA level in the controls; otherwise, it was set to zero. A risk score function (RSF) to predict astrocytoma risk was defined according to a linear combination of the expression level for each miRNA. For example, the RSF for sample i using the information from 9 miRNAs was . In the above equation, sij is the risk score for miRNA j on sample i, and Wj is the weight of the risk score of miRNA j. To determine the Ws, 9 univariate logistic regression models were fitted using the disease status with each of the risk scores. The regression coefficient of each risk score was used as the weight to indicate the contribution of each miRNA to the RSF. Frequency tables and ROC curves were then used to evaluate the diagnostic effects of the profiling and to find the appropriate cutoff point. Moreover, we identified miRNAs in which the expression levels were significantly related to patient survival. The survival curves were estimated using the Kaplan-Meier method with SPSS 13.0 software, and the resulting curves were compared using the log-rank test.
Results
Demographic and Clinical Features of Astrocytoma Patients
Ninety histologically confirmed astrocytoma patients from the Third Affiliated Hospital of Soochow University, ranging from WHO grade II to grade IV, were enrolled in this study. Preoperative serum samples were obtained from all 90 participants, and 110 healthy individuals served as controls. The 90 preoperative participants and 110 healthy controls were randomly assigned to a training set (40 preoperative astrocytoma samples vs 50 healthy controls) or to a validation set (50 preoperative astrocytoma samples vs 60 healthy controls). The demographic and clinical features of the astrocytoma participants and healthy controls are listed in Table 1. There were no significant differences in the demographic factors between the participant samples and the healthy controls or between the participant samples from the training set and the validation set. All 90 astrocytoma participants received follow-up examinations. In the independent validation set, 50 histologically confirmed astrocytoma participants and 50 healthy controls from the Second Affiliated Hospital of Soochow University were enrolled. The demographic and clinical features of the astrocytoma participants and healthy controls are listed in Supplementary Table S1. The paired postoperative serum samples were obtained from 73 patients, including 65 patients from the Third Affiliated Hospital of Soochow University and 8 patients from the Second Affiliated Hospital of Soochow University. Thirty-eight participants (52.0%) were older than 50 years of age, 37 (50.7%) were female, and 13, 34, and 26 samples were characterized as WHO grade II, III or IV astrocytomas, respectively (Table 1).
Selection of Candidate Serum miRNAs for Astrocytoma
To select candidate serum miRNAs for astrocytomas, we employed the TLDA technique to screen the expression levels of 739 miRNAs in pooled serum samples from preoperative astrocytoma participants and healthy controls (each group was pooled from 10 individuals). The results revealed that the serum miRNA expression profiles varied between the preoperative astrocytoma participants and the healthy controls. Correlation and scatter plot analyses revealed that the correlation coefficients were low; the R2 value was 0.4332 between the preoperative astrocytoma participants and the healthy controls (Supplementary Fig. S1). We next narrowed down the list of miRNAs to be used in astrocytoma. The following criteria were used to select the miRNA for further analysis: (i) only upregulated miRNAs were included, and (ii) a increasing ratio >200 was required. Considering that the serum levels of miRNAs are upregulated in patients compared with normal donors in most cancers and other diseases,18 we only studied those upregulated miRNAs in this study. Consequently, 107 miRNAs that met the inclusion criteria were chosen (Supplementary Table S2). In addition to the miRNAs selected following the TLDA analysis, we also investigated miR-181b-5p, which was shown by our group to be downregulated in astrocytoma tissues. Low expression of miR-181b-5p has been significantly associated with poor patient survival.8 In total, 108 miRNAs were selected for further qRT-PCR analysis.
Validation of Differentially Expressed Serum miRNAs by Individual qRT-PCR analysis
In a second step, the 108 candidate miRNAs were individually assayed by qRT-PCR in the 90 preoperative astrocytoma samples and the 110 healthy controls, including the individuals used to create the pools, to validate their differential expression and to investigate whether any miRNA could be used as a biomarker for diagnosis of astrocytoma. Only the miRNAs with a mean fold change >2 and a P value <.05 were selected from the training set for further validation. miRNAs were excluded from further analysis when their expression levels were not significantly altered, the assays were not linear, the detection rates were <50%, or the CT values were higher than 35 in the qRT-PCR assay (Supplementary Table S3). Using these criteria, the levels of 12 miRNAs were significantly increased in the participants relative to the healthy controls (Table 2). To verify the accuracy and specificity of these miRNAs and to refine the number of miRNAs to be used as the astrocytoma serum signature, we further assessed the 12 miRNAs in the validation sample set. The miRNAs were considered significantly altered only when they exhibited a mean fold-change >2 relative to the controls, a P value <.05 and a parallel trend of variation between the training set and the validation set. Our analysis ultimately generated a list of 9 miRNAs that were differentially expressed in the preoperative astrocytoma serum samples compared with the healthy controls. These 9 miRNAs (miR-15b-5p, miR-16-5p, miR-19a-3p, miR-19b-3p, miR-20a-5p, miR-106a-5p, miR-130a-3p, miR-181b-5p, and miR-208a-3p) were shown to be upregulated more than 2-fold (Table 2). The differential expression of the 9 miRNAs in the 90 preoperative astrocytoma serum samples compared with the 110 healthy controls is shown in Supplementary Fig. S2.
Table 2.
Differentially expressed miRNAs in preoperative astrocytoma (As) serum samples compared to healthy control serum samples validated by qRT-PCR
| miRNA | Training Set |
Validation Set |
Training + Validation |
Result | |||
|---|---|---|---|---|---|---|---|
| Mean Fold As/control | P Value | Mean Fold As/control | P Value | Mean Fold As/control | P Value | ||
| miR-15b-5p | 5.317 | 2.89 × 10−3 | 4.100 | 4.61 × 10−6 | 4.803 | 4.23 × 10−5 | Significant |
| miR-16-5p | 3.216 | 2.18 × 10−6 | 2.146 | 3.12 × 10−3 | 2.648 | 6.57 × 10−8 | Significant |
| miR-19a-3p | 3.379 | 4.03 × 10−6 | 2.169 | 3.77 × 10−3 | 2.635 | 2.51 × 10−7 | Significant |
| miR-19b-3p | 2.906 | 5.55 × 10−5 | 2.214 | 7.64 × 10−4 | 2.463 | 3.54 × 10−7 | Significant |
| miR-20a-5p | 3.814 | 4.89 × 10−8 | 2.289 | 8.21 × 10−3 | 2.946 | 5.26 × 10−8 | Significant |
| miR-106a-5p | 2.202 | 2.69 × 10−7 | 2.368 | 2.84 × 10−2 | 2.288 | 1.81 × 10−4 | Significant |
| miR-130a-3p | 3.021 | 3.00 × 10−6 | 3.140 | 2.84 × 10−3 | 3.114 | 2.26 × 10−5 | Significant |
| miR-181b-5p | 2.827 | 4.05 × 10−8 | 4.610 | 3.21 × 10−3 | 3.385 | 2.33 × 10−7 | Significant |
| miR-208a-3p | 3.338 | 6.65 × 10−6 | 2.230 | 2.02 × 10−2 | 2.627 | 2.69 × 10−5 | Significant |
| miR-21-5p | 2.199 | 9.74 × 10−4 | 1.584 | 6.22 × 10−2 | Not significant | ||
| miR-29c-3p | 2.887 | 6.65 × 10−7 | 1.732 | 3.24 × 10−3 | Not significant | ||
| miR-195-5p | 2.063 | 8.26 × 10−4 | 1.463 | 1.85 × 10−2 | Not significant | ||
Selected miRNAs as Potential Biomarkers for Astrocytoma Diagnosis
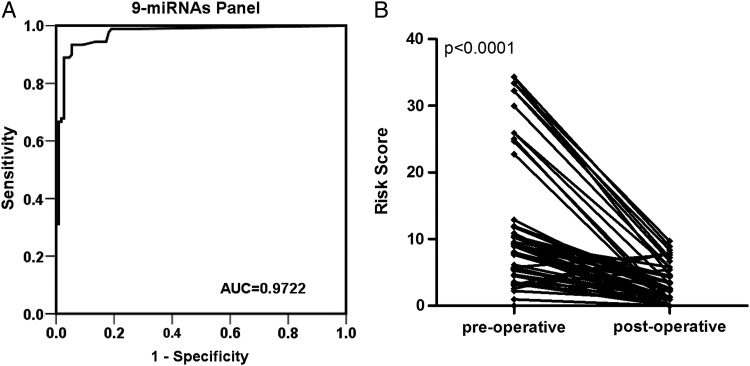
To further evaluate the diagnostic value of this miRNA profiling system, we used a risk score formula to calculate the risk score for the participant samples and control samples. The samples were ranked according to their risk score and then divided into a high-risk group, which represented the predicted astrocytoma cases, and a low-risk group, which represented the predicted control individuals. The frequency table and the ROC curves were then used to evaluate the diagnostic effects of the 9-miRNA panel. The AUC for the combined 9 miRNAs was 0.9722 (95% CI, 0.9501–0.9942) for the astrocytomas and controls (Fig. 2A). With an optimal cutoff value (RSF = 5.649) at which the sum of the sensitivity and specificity was maximal, the sensitivity was 93.3%, and the specificity was 94.5% for astrocytoma. These results demonstrated that the combined analysis of these 9 miRNAs could differentiate astrocytoma patients from healthy controls.
Fig. 2.
Selected miRNAs as potential biomarkers for astrocytoma diagnosis and tumor removal. (A) Receiver-operating characteristic (ROC) curves for the 9-miRNA profile to distinguish the astrocytoma serum samples from the control serum samples. (B) The risk score of the 9 selected serum miRNAs in the astrocytoma patients before and after surgery.
Validation of the Predictive Utility of the 9 miRNA-based Biomarker by a Double-blind Test
We tested another 100 serum samples (50 astrocytomas and 50 controls) in a double-blind fashion to validate the accuracy of the serum miRNA-based biomarker for astrocytoma diagnosis. We used the same risk score formula with a cutoff of 5.649 to calculate the risk score of samples. After analyzing the expression levels of the 9 miRNAs in these serum samples by individual qRT-PCR and classifying them on the basis of a previously built diagnostic model, a clear separation of astrocytoma from controls was observed. The AUC for the 9-miRNA profile was 0.9576 (Supplementary Fig. S3A). The diagnostic sensitivity and specificity obtained for the independent validation set were 94.0% and 92.0%, respectively. Of the 50 astrocytoma cases and 50 controls from the independent validation set, only 3 astrocytoma cases and 4 controls were incorrectly predicted. The accuracy rate of the 9-miRNA profile as the astrocytoma biomarker was 93.0% (Supplementary Fig. S3B, Supplementary Table S4).
Selected miRNAs as Potential Biomarkers for Evaluating Tumor Removal
The expression levels of the 9 miRNAs were analyzed in the paired preoperative and postoperative astrocytoma serum samples from 73 participants who underwent surgical removal of the tumors. The levels of the 9 miRNAs were significantly reduced in the postoperative samples compared with the levels in the preoperative samples (Supplementary Fig. S4). To further evaluate the monitoring value of this miRNA profiling system, a paired t test was used to compare the preoperative- and postoperative risk scores. The postoperative risk score was significantly lower than the preoperative risk score (P < .0001) (Fig. 2B). These findings imply that these serum miRNAs may reflect tumor dynamics and are available as new biomarkers to evaluate tumor removal.
Correlation of Serum miRNA Expression With Demographic and Clinical Factors
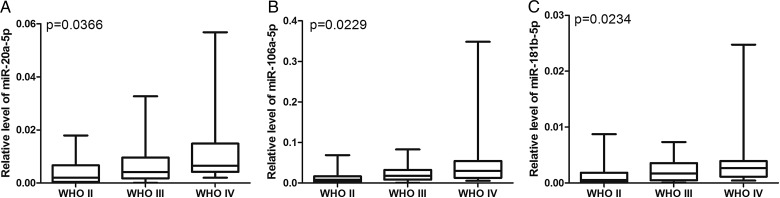
We subsequently investigated whether serum miRNA expression levels represented specific molecular signatures for subsets of astrocytomas. The expression levels of the 9 miRNAs in the 90 preoperative serum samples were stratified using 3 types of clinicopathological parameters (sex, age, and WHO grade). We assessed the relationship between these clinical features and the serum miRNA expression levels. No miRNAs were found to be differentially expressed when the serum samples were stratified by age or sex. However, 3 miRNAs were found to be differentially expressed when the samples were stratified according to tumor grade. As shown in Fig. 3, the expression of miR-20a-5p, miR-106a-5p, and miR-181b-5p increased from WHO grade II to WHO grade IV astrocytomas. This result suggests that the upregulation of miR-20a-5p, miR-106a-5p, and miR-181b-5p is associated with advanced clinical stages of astrocytomas.
Fig. 3.
The levels of miRNA expression in the astrocytoma patients stratified by tumor grade. The relative expression levels of miR-20a-5p, miR-106a-5p, and miR-181b-5p in each group are shown.
Correlation Between miRNA Expression Profiles and the Survival of Astrocytoma Patients
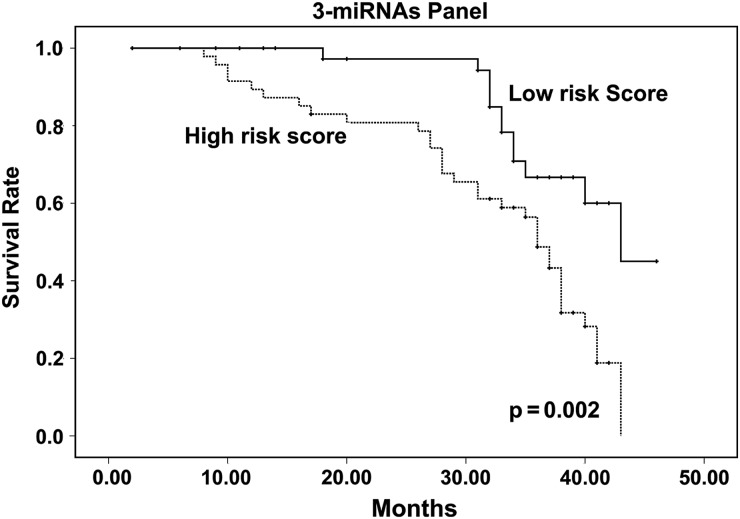
We first performed Kaplan-Meier survival analysis to analyze the influence of clinical variables on the prognosis in astrocytoma patients (Supplementary Fig. S5). Younger participants (aged < 50 years) tended to have a better prognosis than older participants (aged ≥ 50 years) (P < .0001). The participants with a high WHO grade had a poorer survival rate than those with a low WHO grade (P = .010). We next investigated the correlation between the miRNA expression profiles and patient survival using the prospective follow-up data collected from the 90 astrocytoma participants. Due to the observation that 9 miRNAs were differentially expressed between the astrocytoma participants and the controls, these miRNAs were used for the survival analysis. The expression levels of these 9 miRNAs in the astrocytomas were first stratified by the median value. Then, the survival of participants with high miRNA expression levels (≥ median) was compared with the outcomes for participants with low miRNA expression levels (< median), as determined by Kaplan-Meier survival analysis. We first analyzed the follow-up data collected from the 40 participants in the training set. We observed a marginally significant poorer survival rate in astrocytoma patients who expressed high levels of miR-19a-3p (P = .053), miR-106a-5p (P = .036), and miR-181b-5p (P = .031) (Supplementary Fig. S6A–C). To validate the findings from the training set, the 3 candidate prognostic indicators were assessed using the follow-up data collected from the 50 participants in the validation set. miRNAs were considered to be correlated with survival only when they exhibited a parallel trend of variation between the training set and the validation set. We observed a consistent result (Supplementary Fig. S6D–F). More impressively, when we analyzed the follow-up data from the training set and the validation set together, all 3 miRNAs showed a significant correlation with survival (Supplementary Fig. S6G–I). To further evaluate the prognostic value of the 3-miRNA profiling system, Kaplan-Meier survival analysis was used to compare the participants with high-risk and low-risk scores. The participants with high-risk scores had a poorer survival rate than those with low-risk scores (P = .002) (Fig. 4). Taken together, the results suggested that the 3-miRNAs panel could have prognostic value for astrocytoma patients. Next, we performed survival analysis on astrocytoma cohorts stratified by age or WHO grade to evaluate the prognostic value of the 3-miRNA profiling system. In the cohort of participants aged < 50 years, those with high-risk scores had a poorer survival rate than those with low-risk scores (P = .049; Supplementary Fig. S7A), while no significant difference was found between the patients with high-risk scores and those with low-risk scores (P = .105) in the cohort of participants aged ≥ 50 years (Supplementary Fig. S7B). In the cohort of participants with WHO grade II astrocytoma, the patients with high-risk scores had a poorer survival rate than those with low-risk scores (P = .035; Supplementary Fig. S7C), while no significant difference was found between the patients with high-risk scores and those with low-risk scores (P = 0.461, P = 0.241, respectively) in the cohort of participants with WHO grade II or WHO grade III astrocytoma (Supplementary Fig. S7D and E).
Fig. 4.
Kaplan-Meier survival analysis of the astrocytoma patients according to the 3-serum miRNA signature stratified by risk score.
A univariate Cox proportional hazard regression model was performed to determine the influence of risk score as well as clinicopathological characteristics (sex, age and WHO grade) on patient survival. WHO grade II was designated as the low pathological grade, and WHO III-IV was designated as the high pathological grade. This univariate analysis indicated that age, WHO grade, and risk score were significantly related to survival (hazard ratio >2 and P value <0.05 were considered to be statistically significant), whereas sex was not (Table 3). To adjust for the potentially confounding effects of univariate modeling of age, sex, or WHO grade, a multivariable Cox proportional hazard regression was performed analysis using all of these clinicopathological factors. The multivariable analysis revealed that old age and high-risk score were independently associated with decreased survival, whereas the risk score showed a significant prognostic impact on patient survival, as did age (Table 3). These results suggest that the combined 3-miRNAs panel is an important prognostic predictor, independent of other clinicopathological factors.
Table 3.
Survival analysis of astrocytoma patients in relation to the patient's clinicopathological characteristics and 3-miRNAs risk score
| Variable | Subset | Hazard Ratio (95% CI) | P Value |
|---|---|---|---|
| Univariate analysis | |||
| Sex | Female/Male | 0.693 (0.380–1.265) | .232 |
| Age | Age≥50 y/Age<50 y | 4.182 (2.070–8.450) | <.001 |
| WHO | High grade/low grade | 3.300 (1.384–7.850) | .007 |
| Risk Score | High/Low | 3.371 (2.274–5.414) | .006 |
| Multivariate analysis | |||
| Sex | Female/male | 0.768 (0.419–1.409) | .394 |
| Age | Age≥50/Age<50 | 3.367 (1.605–7.060) | .001 |
| WHO | High grade/low grade | 1.596 (0.642–3.966) | .314 |
| Risk score | High/low | 3.117 (2.135–4.949) | .018 |
Discussion
Astrocytoma is a highly invasive tumor of the central nervous system in humans. A unique miRNA molecular diagnostic and prognostic signature in astrocytoma tissues was established in our previous work; however, the use of noninvasive blood-based biomarkers as an important complement to the existing diagnostic tools is strongly preferred for improving astrocytoma diagnosis and prognosis.
Recently, the presence of serum miRNAs has been observed.19 Pilot studies have implied that the unique patterns of serum miRNA may serve as noninvasive biomarkers for the development and prognosis of cancers such as those of breast,20 colon,21 lung,22 prostate,23 hepatocellular,24 gastric,25 and nasopharyngeal26 origin. Several previous reports have suggested that circulating miRNAs are potential noninvasive biomarkers for astrocytoma.9–14 In the current study, our results showed that the combination of multiple serum miRNAs can serve as a potential noninvasive biomarker for the diagnosis and prognosis of astrocytoma. Although there is a small overlap between the proposed relevant miRNAs in these studies and our study, we think that this inconsistency may be due to the differences in study design, sample size, race, and methodology. Compared with these previous studies, our study on serum miRNAs as astrocytoma biomarkers was more comprehensive and more systematic. Malzkorn et al investigated the tissue miRNA expression profile in 4 patients with primary WHO grade II gliomas who spontaneously progressed to WHO grade IV glioblastomas, whereas we studied the serum miRNA expression profile in 140 astrocytoma patients with WHO grades II-IV.9 Roth et al10 and Ilhan-Mutlu et al12,13 only studied WHO grade IV glioblastoma. Wang et al11 selected their target miRNAs for glioma from the literature, whereas we selected our target miRNAs from TLDA, which was more thorough and unbiased. Furthermore, we evaluated the potential of serum miRNAs as biomarkers for prognosis, but those previous studies did not. Nevertheless, all of these studies indicate the potential for circulating miRNAs to be used as novel noninvasive biomarkers for astrocytoma.
In this study, we found that the upregulation of miR-20a-5p, miR-106a-5p, and miR-181b-5p was associated with advanced clinical stages of astrocytoma. This result only showed a trend that these 3 miRNAs were increased from WHO grade II to WHO grade IV astrocytomas, but this does not mean that these miRNAs were qualitatively related to the WHO grade. The underlying mechanism explaining why these 3 miRNAs were correlated with WHO grade in astrocytoma still needs to be explored.
A comparison of the miRNA expression patterns in serum and tissue/cells may provide additional evidence supporting the use of serum miRNAs as reliable diagnostic biomarkers. There are 3 major pathways hypothesized for the entry of miRNAs into the circulation: (i) the energy-free, passive leakage of cellular miRNAs, (ii) the active and selective secretion of microvesicle-free miRNAs in response to various stimuli, and (iii) the active secretion via cell-derived microvesicles.27 Although the current study did not provide direct evidence demonstrating that serum miRNAs are either actively secreted or passively leaked from tumor cells, our data indirectly implied that the miRNAs may originate from the tumors. For example, in our previous study, miR-106a-5p and miR-181b-5p were significantly decreased in astrocytoma tissues,8 and in the present study, miR-106a-5p and miR-181b-5p were significantly increased in astrocytoma serum. However, the number of tumor and plasma samples from the same individual patients in these 2 studies is limited. New, well-designed studies should be performed in the future before a conclusion can be made.
Because miRNAs are known to play crucial roles as oncogenes or tumor suppressors, miRNA deregulation may be involved in the cell proliferation, apoptosis, cell-cycle regulation, invasion, and angiogenesis of astrocytoma. Among the upregulated miRNAs in our study, some miRNAs function as tumor suppressor genes in astrocytoma. For example, miR-15b-5p was reported to reduce cell invasion and angiogenesis by targeting NRP-228 to arrest the cell cycle at G0/G1 and to inhibit cell proliferation by targeting CCNE1 in astrocytoma cells.29 miR-16-5p was found to reduce the cell proliferation, migration, and invasion of astrocytoma cells.30 miR-106a-5p was reported to inhibit cell proliferation and migration and induce apoptosis by targeting FASTK and E2F1.31,32 miR-181b-5p can inhibit cell proliferation, migration, invasion, and tumorigenesis by targeting IGF-1R33 and can enhance temozolomide sensitivity by downregulating MEK1.34,35 Although the roles of miR-19a-3p, miR-19b-3p, miR-20a-5p, miR-130a-3p and miR-208a-3p have been reported in many different types of cancer, there are no direct reports of these miRNAs in astrocytoma to date. Their biological relevance in astrocytoma still needs to be determined in future studies.
In conclusion, our study supports the use of serum miRNAs as noninvasive biomarkers for the diagnosis and prognosis of astrocytomas. This observation will trigger further research to elucidate the functional effects of these miRNAs, which will improve our knowledge regarding the role that these novel biomarkers play in carcinogenesis and expose their true potential as therapeutic agents.
Supplementary Material
Funding
This work was supported by the National Natural Science Foundation of China Projects 31071046 and 81302197; Changzhou Social Development Projects CS20092015 and CS20102010; Changzhou Health Bureau Projects ZD200903 and ZD201007; and Changzhou Science Technology Bureau Guiding Project CY20119004.
Conflict of interest statement. None declared.
Supplementary Material
References
- 1.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 2.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gabayan AJ, Green SB, Sanan A, et al. GliaSite brachytherapy for treatment of recurrent malignant gliomas: a retrospective multi-institutional analysis. Neurosurgery. 2006;58(4):701–709. doi: 10.1227/01.NEU.0000194836.07848.69. [DOI] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 5.Ryan BM, Robles AI, Harris CC. Genetic variation in microRNA networks: the implications for cancer research. Nat Rev Cancer. 2010;10(6):389–402. doi: 10.1038/nrc2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 7.Barbarotto E, Schmittgen TD, Calin GA. MicroRNAs and cancer: profile, profile, profile. Int J Cancer. 2008;122(5):969–977. doi: 10.1002/ijc.23343. [DOI] [PubMed] [Google Scholar]
- 8.Zhi F, Chen X, Wang S, et al. The use of hsa-miR-21, hsa-miR-181b and hsa-miR-106a as prognostic indicators of astrocytoma. Eur J Cancer. 2010;46(9):1640–1649. doi: 10.1016/j.ejca.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Malzkorn B, Wolter M, Liesenberg F, et al. Identification and functional characterization of microRNAs involved in the malignant progression of gliomas. Brain Pathol. 2010;20(3):539–550. doi: 10.1111/j.1750-3639.2009.00328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roth P, Wischhusen J, Happold C, et al. A specific miRNA signature in the peripheral blood of glioblastoma patients. J Neurochem. 2011;118(3):449–457. doi: 10.1111/j.1471-4159.2011.07307.x. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Li P, Li A, et al. Plasma specific miRNAs as predictive biomarkers for diagnosis and prognosis of glioma. J Exp Clin Cancer Res. 2012;31(97):1–10. doi: 10.1186/1756-9966-31-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ilhan-Mutlu A, Wagner L, Wohrer A, et al. Plasma MicroRNA-21 concentration may be a useful biomarker in glioblastoma patients. Cancer Invest. 2012;30(8):615–621. doi: 10.3109/07357907.2012.708071. [DOI] [PubMed] [Google Scholar]
- 13.Ilhan-Mutlu A, Wagner L, Wohrer A, et al. Blood alterations preceding clinical manifestation of glioblastoma. Cancer Invest. 2012;30(9):625–629. doi: 10.3109/07357907.2012.725443. [DOI] [PubMed] [Google Scholar]
- 14.Yang C, Wang C, Chen X, et al. Identification of seven serum microRNAs from a genome-wide serum microRNA expression profile as potential noninvasive biomarkers for malignant astrocytomas. Int J Cancer. 2013;132(1):116–127. doi: 10.1002/ijc.27657. [DOI] [PubMed] [Google Scholar]
- 15.Liu R, Zhang C, Hu Z, et al. A five-microRNA signature identified from genome-wide serum microRNA expression profiling serves as a fingerprint for gastric cancer diagnosis. Eur J Cancer. 2011;47(5):784–791. doi: 10.1016/j.ejca.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C, Wang C, Chen X, et al. Expression profile of microRNAs in serum: a fingerprint for esophageal squamous cell carcinoma. Clin Chem. 2010;56(12):1871–1879. doi: 10.1373/clinchem.2010.147553. [DOI] [PubMed] [Google Scholar]
- 17.Zhi F, Cao X, Xie X, et al. Identification of circulating MicroRNAs as potential biomarkers for detecting acute myeloid leukemia. PLoS One. 2013;8(2):e56718. doi: 10.1371/journal.pone.0056718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zen K, Zhang CY. Circulating microRNAs: a novel class of biomarkers to diagnose and monitor human cancers. Med Res Rev. 2012;32(2):326–348. doi: 10.1002/med.20215. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 20.Chan M, Liaw CS, Ji SM, et al. Identification of circulating microRNA signatures for breast cancer detection. Clin Cancer Res. 2013;19(16):4477–4487. doi: 10.1158/1078-0432.CCR-12-3401. [DOI] [PubMed] [Google Scholar]
- 21.Hofsli E, Sjursen W, Prestvik WS, et al. Identification of serum microRNA profiles in colon cancer. Br J Cancer. 2013;108(8):1712–1719. doi: 10.1038/bjc.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Gu J, Roth JA, et al. Pathway-based serum microRNA profiling and survival in patients with advanced stage non-small cell lung cancer. Cancer Res. 2013;73(15):4801–4809. doi: 10.1158/0008-5472.CAN-12-3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Selth LA, Townley SL, Bert AG, et al. Circulating microRNAs predict biochemical recurrence in prostate cancer patients. Br J Cancer. 2013;109(3):641–650. doi: 10.1038/bjc.2013.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Köberle V, Kronenberger B, Pleli T, et al. Serum microRNA-1 and microRNA-122 are prognostic markers in patients with hepatocellular carcinoma. Eur J Cancer. 2013;49(16):3442–3449. doi: 10.1016/j.ejca.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Song MY, Pan KF, Su HJ, et al. Identification of serum microRNAs as novel non-invasive biomarkers for early detection of gastric cancer. PLoS One. 2012;7(3):e33608. doi: 10.1371/journal.pone.0033608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu N, Cui RX, Sun Y, et al. A four-miRNA signature identified from genome-wide serum miRNA profiling predicts survival in patients with nasopharyngeal carcinoma. Int J Cancer. 2014;134(6):1359–1368. doi: 10.1002/ijc.28468. [DOI] [PubMed] [Google Scholar]
- 27.Redova M, Sana J, Slaby O. Circulating miRNAs as new blood-based biomarkers for solid cancers. Future Oncol. 2013;9(3):387–402. doi: 10.2217/fon.12.192. [DOI] [PubMed] [Google Scholar]
- 28.Zheng X, Chopp M, Lu Y, et al. MiR-15b and miR-152 reduce glioma cell invasion and angiogenesis via NRP-2 and MMP-3. Cancer Lett. 2013;329(2):146–154. doi: 10.1016/j.canlet.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xia H, Qi Y, Ng SS, et al. MicroRNA-15b regulates cell cycle progression by targeting cyclins in glioma cells. Biochem Biophys Res Commun. 2009;380(2):205–210. doi: 10.1016/j.bbrc.2008.12.169. [DOI] [PubMed] [Google Scholar]
- 30.Li X, Ling N, Bai Y, et al. MiR-16–1 plays a role in reducing migration and invasion of glioma cells. Anat Rec (Hoboken) 2013;296(3):427–432. doi: 10.1002/ar.22626. [DOI] [PubMed] [Google Scholar]
- 31.Yang G, Zhang R, Chen X, et al. MiR-106a inhibits glioma cell growth by targeting E2F1 independent of p53 status. J Mol Med. 2011;89(10):1037–1050. doi: 10.1007/s00109-011-0775-x. [DOI] [PubMed] [Google Scholar]
- 32.Zhi F, Zhou G, Shao N, et al. miR-106a-5p Inhibits the proliferation and migration of astrocytoma cells and promotes apoptosis by targeting FASTK. PLoS One. 2013;8(8):e72390. doi: 10.1371/journal.pone.0072390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Z-m, Wang X-f, Qian X, et al. MiRNA-181b suppresses IGF-1R and functions as a tumor suppressor gene in gliomas. RNA. 2013;19(4):552–560. doi: 10.1261/rna.035972.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang J, Sai K, Chen FR, et al. miR-181b modulates glioma cell sensitivity to temozolomide by targeting MEK1. Cancer Chemother Pharmacol. 2013;72(1):147–158. doi: 10.1007/s00280-013-2180-3. [DOI] [PubMed] [Google Scholar]
- 35.Li P, Lu X, Wang Y, et al. MiR-181b suppresses proliferation of and reduces chemoresistance to temozolomide in U87 glioma stem cells. J Biomed Res. 2010;24(6):436–443. doi: 10.1016/S1674-8301(10)60058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.