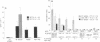Abstract
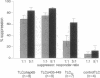
The quality of the response produced by regulatory or helper T (Th) cells presently receives much attention because of its possible implications for vaccine development and immunomodulation. Apart from cytokines and so-called costimulatory signals, antigens and the presenting major histocompatibility complex (MHC) molecules may play a role in determining the type of T-cell response generated toward antigens. To examine the role of antigen and/or HLA in control of T-cell subset activation, we have studied a special case, namely CD4+ suppressor T (Ts) cells in leprosy. Mycobacterium leprae-induced Ts cell clones have been previously isolated from peripheral blood and skin lesions of lepromatous leprosy patients and were shown to specifically down-regulate mycobacterium-specific Th cell responses. Despite considerable effort, the antigens recognized by these Ts cells have thus far not been identified. Here we report that all HLA-DR2-restricted CD4+ Ts cell clones derived from a lepromatous leprosy patient recognize an epitope that maps between the amino acid residues 439 and 448 of the mycobacterial hsp65. The peptide was presented to these Ts cells by HLA-DRB1*1503, a recently discovered HLA-DR2 variant. Non-suppressor T-cell clones derived from the same patient recognized antigens other than the hsp65 and were also stimulated by other HLA-DR2 variants. In independent cloning experiments peptide 435-449 and recombinant hsp65 induced exclusively Ts cells in this lepromatous leprosy patient. The Ts clones recognizing this particular epitope were derived from at least seven different progenitors, as they expressed different T-cell receptor alpha and beta chains. Thus, our data indicate that a specific peptide-HLA class II combination may exclusively activate Ts cells.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom B. R., Godal T. Selective primary health care: strategies for control of disease in the developing world. V. Leprosy. Rev Infect Dis. 1983 Jul-Aug;5(4):765–780. doi: 10.1093/clinids/5.4.765. [DOI] [PubMed] [Google Scholar]
- Bloom B. R., Mehra V. Immunological unresponsiveness in leprosy. Immunol Rev. 1984 Aug;80:5–28. doi: 10.1111/j.1600-065x.1984.tb00493.x. [DOI] [PubMed] [Google Scholar]
- Demopulos J. T., Hodge T. W., Wooten V., Acton R. T. A novel DRB1 allele in DR2-positive American blacks. Hum Immunol. 1991 Jan;30(1):41–44. doi: 10.1016/0198-8859(91)90069-l. [DOI] [PubMed] [Google Scholar]
- Fernandez-Vina M., Moraes J. R., Moraes M. E., Miller S., Stastny P. HLA class II haplotypes in Amerindians and in black North and South Americans. Tissue Antigens. 1991 Nov;38(5):235–237. doi: 10.1111/j.1399-0039.1991.tb01904.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Viña M. A., Gao X. J., Moraes M. E., Moraes J. R., Salatiel I., Miller S., Tsai J., Sun Y. P., An J. B., Layrisse Z. Alleles at four HLA class II loci determined by oligonucleotide hybridization and their associations in five ethnic groups. Immunogenetics. 1991;34(5):299–312. doi: 10.1007/BF00211994. [DOI] [PubMed] [Google Scholar]
- Geluk A., Bloemhoff W., De Vries R. R., Ottenhoff T. H. Binding of a major T cell epitope of mycobacteria to a specific pocket within HLA-DRw17(DR3) molecules. Eur J Immunol. 1992 Jan;22(1):107–113. doi: 10.1002/eji.1830220117. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Godal T., Mustafa A. S., Belehu A., Yemaneberhan T. T-cell conditioned media reverse T-cell unresponsiveness in lepromatous leprosy. Nature. 1983 May 26;303(5915):342–344. doi: 10.1038/303342a0. [DOI] [PubMed] [Google Scholar]
- Hawes G. E., Struyk L., van den Elsen P. J. Differential usage of T cell receptor V gene segments in CD4+ and CD8+ subsets of T lymphocytes in monozygotic twins. J Immunol. 1993 Mar 1;150(5):2033–2045. [PubMed] [Google Scholar]
- Hsieh C. S., Macatonia S. E., Tripp C. S., Wolf S. F., O'Garra A., Murphy K. M. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993 Apr 23;260(5107):547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- Janson A. A., Klatser P. R., van der Zee R., Cornelisse Y. E., de Vries R. R., Thole J. E., Ottenhoff T. H. A systematic molecular analysis of the T cell-stimulating antigens from Mycobacterium leprae with T cell clones of leprosy patients. Identification of a novel M. leprae HSP 70 fragment by M. leprae-specific T cells. J Immunol. 1991 Nov 15;147(10):3530–3537. [PubMed] [Google Scholar]
- Li S. G., Elferink D. G., de Vries R. R. Phenotypic and functional characterization of human suppressor T-cell clones: II. Activation by Mycobacterium leprae presented by HLA-DR molecules to alpha beta T-cell receptors. Hum Immunol. 1990 May;28(1):11–26. doi: 10.1016/0198-8859(90)90098-a. [DOI] [PubMed] [Google Scholar]
- Li S. G., Ottenhoff T. H., Van den Elsen P., Koning F., Zhang L., Mak T., De Vries R. R. Human suppressor T cell clones lack CD28. Eur J Immunol. 1990 Jun;20(6):1281–1288. doi: 10.1002/eji.1830200613. [DOI] [PubMed] [Google Scholar]
- MacDermott R. P., Tritsch G. L., Formeister J. F. Adenosine deaminase and nucleoside phosphorylase activities in normal human blood mononuclear cell subpopulations. Clin Exp Immunol. 1980 Nov;42(2):303–307. [PMC free article] [PubMed] [Google Scholar]
- Mehra V., Brennan P. J., Rada E., Convit J., Bloom B. R. Lymphocyte suppression in leprosy induced by unique M. leprae glycolipid. Nature. 1984 Mar 8;308(5955):194–196. doi: 10.1038/308194a0. [DOI] [PubMed] [Google Scholar]
- Mehra V., Mason L. H., Fields J. P., Bloom B. R. Lepromin-induced suppressor cells in patients with leprosy. J Immunol. 1979 Oct;123(4):1813–1817. [PubMed] [Google Scholar]
- Modlin R. L., Kato H., Mehra V., Nelson E. E., Fan X. D., Rea T. H., Pattengale P. K., Bloom B. R. Genetically restricted suppressor T-cell clones derived from lepromatous leprosy lesions. 1986 Jul 31-Aug 6Nature. 322(6078):459–461. doi: 10.1038/322459a0. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Murray J. S., Madri J., Tite J., Carding S. R., Bottomly K. MHC control of CD4+ T cell subset activation. J Exp Med. 1989 Dec 1;170(6):2135–2140. doi: 10.1084/jem.170.6.2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. S., Pfeiffer C., Madri J., Bottomly K. Major histocompatibility complex (MHC) control of CD4 T cell subset activation. II. A single peptide induces either humoral or cell-mediated responses in mice of distinct MHC genotype. Eur J Immunol. 1992 Feb;22(2):559–565. doi: 10.1002/eji.1830220239. [DOI] [PubMed] [Google Scholar]
- Mutis T., Kraakman E. M., Cornelisse Y. E., Haanen J. B., Spits H., De Vries R. R., Ottenhoff T. H. Analysis of cytokine production by Mycobacterium-reactive T cells. Failure to explain Mycobacterium leprae-specific nonresponsiveness of peripheral blood T cells from lepromatous leprosy patients. J Immunol. 1993 May 15;150(10):4641–4651. [PubMed] [Google Scholar]
- Ottenhoff T. H., Converse P. J., Gebre N., Wondimu A., Ehrenberg J. P., Kiessling R. T cell responses to fractionated Mycobacterium leprae antigens in leprosy. The lepromatous nonresponder defect can be overcome in vitro by stimulation with fractionated M. leprae components. Eur J Immunol. 1989 Apr;19(4):707–713. doi: 10.1002/eji.1830190421. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Elferink D. G., Klatser P. R., de Vries R. R. Cloned suppressor T cells from a lepromatous leprosy patient suppress Mycobacterium leprae reactive helper T cells. 1986 Jul 31-Aug 6Nature. 322(6078):462–464. doi: 10.1038/322462a0. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Haanen J. B., Geluk A., Mutis T., Ab B. K., Thole J. E., van Schooten W. C., van den Elsen P. J., de Vries R. R. Regulation of mycobacterial heat-shock protein-reactive T cells by HLA class II molecules: lessons from leprosy. Immunol Rev. 1991 Jun;121:171–191. doi: 10.1111/j.1600-065x.1991.tb00828.x. [DOI] [PubMed] [Google Scholar]
- Ottenhoff T. H., Walford C., Nishimura Y., Reddy N. B., Sasazuki T. HLA-DQ molecules and the control of Mycobacterium leprae-specific T cell nonresponsiveness in lepromatous leprosy patients. Eur J Immunol. 1990 Oct;20(10):2347–2350. doi: 10.1002/eji.1830201027. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C., Murray J., Madri J., Bottomly K. Selective activation of Th1- and Th2-like cells in vivo--response to human collagen IV. Immunol Rev. 1991 Oct;123:65–84. doi: 10.1111/j.1600-065x.1991.tb00606.x. [DOI] [PubMed] [Google Scholar]
- Powrie F., Coffman R. L. Cytokine regulation of T-cell function: potential for therapeutic intervention. Immunol Today. 1993 Jun;14(6):270–274. doi: 10.1016/0167-5699(93)90044-L. [DOI] [PubMed] [Google Scholar]
- Prasad H. K., Mishra R. S., Nath I. Phenolic glycolipid-I of Mycobacterium leprae induces general suppression of in vitro concanavalin A responses unrelated to leprosy type. J Exp Med. 1987 Jan 1;165(1):239–244. doi: 10.1084/jem.165.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner S. L., Wang Z. E., Hatam F., Scott P., Locksley R. M. TH1 and TH2 cell antigen receptors in experimental leishmaniasis. Science. 1993 Mar 5;259(5100):1457–1460. doi: 10.1126/science.8451641. [DOI] [PubMed] [Google Scholar]
- Ridley D. S., Jopling W. H. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966 Jul-Sep;34(3):255–273. [PubMed] [Google Scholar]
- Rinke de Wit T. F., Bekelie S., Osland A., Miko T. L., Hermans P. W., van Soolingen D., Drijfhout J. W., Schöningh R., Janson A. A., Thole J. E. Mycobacteria contain two groEL genes: the second Mycobacterium leprae groEL gene is arranged in an operon with groES. Mol Microbiol. 1992 Jul;6(14):1995–2007. doi: 10.1111/j.1365-2958.1992.tb01372.x. [DOI] [PubMed] [Google Scholar]
- Salgame P., Abrams J. S., Clayberger C., Goldstein H., Convit J., Modlin R. L., Bloom B. R. Differing lymphokine profiles of functional subsets of human CD4 and CD8 T cell clones. Science. 1991 Oct 11;254(5029):279–282. doi: 10.1126/science.254.5029.279. [DOI] [PubMed] [Google Scholar]
- Salgame P., Convit J., Bloom B. R. Immunological suppression by human CD8+ T cells is receptor dependent and HLA-DQ restricted. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2598–2602. doi: 10.1073/pnas.88.6.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathish M., Bhutani L. K., Sharma A. K., Nath I. Monocyte-derived soluble suppressor factor(s) in patients with lepromatous leprosy. Infect Immun. 1983 Dec;42(3):890–899. doi: 10.1128/iai.42.3.890-899.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sercarz E., Krzych U. The distinctive specificity of antigen-specific suppressor T cells. Immunol Today. 1991 Apr;12(4):111–118. doi: 10.1016/0167-5699(91)90094-A. [DOI] [PubMed] [Google Scholar]
- Struyk L., Kurnick J. T., Hawes G. E., van Laar J. M., Schipper R., Oksenberg J. R., Steinman L., de Vries R. R., Breedveld F. C., van den Elsen P. T-cell receptor V-gene usage in synovial fluid lymphocytes of patients with chronic arthritis. Hum Immunol. 1993 Aug;37(4):237–251. doi: 10.1016/0198-8859(93)90507-w. [DOI] [PubMed] [Google Scholar]
- Van Schooten W. C., Elferink D. G., Van Embden J., Anderson D. C., De Vries R. R. DR3-restricted T cells from different HLA-DR3-positive individuals recognize the same peptide (amino acids 2-12) of the mycobacterial 65-kDa heat-shock protein. Eur J Immunol. 1989 Nov;19(11):2075–2079. doi: 10.1002/eji.1830191116. [DOI] [PubMed] [Google Scholar]