Abstract
Background
Multimodal therapy has improved survival for some childhood CNS tumors. However, whether risk for subsequent neoplasms (SNs) also increases is unknown. We report the cumulative incidence of, and risk factors for, SNs after a childhood primary CNS tumor and determine whether treatment that combines radiation therapy (RT) with chemotherapy increases risk for SNs.
Methods
Analyses included 2779 patients with a primary CNS tumor treated at St Jude Children's Research Hospital between 1985 and 2012. Cumulative incidence and standardized incidence ratios (SIRs) were estimated for SNs confirmed by pathology report. Cumulative incidence among the 237 five-year medulloblastoma survivors treated with multimodal therapy (RT + chemotherapy) was compared with a historical cohort of 139 five-year survivors treated with RT but no chemotherapy in the Childhood Cancer Survivor Study.
Results
Eighty-one survivors had 97 SNs. The cumulative incidence of first SN was 3.0% (95% CI: 2.3%–3.9%) at 10 years, and 6.0% (95% CI: 4.6%–7.7%) at 20 years from diagnosis. Risks were highest for subsequent glioma, all grades (SIR = 57.2; 95% CI: 36.2–85.8) and acute myeloid leukemia (SIR = 31.8; 95% CI: 10.2–74.1). Compared with RT alone, RT + chemotherapy did not increase risk for SNs (hazard ratio: 0.64; 95% CI: 0.38–1.06). Among five-year survivors of medulloblastoma treated with multimodal therapy, cumulative incidence of SN was 12.0% (95% CI: 6.4%–19.5%) at 20 years, no different than survivors treated with RT alone (11.3%, P = .44).
Conclusion
The cumulative incidence of SNs continues to increase with time from treatment with no obvious plateau, but the risk does not appear to be higher after exposure to multimodal therapy compared with RT alone. Continued follow-up of survivors as they age is essential.
Keywords: chemotherapy, childhood CNS tumor survivors, medulloblastoma, radiation, subsequent neoplasms
Historically, primary surgical resection was responsible for early improvements in the overall survival of children diagnosed with CNS tumors. Further improvements in survival have been achieved in more recent decades, with the addition of CNS-directed radiotherapy (RT) and/or systemic chemotherapy.1–3 For example, marked increases in overall survival of children with medulloblastoma were achieved by the addition of craniospinal RT and systemic chemotherapy after primary tumor resection.1,4–7
However, with these improvements in overall survival, it also became clear that survivors of CNS tumors are at increased risk for therapy-related long-term morbidities and mortality.5–8 One of the most serious late effects is the development of subsequent neoplasms (SNs), where risk continues to increase decades following primary cancer therapy.5,9,10 While RT and specific chemotherapeutic agents have both been independently associated with an increased risk for SNs, it is unclear what the magnitude of risk may be among CNS survivors exposed to multimodal therapy. Early studies have suggested that the risk may be high, with reported rates of ∼4% at 10 years.11–15 However, in addition to limited length of follow-up, these studies have lacked direct comparison to populations treated prior to the use of multimodal therapy for medulloblastoma.
Therefore, this study aims to estimate the overall cumulative incidence of SNs in the large population of CNS tumor survivors treated at St Jude Children's Research Hospital (SJCRH) and to explore whether the introduction of multimodal therapy for medulloblastoma has resulted in an increased incidence of SNs.
Patients and Methods
We identified 2779 patients diagnosed and treated for a primary CNS tumor in the SJCRH brain tumor program between January 1, 1985 and December 31, 2012. SNs were identified from the centralized hospital database, electronic medical record, and annual contact through the SJCRH tumor registry. SNs included all benign and malignant neoplasms defined by an International Classification of Diseases for Oncology (ICD-O) behavior code of 0, 1, 2, or 3. The subset of SNs considered as subsequent malignant neoplasms (SMNs) included only malignant diagnoses with an ICD-O behavior code of 3. To be included as an SN, a neoplasm was histologically distinct from the primary CNS tumor. SNs were only included when validated by pathology report (n = 90, 92.8%) or medical record validation of biopsy (n = 5, 5.2%), with the exception of diffuse intrinsic pontine glioma, where standard radiographic and clinical diagnostic criteria were applied. Other SNs suspected by imaging, but unconfirmed by pathology report, were not included. Demographic and treatment characteristics were abstracted from the medical records. Among children who developed SNs, information on underlying genetic predisposition syndromes and cause of death were collected. The study was approved by the institutional review board and the Childhood Cancer Survivor Study steering committee.
Comparison Population: The Childhood Cancer Survivor Study
The Childhood Cancer Survivor Study (CCSS) is a retrospective cohort, with prospective follow-up, of children and adolescents treated for childhood cancer at 26 collaborating institutions in the United States or Canada.12,13 Eligibility for participation in the CCSS included diagnosis of cancer before age 21, initial treatment between January 1, 1970 and December 31, 1986, and survival for at least 5 years from diagnosis. Baseline and follow-up data were collected from survivors or those <18 years of age or those deceased at baseline through their proxies, using self-administered questionnaires or telephone interviews. Treatment information was abstracted from medical records according to a structured protocol.
In the CCSS population there were 140 five-year survivors of medulloblastoma or supratentorial primitive neuroectodermal tumor (PNET) who received RT without chemotherapy, based on abstracted treatment information. One survivor was excluded from the current analysis who had developed an SN prior to entry into the cohort, resulting in 139 evaluable medulloblastoma survivors to serve as a historical comparison group. SNs included new neoplasms (malignant and benign) other than recurrences of the primary childhood malignancies, ascertained through self- or proxy-report questionnaires and/or death certificates. SNs were subsequently confirmed by pathology report or, when not available, by death certificate or other medical records reviewed by study investigators.
Statistical Analysis
The cumulative incidence of SN was estimated for the SJCRH population using the method of Kalbfleisch and Prentice,14 with death treated as a competing risk, and censored at last follow-up. Standardized incidence ratios (SIRs) of observed to expected SMNs were calculated using age-, sex-, and calendar year–specific rates from the Surveillance, Epidemiology, and End Results (SEER) program of the National Cancer Institute for the expected number of malignancies. Nonmelanoma skin cancers, benign meningiomas, and other benign tumors were not included in SIR estimations, as they are not reported to the SEER registry. Person-years of follow-up were calculated from diagnosis to death or last contact, whichever occurred first. Among the 5-year medulloblastoma survivors, comparison of cumulative incidence of SNs between SJCRH and CCSS populations at specific time points was performed using the approach described by Pentilie15 to account for the different lengths of follow-up between cohorts.
The approach by Fine and Gray,16 which utilizes Cox's proportional hazards model but takes into account death as a competing risk, was used to assess the relationship between treatment and demographic factors and the first occurrence of SN. Due to the potential confounding relationship between tumor type and treatment, 2 separate multiple regression analyses were run, one including tumor type and demographic factors, and the other including treatment and demographic factors. To minimize the potential confounding effect of genetic predisposition on the relationship between treatment and SN outcome (children with known tumor predisposition were more likely to receive reduced-intensity therapy or no therapy), children with known cancer predisposition syndromes were removed from the multivariable assessment of risk for SNs.
Results
Among 2779 patients with a primary diagnosis of CNS tumor, 81 survivors had 97 SNs, 64 (66.0%) of which were SMNs. Median follow-up from primary diagnosis was 4.5 years (range, 0.1–28.2 y), and to development of first SN 7.7 years (range, 0.3–21.1 y). The demographic and treatment characteristics of children diagnosed with a primary CNS tumor with and without development of an SN are listed in Table 1.
Table 1.
Demographic and treatment characteristics of children diagnosed at St Jude Children's Research Hospital with a primary CNS tumor, with and without development of an SN
| Characteristics | Overall (n = 2779) | SN (n = 81) n (%) | No SN (n = 2698) n (%) | P |
|---|---|---|---|---|
| Sex | ||||
| Female | 1249 (44.9) | 42 (51.9) | 1207 (44.7) | .20 |
| Male | 1530 (55.1) | 39 (48.1) | 1491 (55.3) | |
| Race | ||||
| Non-Hispanic white | 2084 (75.0) | 65 (80.2) | 2019 (74.8) | .27 |
| Non-Hispanic black | 472 (17.0) | 13 (16.0) | 459 (17.0) | |
| Hispanic | 83 (3.0) | 1 (1.2) | 82 (3.0) | |
| Other | 140 (5.0) | 2 ( 2.5) | 138 (5.1) | |
| Age at diagnosis | ||||
| 0 to <3 y | 663 (23.9) | 29 (35.8) | 634 (23.5) | .023 |
| 3 to <9 y | 1118 (40.2) | 32 (39.5) | 1086 (40.3) | |
| 9 to <15 y | 698 (25.1) | 17 (21.0) | 681 (25.2) | |
| ≥15 y | 300 (10.8) | 3 (3.7) | 297 (11.0) | |
| Tumor type | ||||
| Glioma/astrocytoma | 1340 (48.2) | 25 (30.9) | 1315 (48.7) | <.001 |
| Medulloblastoma/PNET | 597 (21.5) | 32 (39.5) | 565 (20.9) | |
| Ependymoma | 337 (12.1) | 14 (17.3) | 323 (12.0) | |
| Craniopharyngioma | 154 (5.5) | 1 (1.2) | 153 (5.7) | |
| Atypical teratoid rhabdoid tumor | 110 (4.0) | 2 (2.5) | 108 (4.0) | |
| Others | 241 (8.7) | 7 (8.6) | 234 (8.7) | |
| Treatment era | ||||
| 1980–1999 | 1003 (36.1) | 55 (67.9) | 948 (35.1) | <.001 |
| 2000–present | 1776 (63.9) | 26 (32.1) | 1750 (64.9) | |
| Treatment | ||||
| No RT or chemotherapy | 492 (17.7) | 8 (9.9) | 484 (17.9) | .010 |
| RT only | 654 (23.5) | 27 (33.3) | 627 (23.2) | |
| Chemotherapy only | 185 (6.7) | 10 (12.3) | 175 (6.5) | |
| RT + chemotherapy | 1448 (52.1) | 36 (44.4) | 1412 (52.3) | |
| Length of follow-up | ||||
| <1 y | 494 (17.8) | 2 (2.5) | 492 (18.2) | <.001 |
| 1 to <5 y | 970 (34.9) | 6 (7.4) | 964 (35.7) | |
| 5 to <10 y | 540 (19.4) | 22 (27.2) | 518 (19.2) | |
| 10 to <15 y | 373 (13.4) | 21 (25.9) | 352 (13.0) | |
| 15 to <20 y | 227 (8.2) | 16 (19.8) | 211 (7.8) | |
| ≥20 y | 175 (6.3) | 14 (17.2) | 161 (6.0) | |
| Time to first subsequent neoplasm | ||||
| 0 to <5 y | 19 (0.7) | 19 (23.4) | ||
| 5 to <10 y | 37 (1.3) | 37 (45.7) | ||
| 10 to <15 y | 14 (0.5) | 14 (17.3) | ||
| 15 to <20 y | 8 (0.3) | 8 (9.9) | ||
| 20 to <25 y | 3 (0.1) | 3 (3.7) | ||
| Survival status | ||||
| Alive | 1775 (63.9) | 51 (63.0) | 1724 (63.9) | .86 |
| Deceased | 1004 (36.1) | 30 (37.0) | 974 (36.1) | |
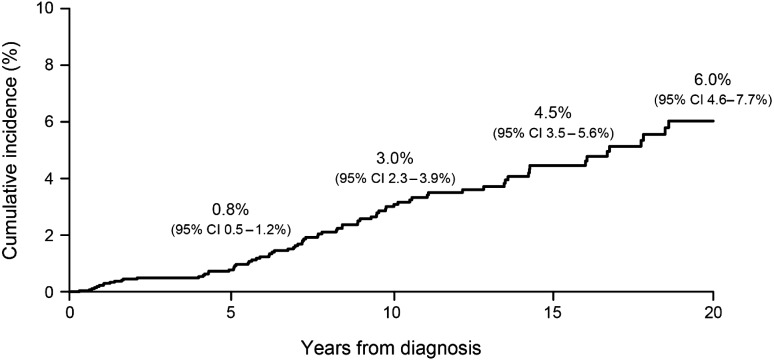
The cumulative incidence of first SN was 3.0% (95% CI: 2.3%–3.9%) at 10 years, and 6.0% (95% CI: 4.6%–7.7%) at 20 years from diagnosis (Fig. 1). In the SJCRH population, 23.4% of SNs occurred in the first 5 years from diagnosis and 45.7% between 5 and 10 years (Table 1). Subsequent CNS tumors accounted for the largest group of SNs (n = 43) and included, most commonly, gliomas (all grades, n = 23) and benign meningiomas (n = 13, Table 2). Other common SNs included hematological or marrow disease (n = 13), nonmelanoma skin cancer (n = 12), thyroid carcinomas (n = 8), and soft tissue sarcomas (n = 6). Eight of 13 (61.5%) hematological SNs occurred within the first 5 years from diagnosis (acute lymphocytic leukemia, n = 2; acute myeloid leukemia, n = 2; chronic myeloid leukemia, n = 1; non-Hodgkin lymphoma, n = 1; myelodysplastic syndrome, n = 1; myeloproliferative neoplasm, n = 1). Hematological malignancies occurred at a higher frequency than CNS malignancies in the first 5 years, whereas the latter were more common beyond 5 years. No hematological malignancies occurred beyond the first 10 years from diagnosis. The cumulative incidence of SNs was highest among survivors of medulloblastoma and ependymoma (Supplementary Fig. S1).
Fig. 1.
Cumulative incidence of SNs among children with primary CNS tumors.
Table 2.
Standardized incidence ratios for occurrence of subsequent neoplasms among children with primary CNS tumors
| n Observed | n Expected | SIR (95% CI) | Median n Years to Occurrence | |
|---|---|---|---|---|
| SNs for which SIR could be calculated (overall) | 64 | 4.55 | 14.1 (10.8–18.0) | 7.3 |
| Sex | ||||
| Female | 29 | 1.98 | 14.6 (9.8–21.0) | 7.3 |
| Male | 35 | 2.56 | 13.7 (9.5–19.0) | 7.2 |
| Age at diagnosis | ||||
| <5 y | 33 | 1.33 | 24.8 (17.1–34.8) | 7.2 |
| 5 to <10 y | 14 | 1.20 | 11.7 (6.4–19.6) | 8.0 |
| ≥10 y | 17 | 2.02 | 8.4 (4.9–13.5) | 5.1 |
| Primary diagnosis | ||||
| Glioma/astrocytoma | 26 | 2.09 | 12.5 (8.1–18.3) | 7.5 |
| Medulloblastoma/PNET | 19 | 1.16 | 16.3 (9.8–25.5) | 7.1 |
| Ependymoma | 12 | 0.53 | 22.6 (11.6–39.4) | 7.7 |
| Craniopharyngioma | 1 | 0.29 | 3.4 (0.1–19.1) | 1.3 |
| ATRT | 2 | 0.06 | 34.9 (3.9–125.9) | 5.7 |
| Others | 4 | 0.42 | 9.5 (2.6–24.4) | 10.6 |
| Type of subsequent malignancy | ||||
| Leukemia | 9 | 0.66 | 13.6 (6.2–25.8) | 4.4 |
| ALL | 3 | 0.44 | 6.9 (1.4–20.1) | 1.1 |
| AML | 5 | 0.16 | 31.8 (10.2–74.1) | 5.8 |
| CML | 1 | 0.04 | 24.1 (0.3–134.3) | 1.7 |
| Lymphoma | 1 | 0.75 | 1.3 (0.02–7.4) | 1.1 |
| CNS (total) | 28 | 0.56 | 49.7 (33.1–71.9) | 7.3 |
| Glioma | 23 | 0.40 | 57.2 (36.2–85.8) | 7.2 |
| Medulloblastoma | 1 | 0.05 | 18.7 (0.2 -104.0) | 5.0 |
| Othersa | 4 | – | – | 12.1 |
| Thyroid | 8 | 0.38 | 20.9 (9.0–41.1) | 14.3 |
| Soft tissue sarcoma | 6 | 0.21 | 29.3 (10.7–63.8) | 4.6 |
| Bone | 3 | 0.18 | 16.3 (3.3–47.5) | 7.1 |
| Testicular | 2 | 0.38 | 5.2 (0.6–18.8) | 8.5 |
| Ovarian | 1 | 0.08 | 13.0 (0.2–72.2) | 16.0 |
| Renal | 1 | 0.12 | 8.6 (0.1–47.6) | 4.2 |
| Othersb | 5 | 1.23 | 4.1 (1.3–9.5) | 10.6 |
| All other SNs for which SIR could not be calculated | 33 | 11.1 | ||
| Meningioma | 13 | 11.1 | ||
| Basal cell carcinoma | 11 | 12.9 | ||
| Myelodysplastic syndrome | 3 | 2.9 | ||
| Myeloproliferative neoplasm/desmoid tumor | 2 | 13.0 | ||
| Squamous cell carcinoma | 1 | 21.1 | ||
| Hemangioma | 1 | 17.7 | ||
| Acoustic neuroma | 1 | 6.2 | ||
| Neurofibroma | 1 | 9.6 | ||
Abbreviations: ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; ATRT, atypical teratoid rhabdoid tumor; CML, chronic myeloid leukemia.
aOthers include malignant meningioma (n = 1), fibrous histiocytoma (n = 1), and dysembryoplastic neuroepithelial tumor (n = 2).
bOthers include adenocarcinoma (n = 1), mammary analogue secretory carcinoma (n = 1), mucoepidermoid carcinoma (n = 1), neuroendocrine tumor (n = 1), and retinoblastoma (n = 1).
Children with a known cancer predisposition syndrome accounted for 22% of the population who developed SNs. Among the 81 survivors, 3 children with Gorlin syndrome developed 11 SNs, 9 children with neurofibromatosis type 1 developed 11 SNs, and 1 SN each was developed by 2 children with Li–Fraumeni syndrome, 1 child with neurofibromatosis type 2, and 1 child each with Lynch syndrome, familial adenomatous polyposis, and Fanconi anemia.
Among 81 patients with SNs, there were 64 subsequent neoplasms for which SIRs could be calculated (Table 2). There were 9 cases of leukemia, including 5 of acute myeloid leukemia (SIR, 31.8; 95% CI: 10.2–74.1). Additionally, children with CNS tumors developed subsequent CNS tumors (SIR, 49.7; 95% CI: 33.0–71.9), soft tissue sarcomas (SIR, 29.3; 95% CI: 10.7–63.8), and thyroid carcinomas (SIR, 20.9; 95% CI: 9.0–41.1) at rates much higher than expected in the general population. Children across all ages had an elevated risk for developing SNs; however, those younger than 5 years at diagnosis (SIR, 24.8; 95% CI: 17.1–34.8) represented the highest risk group compared with the general population.
When children with a genetic predisposition syndrome were excluded, multiple regression analysis identified that risk for SN was 0.64 (95% CI: 0.38–1.06) for children who received RT plus chemotherapy compared with RT alone (Table 3). No significant associations were detected among sex, race, and the development of SN or SMN.
Table 3.
Multivariable assessment of risk factors for subsequent neoplasms and subsequent malignant neoplasms among children diagnosed with a primary CNS tumora
| Characteristics | n | SN | Hazard Ratio for SN (95% CI) | SN, P-value | SMN | Hazard Ratio for SMN (95% CI) | SMN, P-value |
|---|---|---|---|---|---|---|---|
| Sex | |||||||
| Female | 1240 | 33 | 1.0 | .30 | 21 | 1.0 | .92 |
| Male | 1524 | 33 | 0.77 (0.47, 1.26) | 26 | 0.97 (0.54, 1.74) | ||
| Age at diagnosis | |||||||
| ≥9 y | 17 | 1.0 | 15 | 1.0 | |||
| 0 to <3 y | 665 | 21 | 1.78 (0.89, 3.57) | .11 | 12 | 1.17 (0.49, 2.79) | .72 |
| 3 to <9 y | 1114 | 28 | 1.36 (0.75, 2.48) | .31 | 20 | 1.12 (0.58, 2.19) | .73 |
| Race | |||||||
| Non-Hispanic White | 2071 | 52 | 1.0 | .76 | 36 | 1.0 | .94 |
| All others | 693 | 14 | 0.91 (0.50, 1.67) | 11 | 1.03 (0.51, 2.05) | ||
| Treatment received | |||||||
| RT only | 652 | 25 | 1.0 | 19 | 1.0 | ||
| No RT or chemotherapy | 487 | 3 | 0.26 (0.08, 0.87) | .03 | 3 | 0.33 (0.10–1.12) | .08 |
| Chemotherapy only | 180 | 5 | 0.88 (0.30, 2.57) | .82 | 3 | 0.82 (0.20–3.42) | .79 |
| RT + chemotherapy | 1445 | 33 | 0.64 (0.38, 1.06) | .08 | 22 | 0.56 (0.31–1.03) | .06 |
aPatients with known cancer predisposition syndromes removed from analysis.
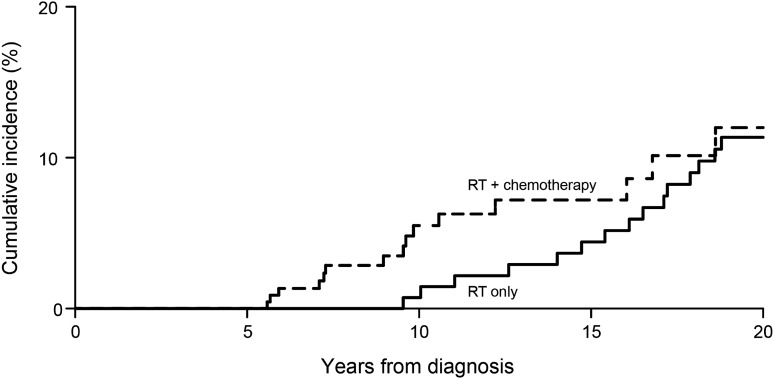
The cumulative incidence of SNs among 5-year survivors of medulloblastoma/PNET treated with multimodal therapy was 5.5% (95% CI: 2.8%–9.6%) at 10 years, and 12.0% (95% CI: 6.4%–19.5%) at 20 years from diagnosis. In contrast, the historical comparison population treated with RT had a cumulative incidence of only 0.7% (95% CI: 0.07%–3.7%; P = .005 for comparison with multimodal therapy) at 10 years, and 11.3% (95% CI: 6.6%–17.4%; P = .44; Fig. 2) at 20 years. Table 5 provides the SNs by year from diagnosis observed in both populations. Those treated with RT only (CCSS population, median follow-up 25.1 y; range, 5.5–37.4 y) had longer follow-up relative to those treated with multimodal therapy (St Jude population, median follow-up 10.3 y; range, 5.1–27.8 y), as would be expected based on historical changes in therapy (Table 4). In addition, within the multimodal therapy population, 3 children developed 4 subsequent malignancies (acute lymphocytic leukemia, acute myeloid leukemia, renal cell carcinoma, and rhabdomyosarcoma) prior to 5-year survival and were not included in the comparison with the RT-only population. All 3 children died of their SMNs. Six 5-year survivors of medulloblastoma with SNs who received multimodal therapy subsequently died; 5 died of SMNs, and only 1 of recurrent disease. The cumulative incidence of SN for medulloblastoma/PNET survivors treated with multimodal therapy including <30 Gy to the neuroaxis was 4.8% (95% CI: 1.5%–11.2%) at 10 years, and 6.8% (95% CI: 2.3%–14.6%) at 20 years, compared with those survivors who received ≥30 Gy, whose rates were 6.5% (95% CI: 2.6%–12.8%) at 10 years, and 13.3% (95% CI: 6.5%–22.5%) at 20 years (P = .11, for comparison of cumulative incidence at 20 y).
Fig. 2.
Cumulative incidence of SNs among 5-year survivors of medulloblastoma or PNET treated with multimodal therapy or RT with no chemotherapy.
Table 5.
Subsequent neoplasms at 5-year interval among 5-year survivors of medulloblastoma or PNET by treatment exposure
| Years From Diagnosis | RT Only, N = 52 in 27 Survivors | RT + Chemotherapy, N = 24 in 16 Survivors |
|---|---|---|
| 5 to <10 y | Basal cell carcinoma (1) | Meningioma (4) |
| High-grade glioma (4) | ||
| Osteosarcoma (1) | ||
| Nerve sheath tumor (1) | ||
| Desmoid tumor (1) | ||
| 10 to <15 y | Meningiomas (2) | Basal cell carcinoma (2) |
| Meningiotheliomatous meningioma (2) | Meningioma (1) | |
| Thyroid carcinoma (1) | Mammary analogue secretory Carcinoma (1) | |
| 15 to <20 y | Basal cell carcinoma (5) | Thyroid carcinoma (2) |
| Meningioma (3) | Basal cell carcinoma (1) | |
| Thyroid carcinoma (2) | Ovarian adenocarcinoma (1) | |
| Meningiotheliomatous meningioma (1) | Atypical meningioma (1) | |
| Sex cord stromal tumor (1) | ||
| Neurilemmoma (1) | ||
| ≥20 y | Basal cell carcinoma (14) | Atypical meningioma (2) |
| Meningioma (10) | Anaplastic meningioma (1) | |
| Thyroid carcinoma (3) | Basal cell carcinoma (1) | |
| High-grade glioma/astrocytoma (2) | ||
| Giant cell sarcoma (1) | ||
| Neurofibrosarcoma (1) | ||
| Neuroendocrine carcinoma (1) | ||
| Plasmacytoma (1) |
Table 4.
Demographic and follow-up characteristics of 5-year survivors of medulloblastoma or supratentorial PNET treated with RT only or multimodal therapy (RT + chemotherapy)
| Characteristics | RT Only,a N = 139 | RT + Chemotherapy,b N = 237 | P |
|---|---|---|---|
| n (%) | n (%) | ||
| Sex | |||
| Female | 75 (54.0) | 89 (37.6) | .002 |
| Male | 64 (46.0) | 148 (62.4) | |
| Race | |||
| Non-Hispanic white | 121 (87.1) | 179 (75.5) | .007 |
| Non-Hispanic black | 8 (5.8) | 34 (14.3) | |
| Hispanic | 3 (2.2) | 9 (3.8) | |
| Others | 7 (5.0) | 15 (6.3) | |
| Age at diagnosis | |||
| 0 to <3 y | 24 (17.3) | 11 (4.6) | .009 |
| 3 to <9 y | 68 (48.9) | 124 (52.3) | |
| 9 to <15 y | 38 (27.3) | 75 (31.6) | |
| ≥15 y | 9 (6.5) | 27 (11.4) | |
| Treatment era | |||
| 1970–1986 | 139 (100) | 11 (4.6) | |
| 1987–1995 | – | 57 (24.1) | |
| 1996 to present | – | 169 (71.3) | |
| Craniospinal RT dose | |||
| >30 Gy | 120 (92.3) | 105 (44.3) | <.001 |
| <30 Gy | 10 (7.7) | 132 (55.7) | |
| Unknown | 9 | ||
| Length of follow-up | |||
| 5 to <10 y | 2 (1.4) | 108 (45.6) | <.001 |
| 10 to <15 y | 7 (5.0) | 57 (24.1) | |
| 15 to <20 y | 9 (6.5) | 32 (13.5) | |
| ≥20 y | 121 (87.1) | 40 (16.8) | |
| SN | |||
| No | 112 (80.6) | 221 (93.3) | <0.001 |
| Yes | 27 (19.4) | 16 (6.7) | |
| Age at first SN | |||
| 0 to <10 y | – | 2 (0.8) | <.001 |
| 10 to 20 y | 4 (2.9) | 8 (3.4) | |
| 20+ y | 23 (16.5) | 6 (2.5) | |
| Time to first SN | |||
| 5 to 10 y | 1 (0.7) | 10 (4.2) | <.001 |
| 10 to <15 y | 5 (3.6) | 2 (0.8) | |
| 15 to <20 y | 9 (6.5) | 3 (1.3) | |
| ≥20 y | 12 (8.6) | 1 (0.4) | |
| Survival status | |||
| Alive | 125 (89.9) | 210 (88.6) | .69 |
| Expired | 14 (10.1) | 27 (11.4) | |
aChildhood Cancer Survivor Study participants; bdiagnosed and treated at St Jude Children's Research Hospital.
Discussion
To our knowledge, this is the first study to compare the cumulative incidence of SNs in survivors of medulloblastoma/PNET treated in the modern era with multimodal therapy (RT + chemotherapy) with a historical cohort treated with RT but no chemotherapy. We observed no evidence for an increase in incidence of SNs in medulloblastoma/PNET survivors treated with multimodal therapy (12% vs 11.3% at 20 y). Furthermore, multivariable analysis of the 2779 children with any type of CNS tumor identified no increase in risk for SNs among survivors who received multimodal therapy compared with those treated with RT but no chemotherapy. This is important because several previous studies have raised concern for an increased risk for SNs with multimodal therapy. In the long-term follow-up of the Children's Oncology Group A9961 randomized trial, 379 children with average risk medulloblastoma were treated with 23.4 Gy craniospinal irradiation and posterior fossa boost plus chemotherapy. They identified a cumulative incidence of SN of 4.3% (95% CI: 1.9%–6.5%) at 10 years that included a number of high-grade intracranial neoplasms.11 Additionally, in a prospective series from Germany of 280 patients who received 35.2 Gy to the craniospinal axis, posterior fossa boost, and sandwich chemotherapy, 12 patients developed secondary tumors between 4.3 and 11.8 years after primary therapy.17 Appropriately, both studies have raised early concern for an increased risk for SNs in survivors who received multimodal therapy, yet neither had a comparison population treated with RT but no chemotherapy.
Among survivors of medulloblastoma/PNET, while the incidence of SNs in children who received multimodal therapy in our study is similar to that of our historical comparison population at 20 years from diagnosis, it is possible that the addition of chemotherapy may alter the pattern, rather than the incidence, of SNs observed. Notably, at 10 years from diagnosis, 5-year survivors of medulloblastoma/PNET treated with multimodal therapy demonstrated a cumulative incidence of SNs of 5.5% (95% CI: 2.8%–9.6%), a rate statistically significantly higher than children who received RT but no chemotherapy. It is noteworthy that the number of high-grade lesions (4 high-grade gliomas, 1 osteosarcoma) in the first 10 years was higher after multimodal therapy than after RT without chemotherapy, or than that reported by others.11 Additionally, by including only 5-year survivors in our analysis (necessary for accurate comparison based on the methodology of the CCSS cohort), we excluded 3 survivors with 4 high-grade malignancies observed in the first 5 years from diagnosis. This pattern of early high-grade neoplasms is consistent with the findings from the long-term follow-up of the Children's Oncology Group 9961 trial.11 Nonetheless, by 20 years of diagnosis we observed no difference in the incidence of SNs.
Nine meningiomas occurred in children who received multimodal therapy for medulloblastoma, 5 of which occurred in the first 15 years. Conversely, most meningiomas in the RT-only comparison population were diagnosed >15 years after their primary tumors, as has been previously reported.18 At our institution, survivors of medulloblastoma were monitored intensively with imaging for at least 10 years postdiagnosis. Thus, based on the historical changes in SN surveillance in more modern eras with use of CT and subsequently MRI, the increased cumulative incidence observed 10 years after multimodal therapy (5.5% vs 0.7%) may be partially attributable to early detection of more benign neoplasms (meningiomas) in the modern era.
The etiology of subsequent neoplasms is multifactorial and includes primary cancer therapy as well as genetic susceptibility.19–21 Our series demonstrated that about 20% of children who develop SN after a primary CNS tumor have a known tumor predisposition syndrome. These are included in our report of cumulative incidence for comparability with other studies, as neither SEER nor CCSS exclude children with these genetic syndromes. Furthermore, children diagnosed with CNS tumors are not uniformly screened for genetic disease unless clinically indicated. Future studies that more proactively identify survivors with genetic predisposition may be warranted to allow early detection of SNs among populations that are at risk. In addition, our knowledge of the full range of cancer predisposition syndromes is not complete. Thus, there may be many survivors in the current study with an increased risk for cancer, not yet attributable to a specifically identified syndrome.
The association of radiation with increased incidence of SNs is well established.22–25 Previous studies have identified that radiation doses as low as 1–2 Gy increase risk for subsequent CNS tumors, including glioma and meningioma.26 Within survivors, increasing RT dose exposure to the CNS is associated with development of both glioma and meningioma in a linear dose-response relationship.18 Our multivariate analysis demonstrated that RT was associated with increased risk for SN development compared with those who received no RT or chemotherapy. As previously noted, the addition of chemotherapy to RT did not increase risk. Among our medulloblastoma/PNET survivors treated at SJCRH, we further demonstrated a nonstatistically significant lower rate of SNs in those who received reduced RT <30 Gy compared with dose >30 Gy (6.8% vs 13.3%).
There are several limitations to our study. Including only SNs validated by pathology or medical record of biopsy is likely to underestimate the number of benign CNS neoplasms that may be identified by imaging only. Second, while systematic clinical assessment is a strength of this single-institution study, reliance on retrospective self-reporting and notification after discharge from SJCRH may result in a lower ascertainment of SN cases beyond 10 years from diagnosis. Third, eligibility for entry into CCSS was limited to 5-year survivors, which restricted our ability to evaluate the incidence among medulloblastoma/PNET survivors (multimodal therapy vs RT without chemotherapy) in the first 5 years. Fourth, as chemotherapy intensity has likely increased in more recent eras, findings from patients treated in past decades may not be generalizable to current regimens. In addition, comparison with a historical cohort results in the inability to control for changes in surveillance practice, such as historical improvement in neuroimaging or changes in the delivery of RT, including use of conformal (systematically used at SJCRH since 1998) and intensity-modulated therapy, resulting in reduction in overall volume of tissue exposure.
Conclusion
In conclusion, in a large single-institution study we demonstrated that survivors of CNS tumors are at significant risk for developing SNs and SMNs with no plateau in the incidence observed. The highest risks observed were among children who received RT and survivors of medulloblastoma and ependymoma. The development of hematological and CNS SNs was most common. However, while adjuvant chemotherapy may have altered the pattern of SN development, there is no firm evidence that the addition of chemotherapy to RT increases the cumulative incidence of SNs. Ongoing follow-up is needed to detect any further difference as this population matures. However, it is not clear whether systematic, annual imaging surveillance across the lifespan for SNs is warranted.27 Prospective longitudinal studies to determine whether early detection of SNs improves outcome should be considered to fully evaluate this important question. Finally, while in the past decade there have been significant advances in the understanding of molecular pathways and subtypes of CNS tumors, the majority of children with CNS tumors will continue to be treated with RT and/or standard chemotherapy agents. This reinforces the need for ongoing efforts to educate adult survivors and improve capture of these events to understand patterns of occurrence as survivors age.
Supplementary Material
Funding
Support to St Jude Children′s Research Hospital was provided by a Cancer Center Support (CORE) grant (CA21765, R. Gilbertson, principal investigator), the Noyes Brain Tumor Foundation, Musicians Against Childhood Cancer, and the American Lebanese-Syrian Associated Charities (ALSAC). This work was also supported by the National Cancer Institute (CA55727, G. T. Armstrong, principal investigator).
This research was previously presented in part at the Children's Oncology Group meeting, Young Investigator poster session, Dallas, Texas, October 10, 2013.
Supplementary Material
Acknowledgments
Conflict of interest statement. None declared.
References
- 1.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- 2.Kortmann RD, Kuhl J, Timmermann B, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT '91. Int J Radiat Oncol Biol Phys. 2000;46(2):269–279. doi: 10.1016/s0360-3016(99)00369-7. [DOI] [PubMed] [Google Scholar]
- 3.Packer RJ, Gajjar A, Vezina G, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average-risk medulloblastoma. J Clin Oncol. 2006;24(25):4202–4208. doi: 10.1200/JCO.2006.06.4980. [DOI] [PubMed] [Google Scholar]
- 4.Gottardo N, Gajjar A. Current therapy for medulloblastoma. Curr Treat Options Neurol. 2006;8(4):319–334. doi: 10.1007/s11940-006-0022-x. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GT, Liu Q, Yasui Y, et al. Long-term outcomes among adult survivors of childhood central nervous system malignancies in the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2009;101(13):946–958. doi: 10.1093/jnci/djp148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer. 2014;14(1):61–70. doi: 10.1038/nrc3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laughton SJ, Merchant TE, Sklar CA, et al. Endocrine outcomes for children with embryonal brain tumors after risk-adapted craniospinal and conformal primary-site irradiation and high-dose chemotherapy with stem-cell rescue on the SJMB-96 trial. J Clin Oncol. 2008;26(7):1112–1118. doi: 10.1200/JCO.2008.13.5293. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong GT, Conklin HM, Huang S, et al. Survival and long-term health and cognitive outcomes after low-grade glioma. Neuro Oncol. 2011;13(2):223–234. doi: 10.1093/neuonc/noq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman DL, Whitton J, Leisenring W, et al. Subsequent neoplasms in 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2010;102(14):1083–1095. doi: 10.1093/jnci/djq238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardous-Ubbink MC, Heinen RC, Bakker PJ, et al. Risk of second malignancies in long-term survivors of childhood cancer. Eur J Cancer. 2007;43(2):351–362. doi: 10.1016/j.ejca.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Packer RJ, Zhou T, Holmes E, et al. Survival and secondary tumors in children with medulloblastoma receiving radiotherapy and adjuvant chemotherapy: results of Children's Oncology Group trial A9961. Neuro Oncol. 2013;15(1):97–103. doi: 10.1093/neuonc/nos267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute–supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27(14):2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: John Wiley; 2002. pp. 163–188. [Google Scholar]
- 15.Pentilie M. Competing Risk: A Practical Perspective. West Sussex, England: John Wiley & Sons; 2006. pp. 62–63. [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 17.Hoff KV, Hinkes B, Gerber NU, et al. Long-term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT ‘91. Eur J Cancer. 2009;45(7):1209–1217. doi: 10.1016/j.ejca.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Neglia JP, Robison LL, Stovall M, et al. New primary neoplasms of the central nervous system in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2006;98(21):1528–1537. doi: 10.1093/jnci/djj411. [DOI] [PubMed] [Google Scholar]
- 19.Travis LB, Ng AK, Allan JM, et al. Second malignant neoplasms and cardiovascular disease following radiotherapy. J Clin Oncol. 2012;30(30):3734–3745. [Google Scholar]
- 20.Kony SJ, de Vathaire F, Chompret A, et al. Radiation and genetic factors in the risk of second malignant neoplasms after a first cancer in childhood. Lancet. 1997;350(9071):91–95. doi: 10.1016/S0140-6736(97)01116-1. [DOI] [PubMed] [Google Scholar]
- 21.Little MP, de Vathaire F, Shamsaldin A, et al. Risks of brain tumour following treatment for cancer in childhood: modification by genetic factors, radiotherapy and chemotherapy. Int J Cancer. 1998;78(3):269–275. doi: 10.1002/(SICI)1097-0215(19981029)78:3<269::AID-IJC1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 22.Bassal M, Mertens AC, Taylor L, et al. Risk of selected subsequent carcinomas in survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006;24(3):476–483. doi: 10.1200/JCO.2005.02.7235. [DOI] [PubMed] [Google Scholar]
- 23.Henderson TO, Whitton J, Stovall M, et al. Secondary sarcomas in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2007;99(4):300–308. doi: 10.1093/jnci/djk052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor AJ, Little MP, Winter DL, et al. Population-based risks of CNS tumors in survivors of childhood cancer: the British Childhood Cancer Survivor Study. J Clin Oncol. 2010;28(36):5287–5293. doi: 10.1200/JCO.2009.27.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meadows AT, Friedman DL, Neglia JP, et al. Second neoplasms in survivors of childhood cancer: findings from the Childhood Cancer Survivor Study cohort. J Clin Oncol. 2009;27(14):2356–2362. doi: 10.1200/JCO.2008.21.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sadetzki S, Chetrit A, Freedman L, et al. Long-term follow-up for brain tumor development after childhood exposure to ionizing radiation for tinea capitis. Radiat Res. 2005;163(4):424–432. doi: 10.1667/rr3329. [DOI] [PubMed] [Google Scholar]
- 27.Bowers DC, Nathan PC, Constine L, et al. Subsequent neoplasms of the CNS among survivors of childhood cancer: a systematic review. Lancet Oncol. 2013;14(8):e321–e328. doi: 10.1016/S1470-2045(13)70107-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.