Abstract
Background
Primary brain tumors are a heterogeneous group of benign and malignant tumors arising from the brain parenchyma and its surrounding structures. The epidemiology of these tumors is poorly understood. The aim of our study is to systematically review the latest literature on the incidence and prevalence of primary brain tumors.
Methods
The systematic review and meta-analysis were conducted according to a predetermined protocol and established guidelines. Only studies reporting on data from 1985 onward were included. Articles were included if they met the following criteria: (i) original research, (ii) population based, (iii) reported an incidence or prevalence estimate of primary brain tumors.
Results
From the 53 eligible studies overall, 38 were included in the meta-analysis. A random-effects model found the overall incidence rate of all brain tumors to be 10.82 (95% CI: 8.63–13.56) per 100 000 person-years. The incidence proportion estimates were heterogeneous, even among the same tumor subtypes, and ranged from 0.051 per 100 000 (germ cell tumors) to 25.48 per 100 000 (all brain tumors). There were insufficient data to conduct a meta-analysis of the prevalence of primary brain tumors.
Conclusions
There is a need for more accurate and comparable incidence and prevalence estimates of primary brain tumors across the world. A standardized approach to the study of the epidemiology of these tumors is needed to better understand the burden of brain tumors and the possible geographical variations in their incidence.
Keywords: brain tumor, incidence, meta-analysis, prevalence, systematic review
Primary brain tumors are a heterogeneous group of benign and malignant tumors arising from the brain parenchyma and its surrounding structures. These tumors are an important cause of morbidity and mortality in both adults and children, often generating severe disabilities and producing high burden in both families and health care systems.1,2 The epidemiology of these tumors is poorly understood, as there is a paucity of data published on their incidence and prevalence across the globe. A solid understanding of the incidence and prevalence of primary brain tumors can help with better planning in the allocation of health resources and a better understanding of geographical differences.
Only one systematic review on the worldwide incidence of brain tumors has been published within the last 20 years.3 The paper, published in 1998, reviewed the methodology and results of studies including not only primary, but also secondary intracranial tumors. The world age-standardized incidence rate for all primary brain tumors reported in this review ranged from 4.3 to 18.6 per 100 000 per year. Unfortunately, this review had methodological limitations. The authors searched only English-language papers published from 1966 to 1995 (including data collected from 1935 to 1991) and included studies that were not population based in the review, which could result in biased estimates. Only one bibliographical database (Medline) was used for the search, and a formal meta-analysis of incidence rates was not performed. There was also marked heterogeneity in the methodology and results of the different studies included in the review.
There is a lack of a comprehensive and up-to-date systematic review of the global incidence and prevalence of brain tumors in the literature. The goal of our study was to systematically review the latest literature on incidence and prevalence of primary brain tumors.
Methods
Search Strategy
The systematic review and meta-analysis were conducted according to a predetermined protocol and established guidelines (Meta-analysis Of Observational Studies in Epidemiology, MOOSE).4 The search strategy (Supplementary Appendix A) was developed by the study authors with expertise in brain tumors and epidemiology and in consultation with a research librarian with systematic review expertise. The search was conducted on December 10, 2010 in Medline and on December 12, 2010 in Embase databases, and references were exported and managed using EndNote X5. Only studies reporting on data from 1985 onward were included, as prior to this date MRI, which has revolutionized the diagnosis of many neurological disorders, was not in clinical use. If a paper reported on data prior to and after 1985, only data exclusively from 1985 or later were included. Articles were included if published in English or French. Review articles on the epidemiology of brain tumors and the reference lists of articles that met inclusion criteria were also hand-searched for additional articles.
Study Selection
Abstracts and titles of all references were screened independently and in duplicate, to identify original research that reported on the prevalence or incidence of all primary brain tumors (malignant or nonmalignant). Those that were clearly not population based were excluded at this stage. Subsequently, 2 reviewers independently screened the full-text articles of abstracts identified in the first phase. Articles were included if they met all of the following criteria: (i) original research, (ii) population based, (iii) reported an incidence or prevalence estimate of primary brain tumors. Disagreements pertaining to the inclusion of articles were resolved by consensus and involvement of a third party as necessary.
Data Extraction
Two reviewers extracted and reached agreement on data from included articles using a standard data collection form. When multiple articles reporting data from the same study population were encountered, the most comprehensive data were used. For example, numerous articles may report on data from the same registry. In cases where the articles reported on different data collection years or subgroups (gender, age), all data (every article) were included. Demographic data were recorded, including age, gender, and study location. Diagnostic data were collected, as were the sources of those data and definitions/diagnostic criteria for brain tumors. Incidence and prevalence estimates of brain tumors from each study were recorded, along with any stratification by age, gender, or year of data collection.
Data Quality
The quality of the included studies was evaluated independently by 2 reviewers, using an assessment tool (Supplementary Appendix B) that was developed based on a previous study quality scoring system and published guidelines.5,6 The quality tool assessed sample representativeness, condition assessment, and statistical methods. Each study was assigned a quality score of 0 to 8 based on fulfillment of the quality criteria.
Data Synthesis and Analysis
To assess for significant between-study heterogeneity, the Cochrane Q statistic was calculated and I2 was used to quantify the magnitude of between-study heterogeneity. It was decided a priori that statistically significant heterogeneity (Q statistic P-value <.05) was absent the pooled estimate and 95% confidence intervals would be calculated using a fixed-effect model. If significant heterogeneity was present, a random-effects model was used. Publication bias was investigated visually using funnel plots and statistically using Begg's and Egger's tests.
For all tests, P < .05 was deemed to be significant. All statistical analyses were carried out in R version 2.14. The “meta” package was used to produce the pooled estimates, forest plots, and publication bias assessment. The “metafor” package was used to conduct the meta-regression using restricted maximum likelihood estimation.
Results
Identification and Description of Studies
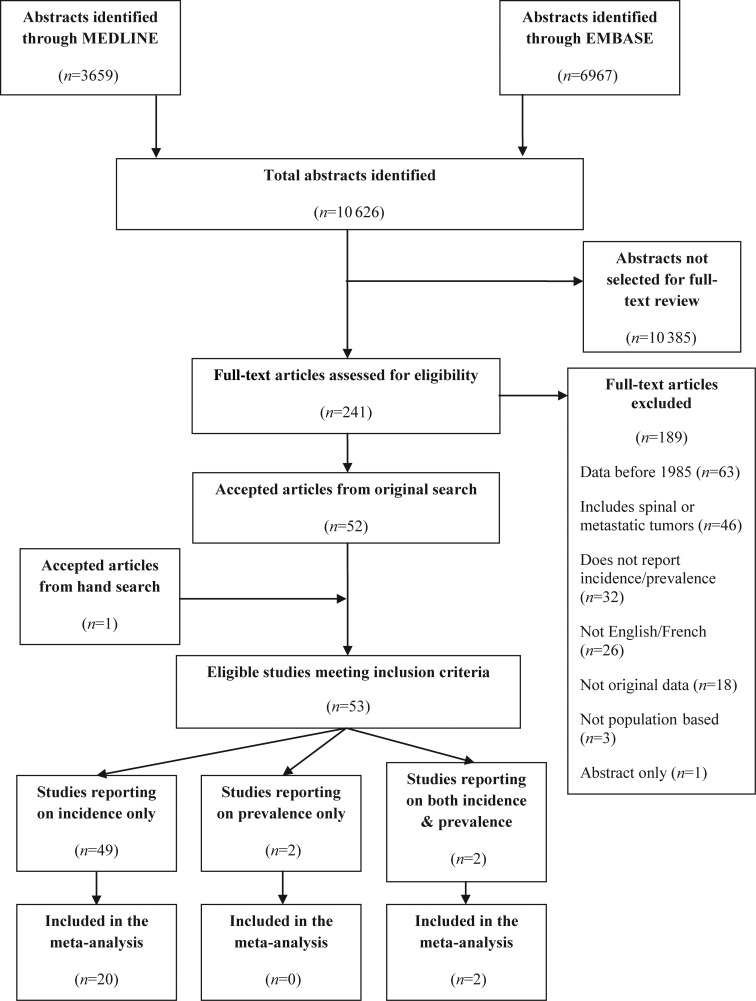
The results of the search strategy yielded a total of 10 626 citations: 3659 from Medline and 6967 from Embase, some of which were duplicates (Fig. 1). After the initial screen, 241 articles met the criteria for full-text review, of which 189 were excluded (63 reported data before 1985, 46 included spinal or metastatic tumors, 32 did not report on the incidence or prevalence of brain tumors, 26 were not in English/French, 18 were not original data, 3 were not population based, and 1 was only an abstract). Hand-searching resulted in the inclusion of 1 additional article. From the 53 eligible studies overall (original search + hand-searching), 22 were included in the meta-analysis; the 16 articles reporting on the incidence proportion of all primary brain tumors were deemed too clinically heterogeneous to pool (1 was included in the incidence rate analyses), there were too few studies reporting on the point (n = 2) and period (n = 2) prevalence estimates to pool (however, these were pooled in the incidence rate analyses), and for the remaining incidence rate articles, 14 were not pooled (12 provided only age-standardized or adjusted estimates, and 2 provided only the estimate, with no confidence intervals or cases). Characteristics of the 53 included studies (reporting on all malignant and nonmalignant primary brain tumors) are shown in Table 1 and Supplementary Tables S1–S11.
Fig. 1.
Brain tumor systematic review study flow diagram.
Table 1.
Prevalence studies of brain tumors
| Study, Date, Reference | Region | Population | Diagnostic Criteria | Data Source | Diagnosis Established by | Tumor Type | Prevalence Date | Overall Prevalence (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Daly, 20067 | Belgium | All ages | Unspecified | Hospital clinic review | Histology; hormonal workup | Pituitary adenoma | September 30, 2005 | 94 (72.2–115.8) per 100 000 |
| Davis, 200120 | United States | All ages | ICD-O; WHO | Registry | Behavior codes; histology | Benign brain; malignant brain; primary brain | 1985–1989 | 97.5 per 100 000 (NA); 29.5 per 100 000 (NA); 130.8 (NA) per 100 000 |
| Fernandez, 20108 | Banbury, Oxfordshire, England | All ages | ICD-O | Administrative database | Histology; hormonal workup | Pituitary adenoma | July 31, 2006 | 77.6 (NA) per 100 000 |
| Porter, 201059 | United States | All ages | ICD-O; ICD-O-1 | Registry | Histology | Primary brain; glioma; meningioma | 2004, 2005 | 221.8 (NA) per 100 000; 6.0 per 100 000; 6.0 per 100 000 |
Abbreviations: ICD-O, International Classification of Diseases for Oncology; WHO, World Health Organization classification of brain tumors; NA, not available.
Of the 53 included studies, 2 reported on the prevalence of brain tumors,7,8 49 reported on the incidence of brain tumors,9–57 and 2 reported on both the incidence and prevalence of brain tumors.58,59 Twenty-nine of the studies reported on data from Europe, 14 from North America, 7 from Asia, 2 from Australia, 1 from Africa, and 0 from South America (some studies report on data from more than one country).
Twenty studies reported on all brain tumors, 15 on gliomas, 28 on medulloblastomas/primitive neuroectodermal tumors (PNETs), 8 on pineal gland tumors, 12 on nerve sheath tumors, 5 on germ cell tumors, 18 on sellar tumors, 2 on hemangioblastomas, 7 on other tumors, 14 on meningiomas, 7 on primary central nervous system lymphomas, and 4 on choroid plexus tumors.
Prevalence of Brain Tumors
Table 1 lists all the prevalence studies of primary brain tumors identified in this review. Two eligible articles reported on the point prevalence of brain tumors but were not included in a meta-analysis, as only overall brain tumors were analyzed. One study reported a point prevalence of pituitary adenoma of 94 per 100 000,7 and the other study reported a point prevalence of pituitary adenoma of 77.6 per 100 000.8
Two eligible articles reported on the period prevalence of brain tumors but were not included in a meta-analysis, as they reported on different types of tumors and only overall brain tumors were analyzed. The total number of participants included was not reported in either paper. One study reported period prevalences of all primary brain tumors of 221.8 per 100 000, gliomas of 6.0 per 100 000, and meningiomas of 6.0 per 100 000.59 The other study of period prevalence reported an estimate of 130.8 per 100 000 for all primary brain tumor types.20
Incidence of Brain Tumors
Supplementary Tables S1–S11 list all of the incidence studies of primary brain tumors identified in this review. Sixteen studies11–15,17,18,48–51,53–57 reported an incidence proportion of brain tumors but were too heterogeneous to be included in a meta-analysis. Five studies reported on all primary brain tumors, 8 on medulloblastomas/PNETs, 3 on gliomas, 3 on sellar tumors, 2 on germ cell tumors, 2 on CNS lymphomas, 2 on pineal tumors, 1 on choroid plexus tumors, 1 on meningiomas, 1 on nerve sheath tumors, and 1 on other tumor types, while some studies reported on multiple tumor types. The incidence proportion estimates ranged from 0.051 per 100 000 (germ cell tumors)50 to 25.48 per 100 000 (all primary brain tumors).17
Twenty-two studies of the 36 identified studies that reported an incidence rate9,10,16,19–47,52,54–59 met all eligibility criteria for inclusion in a meta-analysis of the overall incidence rate of brain tumors. A random-effects model found the overall incidence rate on all primary brain tumors to be 10.82 (95% CI: 8.63–13.56) per 100 000 person-years (Supplementary Fig. S1). The incidence rate estimates ranged from 0.01 per 100 000 person-years (pineal tumors)34 to 25.95 per 100 000 person-years (all primary brain tumors). Significant heterogeneity existed between most estimates.
Sources of Heterogeneity
Gender
The overall incidence rate of all primary brain tumors was 15.80 per 100 000 person-years (95% CI: 10.30–24.24) in females (Supplementary Fig. S2) and 14.33 per 100 000 person-years (95% CI: 10.07–20.38) in males (Supplementary Fig. S3). Meta-regression including gender in the model showed no statistically significant difference by gender on the overall estimate of brain tumors (P > .05). The pooled incidence rate of all meningiomas by gender was borderline significant (P = .05), with the estimate for females (4.21 per 100 000 person-years [95% CI: 2.52–7.04]) being higher than that for males, 2.33 (1.35 per 100 000 person-years [95% CI: 1.35–4.01]). There were no other significant differences by gender for other tumor subtypes (Supplementary Table S13).
Age
The incidence rate of medulloblastomas was significantly higher in children (0.49 per 100 000 person-years [95% CI: 0.42–0.66]) than adults (0.05 per 100 000 person-years [95% CI: 0.03–0.10]27), P < .05, while the incidence rate for all gliomas was higher in adults (14.07 per 100 000 person-years [95% CI: 11.98–16.52]) than in children (0.18 per 100 000 person-years [94% CI: 0.10–0.33]), P < .05. Unfortunately, there were insufficient data to compare the incidence rate of other brain tumor types by age.
Time
Using the study data collection start year, midyear, and end-year (separately) as continuous factors, changes in the incidence rate of all brain tumors were assessed over time using meta-regression. The meta-regression revealed no statistically significant changes in the incidence rate of all brain tumors over time, using the data collection start year (P = .72), midyear (P = .53), or end year (P = .46).
Publication Bias
For the incidence rate of brain tumors overall, there was no statistically significant evidence of publication bias for Begg's (P > .05) and Egger's tests (P > .05); however, upon visual inspection there was slight asymmetry in the funnel plot. Evidence of publication bias was not found for the incidence proportion of brain tumors using Egger's and Begg's test (P > .05); however, the funnel plot also showed slight asymmetry. There was not a sufficient sample size to conduct publication bias analyses for the point and period prevalence of brain tumors.
Study Quality
The median study quality score for studies reporting on the incidence or prevalence of brain tumors was 4/8 (range 2–7). All studies described the target population in detail and either sampled the entire population or used probability sampling (Supplementary Table S12). Thirty-three studies reported a response rate >70%, and of those, 7 articles adequately described the nonresponders. Although population based, the majority of studies did not specifically report on whether their sample was representative of the target population (19/53). Most (32) studies used standardized data collection methods; however, only 7 reported using validated criteria to assess for the presence of brain tumors. Finally, fewer than half of the studies (20) reported estimates with their accompanying confidence intervals or by subgroups.
Discussion
Through a systematic review of the latest literature, we are able to provide pooled estimates of the incidence of different types of primary brain tumors. The overall pooled incidence rate of primary brain tumors was found to be 10.82 per 100 000 person-years. Estimates of incidence rates ranged from 0.01 per 100 000 person-years (pineal tumors) to 25.95 per 100 000 person-years (all primary brain tumors).
The heterogeneous incidence estimates found in our review likely reflect in part the fact that primary brain tumors are a heterogeneous group of disease. This heterogeneity creates difficulties when reporting the epidemiology of overall brain tumors, as reflected in the wide range of incidence estimates found even within studies examining similar tumor types. The differences in incidence estimates can also be a reflection of differences in reporting cases to cancer registries or to the lack of accurate case ascertainment, based on variable diagnostic capabilities of each center or region. In addition, variations in incidence estimates may be due to diverse study methodologies. As an example, Porter et al59 included only tumors with available pathological diagnosis and reported an incidence rate of 6 per 100 000 person-years for all gliomas, while Elia-Pasquet et al2,3 included tumors whether or not confirmed histologically and reported an incidence rate of 14.07 per 100 000 person-years for all gliomas.
In our review, there was one borderline significant difference in the incidence rate of primary brain tumors according to gender. The incidence rate of meningiomas in females was close to being significantly higher than the incidence rate in males (P = .05). When reviewing rate differences according to age, the data were limited. Only the incidence rates of medulloblastomas and of all gliomas were amenable to age-stratified analysis. In our meta-analysis, the incidence rate of medulloblastomas in children was 0.49 per 100 000 person-years, while Giordana et al27 reported it in adults to be 0.05 per 100 000 person-years. Since Giordana et al included only histologically confirmed tumors and the pediatric papers also included a small percentage of nonhistologically confirmed tumors, these differences need to be interpreted cautiously. Unfortunately, there were insufficient data to conduct a meta-analysis of the prevalence of overall primary brain tumors.
The only previous systematic review on the worldwide epidemiology of brain tumors was published in 19983; of the 20 included articles, 17 were either published or collected data from before 1985, an exclusion criterion for the current study. The remaining 3 articles published after this time were included in our review,19,31,54 resulting in a total of 50 new articles included in the present study. In the earlier systematic review, the incidence rate of all primary tumors ranged from 4.3 to 18.6 per 100 000 persons per year, a finding consistent with our current study, where we report a pooled incidence rate of 10.82 per 100 000 persons per year.3 More recently, a systematic review of the epidemiology of brain tumors in Iran was published, and included 9 articles from that country.60 None of the 9 articles included in the Iranian review were included in the present study, as they were not population based according to our prespecified criteria. The review of Iranian studies found an overall incidence rate of primary malignant brain tmors of 2.74 per 100 000 persons per year60; the lower number found in this study compared with our review may be the result of the inclusion of only malignant tumors in the primary analysis.
The worldwide review of brain tumor by Counsell and Grant3 found no evidence of an increase in incidence rate over time, though they used only a dichotomous measure of time (data collection before or during/after the 1980s). We also found no evidence of an increase in the incidence rate of all brain tumors over time, using time as a continuous variable. It has been suggested that the increases reported in some studies over time are entirely artifactual, largely due to an increase in the recognition of brain tumors caused by the rise in the amount of CT scans conducted, an increase in the number of neurologists practicing in the field, and changes to coding practices worldwide.3 As with the review by Counsell and Grant,3 we found a higher incidence rate of medulloblastomas in children compared with adults. The significant differences between males and females in the incidence rate of most tumor types reported in both previous systematic reviews3,60 were not replicated in the current study, which found only a slight gender difference in the incidence rate for meningiomas.
The evidence-based reporting on risk factors for brain tumors is mixed and is likely a reflection of the heterogeneity between tumor types.61,62 Reproductive and hormonal factors have been associated with an increased risk of some tumor types; the risk of meningioma is almost double in women versus men.61 Exposure to therapeutic doses of ionizing radiation is an established (and potentially modifiable) risk factor for some brain tumors, including pituitary adenoma, meningioma, and glioma.61 Other potential risk factors for brain tumor include genetic factors, familial aggregation, and environmental exposures.61,62 Though risk factors for brain tumor were not assessed in the current study, an understanding of them is essential for effective prevention and therapeutic strategies.
Our review has many strengths, including searching more than one bibliographical database (Medline and Embase), incorporating a quality assessment tool for each study, conducting a meta-analysis following a predetermined protocol and guidelines, and completing an assessment of study heterogeneity using a random-effects model. However, our review is not without limitations. We reviewed articles published in only English or French and we cannot rule out the possibility of publication bias, as there was slight asymmetry upon visual inspection of the funnel plot, although this did not reach statistical significance. Few studies reported gender- and/or sex-stratified estimates by tumor type, limiting our ability to calculate pooled estimates for these subgroups. In addition, the median quality score of the included studies was quite low, as many studies did not report estimates with confidence intervals or by subgroups and it was unclear how many used validated criteria to determine whether brain tumors were present. We do not believe this would have an impact on the results presented here, as those studies without confidence intervals would not be pooled. The majority of the study quality issues could be mitigated by more clear and accurate reporting by original study authors. Another limitation is the gap in knowledge regarding the prevalence of brain tumors internationally and by subtypes. Both prevalence and incidence data are needed to truly assess the global burden of brain tumors.
Based on our findings, we can conclude that there is a need to produce more accurate and comparable incidence and prevalence estimates of primary brain tumors across the world. A standardized approach to the study of the epidemiology of these tumors is needed to better understand the burden of the disease and the possible geographical variations in the incidence. Future studies investigating the incidence or prevalence of brain tumors should adopt similar methodologies and use a standardized approach to case ascertainment.
Supplementary Material
Funding
Funding for the study was provided by the Public Health Agency of Canada. The opinions expressed in this publication are those of the authors/researchers and do not necessarily reflect the official views of the Public Health Agency of Canada. K.M.F. and A.D.F. were supported by Alberta Innovates Health Solutions Studentships. N.J. holds a salary award from Alberta Innovates Health Solutions and a Canada Research Chair in Neurological Health Services Research.
Supplementary Material
Acknowledgments
This study is part of the National Population Health Study of Neurological Conditions. We wish to acknowledge the membership of Neurological Health Charities Canada and the Public Health Agency of Canada for their contribution to the success of this initiative. The authors would like to thank Drs Nadia Mansoor and Naomi Popeski for their invaluable assistance.
Conflict of interest statement. The authors have no conflicts of interest to disclose.
References
- 1.Lacy J, Saadati H, Yu J. Complications of brain tumors and their treatment. Hemat Oncol Clin North Am. 2012;26(4):779–796. [DOI] [PubMed] [Google Scholar]
- 2.Jacques G, Cormac O. Central nervous system tumors. Handb Clin Neurol. 2013;112:931–958. [DOI] [PubMed] [Google Scholar]
- 3.Counsell C, Grant R. Incidence studies of primary and secondary intracranial tumors: a systematic review of their methodology and results. J Neurooncol. 1998;37(3):241–250. [DOI] [PubMed] [Google Scholar]
- 4.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. [DOI] [PubMed] [Google Scholar]
- 5.Boyle MH. Guidelines for evaluating prevalence studies. Evid Based Mental Health. 1998;1:37–39. [Google Scholar]
- 6.Loney PL, Chambers LW, Bennett KJ, et al. Critical appraisal of health literature: prevalence or incidence of a health problem. Chronic Dis Can. 1998;19(4):170–176. [PubMed] [Google Scholar]
- 7.Daly AF, Rixhon M, Adam C, et al. High prevalence of pituitary adenomas: a cross-sectional study in the province of Liege, Belgium. J Clin Endocrinol Metab. 2006;91(12):4769–4775. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez A, Karavitaki N, Wass JAH. Prevalence of pituitary adenomas: a community-based, cross-sectional study in Banbury (Oxfordshire, UK). Clin Endocrinol (Oxf). 2010;72(3):377–382. [DOI] [PubMed] [Google Scholar]
- 9.Al-Sheyyab M, Bateiha A, El Kayed S, et al. The incidence of childhood cancer in Jordan: a population-based study. Ann Saudi Med. 2003;23(5):260–263. [DOI] [PubMed] [Google Scholar]
- 10.Alston RD, Newton R, Kelsey A, et al. Childhood medulloblastoma in northwest England 1954 to 1997: incidence and survival. Dev Med Child Neurol. 2003;45(5):308–314. [DOI] [PubMed] [Google Scholar]
- 11.Berger C, Trombert-Paviot B, Mitton N, et al. [Childhood cancer incidence and survival rates in the Rhone-Alpes regional paediatric registry 1987–1999] [in French]. Arch Pediatr. 2006;13(2):121–129. [DOI] [PubMed] [Google Scholar]
- 12.Birch JM, Alston RD, Kelsey AM, et al. Classification and incidence of cancers in adolescents and young adults in England 1979–1997. Br J Cancer. 2002;87(11):1267–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown M, Schrot R, Bauer K, et al. Incidence of first primary central nervous system tumors in California, 2001–2005: children, adolescents and teens. J Neurooncol. 2009;94(2):263–273. [DOI] [PubMed] [Google Scholar]
- 14.Chakrabarti I, Cockburn M, Cozen W, et al. A population-based description of glioblastoma multiforme in Los Angeles County, 1974–1999. Cancer. 2005;104(12):2798–2806. [DOI] [PubMed] [Google Scholar]
- 15.Chan M-Y, Teo W-Y, Seow W-T, et al. Epidemiology, management and treatment outcome of medulloblastoma in singapore. Ann Acad Med Singapore. 2007;36(5):314–318. [PubMed] [Google Scholar]
- 16.Christensen HC, Kosteljanetz M, Johansen C. Incidences of gliomas and meningiomas in Denmark, 1943 to 1997. Neurosurgery. 2003;52(6):1327–1333; discussion 1333–1324. [DOI] [PubMed] [Google Scholar]
- 17.Cordera S, Bottacchi E, D'Alessandro G, et al. Epidemiology of primary intracranial tumors in NW Italy, a population based study: stable incidence in the last two decades. J Neurol. 2002;249(3):281–284. [DOI] [PubMed] [Google Scholar]
- 18.Corn BW, Marcus SM, Topham A, et al. Will primary central nervous system lymphoma be the most frequent brain tumor diagnosed in the year 2000? Cancer. 1997;79(12):2409–2413. [PubMed] [Google Scholar]
- 19.D'Alessandro G, Di Giovanni M, Iannizzi L, et al. Epidemiology of primary intracranial tumors in the Valle d'Aosta (Italy) during the 6-year period 1986–1991. Neuroepidemiology. 1995;14(3):139–146. [DOI] [PubMed] [Google Scholar]
- 20.Davis FG, Kupelian V, Freels S, et al. Prevalence estimates for primary brain tumors in the United States by behavior and major histology groups. Neuro Oncol. 2001;3(3):152–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobec-Meic B, Pikija S, Cvetko D, et al. Intracranial tumors in adult population of the Varazdin County (Croatia) 1996–2004: a population-based retrospective incidence study. J Neurooncol. 2006;78(3):303–310. [DOI] [PubMed] [Google Scholar]
- 22.Dreifaldt AC, Carlberg M, Hardell L. Increasing incidence rates of childhood malignant diseases in Sweden during the period 1960–1998. Eur J Cancer. 2004;40(9):1351–1360. [DOI] [PubMed] [Google Scholar]
- 23.Elia-Pasquet S, Provost D, Jaffre A, et al. Incidence of central nervous system tumors in Gironde, France. Neuroepidemiology. 2004;23(3):110–117. [DOI] [PubMed] [Google Scholar]
- 24.Farinotti M, Ferrarini M, Solari A, et al. Incidence and survival of childhood CNS tumors in the Region of Lombardy, Italy. Brain. 1998;121(Pt 8):1429–1436. [DOI] [PubMed] [Google Scholar]
- 25.Freedman LS, Barchana M, Al-Kayed S, et al. A comparison of population-based cancer incidence rates in Israel and Jordan. Eur J Cancer Prev. 2003;12(5):359–365. [DOI] [PubMed] [Google Scholar]
- 26.Frohlich AM, Sutherland GR. Epidemiology and clinical features of vestibular schwannoma in Manitoba, Canada. Can J Neurol Sci. 1993;20(2):126–130. [DOI] [PubMed] [Google Scholar]
- 27.Giordana MT, Schiffer P, Lanotte M, et al. Epidemiology of adult medulloblastoma. Int J Cancer. 1999;80(5):689–692. [DOI] [PubMed] [Google Scholar]
- 28.Gurney JG, Wall DA, Jukich PJ, et al. The contribution of nonmalignant tumors to CNS tumor incidence rates among children in the United States. Cancer Causes Control. 1999;10(2):101–105. [DOI] [PubMed] [Google Scholar]
- 29.Jakab Z, Balogh E, Kiss C, et al. Epidemiologic studies in a population-based childhood cancer registry in Northeast Hungary. Med Pediatr Oncol. 2002;38(5):338–344. [DOI] [PubMed] [Google Scholar]
- 30.Kaatsch P, Haaf G, Michaelis J. Childhood malignancies in Germany—methods and results of a nationwide registry. Eur J Cancer. 1995;31A(6):993–999. [DOI] [PubMed] [Google Scholar]
- 31.Kaye AH, Giles GG, Gonzales M. Primary central nervous system tumors in Australia: a profile of clinical practice from the Australian Brain Tumor Register. Aust N Z J Surg. 1993;63(1):33–38. [DOI] [PubMed] [Google Scholar]
- 32.Klaeboe L, Lonn S, Scheie D, et al. Incidence of intracranial meningiomas in Denmark, Finland, Norway and Sweden, 1968–1997. Int J Cancer. 2005;117(6):996–1001. [DOI] [PubMed] [Google Scholar]
- 33.Kuratsu J, Takeshima H, Ushio Y. Trends in the incidence of primary intracranial tumors in Kumamoto, Japan. Int J Clin Oncol. 2001;6(4):183–191. [DOI] [PubMed] [Google Scholar]
- 34.Lee CH, Jung KW, Yoo H, et al. Epidemiology of primary brain and central nervous system tumors in Korea. J Korean Neurosurg Soc. 2010;48(2):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liigant A, Asser T, Kulla A, et al. Epidemiology of primary central nervous system tumors in Estonia. Neuroepidemiology. 2000;19(6):300–311. [DOI] [PubMed] [Google Scholar]
- 36.Linabery AM, Ross JA. Trends in childhood cancer incidence in the U.S. (1992–2004). Cancer. 2008;112(2):416–432. [DOI] [PubMed] [Google Scholar]
- 37.Makino K, Nakamura H, Yano S, et al. Population-based epidemiological study of primary intracranial tumors in childhood. Childs Nerv Syst. 2010;26(8):1029–1034. [DOI] [PubMed] [Google Scholar]
- 38.McKinney PA, Parslow RC, Lane SA, et al. Epidemiology of childhood brain tumors in Yorkshire, UK, 1974–95: geographical distribution and changing patterns of occurrence. Br J Cancer. 1998;78(7):974–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNally RJ, Kelsey AM, Cairns DP, et al. Temporal increases in the incidence of childhood solid tumors seen in Northwest England (1954–1998) are likely to be real. Cancer. 2001;92(7):1967–1976. [DOI] [PubMed] [Google Scholar]
- 40.McNeil DE, Cot TR, Clegg L, et al. Incidence and trends in pediatric malignancies medulloblastoma/primitive neuroectodermal tumor: a SEER update. Surveillance Epidemiology and End Results. Med Pediatr Oncol. 2002;39(3):190–194. [DOI] [PubMed] [Google Scholar]
- 41.Nielsen MS, Christensen HC, Kosteljanetz M, et al. Incidence of and survival from oligodendroglioma in Denmark, 1943–2002. Neuro Oncol. 2009;11(3):311–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nilsson B, Gustavasson-Kadaka E, Bengtsson BA, et al. Pituitary adenomas in Sweden between 1958 and 1991: incidence, survival, and mortality. J Clin Endocrinol Metab. 2000;85(4):1420–1425. [DOI] [PubMed] [Google Scholar]
- 43.Pekmezovic T, Golubicic I, Grujicic D, et al. Incidence of primary central nervous system tumors among children in Belgrade (Serbia), 1991–2004. Pediatr Hematol Oncol. 2009;26(5):332–337. [DOI] [PubMed] [Google Scholar]
- 44.Peris-Bonet R, Martinez-Garcia C, Lacour B, et al. Childhood central nervous system tumors—incidence and survival in Europe (1978–1997): report from Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42(13):2064–2080. [DOI] [PubMed] [Google Scholar]
- 45.Pobereskin LH, Chadduck JB. Incidence of brain tumors in two English counties: a population based study. J Neurol Neurosurg Psychiatry. 2000;69(4):464–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quoc NM, Hung NC, Kramarova E, et al. Incidence of childhood cancer in Ho Chi Minh City, Vietnam, 1995–97. Paediatr Perinat Epidemiol. 2000;14(3):240–247. [DOI] [PubMed] [Google Scholar]
- 47.Seedat RY, Claassen AJ, Mol DA. Incidence and management of acoustic neuromas in South Africa. Otol Neurotol. 2002;23(6):996–998. [DOI] [PubMed] [Google Scholar]
- 48.Shugg D, Allen BJ, Blizzard L, et al. Brain cancer incidence, mortality and case survival: observations from two Australian cancer registries. Int J Cancer. 1994;59(6):765–770. [DOI] [PubMed] [Google Scholar]
- 49.Stiller CA, Desandes E, Danon SE, et al. Cancer incidence and survival in European adolescents (1978–1997). Report from the Automated Childhood Cancer Information System project. Eur J Cancer. 2006;42(13):2006–2018. [DOI] [PubMed] [Google Scholar]
- 50.Villano JL, Virk IY, Ramirez V, et al. Descriptive epidemiology of central nervous system germ cell tumors: nonpineal analysis. Neuro Oncol. 2010;12(3):257–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Werner MH, Phuphanich S, Lyman GH. The increasing incidence of malignant gliomas and primary central nervous system lymphoma in the elderly. Cancer. 1995;76(9):1634–1642. [DOI] [PubMed] [Google Scholar]
- 52.Wohrer A, Waldhor T, Heinzl H, et al. The Austrian Brain Tumor Registry: a cooperative way to establish a population-based brain tumor registry. J Neurooncol. 2009;95(3):401–411. [DOI] [PubMed] [Google Scholar]
- 53.Wu XC, Chen VW, Steele B, et al. Cancer incidence in adolescents and young adults in the United States, 1992–1997. J Adolesc Health. 2003;32(6):405–415. [DOI] [PubMed] [Google Scholar]
- 54.Counsell CE, Collie DA, Grant R. Incidence of intracranial tumors in the Lothian region of Scotland, 1989–90. J Neurol Neurosurg Psychiatry. 1996;61(2):143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaatsch P, Rickert CH, Kuhl J, et al. Population-based epidemiologic data on tumors in German children. Cancer. 2001;92(12):3155–3164. [DOI] [PubMed] [Google Scholar]
- 56.Lacour B, Guyot-Goubin A, Guissou S, et al. Incidence of childhood cancer in France: National Children Cancer Registries, 2000–2004. Eur J Cancer Prev. 2010;19(3):173–181. [DOI] [PubMed] [Google Scholar]
- 57.Miltenburg D, Louw DF, Sutherland GR. Epidemiology of childhood brain tumors. Can J Neurol Sci. 1996;23(2):118–122. [DOI] [PubMed] [Google Scholar]
- 58.Davis FG, Malinski N, Haenszel W, et al. Primary brain tumor incidence rates in four United States regions, 1985–1989: A pilot study. Neuroepidemiology. 1996;15(2):103–112. [DOI] [PubMed] [Google Scholar]
- 59.Porter KR, McCarthy BJ, Freels S, et al. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro Oncol. 2010;12(6):520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jazayeri S, Rahimi-Movaghar V, Shokraneh F, et al. Epidemiology of primary CNS tumors in Iran: a systematic review. Asian Pac J Cancer Prev. 2013;14(6):3979–3985. [DOI] [PubMed] [Google Scholar]
- 61.Fisher JL, Schwartzbaum J, Wrensch M, et al. Epidemiology of brain tumors. Neurol Clin. 2007;25(4):867–890. [DOI] [PubMed] [Google Scholar]
- 62.Wrensch M, Minn Y, Chew T, et al. Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro Oncol. 2002;4(4):278–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.