Abstract
Background
Dedicator of cytokinesis 1 (Dock1 or Dock180), a bipartite guanine nucleotide exchange factor for Rac1, plays critical roles in receptor tyrosine kinase–stimulated cancer growth and invasion. Dock180 activity is required in cell migration cancer tumorigenesis promoted by platelet derived growth factor receptor (PDGFR) and epidermal growth factor receptor.
Methods
To demonstrate whether PDGFRα promotes tumor malignant behavior through protein kinase A (PKA)–dependent serine phosphorylation of Dock180, we performed cell proliferation, viability, migration, immunoprecipitation, immunoblotting, colony formation, and in vivo tumorigenesis assays using established and short-term explant cultures of glioblastoma cell lines.
Results
Stimulation of PDGFRα results in phosphorylation of Dock180 at serine residue 1250 (S1250), whereas PKA inhibitors H-89 and KT5720 oppose this phosphorylation. S1250 locates within the Rac1-binding Dock homology region 2 domain of Dock180, and its phosphorylation activates Rac1, p-Akt, and phosphorylated extracellular signal-regulated kinase 1/2, while promoting cell migration, in vitro. By expressing RNA interference (RNAi)–resistant wild-type Dock180, but not mutant Dock180 S1250L, we were able to rescue PDGFRα-associated signaling and biological activities in cultured glioblastoma multiforme (GBM) cells that had been treated with RNAi for suppression of endogenous Dock180. In addition, expression of the same RNAi-resistant Dock180 rescued an invasive phenotype of GBM cells following intracranial engraftment in immunocompromised mice.
Conclusion
These data describe an important mechanism by which PDGFRα promotes glioma malignant phenotypes through PKA-dependent serine phosphorylation of Dock180, and the data thereby support targeting the PDGFRα-PKA-Dock180-Rac1 axis for treating GBM with molecular profiles indicating PDGFRα signaling dependency.
Keywords: Dock180, glioblastomas, PDGFRα, phosphorylation, PKA
Glioblastoma multiforme (GBM) is the most common and lethal primary brain cancer, with a median survival of 16 to 17 months.1,2 The Cancer Genome Atlas project, as applied to the analysis of patient GBM,3,4 has established that there are 3 classes of gene alterations that are common to nearly all of these tumors, one of which involves receptor tyrosine kinase (RTK) activation. One RTK encoding gene of importance is that for platelet derived growth factor receptor α (PDGFRα), which is altered in 13.1% of all GBM, with nearly half of these tumors harboring concurrent epidermal growth factor receptor (EGFR) alterations.3,4 RTKs have proven to be attractive targets for the treatment of a variety of human malignancies, but such targeting, including that directed at PDGFRα, as well as its downstream signaling mediators, including phosphatidylinositol-3 kinase (PI3K)–Akt,5–7 has proven largely unsuccessful in achieving improved outcomes for GBM patients, thereby underscoring the need for improved understanding of PDGFRα interactions, as well as RTK functional redundancies.
Small Rho GTPases, including Rac1, play key regulatory roles in normal development as well as in human disease, including cancer. Dedicator of cytokinesis 1 (Dock1 or Dock180), a guanine nucleotide exchange factor (GEF), activates Rac1 by binding to its partner, engulfment and cell motility protein 1 (ELMO1), which stimulates cell growth and motility in various types of cancer, including glioma.6,8–10 Dock180 contains binding domains of ELMO1, phosphatidylinositol 3,4,5-trisphosphate (Dock homology region 1 [DHR1]), Rac1 (DHR2), and CT10 regulator of kinase (CrK), respectively.11 We and others recently demonstrated that Dock180 activity is required in PDGFR- and EGFR-mediated border cell migration during Drosophila development,12 and serine phosphorylation of Dock180 is involved in EGFR- and PDGFRα-driven glioma tumorigenesis,6,8 as well as breast cancer progression driven by human epidermal growth factor receptor 2.13 Since serine phosphorylation plays a significant role in numerous cellular processes,14 and due to the association between RTK signaling and Dock180 activation, we were motivated to examine whether serine phosphorylation of Dock180 is a key modification in tumors whose molecular profile indicates a PDGFRα dependency.
Protein kinase 1 (PKA; also known as PKAC) is a cAMP-dependent classic serine phosphorylation kinase, important for development and diseases, including glioma.14 PKA is required in prostaglandin E2–stimulated glioma cell proliferation.15 Inhibition of PKA suppressed biotoxin cholera toxin-induced glioma cell differentiation.16 PKA activation is also important in the miR-33a–centered signaling network that promotes glioma-initiating cell growth and self-renewal.17 We recently showed that PKA participated in gliomagenesis driven by EGFR variant III.9 However, the critical roles of PKA with mechanisms remain to be fully investigated.
Here, we report that PKA phosphorylation of serine residue 1250 (p-S1250) of Dock180 is stimulated by PDGFRα in vitro and in vivo. Furthermore, replacement of this serine with leucine (Dock180S1250L) inhibits PDGFRα-stimulated Rac1 activation, glioma cell growth, survival, and invasion in vitro following intracranial engraftment of modified cells in athymic mice. Our results identify Dock180 as an important downstream effector of PDGFRα signaling in GBM, activation of which contributes to the highly malignant behavior of this cancer.
Materials and Methods
Cell Lines
Human embryonic kidney (HEK)293T cells were obtained from American Type Culture Collection. SNB19 and LN444 cells were gifts from Dr Y-H. Zhou at the University of California–Irvine and Dr E. Van Meir at Emory University, respectively. SNB19 and LN444 cell lines were also recently authenticated using short tandem repeat DNA fingerprinting by RADIL (Research Animal Diagnostic and Investigative Laboratory). All cells and primary human GBM cells were cultured and transfected as we previously described.6–9,18 LN444/platelet derived growth factor A (PDGF-A) and SNB19/PDGF-A cell lines that overexpress exogenous PDGF-A were characterized as previously described.6
Antibodies and Reagents
The following antibodies were used in this study: anti-Dock180 (H-4), anti-PDGFRα (C-20), anti–phospho-PDGFRα (Y754), and anti–β-actin (I-19) (Santa Cruz Biotechnology); anti-Rac1 antibody (BD Transduction Laboratories); anti-Flag M2 antibody (Sigma-Aldrich); anti–phospho-Akt (S473, #4051), anti-Akt (#9272), and anti–phospho-EGFR (Y1045, #2237) (Cell Signaling Technology); an anti-Ki67 antigen (NCL-Ki67p, Leica Microsystems); anti–phospho-PKA (Thr197) antigen (D45D3, Cell Signaling Technology); and anti-PKA and anti–pan-phosphoserine (19/pSer, BD Transduction Laboratories). The secondary antibodies were from Vector or Jackson ImmunoResearch Laboratories. Peroxidase blocking reagent was from Dako; AquaBlock was from East Coast Biologics. Various inhibitors were used in this study: GF109203X (Enzo Life Science) and AG1296, PD98059, Roscovitine, H-89, KT5720, and KN-93 (Sigma). Cell culture media and other reagents were from Invitrogen, Sigma-Aldrich, or Thermo Fisher Scientific.
Plasmids
A pcDNA3-EGFP-Rac1-WT (plasmid 12980) was purchased from Addgene.19 The pcDNA3-Flag-Dock180, pLVX-Flag-Dock180, mutant Del1, and Del2 plasmids were constructed and characterized as previously described.6,8,9 Dock180S1198L, Dock180S1250L, and Dock180S1627L point mutations were generated using the Site-Directed Mutagenesis Kit (Invitrogen) by following the manufacturer's protocol.6
Cell Proliferation, Cell Viability, Cell Migration, Immunoprecipitation, and Immunoblotting Assays
Cell proliferation analyses were performed using a WST-1 assay kit (Roche). Cell viability assays were performed using a kit for TUNEL (terminal deoxynucleotidyl transferase deoxyuridine triphosphate nick end labeling; Roche). Cell migration, Rac1 activation, immunoblotting (IB), and immunoprecipitation (IP) assays were performed as we previously described.6,8,9
Rac1 Activation Assay
Rac1 activation was measured using the Rac1 Activation Assay Kit (Millipore-Upstate) according to the manufacturer's instructions.10 Briefly, after treatment with various agonists with or without antagonists for the indicated times, cells were washed with phosphate buffered saline, lysed in ice-cold magnesium lysis buffer, and clarified by centrifugation at 12 000 g for 20 min. Each supernatant was incubated with PAK-1 protein-binding domain agarose beads, pelleted, washed, and resuspended in a Laemmli sample buffer. The samples were resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis on 12% polyacrylamide gels. Guanosine triphosphate (GTP)–bound Rac1 was detected using an anti-Rac1 antibody (BD Transduction Laboratories).
Tumorigenesis Studies
All experiments using animals were performed in accordance with a protocol approved by the Northwestern University Institutional Animal Care and Use Committee. Athymic (Ncr nu/nu) female mice at an age of 6–8 weeks (Taconic Farms) were used for all animal experiments. Various glioma cells (5 × 105 in 5 µL phosphate buffered saline) were stereotactically implanted into the brain of individual mice with 5 mice per group. The glioma-bearing mice were sacrificed between 7 and 8 weeks post-implantation. The brains were removed, processed, and analyzed as we previously described.6,8
Colony Formation Assay
Soft agar colony formation assay was performed as we previously described.7
Statistical Analysis
We used GraphPad Prism version 5.00 software for Windows to do a One-way ANOVA with a Newman–Keuls posttest or a paired two-way Student's t-test as we previously described.20 P < .05 was considered significant.
Results
PDGFRα Stimulates Serine Phosphorylation of Dock180 in Glioma Cells
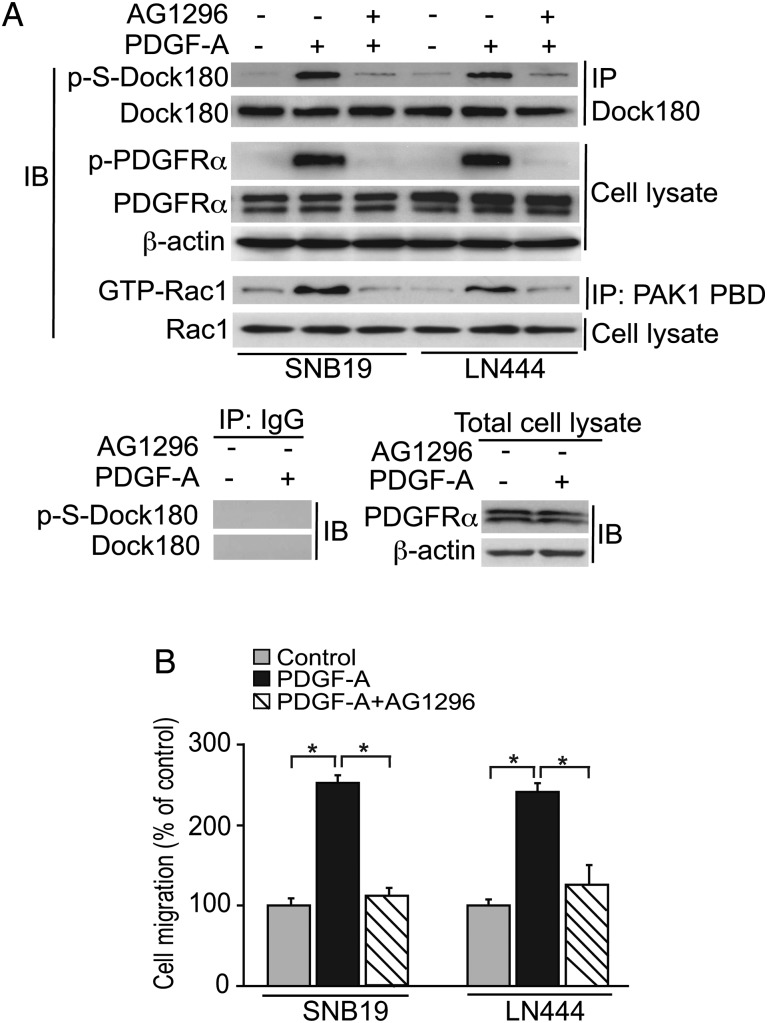
Serine phosphorylation (p-S) of Dock180 has previously been shown to be important for integrin signaling.21 We recently described that EGFR-induced p-S of Dock180 is linked with RTK-stimulated glioma malignant phenotypes.6,8,9 We hypothesized that p-S of Dock180 could also be caused by PDGFRα-initiated signal transduction. To begin addressing this hypothesis, we performed IP of endogenous Dock180 in glioma cells, followed by IB with a pan anti–p-S antibody, working with cell samples (SNB19 and LN444) incubated in the presence or absence of PDGF-A. We found that PDGF-A activated PDGFRα-stimulated p-S of Dock180 and activated Rac1 in these cell lines (Fig. 1A). Furthermore, these molecular events were associated with significantly increased tumor cell invasion, as indicated by results from the use of a modified Boyden chamber assay6,7 (Fig. 1B). To confirm that p-S of Dock180 is dependent on PDGFR activation, serum-starved SNB19 and LN444 cells were treated with or without AG1296, a selective inhibitor of PDGFRα and PDGFRβ,22 for 1 h in advance of 5-min treatments with PDGF-A. AG1296 inhibited PDGFRα autophosphorylation, p-S of Dock180, and Rac1 activation, while significantly reducing PDGF-A–stimulated tumor cell invasion (Fig. 1B).
Fig. 1.
PDGF-A stimulates p-S of Dock180 and promotes glioma cell migration. (A) SNB19 and LN444 cells were serum starved for 24 h and treated with or without PDGF-A and with or without PDGFR inhibitor AG1296 (10 µM), with inhibitor treatments for 1 h prior to 5 min PDGF-A (20 ng/mL) treatment. Rac1 activation was assessed using a Rac1 activation assay kit. IgG was used as a control. Dock180, PDGFRα, β-actin, and Rac1 were used as loading controls. (B) In vitro cell migration assays over a 16-h period using cells subjected to treatments described in (A). Results from PDGF-A and AG1296 treatments are presented in relation to control sample results (6 replicates per treatment per cell line); bars, SD. *P < .05, one-way ANOVA followed by Newman–Keuls post hoc test. The results in (A) and (B) are representative of 3 independent experiments with similar results.
PDGFRα-Stimulated Serine Phosphorylation of Dock180 Depends on Protein Kinase A
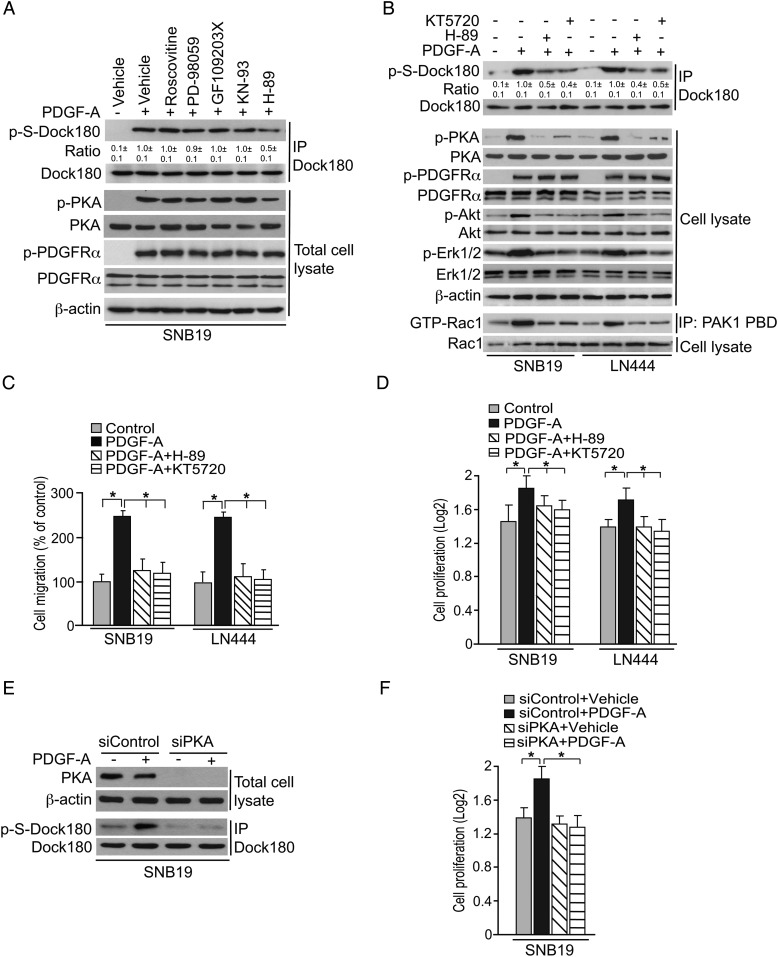
We recently showed that EGFR-stimulated gliomagenesis involves PKA-dependent p-S of Dock180.9 To determine whether this PKA dependency of Dock180 p-S is also involved with PDGFRα signaling, we examined the effects of inhibitors for various known serine/threonine kinases, on PDGF-A–initiated signaling in our glioma cell lines. As shown in Fig. 2A, treatment with H-89, an inhibitor of PKA, inhibited PDGF-A–stimulated p-S of Dock180. In contrast, GF109203X, Roscovitine, KN-93, and PD-98059, inhibitors of protein kinase C, cyclin-dependent kinase 5, calmodulin, and mitogen-activated protein kinase kinase (MEK), respectively, had little or no effect on Dock180 p-S. To further confirm PKA as an intermediate of PDGF-A–induced Dock180 p-S, as well as to address PKA inhibitor effects on Rac1, a downstream effector of Dock180, we treated SNB19 and LN444 cells separately with or without H-89 or a second PKA inhibitor, KT5720.9 As shown in Fig. 2B, treatment with either PKA inhibitor markedly attenuated PDGF-A stimulation of Dock180 p-S, as well as Rac1 activation, and additionally inhibited Akt and extracellular signal-regulated kinase (ERK)1/2 phosphorylation. Moreover, each inhibitor prevented PDGF-A from stimulating glioma cell invasion (Fig. 2C) as well as cell proliferation (Fig. 2D). To further support this result, we knocked down the endogenous PKA using short interfering RNAs (siRNAs) (Fig. 2E). Compared with the control, depletion of PKA inhibited p-S of Dock180 and cell proliferation stimulated by PDGF-A in SNB19 cells (Fig. 2E and F).
Fig. 2.
PDGF-A–stimulated p-S of Dock180 is PKA dependent. (A) IP and IB analysis of serine/threonine kinase inhibitor effects on PDGF-A–induced p-S of Dock180. Serum-starved SNB19 cells were pretreated with or without PKC inhibitor GF109203X (10 µM), Cdk5 inhibitor Roscovitine (20 µM), calmodulin-dependent kinase inhibitor KN-93 (250 µM), MEK inhibitor PD-98059 (50 µM), PKA inhibitor H-89 (20 µM), or vehicle (0.1% dimethyl sulfoxide) for 1 h. Cells were then treated with PDGF-A (50 ng/mL) or vehicle for 5 min. Total Dock180, PKA, PDGFRα, and β-actin were used as loading controls. Intensity ratios of p-S of Dock180 to total Dock180 were calculated using NIH ImageJ software, and are indicated beneath their corresponding p-S IB results. (B) IP and IB analysis of PKA inhibitor effects on PDGF-A–stimulated p-S of Dock180, p-Akt, p-ERK1/2, p-PDGFRα, and Rac1 activity. SNB19 and LN444 cells were serum starved for 24 h then treated with or without PKA inhibitors KT5720 (10 µM), H-89 (25 µM), or vehicle, for 1 h. Cells were then stimulated with PDGF-A (50 ng/mL) or vehicle for 5 min. Dock180, PKA, PDGFRα, β-actin, Akt, ERK1/2, and Rac1 were used as loading controls. Intensity ratios of p-S Dock180 to total Dock180 were calculated as described in (A). PBD, protein-binding domain. (C) In vitro Boyden chamber cell invasion assay results, using cells treated as described in (B). PKA inhibitor treatment results are presented in relation to PDGF-A treated sample. (D) A total of 4000 cells, subjected to treatments as indicated in (B), were seeded in 96-well plates with Dulbecco's modified Eagle's medium containing PDGF-A (20 ng/mL), together with PKA inhibitor KT5720 (10 µM), H-89 (25 µM), or vehicle for 3 days. Cell proliferation effects were determined by 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Results have been normalized to the mean MTT values of untreated cells at day 0. (E) IP and IB analysis of effect of PKA knockdown on PDGF-A–induced p-S of Dock180. A PKA siRNA pool (siPKA) or a control siRNA (siControl) was transiently transfected into SNB19 cells. Dock180 and β-actin were used as loading controls. (F) Cell proliferation assay as shown in (D). Cells were come from (E). Data in (A)–(F) are representative of 3 independent experiments with similar results.
PKA-Dependent Serine Phosphorylation of Dock180 Is Important for Cell Migration and Colony Formation of GBM Xenograft Explant Cultures
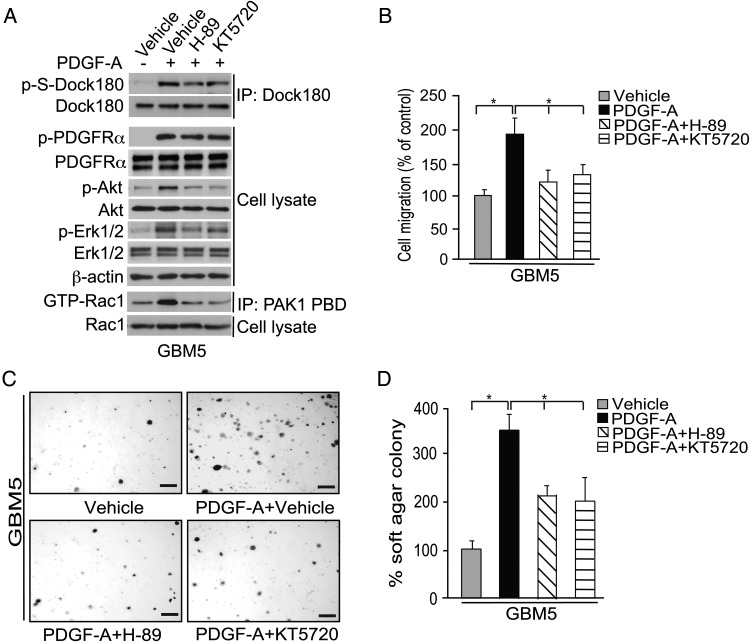
Next, we examined whether PKA function is important for GBM cell migration, using short-term explant cultures from GBM5 xenografts, which retain high-level expression of endogenous PDGFRα.6 As was the case with the established cell lines (Fig. 2), stimulation of GBM5 cells with PDGF-A markedly induced phosphorylation of PDGFRα, Dock180, Akt, and ERK1/2 and heightened Rac1 activity (Fig. 3A). In addition, PDGF-A treatment of GBM5 cells stimulated their migration (Fig. 3B) and increased their ability to form colonies in soft agar (Fig. 3C and D). Use of the same PKA inhibitors as before, H-89 and KT5720, caused appreciable decreases in PDGF-A–stimulated signal transduction, cell migration, and colony formation for GBM5 cells (Fig. 3A–D).
Fig. 3.
PKA is required for PDGFRα-associated GBM cell migration and colony formation. (A) IP and IB of effect of PKA inhibitors on p-S of Dock180, p-Akt, p-ERK1/2, p-PDGFRα, and Rac1 activity, following PDGF-A treatment of explant cultures from GBM5 xenografts. GBM5 cells were serum starved for 24 h then treated with KT5720 (10 µM), H-89 (25 µM), or vehicle for 1 h. Cells were then stimulated with PDGF-A (50 ng/mL) or vehicle for 5 min. Total Dock180, PDGFRα, Akt, ERK1/2, β-actin, and Rac1 were used as loading controls. (B) In vitro cell migration assay results, using cells treated as described in (A). Data are presented as percentage of treated cells compared with controls, from 6 replicates per cell line and per treatment. Bars, SD; *P < .05, one-way ANOVA followed by Newman–Keuls post hoc test. (C) Representative images of colony formation results. Four thousand GBM5 cells were seeded on soft agar with or without PDGF-A (20 ng/mL) and with or without KT5720 (10 µM), H-89 (25 µM), or vehicle for 7 days. Scale bars, 1 mm. (D) Quantification of colony formation results. Bars, SD; *P < .05, one-way ANOVA followed by Newman–Keuls post hoc test. Results shown in (A) to (D) are representative of results from 3 independent experiments.
PDGFRα Signaling Results in Phosphorylation of Serine 1250 of Dock180
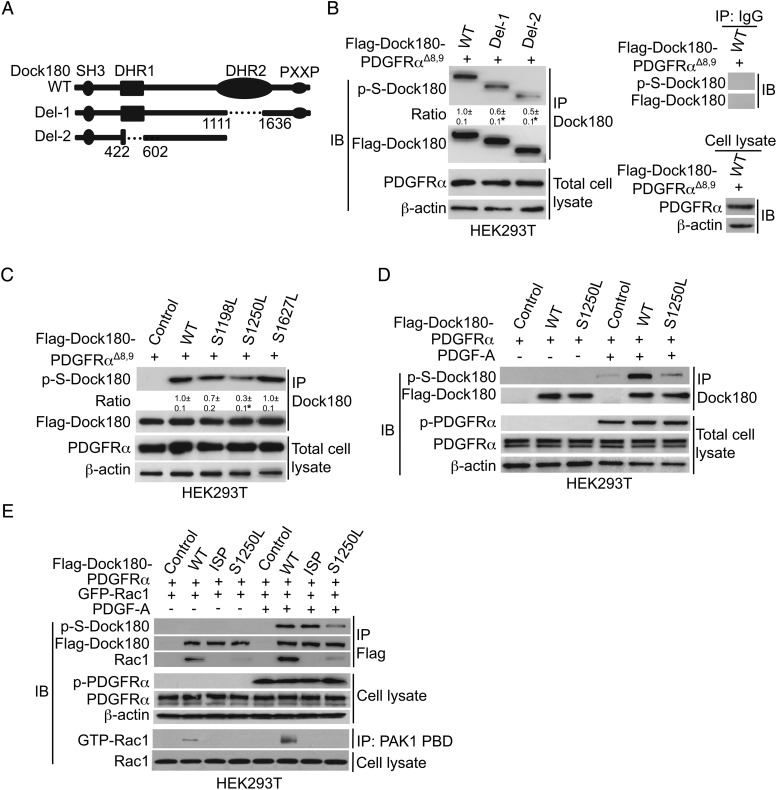
We next directed our investigation toward identifying the Dock180 serine residue(s) that are phosphorylated in association with PDGF-A–initiated signaling. To identify the putative phosphorylated serine(s) of Dock180, we constructed 2 Flag-tagged Dock180 deletion mutants, Del1 (deletion of amino acid [AA] residues 1111 and 1636 in the DHR2 domain) and Del2 (deletion of AA 422 to 602 in the DHR1 domain and C-terminus including the CrK domain), and additionally constructed Flag-tagged wild-type (WT) Dock180 (Dock180WT; Fig. 4A). Each Dock180 construct was cotransfected with PDGFRαΔ8,9, a glioma-derived and constitutively active PDGFRα mutant6 in HEK293T cells. As shown in Fig. 4B, coexpression of PDGFRαΔ8,9 with WT Dock180 promoted Dock180 p-S, in relation to Dock180 p-S in Del1 or Del2 mutants (Fig. 4B). This result suggests that there are p-S sites of Dock180 located in the DHR1 domain between AA 442 and 602 and in DHR2 between AA 1111 and 1636 and/or C-terminus (Fig. 4A). Since the DHR1 domain and C-terminus lack the docking site for Rac1, while the DHR2 domain interacts with Rac1, resulting in Rac1 activation,11 we focused our attention on the DHR2 domain (AA 1111–1636). Specifically, we chose S1250, which we previously found to be phosphorylated in association with PKA activation downstream of EGFR-initiated signaling,9 and 2 serine residues, S1198 and S1627, which are in the DHR2 region and next to S1250. Thus, we coexpressed each of 3 Flag-tagged Dock180 leucine-for-serine substitution mutants, Dock180S1250L, Dock180S1198L, and Dock180S1627L, with constitutively active PDGFRαΔ8,9 in HEK293T cells. We found that the cells expressing Dock180S1250L showed markedly decreased PDGFRαΔ8,9-induced p-S, whereas minimal reduction of p-S was evident when coexpressing Dock180S1198L or Dock180S1627L with activated PDGFRαΔ8,9 (Fig. 4C). We further confirmed this observation by coexpression of Dock180WT and Dock180S1250L with PDGFRαWT in HEK293T cells that were treated with PDGF-A. As shown in Fig. 4D, PDGF-A treatment of the transduced 293T cells stimulated significant phosphorylation of PDGFRα and p-S of Dock180WT, whereas a much lesser p-S increase was apparent for cells expressing p-Dock180S1250L.
Fig. 4.
Dock180S1250 is a major PKA p-S site following PDGFRα stimulation. (A) Schematics of wild-type and Dock180 mutant Flag-tagged constructs. (B) IP and IB for the effect of Dock180 deletions on p-S of Dock180 in HEK293T cells. Flag-tagged Dock180 WT or mutants Del1 or Del2 were transiently transfected together with or without constitutively active PDGFRαΔ8,9 into HEK293T cells. After 48 h, serum-starved cells were lysed and then analyzed by IP and IB. Flag-Dock180, total PDGFRα, and β-actin were used as loading controls. IgG was used as a control for IP. (C) IP and IB for effect of Dock180 serine substitution mutants on p-S of Dock180 in HEK293T cells. Flag-tagged Dock180 WT or mutants S1198L, S1250L, or S1627L were transiently transfected with constitutively activated PDGFRαΔ8,9 into HEK293T cells. Flag-Dock180, PDGFRα, and β-actin are loading controls. (D) IP and IB for effect of Dock180 S1250L mutation on p-S of Dock180 in PDGF-A–treated HEK293T cells. Flag-tagged Dock180WT, Dock180S1250L mutant, or vector control was transiently transfected with WT PDGFRα into HEK293T cells. After 48 h, serum-starved cells were stimulated with or without PDGF-A for 5 min and then analyzed by IP and IB. Flag-Dock180, PDGFRα, and β-actin are loading controls. (E) PKA-dependent p-S1250 of Dock180 enhances Dock180 association with Rac1; cDNAs of Flag-tagged Dock180WT, Dock180ISP mutant, Dock180S1250L mutant, or empty vector control was cotransfected with cDNAs for PDGFRα and GFP-Rac1 into HEK293T cells. After 48 h, serum-starved cells were stimulated with or without PDGF-A for 5 min and then lysed in the presence of 10 mM EDTA followed by IP and IB. Flag-Dock180, total PDGFRα, Rac1, and β-actin were used as loading controls. Data in (B) to (E) are representative of results from 3 independent experiments with similar results.
We next analyzed the effect of p-Dock180S1250 on Rac1 interaction. For this analysis, another Dock180 mutant, in which Ile-Ser-Pro (ISP) residues at AA 1487–1489 had been replaced with Ala-Ala-Ala (Dock180ISP), was used as a control because this substitution of 3 amino acids prevented Dock180–Rac1 interaction.23 This mutant (Dock180ISP) as well as Dock180WT, Dock180S1250L, and empty vector were transiently transfected into HEK293T cells with PDGFRα and green fluorescent protein–Rac1, with transfectant cells subsequently serum starved then stimulated with PDGF-A. Flag-tagged Dock180 proteins were then immunoprecipitated and analyzed for Rac1 activity and Dock180 p-S. As shown in Fig. 4E, PDGF-A treatment promoted p-S of Dock180WT and Rac1 activity, as well as Dock180WT association with Rac1. In contrast, PDGF-A treatment resulted in a lesser increase in p-S of Dock180S1250L and substantially less Rac1 activity and Dock180ISP interaction with Rac1 (Fig. 4E). As expected, Dock180ISP showed no effect on activation of Rac1 and was incapable of interacting with Rac1, despite a PDGF-A–stimulated increase in p-S of this mutant. Taken together, these results indicate that PKA-dependent phosphorylation of S1250 is important for the activation of Rac1 in PDGF-A–stimulated glioma cells.
Phosphorylation of serine residue of Dock180S1250 is important for PDGFRα-driven glioma cell proliferation, colony formation, survival, and migration in vitro.
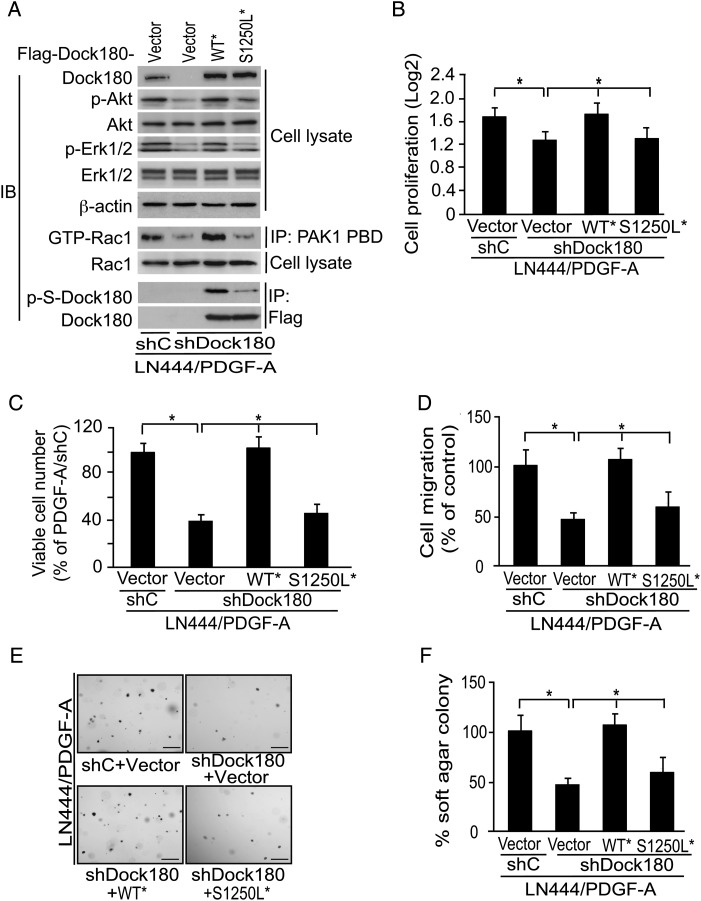
To examine the importance of Dock180 p-S1250 for linking PDGFRα activation with glioma cell biologic activities, we performed the following. First, we stably expressed small hairpin (sh)RNA–resistant Dock180WT* or Dock180S1250L* in LN444 cells that had been modified for expression of exogenous PDGF-A and for expression of shRNA to suppress endogenous Dock180 (shDock180). The expression of exogenous Dock180WT* or Dock180S1250L* proteins were at levels comparable to that of endogenous Dock180 in LN444/PDGF-A cells modified with control shRNA (shC in Fig. 5A). Anti–p-S IB analysis revealed a higher level of Dock180 p-S in Dock180WT* than in Dock180S1250L* cells. Moreover, Dock180WT*, but not Dock180S1250L*, rescued PDGF-A–stimulated p-Akt, p-ERK1/2, and Rac1 activity in LN444/PDGF-A/shDock180 cells with shRNA depleted endogenous Dock180 expression (Fig. 5A). Expression of Dock180WT*, but not Dock180S1250L*, also rescued PDGF-A–stimulated cell growth, viability, migration, and colony formation of LN444/PDGF-A/shDock180 cells (Fig. 5B–E). In consideration of our previous findings that ablation of the PI3K-binding site of PDGFRα or inhibition of Akt or ERK1/2 attenuated PDGF-A stimulation of glioma cell proliferation and survival in vitro and tumor growth in vivo,7 our results support that p-S1250 of Dock180 promotes glioma cell migration and proliferation via Rac1-Akt and -ERK1/2 signaling.
Fig. 5.
Dock180 S1250 is important for PDGFRα-stimulated glioma cell migration and survival in vitro. (A) Retroviral encoded shRNA-resistant Dock180WT*, Dock180S1250L*, or empty vector was used to transduce LN444/PDGF-A/shC or LN444/PDGF-A/shDock180 cells. Infected cells were subjected to IP and IB analysis for effects of Dock180WT* or Dock180S1250L* expression on PDGFRα-initiated intracellular signaling. Flag-Dock180, total ERK1/2, total Akt, total Rac1, and β-actin were used as loading controls. (B) MTT assays of cell proliferation using the cells treated as described in (A). (C) Serum-starved cells were seeded in 8-well chamber slides with Dulbecco's modified Eagle's medium plus 0.5% fetal bovine serum. After 48 h, cell apoptosis was determined by TUNEL analysis of 1000 cells/treatment. (D) Boyden chamber cell migration assay results using cells treated as described in (A). Data are presented as the percentage of migrated cells in relation to vector-only control cells from 6 replicates per modified cell line. (E) Images from colony formation assay experiment. LN444/PDGF-A cells modified or expression of the indicated shRNA and Dock180 constructs were seeded on soft agar for 7 days in triplicates. Scale bars, 1 mm. (F) Quantifications of colony formation assay results. In (B), (C), (D), and (F), bars, SD; *P < .05, one-way ANOVA followed by Newman–Keuls post hoc test. Data shown in in (A) to (F) are representative of results from 3 independent experiments.
PDGFRα-Driven Phosphorylation of Dock180S1250 Is Required for Glioma Cell Growth and Invasion in the Brain
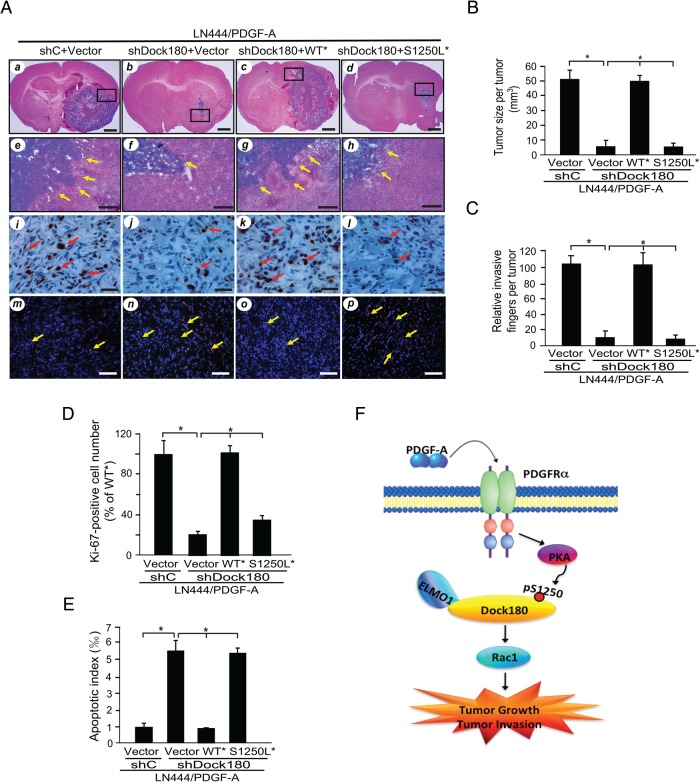
To address the importance of Dock180S1250 p-S in PDGFRα-associated glioma tumorigenesis and invasion in vivo, we implanted LN444/PDGF-A cells transduced with sh vector control (shC), shDock180, and either Dock180WT* or Dock180S1250L* into the brains of mice. As shown in Fig. 6A,6,7 mice that received LN444/PDGF-A modified with shC developed large and highly invasive gliomas at 60 days post-implantation (Fig. 6A, panels a, e, Fig. 6B and C), whereas tumor growth and invasion were substantially inhibited in LN444/PDGF-A intracranial gliomas modified with shDock180 (Fig. 6A, panels b, f, Fig. 6B and C). The expression of Dock180WT* (Fig. 6A, panels c, g, Fig. 6B and 6c), but not Dock180S1250L* (Fig. 6A, panels d, h, Fig. 6B and 6c), in LN444/PDGF-A/shDock180 cells rescued tumor growth and invasion, as well as tumor proliferation and apoptotic resistance (Fig. 6A, panels i, m, k, o). Rescue of these phenotypes was not evident in cells modified for expression of Dock180S1250L* (see Fig. 6A, panels j, n, l, and p).
Fig. 6.
Dock180 S1250 is critical for PDGFRα-driven glioma tumor growth and invasion in vivo. (A) Effect of expressing shRNA-resistant Dock180WT* or Dock180S1250L* on glioma growth, invasion, proliferation, and apoptosis in vivo. Representative hematoxylin/eosin (H&E) and immunohistochemistry images of brain sections from mice receiving intracranial injection with LN444/PDGF-A glioma cells modified with the indicated shRNA and Dock180 expression constructs, at 7 to 8 weeks post-injection. Panels a, e, i , and m, LN444/PDGF-A shC tumors; b, f, j, and n, shDock180 + vector tumors; c, g, h, and o, shDock180 + Dock180WT* tumors; d, h, i, and p, shDock180 + Dock180S1250L* tumors. H&E staining, panels a to h. Panels e to h are enlarged areas from a to d that are marked with squares, with arrows indicating invasive tumor cells. Panels i to l, Ki67 staining. Panels m to p, TUNEL staining; in i to l and in m to p, Ki67 and TUNEL positive cells, respectively, are indicated by arrows. Scale bars in a to d, 1 mm, in e to h, 200 µm, in i to l, 50 µm, and in m to p, 100 µm. (B and C) Quantification of tumor size and relative invasive fingers per tumor. (B) Tumor volumes were determined by microscopically measuring tumor areas followed by estimation of the tumor volume as an oval-shaped sphere. (C) Relative invasive figures were estimated microscopically by counting protruded tumor tissue figures and disseminated areas as shown in panels a and e. (D and E) Quantifications of Ki67 and TUNEL staining, respectively. (F) A working model for the PDGFRα-PKA-p-Dock180S1250-Rac1 signaling in glioma cells. PDGF-A activation of PDGFRα induces PKA phosphorylation of Dock180 at S1250 residue and then promotes Rac1 activation, resulting in increased glioma growth and invasion. In (B) to (E), results are based on 5 tumors per group from 2 independent experiments. Bars, SD. *P < .05, one-way ANOVA followed by Newman–Keuls post hoc test. Data in (A) to (E) represent 2 independent experiments with similar results.
Discussion
In this study, we report that PDGFRα stimulates p-S of Dock180 in glioma cells, which is associated with increased glioma cell proliferation and migration in vitro and in vivo. Through the use of pharmacologic approaches, we interpret p-S of Dock180 as being primarily mediated by PKA in PDGF-A–stimulated glioma cells.
PKA is a member of the AGC family, a subgroup of Ser/Thr protein kinases that are most related to PKA, cyclic guanosine monophosphate–dependent protein kinase (PKG; also known as CGK1α), and protein kinase C (PKC). PKA is essential for various cell biologic properties, including proliferation and migration, and influences these activities through phosphorylation of GEFs for Rac1, Cdc42, and Rho, following signal initiation by RTKs, including PDGFRα.14 In PDGF-induced membrane ruffling that is critical for cell movement, PKA regulates dynamics of PIP3 (phosphatidylinositol (3,4,5)-triphosphate) and Rac1 activation,24 and the inhibition of PKA activity markedly attenuates PDGF-induced membrane ruffling and Rac activation. The expression of constitutively active Rac1 rescues membrane ruffling in PKA-inhibited cells, even in the absence of PI3K signaling.24 Our results, showing PKA as an intermediate in PDGFRα-initiated signal transduction, resulting in activation of the Rac1 GEF, Dock180, and attendant stimulation of cell motility, are consistent with these studies. Through use of a pharmacologic approach, specifically by treating tumor cells with H-89 and KT5720 inhibitors, as well as by employing a genetic approach in which we modified tumor cells for expression of exogenous Dock180S1250L mutant, we have demonstrated a signaling cascade involving PDGFRα, PKA, Dock180, and Rac1, which promotes GBM cell migration and cell proliferation in vitro, as well as GBM xenograft growth and invasion in the brains of immunocompromised mice. Our results, therefore, demonstrate the importance of PKA-dependent protein phosphorylation of GEFs in RTK-driven GBM growth and invasion.
In this study, we show that S1250, of Dock180, is a major phosphorylation site of PKA in PDGFRα-stimulated glioma cells. This phosphorylation site is located in a DHR2 domain that promotes Rac1-GTP and Rac1–guanosine diphosphate exchange during Rac1 activation,11,25 which, in turn, is linked with key glioma cell biologic properties, including proliferation and invasion. Expression of shRNA-resistant Dock180WT*, but not Dock180S1250L*, rescued PDGFRα-stimulated glioma growth and proliferation invasion in the brain (Fig. 6). Of note, both S1250 and ISP are localized in the DHR2 domain and are important for Rac1 binding and Rac-GTP loading.9 Although Dock180-ISP did not affect the p-S1250 of Dock180, Dock180-S1250L may function similarly to Dock180-ISP on impairing the interaction of DHR2 with Rac1.23 Alternatively, p-S1250 of Dock180 is required for Dock180 to acquire or maintain an active conformation as described for Vav1.26 Moreover, further investigation is warranted to test this hypothesis. Taken together, our results indicate that PKA-mediated phosphorylation of Dock180S1250 plays a critical role in PDGFRα-driven gliomagenesis.
Although both ERK1/2 and Akt are the targets of Rac1, the impacts of Rac1 inhibition on ERK1/2 and Akt activations are different in different types of cells. In vascular endothelial cells, knockdown of Rac1 inhibited cell migration and proliferation through suppression of sphingosine-1 phosphate activation of PI3K/Akt.27 In lung epithelial cells, a dominant negative (DN) Rac1 inhibited cell migration through attenuation of thrombin activation of PI3K/Akt.28 In glioma cells, a DN Rac1 induced cell apoptosis through inhibition of ERK1/2 but not Akt activities.29 We showed that inhibition of PI3K by LY294002 (thereby inhibiting the induced p-Akt) and inhibition of MEK by PD98059 (thereby inhibiting the induced p-ERK1/2) abrogated PDGF-A–stimulated cell growth and survival of glioma cells.7 In glioma SNB19, LN444, and primary GBM5 cells that express endogenous PDGFRα and Dock180, inhibition of Dock180 suppressed PDGF-A activation of Akt and ERK1/2 and cell migration, survival, and proliferation.6 Here, we show that Dock180-S1250L mutant inhibits PDGFRα stimulation of Akt and ERK1/2, thereby collaborating with the role of p-Akt and p-ERK1/2 in mediating PDGFRα-Dock180-Rac1 stimulation of glioma tumorigenesis.
Intratumoral heterogeneity of GBM has been implicated in tumor growth and progression, as well as GBM resistance to therapy.30,31 GBM is often driven by aberrant activation of RTK signaling, with 50% of GBM tumors showing amplification of an RTK.4,5,32,33 Furthermore, using multicolor fluorescence in situ hybridization, as many as 3 different RTKs (EGFR, MET, PDGFRA) were found amplified in a single GBM tumor, and in some cases EGFR and PDGFRA are found amplified in the same tumor cells, which potentially accounts for the relative lack of success for treatments targeting single RTKs; that is, the inhibition of one RTK can potentially be compensated for by the increased activity of another RTK.31,33 Recently we reported that Src-dependent tyrosine phosphorylation of Dock180 is also important to EGFR- and PDGFRα-stimulated GBM growth and invasion in humans and in mice.6,8 Collectively, our studies highlight Dock180 phosphorylation, by Src and PKA, as essential downstream activities of RTK-driven glioma tumorigenesis.31,34 Moreover, our results suggest that therapeutic targeting of the p-Dock180–Rac1 interaction could benefit GBM patients, as well as possibly patients with other types of cancer for which aberrant activation of PDGFRα signaling has been implicated.
Funding
This work was supported in part by NIH grants CA130966 and CA158911; a Zell Scholar Award from the Zell Family Foundation and funds from the Northwestern Brain Tumor Institute and Department of Neurology at Northwestern University Feinberg School of Medicine to S-Y.C.; a Brain Cancer Research Award from the James S. McDonnell Foundation to B.H. Support to H.F. was from the National Natural Science Foundation of China (no. 81372704), the Program for Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, Innovation Program of Shanghai Municipal Education Commission (no. 14ZZ111), and the State Key Laboratory of Oncogenes and Related Genes in China (no. 90-14-04). Support to Y.L. was from the National Natural Science Foundation of China (nos. 81470315 and 81330015), the Pujiang Talent Plan of Shanghai City, China (no. 14PJ1406500), Natural Science Foundation of Tianjin City, China (no. 13JCYBJC39400). Support to W-Q.G. was from the Chinese Ministry of Science and Technology (2012CB966800), the National Natural Science Foundation of China (81130038 and 81372189), Key Discipline and Specialty Foundation of Shanghai Health Bureau, and the KC Wong Foundation. Support to J.N.S. was from the Mayo Brain Tumor SPORE (CA108961) and Mayo Clinic. Support to C.D.J. was from NIH grants NS080619 and CA159467. Support to A.T.P. was from the Michael J. Marchese Endowed Chair in Neurological Surgery at Northwestern University.
Acknowledgments
The authors thank M. Matsuda, K. Vuori, R. Tsien, E. Van Meir, and Y. Zhou for providing reagents, and Angel Alvarez for proofreading of this manuscript.
Conflict of interest statement. All authors declare no conflict of interest.
References
- 1.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–722. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Network CGAR. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennan CW, Verhaak RG, McKenna A, et al. The somatic genomic landscape of glioblastoma. Cell. 2013;155(2):462–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21(21):2683–2710. [DOI] [PubMed] [Google Scholar]
- 6.Feng H, Hu B, Liu KW, et al. Activation of Rac1 by Src-dependent phosphorylation of Dock180Y1811 mediates PDGFRalpha-stimulated glioma tumorigenesis in mice and humans. J Clin Invest. 2011;121(12):4670–4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu KW, Feng H, Bachoo R, et al. SHP-2/PTPN11 mediates gliomagenesis driven by PDGFRA and INK4A/ARF aberrations in mice and humans. J Clin Invest. 2011;121(3):905–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng H, Hu B, Jarzynka MJ, et al. Phosphorylation of dedicator of cytokinesis 1 (Dock180) at tyrosine residue Y722 by Src family kinases mediates EGFRvIII-driven glioblastoma tumorigenesis. Proc Natl Acad Sci U S A. 2012;109(8):3018–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng H, Hu B, Vuori K, et al. EGFRvIII stimulates glioma growth and invasion through PKA-dependent serine phosphorylation of Dock180. Oncogene. 2013;33(19):2504–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarzynka MJ, Hu B, Hui KM, et al. ELMO1 and Dock180, a bipartite Rac1 guanine nucleotide exchange factor, promote human glioma cell invasion. Cancer Res. 2007;67(15):7203–7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurin M, Cote JF. Insights into the biological functions of Dock family guanine nucleotide exchange factors. Genes Dev. 2014;28(6):533–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bianco A, Poukkula M, Cliffe A, et al. Two distinct modes of guidance signalling during collective migration of border cells. Nature. 2007;448(7151):362–365. [DOI] [PubMed] [Google Scholar]
- 13.Laurin M, Huber J, Pelletier A, et al. Rac-specific guanine nucleotide exchange factor DOCK1 is a critical regulator of HER2-mediated breast cancer metastasis. Proc Natl Acad Sci U S A. 2013;110(18):7434–7439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010;11(1):9–22. [DOI] [PubMed] [Google Scholar]
- 15.Payner T, Leaver HA, Knapp B, et al. Microsomal prostaglandin E synthase-1 regulates human glioma cell growth via prostaglandin E(2)-dependent activation of type II protein kinase A. Mol Cancer Ther. 2006;5(7):1817–1826. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Yin W, Wang X, et al. Cholera toxin induces malignant glioma cell differentiation via the PKA/CREB pathway. Proc Natl Acad Sci U S A. 2007;104(33):13438–13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang H, Sun T, Hu J, et al. MiR-33a promotes glioma-initiating cell self-renewal via PKA and NOTCH pathways. J Clin Invest. 2014;124(10):4489–4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng H, Liu KW, Guo P, et al. Dynamin 2 mediates PDGFRalpha-SHP-2-promoted glioblastoma growth and invasion. Oncogene. 2012;31(21):2691–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraynov VS, Chamberlain C, Bokoch GM, et al. Localized Rac activation dynamics visualized in living cells. Science. 2000;290(5490):333–337. [DOI] [PubMed] [Google Scholar]
- 20.Yiin JJ, Hu B, Schornack PA, et al. ZD6474, a multitargeted inhibitor for receptor tyrosine kinases, suppresses growth of gliomas expressing an epidermal growth factor receptor mutant, EGFRvIII, in the brain. Mol Cancer Ther. 2010;9(4):929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiyokawa E, Hashimoto Y, Kurata T, et al. Evidence that DOCK180 up-regulates signals from the CrkII-p130(Cas) complex. J Biol Chem. 1998;273(38):24479–24484. [DOI] [PubMed] [Google Scholar]
- 22.Kovalenko M, Gazit A, Bohmer A, et al. Selective platelet-derived growth factor receptor kinase blockers reverse sis-transformation. Cancer Res. 1994;54(23):6106–6114. [PubMed] [Google Scholar]
- 23.Brugnera E, Haney L, Grimsley C, et al. Unconventional Rac-GEF activity is mediated through the Dock180-ELMO complex. Nat Cell Biol. 2002;4(8):574–582. [DOI] [PubMed] [Google Scholar]
- 24.Deming PB, Campbell SL, Baldor LC, et al. Protein kinase A regulates 3-phosphatidylinositide dynamics during platelet-derived growth factor-induced membrane ruffling and chemotaxis. J Biol Chem. 2008;283(50):35199–35211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cote JF, Motoyama AB, Bush JA, et al. A novel and evolutionarily conserved PtdIns(3,4,5)P3-binding domain is necessary for DOCK180 signalling. Nat Cell Biol. 2005;7(8):797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nat Rev Mol Cell Biol. 2005;6(2):167–180. [DOI] [PubMed] [Google Scholar]
- 27.Gonzalez E, Kou R, Michel T. Rac1 modulates sphingosine 1-phosphate-mediated activation of phosphoinositide 3-kinase/Akt signaling pathways in vascular endothelial cells. J Biol Chem. 2006;281(6):3210–3216. [DOI] [PubMed] [Google Scholar]
- 28.Lin CH, Cheng HW, Ma HP, et al. Thrombin induces NF-kappaB activation and IL-8/CXCL8 expression in lung epithelial cells by a Rac1-dependent PI3 K/Akt pathway. J Biol Chem. 2011;286(12):10483–10494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Senger DL, Tudan C, Guiot MC, et al. Suppression of Rac activity induces apoptosis of human glioma cells but not normal human astrocytes. Cancer Res. 2002;62(7):2131–2140. [PubMed] [Google Scholar]
- 30.Sottoriva A, Spiteri I, Piccirillo SG, et al. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci U S A. 2013;110(10):4009–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snuderl M, Fazlollahi L, Le LP, et al. Mosaic amplification of multiple receptor tyrosine kinase genes in glioblastoma. Cancer Cell. 2011;20(6):810–817. [DOI] [PubMed] [Google Scholar]
- 32.Liu KW, Hu B, Cheng SY. Platelet-derived growth factor signaling in human malignancies. Chin J Cancer. 2011;30(9):581–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wykosky J, Fenton T, Furnari F, et al. Therapeutic targeting of epidermal growth factor receptor in human cancer: successes and limitations. Chin J Cancer. 2011;30(1):5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szerlip NJ, Pedraza A, Chakravarty D, et al. Intratumoral heterogeneity of receptor tyrosine kinases EGFR and PDGFRA amplification in glioblastoma defines subpopulations with distinct growth factor response. Proc Natl Acad Sci U S A. 2012;109(8):3041–3046. [DOI] [PMC free article] [PubMed] [Google Scholar]