Abstract
Background
Panobinostat is a histone deacetylase inhibitor with antineoplastic and antiangiogenic effects in glioma that may work synergistically with bevacizumab. We conducted a multicenter phase II trial of panobinostat combined with bevacizumab in patients with recurrent high-grade glioma (HGG).
Methods
Patients with recurrent HGG were treated with oral panobinostat 30 mg 3 times per week, every other week, in combination with bevacizumab 10 mg/kg every other week. The primary endpoint was a 6-month progression-fee survival (PFS6) rate for participants with recurrent glioblastoma (GBM). Patients with recurrent anaplastic glioma (AG) were evaluated as an exploratory arm of the study.
Results
At interim analysis, the GBM arm did not meet criteria for continued accrual, and the GBM arm was closed. A total of 24 patients with GBM were accrued prior to closure. The PFS6 rate was 30.4% (95%, CI 12.4%–50.7%), median PFS was 5 months (range, 3–9 months), and median overall survival (OS) was 9 months (range, 6–19 months). Accrual in the AG arm continued to completion, and a total of 15 patients were enrolled. The PFS6 rate was 46.7% (range, 21%–73%), median PFS was 7 months (range, 2–10 months), and median OS was 17 months (range, 5 months–27 months).
Conclusions
This phase II study of panobinostat and bevacizumab in participants with recurrent GBM did not meet criteria for continued accrual, and the GBM cohort of the study was closed. Although it was reasonably well tolerated, the addition of panobinostat to bevacizumab did not significantly improve PFS6 compared with historical controls of bevacizumab monotherapy in either cohort.
Keywords: anaplastic glioma; antiangiogenesis,; bevacizumab; glioblastoma; panobinostat
High-grade gliomas, which include glioblastomas (GBMs) and anaplastic gliomas (AGs), are the most common malignant primary brain tumors in adults1 and are associated with poor survival despite maximal surgery, radiation, and chemotherapy.2–4 Bevacizumab, a humanized monoclonal antibody against vascular endothelial growth factor (VEGF), is frequently used to treat recurrent high-grade glioma (HGG). Bevacizumab monotherapy received accelerated approval for recurrent GBM by the United States Food and Drug Administration in 2009 on the basis of phase II clinical trials demonstrating response rates of 20%–26%, 6-month progression-free survival (PFS6) rates of 29%–42.6%, and median overall survival (OS) of 7.1–9.2 months.5,6 Phase II studies in recurrent AG suggest that bevacizumab plus irinotecan also has activity in this patient population with response rates of 55%–66% and PFS6 rates of 56%–61%.7,8 However, responses to bevacizumab are not durable, and some patients fail to benefit.9
Panobinostat is a potent, small-molecule inhibitor of classes I, II, and IV histone deacetylases (HDACs) with greater potency than vorinostat.10 HDAC inhibitors, including panobinostat, may inhibit angiogenesis by reducing VEGF secretion and modulating the expression of other VEGF family members via inhibition of HIF-1α.11–14 In addition, the SDF-1α/CXCR4 pathway has been implicated in bevacizumab resistance,15–17 and panobinostat depletes CXCR4 levels and signaling.18 A phase I study of panobinostat in combination with bevacizumab for recurrent HGG suggested that oral panobinostat 30 mg 3 times per week, every other week, can be safely combined with bevacizumab 10 mg/kg every other week.19 We conducted a multicenter, phase II trial of panobinostat in combination with bevacizumab in patients with recurrent GBM. We also examined the same combination of agents in patients with recurrent AG as an exploratory arm.
Materials and Methods
The study was approved by the local institutional review boards at each of the participating institutions. All patients gave signed informed consent per institutional guidelines. Two arms were enrolled: recurrent GBM as the primary study population and recurrent AG as an exploratory cohort. This study was registered on www.clinicaltrials.gov (NCT00859222).
Patient Eligibility
Eligibility criteria included histologically confirmed GBM or gliosarcoma for the GBM arm or histologically confirmed anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), or anaplastic oligoastrocytoma (AOA) for the AG arm. Additional criteria for both cohorts included age ≥ 18 years old, KPS ≥ 60%, no more than 2 prior relapses, and adequate bone marrow reserve and organ function. Prior treatments with VEGF-targeted therapies and/or HDAC inhibitors were not permitted. Patients on enzyme-inducing anticonvulsants, valproic acid, warfarin, or QT-prolonging medications were excluded, as were patients with clinically significant cardiovascular events, cardiac arrhythmias, QT-prolonging conditions, a history of grade 3 thrombocytopenia on any prior regimen, presence of ≥ grade 2 peripheral neuropathy, bleeding diathesis or coagulopathy, history of abdominal fistula or gastrointestinal perforation, serious nonhealing wounds, or significant intratumoral or peritumoral hemorrhage. Recent resection for recurrent disease was allowed ≥ 4 weeks after surgery; residual disease after resection was not required. At least 15 unstained fresh-frozen, paraffin-embedded slides from a prior surgery were required to participate in the study.
Treatment and Study Design
Panobinostat was supplied by Novartis Pharmaceuticals Corporation, and bevacizumab was supplied by Genentech. Doses were chosen based on results from a phase I study of recurrent HGG.19 Participants received panobinostat orally 30 mg 3 times per week, every other week (days 1, 3, 5 and 15, 17, 19 of every 28-day cycle) as well as bevacizumab intravenously at 10 mg/kg on days 1 and 15 of every 28-day cycle. Participants were evaluated every 2 weeks and as clinically indicated while on the study. Brain MRIs with and without contrast were obtained every 8 weeks. The primary endpoint was PFS6. Secondary objectives included median progression free survival (PFS), OS, overall response rate, and safety. Toxic effects were graded according to the National Cancer Institute Common Toxicity Criteria, version 4.0. Radiographic assessments were performed by the local investigator and were based on the Response Assessment in Neuro-Oncology (RANO) criteria.20 Survival analysis was based on Kaplan-Meier estimates.
Statistical Analysis
The primary clinical objective of this phase of the study was to determine if bevacizumab in combination with panobinostat could significantly delay progression in participants with recurrent GBM. Based on Friedman et al5, the historical PFS6 was 35% for the bevacizumab-alone arm. This trial was powered to discriminate between 35% and 55% PFS6 rates. With accrual of 41 GBM participants, the trial would have been considered a success if at least 20 GBM participants were progression free by 6 months. This design had at least 85% power and a .07 significance level to predict a difference between the null hypothesis of 35% PFS6 rate and the alternative hypothesis of 55% PFS6 rate.
The protocol specified a planned interim analysis after the first 21 participants had been accrued. If 12 or more of those patients had been observed to have died or had disease progression/relapse within 6 months of initiating treatment, accrual was to be suspended and the data carefully reviewed before proceeding with additional patient accrual. Participants who were removed from active treatment for toxicity prior to reaching 6 months on treatment were not included in this interim analysis. Participants with recurrent GBM enrolled in the phase I study at the maximum tolerated dose (ie, the phase II dose) were eligible for inclusion in the interim analysis.
Immunohistochemistry for Isocitrate Dehydrogenese 1 Mutation
The tumors of participants enrolled in the AG arm were tested for R132H IDH1 mutation status by immunohistochemistry on formalin-fixed, paraffin-embedded sections as previously described.21 Isocitrate dehydrogenese 1 (IDH1) status was not tested in the GBM arm.
Results
Interim Analysis
Thirteen of the first 21 participants in the GBM arm progressed within 6 months. The study did not meet criteria for continued accrual, and the GBM arm of the study was closed following interim analysis. The data from the GBM arm presented here are based on all participants included in the interim analysis. Accrual to the exploratory AG arm of the study was not stopped despite the interim analysis results; therefore, accrual in this exploratory arm continued to completion.
Patient Characteristics
A total of 24 participants with GBM were accrued prior to study closure (Table 1). Median age was 53 years (range, 22–66 years), median KPS was 85% (range, 60%–100%), and median number of prior relapses was 1 (range, 1–2). In the AG arm, 15 patients were enrolled with a median age of 48 years (range, 31–69 years), median KPS 85% (range, 70%-100%), and median number of prior relapses of 1 (range, 1–4). Histologies in the AG arm included 8 participants with AA (53.3%), 5 with AO (33.3%), and 2 with AOA (13.3%).
Table 1.
Participant characteristics
| Characteristic | GBM Arm (N = 24) | AG Arm (N = 15) |
|---|---|---|
| Age, years, median (range) | 53 (22–66) | 48 (31–69) |
| KPS, median (range) | 85 (60–100) | 85 (70–100) |
| Sex, female, N (%) | 10 (41.7%) | 5 (33.3%) |
| Race, N (%) | ||
| Caucasian | 16 (66.7%) | 14 (93.3%) |
| Multiracial | 2 (8.33%) | 0 |
| Asian | 1 (4.2%) | 0 |
| Other | 5 (20.8%) | 1 (6.7%) |
| Number of prior relapses, median (range) | 1 (1–2) | 1 (1–4) |
| 1, N (%) | 15 (62.5%) | 7 (46.7%) |
| 2, N (%) | 9 (37.5%) | 4 (26.7%) |
| 3, N (%) | 0 | 3 (20%) |
| 4, N (%) | 0 | 1 (6.7%) |
| Histology, N (%) | ||
| GBM | 24 (100%) | N/A |
| AA | N/A | 8 (53.3%) |
| AO | N/A | 5 (33.3%) |
| AOA | N/A | 2 (13.3%) |
| R132H IDH1 mutation by immunohistochemistry, N (%) | N/A | 10 (66.7%) |
Abbreviations: AA, anaplastic astrocytoma; AG, anaplastic glioma; AO, anaplastic oligodendroglioma; AOA, anaplastic oligoastrocytoma; GBM, glioblastoma.
Outcomes
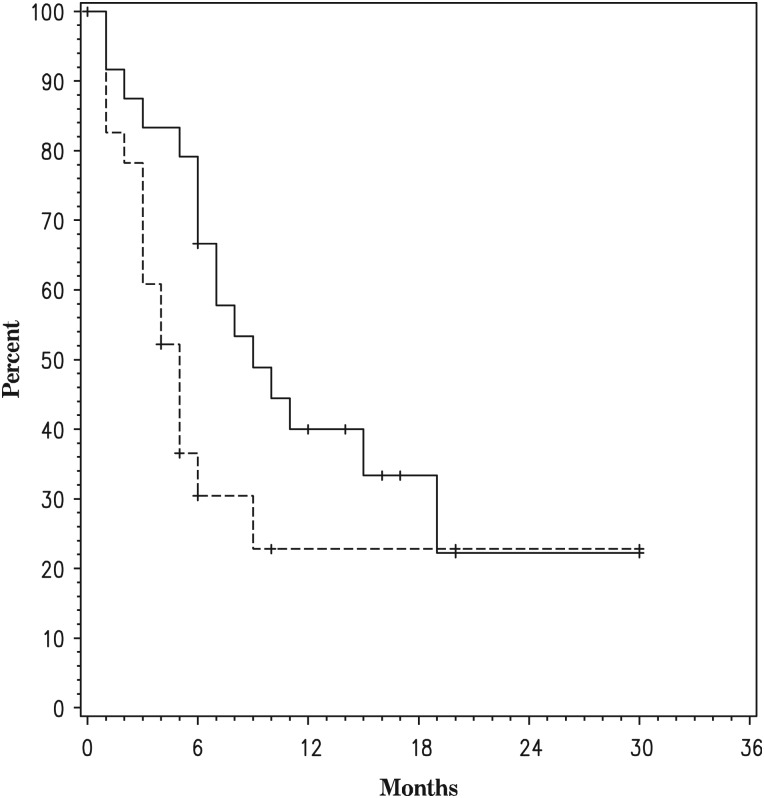
In the GBM arm, the PFS6 rate was 30.4% (95% CI, 12.4%–50.7%), median PFS was 5 months (95% CI, 3–9 months), and median OS was 9 months (95% CI, 6 months–19 months) (Table 2, Fig. 1). Radiographic responses by RANO criteria included 7 partial responses (29.2%), 14 stable disease (58.3%), and 3 progressive disease (12.5%). In the AG arm, the PFS6 rate was 46.7% (range, 21%–73%), median PFS was 7 months (range, 2–10 months), and median OS was 17 months (range, 5–27 months). Radiographic responses by RANO criteria included 4 partial responses (26.7%), 9 stable disease (60.0%), and 2 progressive disease (13.3%).
Table 2.
Outcomes
| GBM Arm (N = 24) | AG Arm (N = 15) | |
|---|---|---|
| Median OS (95% CI) | 9 months (6, 19) | 17 months (5, 27) |
| IDH1 wild-type by IHC | – | 5 months (2,9) |
| IDH1 mutant by IHC | – | 22 months (7, NR) |
| Median PFS (95% CI) | 5 months (3, 9) | 7 months (2, 10) |
| IDH1 wild-type by IHC | – | 3 months (1, NR) |
| IDH1 mutant by IHC | – | 9 months (1, NR) |
| PFS6 rate (95% CI) | 30.4% (12.4%, 50.7%) | 46.7% (21%, 73%) |
| IDH1 wild-type by IHC | – | 20% (0.8%, 58%) |
| IDH1 mutant by IHC | – | 70% (33%, 90%) |
| Best radiographic response (RANO) | ||
| Complete response | 0 | 0 |
| Partial response | 7 (29.2%) | 4 (26.7%) |
| Stable disease | 14 (58.3%) | 9 (60.0%) |
| Progressive disease | 3 (12.5%) | 2 (13.3%) |
Abbreviations: AG, anaplastic glioma; GBM, glioblastoma; IHC, immunohistochemistry; OS, overall survival; PFS, progression-free survival; RANO, Response Assessment in Neuro-Oncology.
Fig. 1.
Overall survival (solid line) and progression-free survival (dashed line) in the glioblastoma arm.
Toxicity
In the GBM arm, the most common grade 3 or higher toxicities attributed to panobinostat and/or bevacizumab included hypophosphatemia (12.5%), thrombocytopenia (12.5%), lymphopenia (8.3%), neutropenia (8.3%), and alanine amino transferase elevation (8.3%) (Table 3). There was one grade 4 CNS hemorrhage (4.2%) and one grade 4 pulmonary embolism (4.2%). In the AG arm, the most common grade 3 or 4 toxicities attributed to panobinostat and/or bevacizumab included thrombocytopenia (20.0%) and hypophosphatemia (13.3%). There were no deaths related to study treatment in either arm of the study.
Table 3.
Grade 3 or higher toxicities possibly, probably, or definitely associated with panobinostat and/or bevacizumab
| Toxicity | GBM Arm (N = 24) |
AG Arm (N = 15) |
||
|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | |
| Hematologic | ||||
| Lymphopenia | 2 (8.3%) | 0 | 0 | 0 |
| Neutropenia | 2 (8.3%) | 0 | 0 | 1 (6.7%) |
| Thrombocytopenia | 1 (4.2%) | 2 (8.3%) | 3 (20.0%) | 0 |
| Nonhematologic | ||||
| ALT elevation | 2 (8.3%) | 0 | 0 | 0 |
| CNS hemorrhage | 0 | 1 (4.2%) | 0 | 0 |
| Fatigue | 1 (4.2%) | 0 | 1 (6.7%) | 0 |
| Hypertension | 1 (4.2%) | 0 | 0 | 0 |
| Hypophosphatemia | 3 (12.5%) | 0 | 0 | 2 (13.3%) |
| QT interval prolongation | 1 (4.2%) | 0 | 0 | 0 |
| Pulmonary embolism | 0 | 1 (4.2%) | 0 | 0 |
| Weight loss | 1 (4.2%) | 0 | 0 | 0 |
Abbreviations: AG, astrocytic glioma; ALT, alanine amino transferase; GBM, glioblastoma.
IDH1 Mutations in the Anaplastic Glioma Arm
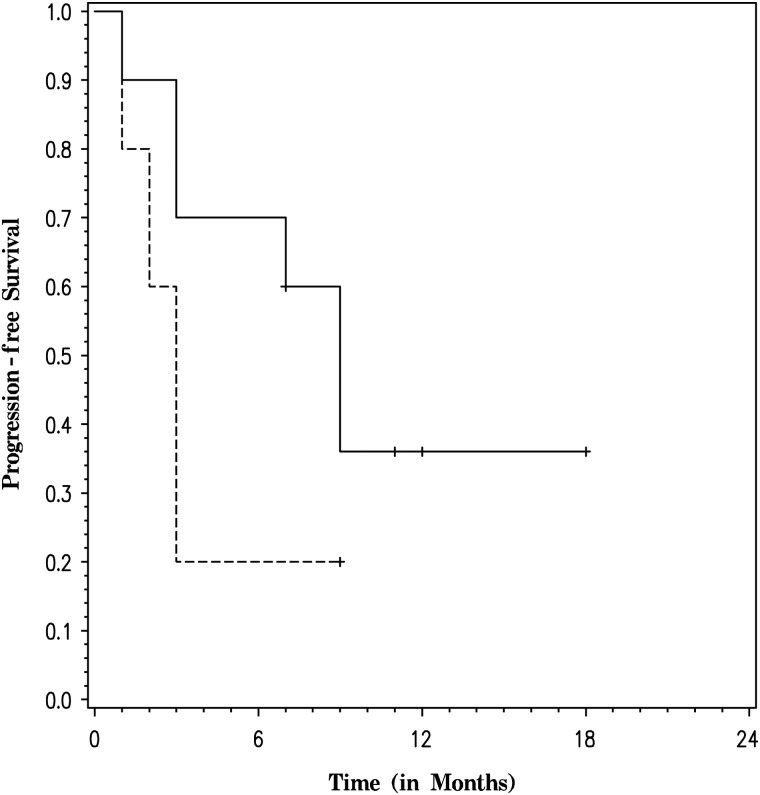
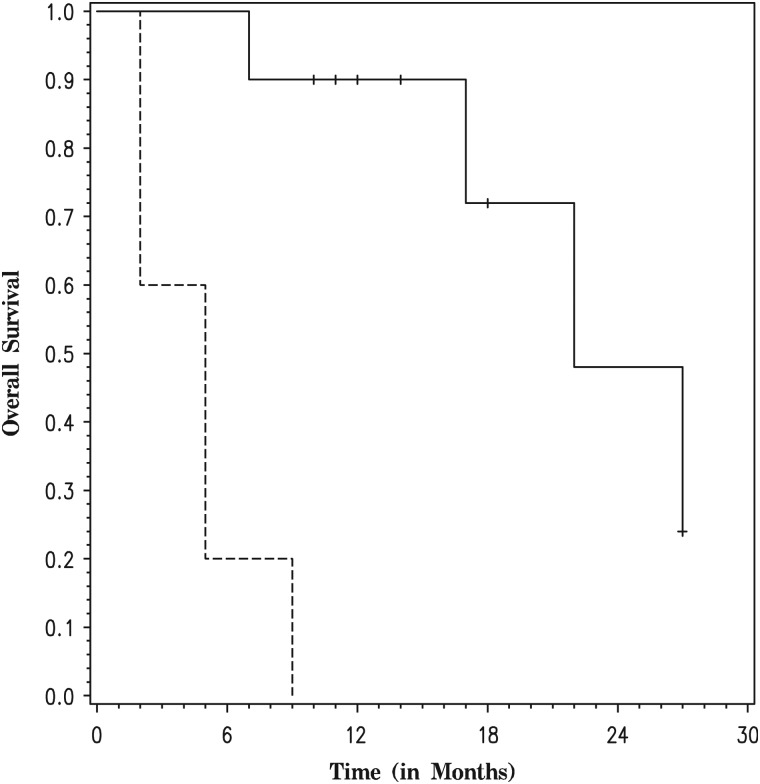
IDH1 R132H mutation status was examined in the AG arm. Ten participants (66.7%) demonstrated IDH1 positivity by immunohistochemistry (IHC) (Table 2). There was no significant difference in PFS (P=.2) between participants with IDH1 mutant tumors and those with wild-type tumors (Fig. 2). However, there was a significant difference in OS (P = .0001) favoring participants with IDH1 mutant tumors (Fig. 3).
Fig. 2.
Progression-free survival in the anaplastic glioma arm by R132H IDH1 mutation status (dashed line for participants with negative staining for R132H IDH1 mutation and solid line for participants with positive staining for R132H IDH1 mutation).
Fig. 3.
Overall survival in the anaplastic glioma arm by R132H IDH1 mutation status (dashed line for participants with negative staining for R132H IDH1 mutation and solid line for participants with positive staining for R132H IDH1 mutation).
Discussion
Preclinical evidence suggests that class I and class II HDAC inhibitors, such as panobinostat, may be useful antiangiogenesis22 and antitumor23–26 agents, hence providing a rationale for the combination of panobinostat and bevacizumab in recurrent GBM. Interim analysis of participants in the recurrent GBM arm of the study revealed a PFS6 rate of 30.4%. This is similar to the Kreisl et al study of bevacizumab monotherapy in recurrent GBM, in which the PFS6 rate was 29% but was worse than the bevacizumab monotherapy arm of Friedman et al, in which the PFS6 rate was 42.6%. Compared with Friedman et al, in which 80% of participants were treated at first relapse, our participant population may represent a more heavily pretreated population with 62.5% in first relapse and 37.5% in second relapse, potentially explaining the differences in PFS6 rates. When compared with historical bevacizumab controls, the addition of panobinostat to bevacizumab in recurrent GBM did not significantly improve PFS6, and the GBM arm of the study was closed at planned interim analysis.
In the AG arm, the PFS6 rate of 46.7% and median PFS of 7 months were similar to prior phase II studies of bevacizumab and irinotecan in recurrent AG.7,8 This again suggests that the addition of panobinostat to bevacizumab may not delay progression compared with historical bevacizumab controls. However, the median OS of 17 months (74 weeks) appears to be longer compared with the median OS of 65 weeks in the Dejsardins et al study. Our study had a slightly higher percentage of participants with AO or AOA (46.6%) compared with Desjardins et al (24%), which may account for this longer median OS. In addition, we examined IDH1 R132H mutation status by IHC in our AG cohort and found that 66.7% had an IDH1 R132H mutation. Although the frequencies of IDH1 mutation in the Desjardin et al and Vredenburgh et al studies are not known, the high percentage of IDH1 mutant tumors in our study may account for the prolonged OS compared with historical controls. It is unlikely that treatment with panobinostat and bevacizumab resulted in increased OS in the IDH1 mutant group since there was no difference in PFS.
There are several potential reasons for the lack of activity with this combination. The dose of panobinostat used in this study was the maximum tolerated dose determined in our preliminary phase I study.19 Even though this dose is being explored in hematologic malignancies, it may be too low to achieve adequate tumor concentrations in the brain. This issue is compounded by the limited penetration of panobinostat across the blood-tumor barrier, a problem that is further exacerbated by combining it with bevacizumab, which reduces vascular permeability. In contrast, lomustine, which does penetrate the blood-tumor barrier, was successfully combined with bevacizumab for treatment of recurrent GBM.27 This difference underscores the need to combine bevacizumab with agents that have good blood-tumor barrier penetration. In addition, there are no preclinical data exploring the activity of the combination of panobinostat and bevacizumab in GBM models.
Conclusions
Although it was reasonably well tolerated, the addition of panobinostat to bevacizumab in recurrent GBM and recurrent AG did not significantly improve PFS6 compared with historical controls. Treatment with this combination is not recommended. A role may still exist for combining an HDAC inhibitor with chemoradiation, and there is an ongoing clinical trial combining vorinostat with radiation and temozolomide in newly diagnosed GBM.
Funding
This work is supported by Novartis and Genentech.
References
- 1.Ostrom QT, Gittleman H, Liao P, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2007–2011. Neuro Oncol. 2014;16:Suppl 4:iv1–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 3.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009;27(35):5874–5880. [DOI] [PubMed] [Google Scholar]
- 4.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. [DOI] [PubMed] [Google Scholar]
- 5.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. [DOI] [PubMed] [Google Scholar]
- 6.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27(5):740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. [DOI] [PubMed] [Google Scholar]
- 8.Desjardins A, Reardon DA, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent WHO grade 3 malignant gliomas. Clin Cancer Res. 2008;14(21):7068–7073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norden AD, Drappatz J, Wen PY. Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol. 2008;7(12):1152–1160. [DOI] [PubMed] [Google Scholar]
- 10.Anne M, Sammartino D, Barginear MF, et al. Profile of panobinostat and its potential for treatment in solid tumors: an update. Onco Targets Ther. 2013;6:1613–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deroanne CF, Bonjean K, Servotte S, et al. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene. 2002;21(3):427–436. [DOI] [PubMed] [Google Scholar]
- 12.Qian DZ, Kato Y, Shabbeer S, et al. Targeting tumor angiogenesis with histone deacetylase inhibitors: the hydroxamic acid derivative LBH589. Clin Cancer Res. 2006;12(2):634–642. [DOI] [PubMed] [Google Scholar]
- 13.Qian DZ, Wang X, Kachhap SK, et al. The histone deacetylase inhibitor NVP-LAQ824 inhibits angiogenesis and has a greater antitumor effect in combination with the vascular endothelial growth factor receptor tyrosine kinase inhibitor PTK787/ZK222584. Cancer Res. 2004;64(18):6626–6634. [DOI] [PubMed] [Google Scholar]
- 14.Sawa H, Murakami H, Ohshima Y, et al. Histone deacetylase inhibitors such as sodium butyrate and trichostatin A inhibit vascular endothelial growth factor (VEGF) secretion from human glioblastoma cells. Brain Tumor Pathol. 2002;19(2):77–81. [DOI] [PubMed] [Google Scholar]
- 15.Kioi M, Vogel H, Schultz G, et al. Inhibition of vasculogenesis, but not angiogenesis, prevents the recurrence of glioblastoma after irradiation in mice. J Clin Invest. 2010;120(3):694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rubin JB, Kung AL, Klein RS, et al. A small-molecule antagonist of CXCR4 inhibits intracranial growth of primary brain tumors. Proc Natl Acad Sci USA. 2003;100(23):13513–13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batchelor TT, Sorensen AG, di Tomaso E, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11(1):83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mandawat A, Fiskus W, Buckley KM, et al. Pan-histone deacetylase inhibitor panobinostat depletes CXCR4 levels and signaling and exerts synergistic antimyeloid activity in combination with CXCR4 antagonists. Blood. 2010;116(24):5306–5315. [DOI] [PubMed] [Google Scholar]
- 19.Drappatz J, Lee EQ, Hammond S, et al. Phase I study of panobinostat in combination with bevacizumab for recurrent high-grade glioma. J Neurooncol. 2012;107(1):133–138. [DOI] [PubMed] [Google Scholar]
- 20.Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 21.Capper D, Reuss D, Schittenhelm J, et al. Mutation-specific IDH1 antibody differentiates oligodendrogliomas and oligoastrocytomas from other brain tumors with oligodendroglioma-like morphology. Acta Neuropathol. 2011;121(2):241–252. [DOI] [PubMed] [Google Scholar]
- 22.New M, Olzscha H, La Thangue NB. HDAC inhibitor-based therapies: can we interpret the code?. Mol Oncol. 2012;6(6):637–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asklund T, Kvarnbrink S, Holmlund C, et al. Synergistic killing of glioblastoma stem-like cells by bortezomib and HDAC inhibitors. Anticancer Res. 2012;32(7):2407–2413. [PubMed] [Google Scholar]
- 24.Eyupoglu IY, Hahnen E, Buslei R, et al. Suberoylanilide hydroxamic acid (SAHA) has potent anti-glioma properties in vitro, ex vivo and in vivo. J Neurochem. 2005;93(4):992–999. [DOI] [PubMed] [Google Scholar]
- 25.Ugur HC, Ramakrishna N, Bello L, et al. Continuous intracranial administration of suberoylanilide hydroxamic acid (SAHA) inhibits tumor growth in an orthotopic glioma model. J Neurooncol. 2007;83(3):267–275. [DOI] [PubMed] [Google Scholar]
- 26.Yin D, Ong JM, Hu J, et al. Suberoylanilide hydroxamic acid, a histone deacetylase inhibitor: effects on gene expression and growth of glioma cells in vitro and in vivo. Clin Cancer Res. 2007;13(3):1045–1052. [DOI] [PubMed] [Google Scholar]
- 27.Taal W, Oosterkamp HM, Walenkamp AME, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 2014;15(9):943–953. [DOI] [PubMed] [Google Scholar]