Abstract
Background
Aurora Kinase A (AURKA) encodes a protein that regulates the formation and stability of the mitotic spindle and is highly active in atypical teratoid rhabdoid tumors (ATRT) through loss of the INI1 tumor suppressor gene. Alisertib (MLN8237) inhibits AURKA in vitro and in vivo. Given the strong preclinical data supporting the use of alisertib for ATRT patients, we sought and obtained permission to use alisertib in single patient treatment plans for 4 recurrent pediatric ATRT patients.
Methods
Patients with recurrent or progressive ATRT received alisertib 80 mg/m2 by mouth once daily for 7 days of a 21-day treatment cycle. Disease evaluation (MRI of brain and spine and lumbar puncture) was done after 2 cycles of alisertib and every 2–3 cycles thereafter for as long as the patients remained free from tumor progression.
Results
Four patients with median age of 2.5 years (range, 1.39–4.87 y) at diagnosis received alisertib 80 mg/m2 by mouth once daily for 7 days of a 21-day treatment cycle, and all 4 patients had disease stabilization and/or regression after 3 cycles of alisertib therapy. Two patients continued to have stable disease regression for 1 and 2 years, respectively, on therapy.
Conclusions
Single-agent alisertib produced marked and durable regression in disease burden, as detected by brain and spine MRI and by evaluation of spinal fluid cytology. Alisertib has moderate but manageable toxicities, and its chronic administration appears feasible in this pediatric population. These novel data support the incorporation of alisertib in future therapeutic trials for children with ATRT.
Keywords: ATRT, Aurora kinase A, brain tumor, pediatric, targeted therapy
Malignant rhabdoid tumors (MRTs) are rare, highly aggressive tumors that occur in young children, which were initially reported in association with the kidney but have also been found in liver, thymus, and other soft tissue sites as well as within the central nervous system (CNS).1,2 The most common location for nonrenal MRT is the CNS, and these tumors are referred to as atypical teratoid/rhabdoid tumors (ATRTs).3 ATRT, along with primitive neuroectodermal tumor, is the most common type of tumor arising in children younger than aged 1 year.4,5 While there is no standard or effective therapy for ATRT, a subset of patients may benefit from intensive multimodal therapy.6–8 For most patients, the median event-free survival with surgery, chemotherapy, and radiotherapy is less than one year.5,9
Early cytogenetic studies revealed that these tumor cells carried few or no detectable chromosomal rearrangements and that monosomy 22 was a nonrandom chromosomal abnormality in ATRT.2,10,11 Researchers found that disruption of the locus at 22q11.2 resulted in loss of function of the switch/sucrose nonfermentable (SWI/SNF)-related ATP-dependent chromatin-remodeling complex.12,13 This was one of the first chromatin remodeling complexes to be identified and comprises 12–15 protein subunits in mammalian cells.14 Inactivating mutations have been identified in all 9 exons of SMARCB1 (also known as INI1, hSNF5, and BAF47) gene products, which are pathognomonic for of ATRT.15–17 Rhabdoid tumors may be distinguished from other tumor entities by characteristic histopathological features and immunohistochemical analysis of INI1/Baf47.
Aurora Kinase A (AURKA) is a gene that encodes cell cycle-associated serine/threonine kinase that regulates centrosome maturation and mitosis.18 Inhibition of AURKA leads to mitotic delays and severe chromosome alignment and segregation defects, followed by cell death.19 AURKA is highly expressed in malignant rhabdoid tumors through the characteristic loss of the INI1 tumor suppressor gene.20 INI1 directly represses AURKA in a cell-type specific manner, and knockdown of AURKA in MRT cells induces mitotic arrest and apoptosis in MRT but not normal diploid cells. Although INI1 represses AURKA in tumor and normal cells, its repression of AURKA in normal diploid cells does not induce cleavage of caspase 3 and does not lead to decreased survival.20 This is an extremely exciting result with important clinical implications, particularly for the treatment of ATRTs that arise within the developing nervous system of young children.
Alisertib (MLN8237) is a selective, potent, and orally bioavailable small-molecule inhibitor of AURKA, which has shown antitumor activity in vitro and in vivo models through the Pediatric Preclinical Testing Program (PPTP) and provided the preclinical rationale for development of alisertib in childhood cancer.21 Alisertib has been evaluated in adults with recurrent solid tumors and has been found to be tolerable and to have some indication of activity with tumor stabilization and one partial response.22 Recently, alisertib was evaluated in 33 pediatric patients with recurrent/refractory solid tumors (excluding CNS tumors) in a phase 1 study through Children's Oncology Group (COG).23 The maximum tolerated dose (MTD) in this cohort was 80 mg/m2/day administered orally once daily for 7 days out of a cycle of 21 days.
Due to the lack of any curative therapy for ATRT, the strong biological rationale for AURKA inhibition in ATRT, and the safety and tolerability profile of alisertib in the pediatric phase 1 study, we conducted single patient treatment plans for 4 patients with recurrent/refractory ATRT at St. Jude Children's Research Hospital between July 2012 and August 2014.
Patients and Methods
Patients
Four (4) patients with recurrent/refractory ATRT were each enrolled on a single patient treatment plan (SPTP) at St. Jude Children's Research Hospital between July 2012 and June 2013. The institutional review board and FDA approved an individual treatment plan before each patient was enrolled, and continuing approval was maintained throughout the study. Written informed consent for participation was obtained from the patients' parents or legal guardians, and patient assents were obtained when appropriate.
Alisertib was administered orally on an empty stomach (at least one hour before or 2 hours after food or drink except for water) at the recommended phase 2 dose of 80 mg/m2 once daily on days 1–7 of a 21-day course.23 Enteric-coated tablets were swallowed whole. If emesis occurred after a dose of alisertib, the dose was not repeated. We encouraged parents to give the medication prior to bedtime and without any other medications to minimize daytime somnolence. Drug doses were adjusted based upon the body surface area within one week prior to the beginning of each cycle. If the proposed dose of 80 mg/m2/day was not tolerated, the dose was reduced to 60 mg/m2/day. If a participant had an increase in tumor size of >25% in 2-dimensional area as measured on MRI or the appearance of tumor cells in cerebral spinal fluid (CSF), he or she was considered to have progressive disease and was taken off study. Cycles were repeated up to 34 times (35 cycles) for a duration of 24 months of therapy.
Prior Therapy
All participants were treated with several cycles of platinum-based chemotherapy at St. Jude Children's Research Hospital. Participant #1 was treated on Pediatric Brain Tumor Consortium Study 001,24 and participants #2 and #3 were treated on the average risk arm of the St. Jude institutional study for newly diagnosed patients with embryonal brain tumors that comprises risk-adapted craniospinal radiation therapy, followed by 4 cycles of dose-intensive chemotherapy with stem cell support.25 At the time of recurrence, participant #3 received 4 months of cyclophosphamide and etoposide before starting on alisertib therapy. At diagnosis, participant #4 was treated on the St. Jude institutional protocol for infants with newly diagnosed embryonal brain tumors, which comprises 4 cycles of induction therapy (methotrexate, cisplatin, cyclophosphamide, etoposide), followed by focal proton radiotherapy, followed by 6 months of maintenance chemotherapy with oral cyclophosphamide and topotecan alternating with oral etoposide.
Assessments
Prior to enrollment and approximately every 2 to 3 cycle intervals, participants had MRI of the brain and spine and lumbar puncture for assessment of disease status. Complete blood counts and blood chemistries were monitored as needed for optimal patient care with the following minimum timing of observations: physical exam, height, weight, complete blood count, differential, complete metabolic panel including ALT, AST, and bilirubin, were done weekly during first 3 weeks of therapy and prior to starting each cycle. Criteria for starting the subsequent cycle included ANC ≥ 750/mm3, platelet count >50 000/mm3, hemoglobin ≥8 g/dL. Transfusions were permitted to meet both the platelet and hemoglobin criteria. Cycles were repeated up to 34 times (35 cycles) for a duration of 24 months of therapy.
Immunohistochemistry
Immunohistochemistry was performed on deparaffinized sections from patient samples and a tissue microarray containing 2 cores each from 30 ATRTs collected with appropriate institutional review board approval. In brief, tissue sections were probed using antibodies to AURKA (Abcam, clone EP1008Y, 1:100), BAF47 (BT Transduction Laboratories, clone 25/BAF47, 1:200), and Ki67 (Dako, MIB-1,1:200). Reaction product was detected using the Bond refine polymer detection kit (Leica Biosystems). Immunohistochemical preparations were evaluated by a neuropathologist (B.A.O). AURKA immunoreactivity was scored as positive in the presence of nuclear and cytoplasmic immunoreactivity in tumor cells and immunonegative in the absence of specific staining.
Statistical Analysis
Kaplan-Meier estimates for progression-free survival (PFS) and overall survival (OS) based on the 4 participants are provided and serve as more of a tool for showing how many events have happened and when the events happened rather than estimating survival rates. PFS was measured from the start of alisertib to the earliest date of disease progression, defined as an increase in 2 dimensional area >25% or a new lesion as noted on MRI or the appearance of tumor cells in CSF. For patients without disease progression, PFS was censored at the last follow-up date. OS was measured from the start of alisertib to the date of death or date of last contact.
Results
Participant Demographics and Baseline Characteristics
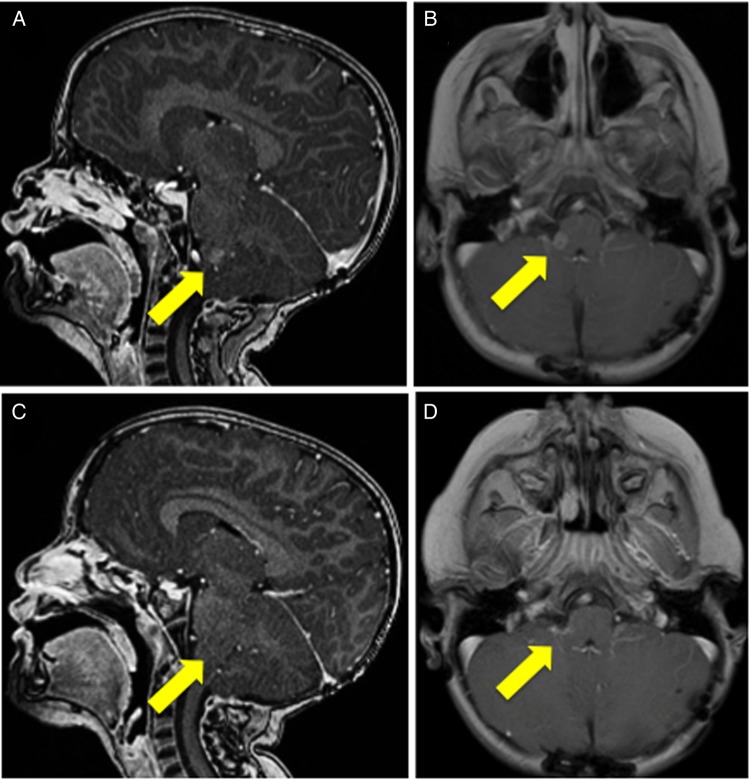
Each participant developed new sites of progressive disease after definitive surgery, radiation therapy, and at least one prior regimen of chemotherapy. Participants’ demographics and disease characteristics are summarized in Table 1. Each of the 4 participants had disease stabilization or decrease in tumor size/burden after 1–2 cycles (approximately 3–6 weeks) of alisertib therapy. The median duration of stable disease was 11.0 months (range, 6.3–11.7 months). As of August 5, 2014, these participants had received 5, 18, 14, and 31 cycles of Alisertib, respectively. One participant had increasing numbers of malignant cells in her CSF on 2 lumbar punctures done 2 weeks apart prior to initiating therapy. She had clearance of the malignant cells on a subsequent lumbar puncture done 10 days after starting alisertib as part of a work-up for fever and neutropenia. Subsequent sampling of CSF at times of disease evaluation repeatedly confirmed the absence of malignant cells. The best response was noted in participant #4, who had a regression of the lesion at the right cerebellar pontine angle first noted after 3 cycles of alisertib therapy that was sustained for more than one year (Fig. 1). Two participants progressed, were taken off alisertib therapy, and died months later from progressive disease. The other 2 participants remained on treatment at the time of analysis.
Table 1.
Patient demographics and treatment
| Patient | Age at Diagnosis | Sex | Tumor Location/ Extent of Resection | RT | Site of Recurrence | Age at Recurrence | Treatment Duration (cycles) | PFS (months) | OS (months) | Best Response |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1.39 | Male | RF/STR | F 55.8 Gy | R thalamus | 6.92 | 5 | 3.2 | 8.4 | SD |
| 2 | 4.87 | Female | PF/GTR | CSI+ | Spine | 9.04 | 23 | 16.4a | 16.4a | PR |
| 3 | 3.26 | Female | PF/GTR | CSI+ | Spine, CSF | 4.75 | 14 | 15.1 | 21.8 | PR (CSF cleared & spinal lesion decreased in size) |
| 4 | 1.75 | Male | Pineal/GTR | FP 59.4 Gy | Brainstem | 3.83 | 34 | 23.6a | 25.6a | CR |
Abbreviations: CSI+, craniospinal radiation 23.4 Gy with boost to tumor bed to 55.8 Gy; F, focal irradiation with photons; FP, focal irradiation with protons; GTR, gross total resection; OS, overall survival; PF, posterior fossa; PFS, progression-free survival; RF, right frontal lobe; RT, radiation therapy; SD, stable disease; STR, subtotal resection.
aCensored.
Fig. 1.
MRI of participant #4 from one week prior to initiating alisertib therapy (A) sagittal T1 with contrast, (B) axial T2 with contrast; (C) sagittal T1 with contrast one year on single-agent alisertib showing regression of tumor; (D) axial T2 with contrast one year on single-agent alisertib therapy showing regression of tumor that had persisted for 11.7 months.
Expression of Aurora Kinase A in Atypical Teratoid RhabdoidTumor
To confirm that ATRTs expressed AURKA, we evaluated a tissue microarray containing 30 ATRTs for AURKA protein expression using immunohistochemistry. While AURKA expression was not detected in control brain sections, 29 of 30 (97%) ATRTs demonstrated AURKA protein expression (Supplementary material, Fig. 1). Immunoreactivity was restricted to a variable proportion of tumor cells, and protein expression was localized to both the cytoplasm and nuclear compartments in immunopositive cells. The density of immunopositive cells varied from sparse (Supplementary material, Fig. 1C) to more dense (Supplementary material, Fig. 1D).
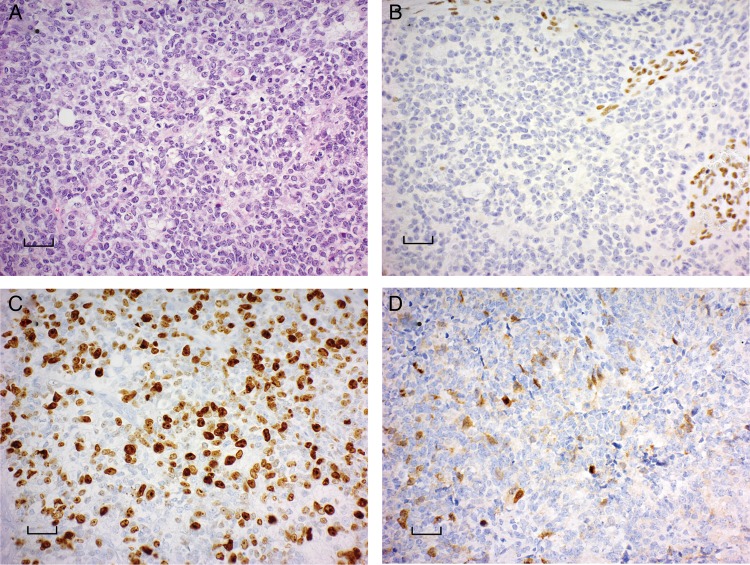
For each participant, the diagnosis of ATRT was confirmed by histopathological review, and each tumor demonstrated loss of BAF47/INI1 immunoreactivity within tumor cells (Fig. 2A and B). Immunohistochemistry was performed for AURKA on patient samples prior to receiving Alisertib and demonstrated robust AURKA expression as well as high proliferative index as noted by Ki-67 immunolabeling (Fig. 2C and D).
Fig. 2.
ATRT tumors express aurora kinase A by immunohistochemistry. (A) Hematoxylin and eosin-stained section of ATRT demonstrated predominantly small cell morphology with only rare rhabdoid cells. (B) Loss of BAF47/INI1 immunoreactivity in tumor cells with retained staining in blood vessel walls. (C) The tumor cells demonstrated high frequency immunolabeling with Ki67 reflecting a high proliferative fraction. (D) The tumor demonstrated immunoreactivity with antibodies to Aurora Kinase A in a cytoplasmic and nuclear distribution. Scale bar represents 200 µM.
Safety
Alisertib-related nonhematological toxicities observed were mild to moderate and included somnolence and alopecia. Laboratory abnormalities included neutropenia, decreased total WBCs, thrombocytopenia, and anemia (Supplementary material, Table 1). A total of 261 episodes of adverse events were observed in this patient cohort. Among these events, 42 were grade 4 and were experienced by 3 of the 4 participants. Participant #4 did not experience any grade 4 toxicity and did not experience any adverse events until the ninth cycle of treatment. Among the grade 4 toxicities, 29 episodes comprised a decrease in neutrophil counts (observed in 3 participants), 11 episodes of grade 4 decrease in WBCs (observed in 2 participants), and 2 episodes of grade 4 decrease in platelet counts (observed in one participant) (Table 2). Participant #3 had prolonged neutropenia lasting longer than one week on more than one occasion and was reduced to 60 mg/m2.
Table 2.
Grade 4 toxicities observed in this patient cohort throughout the treatment duration
| Table of Grade 4 Toxicities | ||||
|---|---|---|---|---|
| Patient | Event Name |
|||
| Frequency | Neutrophil Count Decreased | Platelet Count Decreased | WBC Decreased | Total |
| 1 | 1 | 0 | 0 | 1 |
| 2 | 13 | 0 | 5 | 18 |
| 3 | 15 | 2 | 6 | 23 |
| 4 | 0 | 0 | 0 | 0 |
| Total | 29 | 2 | 11 | 42 |
Among these events, 42 were grade 4 and were experienced by 3 of the 4 patients.
Discussion
While ATRT is a rare and particularly aggressive brain tumor that arises in early childhood and has been historically an underrecognized entity,26,27 recent studies have demonstrated that inactivating mutations of the SWI/SNF chromatin remodeling complex underlie the initiation and progression of several more prevalent types of cancer. It is estimated that SWI/SNF is the most frequently mutated chromatin-regulatory complex, and at least one member of this complex is mutated in 20% of all human tumors and is an attractive candidate drug target.28,29 Reactivation of a tumor suppressor gene, such as INI1, is not biologically feasible, yet the possibility of targeting the molecular consequence of INI1 loss within the tumor cells (ie, de-repression of AURKA) is what we sought to achieve with alisertib. The essential role of AURKA in mitotic progression and its dysregulation in pediatric tumor xenografts also make it an attractive therapeutic target in ATRT.21 Given the high expression of AURKA that arises from loss of the SWI/SNF complex in this type of tumor, we sought and obtained IRB and FDA permission to treat each of 4 recurrent ATRT patients with alisertib on single patient treatment plans. Initially, we were planning on treating a single patient with alisertib on a compassionate use basis. We did not anticipate that alisertib would be so well tolerated in the heavily pretreated population and that it would have such an indication of clinical efficacy. However, after the first couple of patients, other families heard about the single patient treatment option and requested treatment as well. All participants demonstrated disease stabilization or regression on single-agent alisertib over a period of >3 months duration.
The relatively long interval between completion of radiation therapy and the appearance of new lesions (between 2 and almost 6 years) is supportive of true tumor recurrence rather than pseudoprogression in our patients. Participant #2 presented with progressive back pain and underwent MRI evaluation. She experienced a decrease in back pain after one cycle of alisertib and had disease stabilization on spine MRI after 2 cycles of alisertib. After 4 cycles, this patient had a partial response and >50% decrease in size of the primary spinal lesion that was maintained for 16 months. A smaller nodule adjacent to the larger lesion remained stable in size. In each case, the participants were considered to have progressive disease due to the appearance of a new lesion in a place distant from the original tumor and, in the case of participant #3, had increasing numbers of tumor cells on lumbar punctures done 2 weeks apart prior to initiating alisertib therapy. During a workup for fever and neutropenia 10 days after starting alisertib therapy, lumbar puncture revealed a clearance of tumor cells from her spinal fluid. This participant also had a decrease in the leptomeningeal enhancement of a concurrent spinal cord lesion.
Alisertib, given as a single daily dose of 80 mg/m2 for 7 out of 21 days, was well tolerated by our patients, and the most prevalent toxicity was WBC suppression. The recommended pediatric phase 2 dose of alisertib differs from the adult MTD, being approximately 1.5 times the MTD of 50 mg p.o. twice daily that was established in the adult studies.22,30 Neurological toxicities, including somnolence and dizziness, were reported in 50% of adult patients and resolved spontaneously during the 14 day period off alisertib. Alisertib has structural similarity to benzodiazepines and potentiates binding at the GABA receptor (Millennium Pharmaceuticals Investigators Brochure22,23). Benzodiazepine-like effects (eg, somnolence, confusion, and memory loss) have been observed with the onset of maximal plasma concentration. CNS effects associated with peak plasma levels of the enteric-coated tablet formulation in adults have generally been managed by administering divided doses, although dose reductions have sometimes been required. While CNS effects attributed to alisertib are generally reversible and manageable by dose delay or reduction, the causal relationship approach to management can be confounded by factors including concomitant medications (eg, narcotic analgesics, antianxiety medications), comorbidities (eg, infection, anemia, electrolyte abnormalities), and progressive malignancy (eg, brain metastases).
Although ATRT is characterized by the disruption of the SWI/SNF complexes, other yet-to-be-identified molecular differences likely influence the heterogeneity of clinical presentation and variable response to therapy. The burgeoning molecular insight into these tumors has revealed that, while there are very few somatic mutations, the epigenetic landscape is complex and there is heterogeneity suggesting the likelihood of several molecular subtypes of ATRT (similar to findings reported for embryonal brain tumors).26 Approximately one-third of patients with ATRT have an underlying germline mutation in the SWI/SNF complex that is now recognized as a cancer-predisposition syndrome.31 Not surprisingly, the median age at diagnosis of patients with germline mutation was much lower (6 months) than those with somatic mutation (18 months).32 Additionally, the younger patients were more likely to have disseminated or concurrent tumors in other organs and poorer prognosis.4,33 Initiation of radiotherapy in patients with localized disease within one month of surgical resection was associated with a lower incidence of progressive disease and improved prognosis.34
In conclusion, the treatment and cure of tumors that arise within the developing nervous system continue to be challenging. In this report, we present novel data indicating that patients with recurrent/refractory ATRT have a high chance of responding to alisertib with disease stabilization and/or regression even when heavily pretreated with prior chemotherapy and radiation therapy. Aurora kinase inhibitors are one example of next-generation antimitotic agents for targeted cancer therapy. A variety of molecularly targeted agents, such as alisertib, are being developed that have more specific activity against tumor cells than standard cytotoxic agents, which would avoid many of the typical toxicities of conventional cytotoxic chemotherapy. Alisertib was well tolerated in this population of young pediatric patients without significant cumulative toxicities. This study is the first to evaluate alisertib for patients with refractory ATRT and represents the longest duration that such patients have remained on the medication with stable tumor regression. The safety profile demonstrates that prolonged treatment with alisertib in pediatric patients can be tolerated with appropriate monitoring and supportive care.
Supplementary Material
Funding
This study was supported, in part, by the National Institutes of Health Cancer Center Support (CORE) Grant P30 CA21765, Musicians Against Childhood Cancer (MACC), and the America Lebanese Syrian Associated Charities (ALSAC).
Supplementary Material
Acknowledgments
To Millennium Pharmaceuticals/the Takeda Company, especially to Theresa Bucher, Ely Benaim, and Jay Hunt for the donation of alisertib for our patients and for their support of this compassionate use request. A portion of these data were presented in abstract form at a working group meeting: Rhabdoid Tumors: Integrating Biological Insights With Clinical Successes. Institut Curie, Paris, France, November 2013.
Conflict of interest statement. The authors disclose no potential conflicts of interest.
References
- 1.Biggs PJ, Garen PD, Powers JM, et al. Malignant rhabdoid tumor of the central nervous system. Hum Pathol. 1987;18(4):332–337. [DOI] [PubMed] [Google Scholar]
- 2.Burger PC, Yu IT, Tihan T, et al. Atypical teratoid/rhabdoid tumor of the central nervous system: a highly malignant tumor of infancy and childhood frequently mistaken for medulloblastoma: a Pediatric Oncology Group study. Am J Surg Pathol. 1998;22(9):1083–1092. [DOI] [PubMed] [Google Scholar]
- 3.Parham DM, Weeks DA, Beckwith JB. The clinicopathologic spectrum of putative extrarenal rhabdoid tumors. An analysis of 42 cases studied with immunohistochemistry or electron microscopy. Am J Surg Pathol. 1994;18(10):1010–1029. [DOI] [PubMed] [Google Scholar]
- 4.Buscariollo DL, Park HS, Roberts KB, et al. Survival outcomes in atypical teratoid rhabdoid tumor for patients undergoing radiotherapy in a Surveillance, Epidemiology, and End Results analysis. Cancer. 2011;11817:4212–4219. [DOI] [PubMed] [Google Scholar]
- 5.Hilden JM, Meerbaum S, Burger P, et al. Central nervous system atypical teratoid/rhabdoid tumor: results of therapy in children enrolled in a registry. J Clin Oncol. 2004;22(14):2877–2884. [DOI] [PubMed] [Google Scholar]
- 6.Benesch M, Bartelheim K, Fleischhack G, et al. High-dose chemotherapy (HDCT) with auto-SCT in children with atypical teratoid/rhabdoid tumors (AT/RT): a report from the European Rhabdoid Registry (EU-RHAB). Bone Marrow Transplant. 2014;49(3):370–375. [DOI] [PubMed] [Google Scholar]
- 7.Chi SN, Zimmerman MA, Yao X, et al. Intensive multimodality treatment for children with newly diagnosed CNS atypical teratoid rhabdoid tumor. J Clin Oncol. 2009;27(3):385–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finkelstein-Shechter T, Gassas A, Mabbott D, et al. Atypical teratoid or rhabdoid tumors: improved outcome with high-dose chemotherapy. J Pediatr Hematol Oncol. 2010;32(5):e182–e186. [DOI] [PubMed] [Google Scholar]
- 9.Tekautz TM, Fuller CE, Blaney S, et al. Atypical teratoid/rhabdoid tumors (ATRT): improved survival in children 3 years of age and older with radiation therapy and high-dose alkylator-based chemotherapy. J Clin Oncol. 2005;23(7):1491–1499. [DOI] [PubMed] [Google Scholar]
- 10.Biegel JA, Rorke LB, Emanuel BS. Monosomy 22 in rhabdoid or atypical teratoid tumors of the brain. N Engl J Med. 1989;321(13):906. [DOI] [PubMed] [Google Scholar]
- 11.Packer RJ, Biegel JA, Blaney S, et al. Atypical teratoid/rhabdoid tumor of the central nervous system: report on workshop. J Pediatr Hematol Oncol. 2002;24(5):337–342. [DOI] [PubMed] [Google Scholar]
- 12.Kalpana GV, Marmon S, Wang W, et al. Binding and stimulation of HIV-1 integrase by a human homolog of yeast transcription factor SNF5. Science. 1994;266(5193):2002–2006. [DOI] [PubMed] [Google Scholar]
- 13.Versteege I, Sevenet N, Lange J, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394(6689):203–206. [DOI] [PubMed] [Google Scholar]
- 14.Wilson BG, Roberts CW. SWI/SNF nucleosome remodellers and cancer. Nat Rev Cancer. 2011;11(7):481–492. [DOI] [PubMed] [Google Scholar]
- 15.Biegel JA, Tan L, Zhang F, et al. Alterations of the hSNF5/INI1 gene in central nervous system atypical teratoid/rhabdoid tumors and renal and extrarenal rhabdoid tumors. Clin Cancer Res. 2002;8(11):3461–3467. [PubMed] [Google Scholar]
- 16.Jackson EM, Sievert AJ, Gai X, et al. Genomic analysis using high-density single nucleotide polymorphism-based oligonucleotide arrays and multiplex ligation-dependent probe amplification provides a comprehensive analysis of INI1/SMARCB1 in malignant rhabdoid tumors. Clin Cancer Res. 2009;15(6):1923–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieran MW, Roberts CW, Chi SN, et al. Absence of oncogenic canonical pathway mutations in aggressive pediatric rhabdoid tumors. Pediatr Blood Cancer. 2012;59(7):1155–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nikonova AS, Astsaturov I, Serebriiskii IG, et al. Aurora A kinase (AURKA) in normal and pathological cell division. Cell Mol Life Sci. 2013;70(4):661–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasai K, Parant JM, Brandt ME, et al. Targeted disruption of Aurora A causes abnormal mitotic spindle assembly, chromosome misalignment and embryonic lethality. Oncogene. 2008;27(29):4122–4127. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Cimica V, Ramachandra N, et al. Aurora A is a repressed effector target of the chromatin remodeling protein INI1/hSNF5 required for rhabdoid tumor cell survival. Cancer Res. 2011;71(9):3225–3235. [DOI] [PubMed] [Google Scholar]
- 21.Carol H, Boehm I, Reynolds CP, et al. Efficacy and pharmacokinetic/pharmacodynamic evaluation of the Aurora kinase A inhibitor MLN8237 against preclinical models of pediatric cancer. Cancer Chemother Pharmacol. 2011;68(5):1291–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dees EC, Cohen RB, von Mehren M, et al. Phase I study of aurora A kinase inhibitor MLN8237 in advanced solid tumors: safety, pharmacokinetics, pharmacodynamics, and bioavailability of two oral formulations. Clin Cancer Res. 2012;18(17):4775–4784. [DOI] [PubMed] [Google Scholar]
- 23.Mosse YP, Lipsitz E, Fox E, et al. Pediatric phase I trial and pharmacokinetic study of MLN8237, an investigational oral selective small-molecule inhibitor of Aurora kinase A: A Children's Oncology Group Phase I Consortium study. Clin Cancer Res. 2012;18(21):6058–6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaney SM, Kocak M, Gajjar A, et al. Pilot study of systemic and intrathecal mafosfamide followed by conformal radiation for infants with intracranial central nervous system tumors: a pediatric brain tumor consortium study (PBTC-001). J Neurooncol. 2012;109(3):565–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. [DOI] [PubMed] [Google Scholar]
- 26.Spence T, Sin-Chan P, Picard D, et al. CNS-PNETs with C19MC amplification and/or LIN28 expression comprise a distinct histogenetic diagnostic and therapeutic entity. Acta Neuropathol. 2014;128(2):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller S, Ward JH, Rogers HA, et al. Loss of INI1 protein expression defines a subgroup of aggressive central nervous system primitive neuroectodermal tumors. Brain Pathol. 2013;23(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hohmann AF, Vakoc CR. A rationale to target the SWI/SNF complex for cancer therapy. Trends Genet. 2014;30(8):356–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kadoch C, Hargreaves DC, Hodges C, et al. Proteomic and bioinformatic analysis of mammalian SWI/SNF complexes identifies extensive roles in human malignancy. Nat Genet. 2013;45(6):592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cervantes A, Elez E, Roda D, et al. Phase I pharmacokinetic/pharmacodynamic study of MLN8237, an investigational, oral, selective aurora a kinase inhibitor, in patients with advanced solid tumors. Clin Cancer Res. 2012;18(17):4764–4774. [DOI] [PubMed] [Google Scholar]
- 31.Eaton KW, Tooke LS, Wainwright LM, et al. Spectrum of SMARCB1/INI1 mutations in familial and sporadic rhabdoid tumors. Pediatr Blood Cancer. 2011;56(1):7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bourdeaut F, Lequin D, Brugieres L, et al. Frequent hSNF5/INI1 germline mutations in patients with rhabdoid tumor. Clin Cancer Res. 2011;17(1):31–38. [DOI] [PubMed] [Google Scholar]
- 33.Bruggers CS, Bleyl SB, Pysher T, et al. Clinicopathologic comparison of familial versus sporadic atypical teratoid/rhabdoid tumors (AT/RT) of the central nervous system. Pediatr Blood Cancer. 2011;56(7):1026–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pai Panandiker AS, Merchant TE, Beltran C, et al. Sequencing of local therapy affects the pattern of treatment failure and survival in children with atypical teratoid rhabdoid tumors of the central nervous system. Int J Radiat Oncol Biol Phys. 2012;82(5):1756–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.