Abstract
Objective
To determine if biomarkers of oxidized lipoproteins are genetically determined. Lipoprotein(a) [Lp(a)] is a heritable risk factor and carrier of oxidized phospholipids (OxPL).
Approach and Results
We measured OxPL-apoB, Lp(a), IgG and IgM autoantibodies to malondialdehyde-modified low density lipoprotein (MDA-LDL), copper oxidized LDL (CuOxLDL) and apoB-immune complexes (ApoB-IC) in 386 monozygotic and dizygotic twins to estimate trait heritability (h2) and determine specific genetic effects among traits. A genome wide linkage study followed by genetic association was performed. The h2 (scale:0-1) for Lp(a) was 0.91±0.01 and for OxPL-apoB 0.87±0.02, which were higher than physiologic, inflammatory, or lipid traits. h2 of IgM MDA-LDL, CuOxLDL and ApoB-IC were 0.69±0.04, 0.67±0.05, and 0.80±0.03, respectively, and for IgG MDA-LDL, CuOxLDL and apoB-IC 0.62±0.05, 0.52±0.06, and 0.53±0.06, respectively. There was an inverse correlation between the major apo(a) isoform and OxPL-apoB (R=-0.49, p<0.001), and Lp(a) (R=-0.48, p<0.001) and OxPL-apoB was modestly correlated with Lp(a) (ρ=0.57, p<0.0001). The correlation in major apo(a) isoform size was concordant (R=1.0, p<0.001) among monozygotic twins but not dizygotic twins (R=0.40, p=0.055). Lp(a) and OxPL-apoB shared genetic co-determination (genetic covariance: ρG = 0.774±0.032, p=1.09×10-38), though not environmental determination (environmental covariance: ρE= 0.081±0.15, p=0.15). In contrast, Lp(a) shared environmental but not genetic co-determination with autoantibodies to MDA-LDL and CuOxLDL and ApoB-IC. Sib-pair genetic linkage of the Lp(a) trait revealed that SNP rs10455872 was significantly associated with OxPL-apoB after adjusting for Lp(a).
Conclusions
OxPL-apoB and other biomarkers of oxidized lipoproteins are highly heritable cardiovascular risk factors that suggest novel genetic origins of atherothrombosis.
Keywords: heritability, lipoproteins, oxidation, atherosclerosis, thrombosis
Introduction
Cardiovascular disease (CVD) due to atherosclerosis is mediated by a variety of contributing pathways including dyslipidemia, oxidation of lipoproteins, inflammation, and thrombosis. It is well established that after entering the vessel wall, low density lipoproteins (LDL) may undergo lipid peroxidation generating oxidized LDL (OxLDL), which is taken up by macrophages leading to foam cell formation. In addition, many bioactive oxidized lipids and oxidized lipid protein adducts are formed that we have termed “oxidation-specific epitopes” (OSE).1 In addition to providing ligands on OxLDL mediating uptake in macrophages, OSE are major intermediaries between dyslipidemia and inflammation. OSE interact with cells in the vessel wall, including endothelial cells, smooth muscle cells and macrophages, to upregulate inflammatory gene expression and generate pro-inflammatory cytokines,2 leading to development of atherosclerosis and vulnerable plaques.3
It has been well documented that measurements of circulating biomarkers of oxidized lipoproteins provide insights into the pathophysiology of CVD and clinical risk prediction.4-6 These biomarkers include direct measures, such as oxidized phospholipids on apoB containing lipoproteins (OxPL-apoB)4 and OxLDL,5, 7 as well as indirect measures such as circulating autoantibodies to malondialdehyde-modified LDL (MDA-LDL), copper-oxidized LDL (CuOxLDL) and apoB-immune complexes (ApoB-IC).8, 9 The measurement of circulating OxPL-apoB has been associated with anatomical CVD, prediction of the presence and progression of atherosclerosis in a variety of arterial beds as well as with death, myocardial infarction and stroke in unselected populations (reviewed in Taleb et al4). In addition, it allows reclassification of intermediate Framingham risk patients into higher or lower risk categories.4, 10, 11 Autoantibodies to OxLDL can predict both CVD and cardiovascular events.8, 11 For example, several studies have documented that circulating IgM autoantibodies to OxLDL, which may reflect non-mutated natural antibodies, are inversely associated with CVD,11-13 possibly through protective functions of neutralization and clearance of these OSE as found on OxLDL and apoptotic cells.1 In contrast IgG autoantibodies to oxidized LDL, which are generally acquired antibodies rather than natural antibodies, are associated with higher cardiovascular risk.11, 14, 15
With the expanding data on these biomarkers, it would be important to know if they are associated with a genetic contribution to cardiovascular risk. In this study, utilizing twin pairs that allow us to address this question, we hypothesize that biomarkers of oxidized lipoproteins have a heritable component that may underlie their association with CVD.
Methods
Results
Baseline Characteristics of Study Group
Table 1 displays the baseline characteristics of the study group, representing 386 subjects and 193 twin pairs; 129 monozygotic pairs (25 male/male and 104 female/female pairs) and 64 dizygotic pairs (13 male/male, 38 female/female, and 13 male/female pairs) with mean age of 40.3, ranging from 15-84 years. The majority (n=322, 83%) of twins were of white (European or Hispanic) ancestry. The physical, physiological, biochemical and lipid parameters are typical of this age group. The median Lp(a) levels were 3.1 mg/dl, which is somewhat lower than other reports for European Caucasians with median Lp(a) levels ∼10-15 mg/dl.16 The size of the major and minor apo(a) isoforms are presented and trend to be medium to large in size, consistent with the lower Lp(a) levels.
Table 1. Characteristics of study group.
| Trait | N | Mean |
|---|---|---|
| Demographics: | ||
| Sex | 386 | |
| Male | 92 | |
| Female | 294 | |
| Ancestry | 386 | |
| European Caucasian | 322 | |
| Non-European (non-Caucasian) | 64 | |
| Age, years | 386 | 40.3 (16.9) |
| Physical: | ||
| Height (meters) | 386 | 1.67 (0.90) |
| Body weight (kilograms) | 386 | 70.0 (16.2) |
| Body Mass Index (kg/m2) | 386 | 24.9 (4.91) |
| Physiological: | ||
| Systolic Blood Pressure (mmHg) | 385 | 117.7 (17.8) |
| Diastolic Blood Pressure (mmHg) | 384 | 63.4 (11.7) |
| Heart rate (beats/min) | 385 | 71.6 (12.3) |
| Biochemical: | ||
| Plasma glucose (mg/dL) | 386 | 82.9 (20.1) |
| Plasma insulin (μUnit/ml) | 384 | 14.6 (21.9) |
| C-reactive protein (mg/L) | 375 | 0.92 (0.38-2.8) |
| Lipid values | ||
| Total cholesterol (mg/dl) | 386 | 176.7 (35.3) |
| Triglycerides (mg/dl) | 386 | 85.5 (71.7-129) |
| HDL-C (mg/dl) | 386 | 51.4 (15.3) |
| LDL-C (mg/dl) | 384 | 103.6 (30.6) |
| Apolipoprotein A-1 (mg/dl) | 386 | 136.2 (27.4) |
| Apolipoprotein B (mg/dl) | 385 | 75.8 (20.7) |
| Oxidative Biomarkers | ||
| Lp(a) (mg/dl) | 386 | 3.1 (2.0-5.5) |
| OxPL-apoB, RLU | 386 | 4196 (2819-6856) |
| IgG MDA-LDL, RLU | 386 | 5606 (4102-7462) |
| IgM MDA-LDL, RLU | 386 | 18802 (13664-27790) |
| IgG Cu-OxLDL, RLU | 386 | 4368 (3233-5758) |
| IgM Cu-OxLDL, RLU | 386 | 7627 (5207-11774) |
| IgG ApoB-IC, RLU | 386 | 15026 (10782-19181) |
| IgM ApoB-IC, RLU | 386 | 4415 (4594-9105) |
| Major apo(a) isoform, # KIV repeats all twins | 235 | 25 (20-27) |
| Minor apo(a) isoform, # KIV repeats all twins | 224 | 29 (29-34) |
| Major apo(a) Isoform, # KIV repeats | ||
| Monozygotic twins | 167 | 26 (22-30) |
| Dizygotic twins | 48 | 25 (20-28) |
| Minor apo(a) Isoform, # KIV repeats | ||
| Monozygotic twins | 157 | 32 (27-34) |
| Dizygotic twins | 48 | 31 (27-33) |
Results are given as mean value +/- SD, or as median (inter-quartile range) if not normally distributed.
RLU is relative light units.
Heritability (h2) of Lp(a), OxPL-apoB and Other Biomarkers of Oxidized Lipoproteins
The fraction of trait variance accounted for by genetic variation, h2, can be estimated by variance components in twin pairs (monozygotic versus dizygotic). We evaluated h2 on physical (BMI), physiologic (BP), inflammatory (hsCRP), lipid and apolipoproteins (LDL-C, Lp[a]), and oxidative biomarker (OxPL-apoB, and IgG and IgM autoantibodies to MDA-LDL and CuOxLDL and ApoB-IC) variables. Lp(a) and OxPL-apoB displayed high h2, at ∼87-91% of trait variance (Table 2). IgM autoantibodies to MDA-LDL and CuOxLDL and IgM apoB-IC had h2 in range of 0.67-0.80, while IgG autoantibodies to MDA-LDL and CuOxLDL and IgM apoB-IC had h2 in range of 0.52-0.62. Similar high heritability for these parameters was noted when restricting the analyses to European/Caucasian subjects (Supplementary Table 1).
Table 2. Heritability, environmental and genetic covariance of various traits in the UCSD Twin group.
| Trait | Heritability | Environmental covariance (with Lp[a]) | Genetic covariance (with Lp[a]) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | h2 | SEM | p | Rho E | SEM | p | Rho G | SEM | p | |
|
| ||||||||||
| Physical | ||||||||||
| Body mass index, g/m2 | 386 | 0.86 | 0.02 | <0.0001 | -0.10 | 0.10 | 0.35 | -0.08 | 0.09 | 0.40 |
| Physiological | <0.0001 | |||||||||
| SBP (mmHg) | 380 | 0.46 | 0.06 | <0.0001 | -0.17 | 0.10 | 0.085 | -0.14 | 0.11 | 0.22 |
| DBP (mmHg) | 380 | 0.52 | 0.06 | <0.0001 | -0.19 | 0.10 | 0.072 | -0.06 | 0.11 | 0.57 |
| Metabolic | ||||||||||
| Plasma glucose, mg/dl | 380 | 0.47 | 0.07 | <0.0001 | -0.40 | 0.09 | 0.0001 | -0.18 | 0.11 | 0.11 |
| Plasma insulin, μUnit/ml | 380 | 0.50 | 0.09 | <0.0001 | -0.23 | 0.09 | 0.018 | -0.19 | 0.13 | 0.15 |
| Biochemical and lipid | ||||||||||
| C-reactive protein, mg/L | 375 | 0.60 | 0.05 | <0.0001 | 0.15 | 0.10 | 0.17 | 0.05 | 0.11 | 0.62 |
| Total cholesterol, mg/dL | 386 | 0.40 | 0.08 | <0.0001 | 0.31 | 0.09 | 0.0023 | -0.05 | 0.11 | 0.68 |
| Triglycerides, mg/dL | 386 | 0.64 | 0.06 | <0.0001 | -0.14 | 0.09 | 0.13 | -0.15 | 0.12 | 0.19 |
| HDL-C, mg/dL | 386 | 0.68 | 0.05 | <0.0001 | 0.27 | 0.10 | 0.013 | 0.27 | 0.15 | 0.08 |
| LDL-C, mg/dL | 386 | 0.40 | 0.08 | <0.0001 | 0.31 | 0.09 | 0.0012 | 0.02 | 0.10 | 0.85 |
| ApoA1, mg/dL | 386 | 0.62 | 0.06 | <0.0001 | 0.20 | 0.10 | 0.046 | 0.08 | 0.10 | 0.40 |
| ApoB, mg/dL | 386 | 0.47 | 0.08 | <0.0001 | 0.30 | 0.09 | 0.0035 | 0.01 | 0.10 | 0.90 |
| Oxidation-Specific Biomarkers | ||||||||||
| Lp(a), mg/dl | 386 | 0.91 | 0.01 | 1.65E-55 | N/A | N/A | N/A | N/A | N/A | N/A |
| OxPL-apoB, RLU | 386 | 0.87 | 0.02 | 1.22E-12 | 0.118 | 0.081 | 0.15 | 0.774 | 0.032 | 1.09E-38 |
| IgG ApoB-IC, RLU | 386 | 0.53 | 0.06 | 5.55E-34 | 0.289 | 0.076 | 0.0001 | 0.141 | 0.086 | 0.108 |
| IgM ApoB-IC, RLU | 386 | 0.80 | 0.03 | 2.64E-17 | 0.178 | 0.082 | 0.034 | 0.049 | 0.074 | 0.512 |
| IgG MDA-LDL, RLU | 386 | 0.62 | 0.05 | 1.93E-21 | 0.233 | 0.080 | 0.005 | 0.006 | 0.082 | 0.941 |
| IgM MDA-LDL, RLU | 386 | 0.69 | 0.04 | 2.62E-12 | 0.184 | 0.082 | 0.030 | -0.002 | 0.079 | 0.983 |
| IgG Cu-OxLDL, RLU | 386 | 0.52 | 0.06 | 3.62E-20 | 0.197 | 0.080 | 0.017 | 0.115 | 0.088 | 0.194 |
| IgM Cu-OxLDL, RLU | 386 | 0.67 | 0.05 | 1.30E-47 | 0.207 | 0.082 | 0.014 | 0.025 | 0.080 | 0.751 |
The environmental and genetic covariance estimates refer to co-determination with the Lp(a) trait.
N/A=not applicable as this is the comparison trait
Genetic vs. Environmental Covariance of Lp(a), OxPL-apoB and Biomarkers of Oxidized Lipoproteins
When two traits are highly correlated, the basis of the correlation may be either shared genetic determination (genetic covariance, or pleiotropy; ρG), or shared environmental determination (environmental covariance; ρE). Such distinctions can be approached by variance components in the classical twin design. Thus, we studied such co-determinations for the Lp(a) trait in the twin series (Table 2, Figure 1) and found that the sole Lp(a) correlation based on genetic covariance (ρG=0.774±0.032, p=1.09×10-38) was for OxPL-apoB. By contrast, Lp(a) correlations with other risk traits, including IgG and IgM autoantibodies to MDA-LDL and CuOxLDL and apoB-IC, were mediated largely by environmental covariance (ρE) (Figure 1).
Figure 1. Relationship of Lp(a) to shared genetic and environmental determinants.
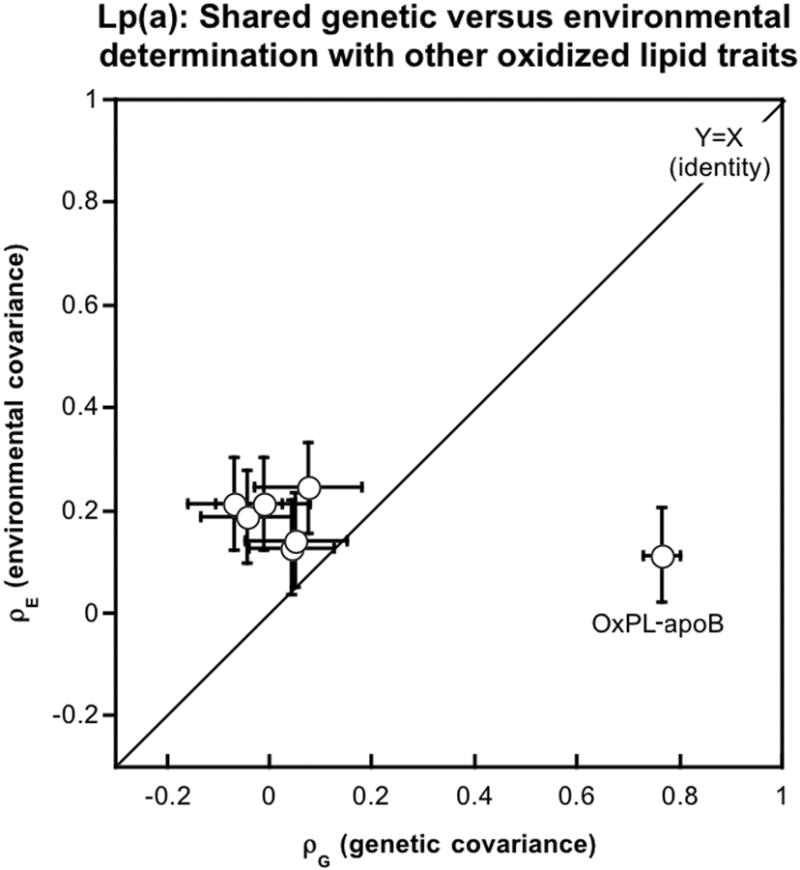
Genetic vs. environmental covariance of Lp(a) with OxPL-apoB, or other biomarkers of oxidized lipoproteins (IgG and IgM autoantibodies to MDA-LDL and CuOxLDL and apoB-IC). Results are shown as the estimate +/- standard error(SE). The diagonal is a line of identity (Y=X). Covariance estimates and their SE values are found in Table 2.
Relationship between size of apo(a) isoforms among monozygotic and dizygotic twins
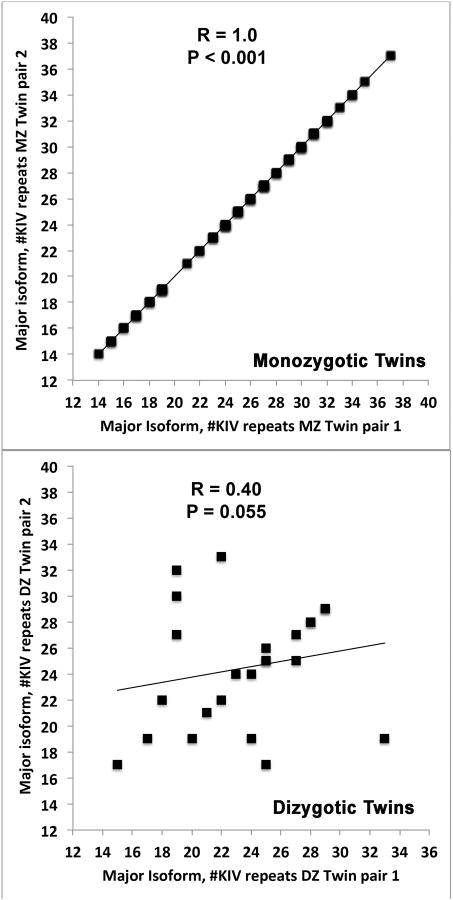
As expected, the correlation in major apo(a) isoform size among monozygotic twin pairs was completely concordant (R=1.0, p<0.001). In contrast, the correlation size among dizygotic twin pairs was lower and of borderline significance (R=0.40, p=0.055) (Figure 2). For the minor isoform, the correlation among monozygotic twins was also completely concordant (R=1.0, p<0.001), but the correlation size among dizygotic twins was higher than the correlation of the major isoform (R=0.74, p=0<0.001).
Figure 2. Relationship of apo(a) isoforms in monozygotic and dizygotic twins.
Correlation of the major apo(a) isoform as measured by number of K4 repeats within monozygotic (A) and dizygotic (B) twin pairs.
Correlations Between Major and Minor apo(a) isoforms, OxPL-apoB, Lp(a) and Biomarkers of Oxidized Lipoproteins
A correlation analysis was carried in out in twins pairs with complete data on isoforms, OxPL-apoB and other biomarkers of oxidized lipoproteins. A modest positive correlation was noted between the major and minor apo(a) isoforms (R=0.48, p<0.001). There was an inverse correlation between the major isoform and OxPL-apoB (R=-0.49, p<0.001), and Lp(a) (R=-0.48, p<0.001) (Table 3). Neither major or minor apo(a) isoforms correlated with other oxidative biomarkers. Lp(a) was modestly correlated with OxPL-apoB (R=0.57, p<0.001) and weakly with IgG apoB-IC, IgG MDA-LDL and Cu-OxLDL but not with IgM MDA-LDL and Cu-OxLDL or apoB-IC. Modest to strong correlations were noted among the IgG and IgM classes of oxidative biomarkers.
Table 3. Correlations Among Major and Minor Apo(a) Isoforms, OxPL-apoB, Lp(a) and Other Biomarkers of Oxidized Lipoproteins.
| Trait | Spearman's rho | Major isoform |
Minor Isoform |
Lp(a) | OxPL-apoB | IgG ApoB-IC |
IgM ApoB-IC |
IgG MDA-LDL |
IgM MDA-LDL |
IgG CuOxLDL |
IgM CuOxLDL |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Major isoform | CorrCoeff | 0.48 | -0.48 | -0.49 | -0.02 | 0.02 | 0.02 | 0.08 | -0.02 | 0.07 | |
| P-Value | <0.001 | <0.001 | <0.001 | 0.74 | 0.83 | 0.82 | 0.21 | 0.78 | 0.30 | ||
|
| |||||||||||
| Minor Isoform | CorrCoeff | -0.015 | -0.30 | -0.05 | -0.11 | 0.07 | 0.05 | 0.10 | 0.02 | ||
| P-Value | 0.021 | <0.001 | 0.45 | 0.12 | 0.29 | 0.48 | 0.13 | 0.74 | |||
|
| |||||||||||
| Lp(a) | CorrCoeff | 0.57 | 0.20 | 0.07 | 0.10 | 0.06 | 0.16 | 0.08 | |||
| P-Value | <0.001 | <0.001 | 0.19 | 0.05 | 0.26 | 0.001 | 0.14 | ||||
|
| |||||||||||
| OxPL-apoB | CorrCoeff | 0.26 | 0.11 | 0.06 | 0.09 | 0.09 | 0.09 | ||||
| P-Value | <0.001 | 0.036 | 0.29 | 0.09 | 0.08 | 0.10 | |||||
|
| |||||||||||
| IgG ApoB-IC | CorrCoeff | 0.22 | 0.69 | 0.13 | 0.59 | 0.14 | |||||
| P-Value | <0.001 | <0.001 | 0.010 | <0.001 | 0.008 | ||||||
|
| |||||||||||
| IgM ApoB-IC | CorrCoeff | 0.22 | 0.69 | 0.24 | 0.72 | ||||||
| P-Value | <0.001 | <0.001 | <0.001 | <0.001 | |||||||
|
| |||||||||||
| IgG MDA-LDL | CorrCoeff | 0.23 | 0.80 | 0.21 | |||||||
| P-Value | <0.001 | <0.001 | <0.001 | ||||||||
|
| |||||||||||
| IgM MDA-LDL | CorrCoeff | 0.25 | 0.88 | ||||||||
| P-Value | <0.001 | <0.001 | |||||||||
|
| |||||||||||
| IgG CuOxLDL | CorrCoeff | 0.28 | |||||||||
| P-Value | <0.001 | ||||||||||
The correlation analysis was carried in out in twins pairs with complete data on isoforms, OxPL-apoB and other biomarkers of oxidized lipoproteins (n=224).
In the monozygotic twins, there was an inverse correlation between the major isoform and OxPL-apoB (R=-0.51, p<0.001), and Lp(a) (R=-0.46, p<0.001) and between the minor isoform and OxPL-apoB (R=-0.36, p<0.001), but not Lp(a) (R=-0.12, p=0.12). In the dizygotic twins, there was a similar inverse correlation between the major isoform and OxPL-apoB (R=-0.49, p<0.001), and Lp(a) (R=-0.49, p<0.001) but not between the minor isoform and OxPL-apoB (R=-0.14, p=0.32), and Lp(a) (R=-0.17, p=0.23).
Genetic Linkage and Association Analyses of the Lp(a) trait
Genetic linkage
To probe specific genomic regions that might underlie the heritability of such traits, we first turned to genome-wide microsatellite linkage (or meiotic co-segregation) in a series of sibling pairs derived from the twins and sibships. For the Lp(a) trait, we noticed a broad (∼40 cM) biphasiclinkageregion (with peak LOD=4.36) on chromosome 6q (Figure 3A), whose confidence interval included the LPA (Lp(a]) locus on chromosome 6q26. Other chromosomes did not display significant LOD peaks. No genome wide significant linkages were noted for the other oxidative biomarkers.
Figure 3. Genetic linkage and association analysis of Lp(a).
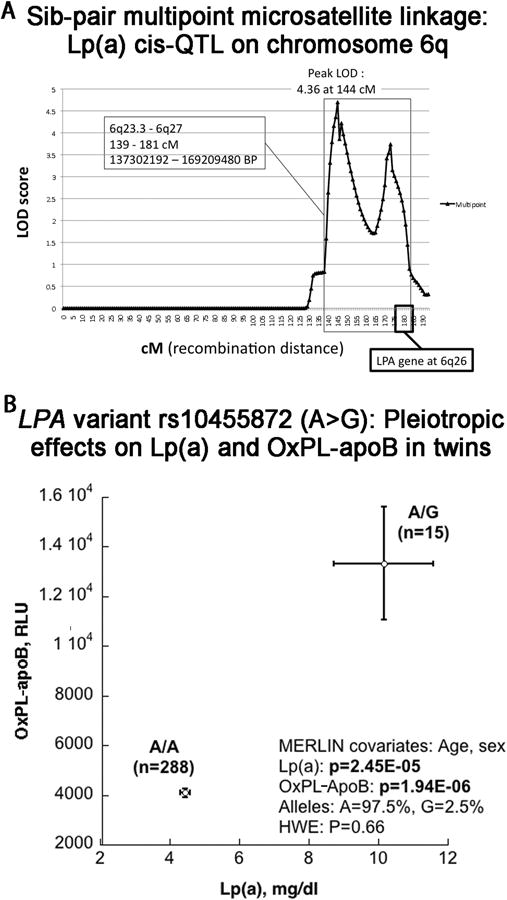
3A. Genetic linkage (meiotic cosegregation of marker and Lp(a) trait) in sibling pairs, plotted on chromosome 6 (harboring the LPA locus on 6q26). Genetic (meiotic recombination) distance is in cM (horizontal axis). LOD score (vertical axis) indicates the Log[10] of the odds ratio for linkage. A LOD=4.36 is considered significant at the genome-wide level.
3B. Genetic association (during dense SNP genotyping) for the Lp(a) and OxPL-apoB traits in individuals, plotted (mean +/- SEM) as a function of the peak associated SNP at the LPA locus for each trait: intronic variant rs10555872 (A>G). Minor (G) allele carriers display coordinate elevations of both Lp(a) and OxPL-apoB. HWE: Hardy Weinberg Equilibrium.
Genetic association
To further investigate the LPA locus itself as a source of genetic variation that might influence circulating Lp(a) concentration, we conducted dense SNP genotyping across the chromosome 6q26 region harboring LPA. The entire ∼135 kbp LPA locus (RefSeq NM_005577.2), as well as its proximal promoter, is contained within one linkage disequilibrium (LD) block in our subjects, as defined by local cM/Mb (recombination rate) boundaries. Dense SNP genotyping across the LPA region, including 76 SNPs (Supplementary Table II), demonstrated that rs10455872 [A (n=288) > G (n=15)], with frequency of the G allele at 2.5%, displays the peak association with significantly increased levels of both Lp(a) (p=2.45×10-5) and OxPL-apoB (p=1.94×10-6) (Figure 3B). To test if the effect of rs10455872 on OxPL-apoB was independent of its association with Lp(a), Lp(a) levels were adjusted in secondary analyses. After adjustment, the association of rs10455872 with OxPL-apoB was still significant (beta=-0.25, SE=0.09, p=0.006).
A cartoon of the physical relationships of Lp(a) and OxPL summarizing the specific pleiotropic effect of one gene (LPA, snp rs10455872) on two traits: Lp(a) and OxPL-apoB is shown in Figure 4.
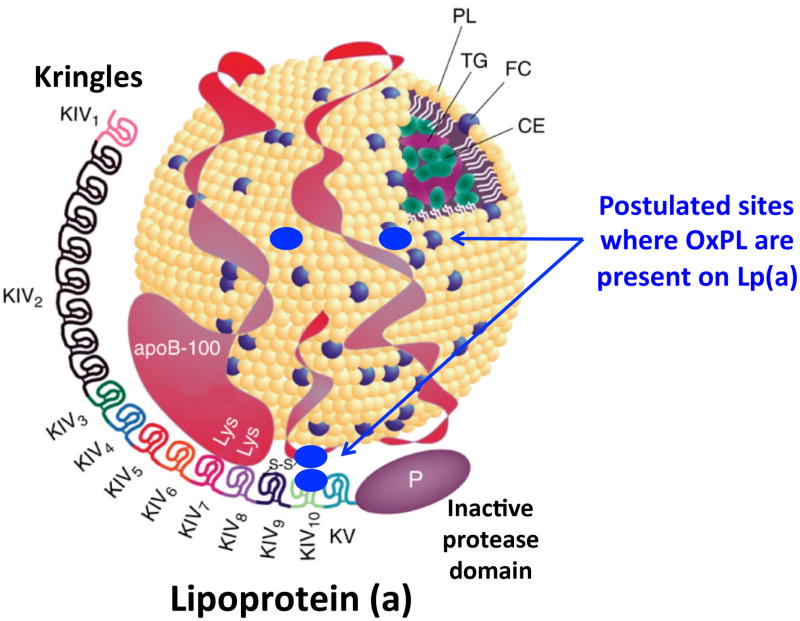
Figure 4. Postulated sites of OxPL binding to Lp(a).
Cartoon of the proposed relationship of OxPL to Lp(a) denoting that OxPL is present covalently bound to apo(a) and also in the lipid phase of apoB. The figure was modified from Albers et al 41 with permission.
Discussion
This study demonstrates that biomarkers of oxidized lipoproteins are highly heritable cardiovascular risk factors. Importantly, the heritability of OxPL-apoB, autoantibodies to OxLDL and apoB-immune complexes were substantially higher than other physiologic, inflammatory, and lipid traits measured in the same subjects. These observations suggest previously unexplored genetic origins for atherosclerosis mediated by oxidized lipoproteins.
Since Lp(a) is the major lipoprotein carrier of OxPL,24,25 the high heritability of the biomarker OxPL-apoB as a cardiovascular risk factor, as well as its genetic covariance (shared heritability) with Lp(a), naturally follows.17, 18 However, it was also demonstrated that SNP rs10458872 correlates with OxPL-apoB even after adjusting for Lp(a) levels, suggesting pleiotropic effects on OxPL-apoB and Lp(a). This may be due to the fact that rs10458872 is associated with smaller apo(a) isoforms that mediate higher Lp(a) levels,19 which are particularly enriched in OxPL as demonstrated in the Dallas Heart Study.20 Thus, rs10458872 may reflect the presence of the most atherogenic and OxPL-enriched Lp(a) particles. It is now well accepted that Lp(a) is a highly heritable,21, 22 and likely causal risk factor for CVD, particularly with the recent strong supporting data from epidemiological, genome wide association and mendelian randomization studies demonstrating its independent predictive role in CVD, myocardial infarction and aortic stenosis and calcification.19, 23-26 The OxPL-apoB assay quantitates OxPL on apoB-containing lipoproteins, but this primarily reflects the OxPL bound to Lp(a), both covalently to apo(a) and in the lipid phase of apoB17, 18 (i.e.,Lp(a)-apoB particles), as shown in Figure 4. We have previously shown that the correlation of OxPL-apoB with Lp(a) is variable, with Spearman range of r=0.13-0.88, depending on racial differences, the underlying genetics of the LPA gene, and specifically in the number of kringle IV repeats.20 In general, the OxPL-apoB correlates best with Lp(a) plasma levels when small apo(a) isoforms are present in setting of elevated plasma Lp(a) levels, irrespective of race.20, 27 In the present study, the relatively modest correlation between OxPL-apoB and Lp(a) levels (Spearman ρ=0.57, p<0.0001) reflects the fact that this twin cohort tended to have medium to large isoforms and corresponding lower Lp(a) levels. This variability between OxPL-apoB levels and Lp(a) likely reflects the fact that in some studies OxPL-apoB remains an independent predictor of CVD risk even when adjusted for Lp(a) levels, similar to this study with the SNP rs10455872.28, 29 We previously also showed that the LPA SNP rs3798220, which is associated with elevated Lp(a) levels was also associated with elevated OxPL-apoB levels.30 In aggregate, the current genetic data reinforce the hypothesis that the content of OxPL on Lp(a) is an important biological mediator of the enhanced atherogenicity of Lp(a).
In support of the relationship between Lp(a) and OxPL-apoB and the fact that Lp(a) strongly binds OxPL,17, 18 these analyses show that the association between Lp(a) and OxPL-apoB is mainly determined by genetic factors, as opposed to environmental factors. This is supported by the fact that there is overwhelmingly genetic codetermination (measured by the genetic covariance of ρG0.77±0.03) but not environmental codetermination (measured by ρE) 0.12±0.08 and by the strong correlation between Lp(a) and OxPL-apoB (Spearman ρ=0.56). In contrast, although individually highly heritable traits, the associations between Lp(a) and autoantibodies to OxLDL and apoB-IC are determined by environmental factors, as supported by weak or absent correlations, as opposed to pleiotropic genetic effects. Thus, although autoantibodies to OxLDL and apoB-IC are also highly heritable, they do not share significant heritability with Lp(a), or even with OxPL-apoB consistent with different genetic origins of heritability. Interestingly, the relationship between the major apo(a) isoform size and OxPL-apoB and Lp(a) was similar, with Spearman ρ in the range of r=-0.50. This is consistent with prior observations from the Dallas Heart Study in Blacks, Whites and Hispanics and supports the notion that the size of the apo(a) isoform can explain approximately 25% of both Lp(a) and OxPL-apoB levels.20 In contrast, the minor apo(a) isoform had significantly weaker or absent correlation with both OxPL-apoB and Lp(a), consistent with its lesser role in mediating plasma Lp(a) levels.
The perfect concordance of the major and minor apo(a) isoforms within monozygotic twin pairs is expected based on these subjects carrying identical alleles. Interestingly, in dizygotic twins the correlation coefficient ranged from 0.40-0.74, explaining 16-50% of this relationship, providing a reflection of the genetic variation at the LPA locus. However, this variability only reflects genetic differences in kringle IV repeats and does not take into account snps or regulatory elements in the LPA gene mediating expression of Lp(a).31 Microsatellite linkage in a series of sibling pairs derived from the twins and sibships isolated Lp(a) trait determination to chromosome 6q26, encompassing the known location of the LPA gene. Further dense SNP genotyping revealed that LPA intronic SNP rs10455872 was associated with elevated levels of both Lp(a) and OxPL-apoB, and was independently associated with OxPL-apoB when adjusting for Lp(a) levels. The lack of genome wide significance for an association of plasma levels of OxPL-apoB and indirect oxidative biomarkers suggests either low power or multifactorial etiologies of their respective plasma levels.
This study additionally demonstrates that IgG and IgM autoantibodies to OxLDL and apoB-immune complexes are highly heritable biomarkers. As the experimental and clinical database grows on the role of autoantibodies to OxLDL, it has become evident that IgG and IgM titers reflect different clinical risk predictions. In general, IgG autoantibody levels to OxLDL are associated with higher CVD risk, whereas IgM autoantibodies are associated with lower risk.8, 11, 13 In the Bruneck study with prospective 15 year follow-up, IgM autoantibodies to MDA-LDL had a fully adjusted hazard ratio 0.69 for death, MI and stroke, consistent with an overall protective effect, whereas IgG autoantibodies to MDA-LDL had a hazard ratio of 1.2 for predicting a death, MI and stroke.11 These findings are consistent with the only other study evaluating the genetic role of such biomarkers by Paavola et al32 showing h2=0.28-0.65 for IgG, IgM and IgA autoantibodies to OxLDL but studied only in subjects with low HDL-C. Their heritability study also included patients with a high prevalence of CHD (39%) and on statin therapy, which are known to affect levels of such biomarkers.8 In contrast, our twin study had no patients with CAD or on statin therapy, and therefore has fewer confounding variables and is thus a more robust assessment of the h2 of these biomarkers. However, both studies show a surprisingly strong genetic basis for antibody responses to OSE. In part, this may reflect the known genetic underpinnings of CAD, as well as a hereditary influence on immune responses.
In support of the clinical outcomes data, experimental studies in IgM-/-/Ldlr-/-negative mice, lacking IgM antibodies, demonstrate a 7-fold acceleration in atherosclerosis compared to Ldlr-/- controls.33, 34 In fact, it has also demonstrated in both mice and humans that IgM autoantibodies to OSE represent ∼15-30% of the total IgM repertoire, suggesting important homeostatic functions.35 The mechanisms through which IgM antibodies may be protective are not fully defined, but for the most part they reflect the presence of “natural” antibodies that are present at birth and are presumed to be positively selected for their overall benefit to maintain homeostasis. These may function in housekeeping roles such as neutralization and/or clearance of OSE present on apoptotic cells and oxidized lipoproteins, and in binding to and preventing pro-inflammatory effects of OSE.1
Limitations of this study are that this cohort of patients is relatively young, healthy and primarily of European Caucasian descent, with relatively low Lp(a) levels. Therefore, there are no cardiovascular outcomes associated to link the heritability of these measures to clinical outcomes. However lack of CVD allows us to more cleanly interpret the findings of heritability, and provides evidence that heritability of these components contributes to the innate and adaptive immune response in atherogenesis.
Significance
These findings have clinical implications. First, they support the epidemiological and trial data that OxPL-apoB is a strong risk factor for CVD and that it reflects the clinical risk mediated by the most atherogenic Lp(a) particles.4, 11, 36, 37 Second, the data imply that natural antibodies to OSE, may be useful as diagnostic agents in biomarker assays,4 molecular imaging approaches targeting oxidation-rich lesions38 and therapeutic applications using passive immunization approaches.39 Finally, further understanding the potential genetic determinants of CVD may enable insights into the functions of the adaptive and innate immune systems, allowing novel immunomodulatory approaches to optimize anti-atherogenic strategies to reduce CVD risk or events.1, 40
Conclusion
In this twin study, we demonstrate that biomarkers of oxidized lipoproteins are highly heritable cardiovascular risk factors. Advantages of this study include the inclusion of twins allowing estimation of heritability, the presence of multiple phenotypes allowing estimation of genetic and environmental covariance and presence of multiple ethnicities. This observation along with the clinical data that these biomarkers predict CVD risk11 indicates novel genetic origins to be probed for risk of atherothrombosis mediated by oxidized lipoproteins and Lp(a).
Supplementary Material
Acknowledgments
The authors wish to dedicate this work to the memory Daniel T. O'Connor, whose body of work, particularly with the UCSD Twins cohort, and passion
Sources of Funding: NIH R01-HL119828, P01-HL088093, P01-HL055798, R01-HL093767 R01-HL086599 and Fondation Leducq (ST, JLW), DK094894 and HL58120 (DTO'C), and P30 DK079337 (CMN, DTO'C).
Abbreviations
- MDA-LDL
malondialdehyde-modified low density lipoprotein
- CuOxLDL
copper oxidized LDL
- ApoB-IC
apoB-immune complexes
- OxPL
oxidized phospholipids
- OxLDL
oxidized LDL
- OSE
oxidation-specific epitopes
Footnotes
Disclosure: Drs. Tsimikas and Witztum are co-inventors of and receive royalties from patents owned by the University of California San Diego on oxidation-specific antibodies and mimotopes. Dr. Tsimikas has a dual appointment at UCSD and as an employee at Isis Pharmaceuticals, Inc. Dr. Witztum has received honoraria for consulting for Isis, CymaBay and Intercept Pharmaceuticals. The other authors report no conflicts.
References
- 1.Miller YI, Choi SH, Wiesner P, Fang L, Harkewicz R, Hartvigsen K, Boullier A, Gonen A, Diehl CJ, Que X, Montano E, Shaw PX, Tsimikas S, Binder CJ, Witztum JL. Oxidation-specific epitopes are danger-associated molecular patterns recognized by pattern recognition receptors of innate immunity. Circ Res. 2011;108:235–248. doi: 10.1161/CIRCRESAHA.110.223875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee S, Birukov KG, Romanoski CE, Springstead JR, Lusis AJ, Berliner JA. Role of phospholipid oxidation products in atherosclerosis. Circ Res. 2012;111:778–799. doi: 10.1161/CIRCRESAHA.111.256859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Dijk RA, Kolodgie F, Ravandi A, Leibundgut G, Hu PP, Prasad A, Mahmud E, Dennis E, Curtiss LK, Witztum JL, Wasserman BA, Otsuka F, Virmani R, Tsimikas S. Differential expression of oxidation-specific epitopes and apolipoprotein(a) in progressing and ruptured human coronary and carotid atherosclerotic lesions. J Lipid Res. 2012;53:2773–2790. doi: 10.1194/jlr.P030890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taleb A, Witztum JL, Tsimikas S. Oxidized phospholipids on apolipoprotein b-100 (oxpl/apob) containing lipoproteins: A biomarker predicting cardiovascular disease and cardiovascular events. Biomarkers Med. 2011;5:673–694. doi: 10.2217/bmm.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Itabe H. Oxidized low-density lipoprotein as a biomarker of in vivo oxidative stress: From atherosclerosis to periodontitis. J Clin Biochem Nutr. 2012;51:1–8. doi: 10.3164/jcbn.11-00020R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsimikas S, Hall JH. Lipoprotein(a) as a potential causal genetic risk factor of cardiovascular disease: A rationale for increased efforts to understand its pathophysiology and develop targeted therapies. J Am Coll Cardiol. 2012;60:716–721. doi: 10.1016/j.jacc.2012.04.038. [DOI] [PubMed] [Google Scholar]
- 7.Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR., Jr Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299:2287–2293. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gounopoulos P, Merki E, Hansen LF, Choi SH, Tsimikas S. Antibodies to oxidized low density lipoprotein: Epidemiological studies and potential clinical applications in cardiovascular disease. Minerva Cardioangiol. 2007;55:821–837. [PubMed] [Google Scholar]
- 9.Lopes-Virella MF, Virella G. Clinical significance of the humoral immune response to modified LDL. Clin Immunol. 2010;134:55–65. doi: 10.1016/j.clim.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bertoia ML, Pai JK, Lee JH, Taleb A, Joosten MM, Mittleman MA, Yang X, Witztum JL, Rimm EB, Tsimikas S, Mukamal KJ. Oxidation-specific biomarkers and risk of peripheral artery disease. J Am Coll Cardiol. 2013;61:2169–2179. doi: 10.1016/j.jacc.2013.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsimikas S, Willeit P, Willeit J, Santer P, Mayr M, Xu Q, Mayr A, Witztum JL, Kiechl S. Oxidation-specific biomarkers, prospective 15-year cardiovascular and stroke outcomes, and net reclassification of cardiovascular events. J Am Coll Cardiol. 2012;60:2218–2229. doi: 10.1016/j.jacc.2012.08.979. [DOI] [PubMed] [Google Scholar]
- 12.Ravandi A, Boekholdt SM, Mallat Z, Talmud PJ, Kastelein JJ, Wareham NJ, Miller ER, Benessiano J, Tedgui A, Witztum JL, Khaw KT, Tsimikas S. Relationship of IgG and IgmM autoantibodies and immune complexes to oxidized LDL with markers of oxidation and inflammation and cardiovascular events: Results from the epic-norfolk study. J Lipid Res. 2011;52:1829–1836. doi: 10.1194/jlr.M015776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karvonen J, Paivansalo M, Kesaniemi YA, Horkko S. Immunoglobulin M type of autoantibodies to oxidized low-density lipoprotein has an inverse relation to carotid artery atherosclerosis. Circulation. 2003;108:2107–2112. doi: 10.1161/01.CIR.0000092891.55157.A7. [DOI] [PubMed] [Google Scholar]
- 14.Mustafa A, Nityanand S, Berglund L, Lithell H, Lefvert AK. Circulating immune complexes in 50-year-old men as a strong and independent risk factor for myocardial infarction. Circulation. 2000;102:2576–2581. doi: 10.1161/01.cir.102.21.2576. [DOI] [PubMed] [Google Scholar]
- 15.Rossi GP, Cesari M, De Toni R, Zanchetta M, Maiolino G, Pedon L, Ganzaroli C, Maiolino P, Pessina AC. Antibodies to oxidized low-density lipoproteins and angiographically assessed coronary artery disease in white patients. Circulation. 2003;108:2467–2472. doi: 10.1161/01.CIR.0000097122.19430.48. [DOI] [PubMed] [Google Scholar]
- 16.Nordestgaard BG, Chapman MJ, Ray K, et al. European Atherosclerosis Society Consensus P. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur Heart J. 2010;31:2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergmark C, Dewan A, Orsoni A, Merki E, Miller ER, Shin MJ, Binder CJ, Horkko S, Krauss RM, Chapman MJ, Witztum JL, Tsimikas S. A novel function of lipoprotein [a] as a preferential carrier of oxidized phospholipids in human plasma. J Lipid Res. 2008;49:2230–2239. doi: 10.1194/jlr.M800174-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Leibundgut G, Scipione C, Yin H, Schneider M, Boffa MB, Green S, Yang X, Dennis EA, Witztum JL, Koschinsky ML, Tsimikas S. Determinants of binding of oxidized phospholipids on apolipoprotein (a) and lipoprotein (a) J Lipid Res. 2013;54:2815–2830. doi: 10.1194/jlr.M040733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke R, Peden JF, Hopewell JC, et al. Genetic variants associated with lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361:2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 20.Tsimikas S, Clopton P, Brilakis ES, Marcovina SM, Khera A, Miller ER, de Lemos JA, Witztum JL. Relationship of oxidized phospholipids on apolipoprotein b-100 particles to race/ethnicity, apolipoprotein(a) isoform size, and cardiovascular risk factors: Results from the dallas heart study. Circulation. 2009;119:1711–1719. doi: 10.1161/CIRCULATIONAHA.108.836940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Austin MA, Sandholzer C, Selby JV, Newman B, Krauss RM, Utermann G. Lipoprotein(a) in women twins: Heritability and relationship to apolipoprotein(a) phenotypes. Am J Hum Genet. 1992;51:829–840. [PMC free article] [PubMed] [Google Scholar]
- 22.Lamon-Fava S, Jimenez D, Christian JC, Fabsitz RR, Reed T, Carmelli D, Castelli WP, Ordovas JM, Wilson PW, Schaefer EJ. The nhlbi twin study: Heritability of apolipoprotein a-i, b, and low density lipoprotein subclasses and concordance for lipoprotein(a) Atherosclerosis. 1991;91:97–106. doi: 10.1016/0021-9150(91)90191-5. [DOI] [PubMed] [Google Scholar]
- 23.Kronenberg F, Utermann G. Lipoprotein(a): Resurrected by genetics. J Int Med. 2013;273:6–30. doi: 10.1111/j.1365-2796.2012.02592.x. [DOI] [PubMed] [Google Scholar]
- 24.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301:2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 25.Erqou S, Kaptoge S, Perry PL, Di Angelantonio E, Thompson A, White IR, Marcovina SM, Collins R, Thompson SG, Danesh J. Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302:412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thanassoulis G, Campbell CY, Owens DS, et al. Genetic associations with valvular calcification and aortic stenosis. N Engl J Med. 2013;368:503–512. doi: 10.1056/NEJMoa1109034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kiechl S, Willeit J, Mayr M, Viehweider B, Oberhollenzer M, Kronenberg F, Wiedermann CJ, Oberthaler S, Xu Q, Witztum JL, Tsimikas S. Oxidized phospholipids, lipoprotein(a), lipoprotein-associated phospholipase a2 activity, and 10-year cardiovascular outcomes: Prospective results from the bruneck study. Arterioscler Thromb Vasc Biol. 2007;27:1788–1795. doi: 10.1161/ATVBAHA.107.145805. [DOI] [PubMed] [Google Scholar]
- 28.Tsimikas S, Brilakis ES, Miller ER, McConnell JP, Lennon RJ, Kornman KS, Witztum JL, Berger PB. Oxidized phospholipids, lp(a) lipoprotein, and coronary artery disease. N Engl J Med. 2005;353:46–57. doi: 10.1056/NEJMoa043175. [DOI] [PubMed] [Google Scholar]
- 29.Tsimikas S, Mallat Z, Talmud PJ, Kastelein JJ, Wareham NJ, Sandhu MS, Miller ER, Benessiano J, Tedgui A, Witztum JL, Khaw KT, Boekholdt SM. Oxidation-specific biomarkers, lipoprotein(a), and risk of fatal and nonfatal coronary events. J Am Coll Cardiol. 2010;56:946–955. doi: 10.1016/j.jacc.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 30.Arai K, Luke MM, Koschinsky ML, Miller ER, Pullinger CR, Witztum JL, Kane JP, Tsimikas S. The i4399m variant of apolipoprotein(a) is associated with increased oxidized phospholipids on apolipoprotein b-100 particles. Atherosclerosis. 2010;209:498–503. doi: 10.1016/j.atherosclerosis.2009.09.077. [DOI] [PubMed] [Google Scholar]
- 31.Hoover-Plow J, Huang M. Lipoprotein(a) metabolism: Potential sites for therapeutic targets. Metabolism: clinical and experimental. 2013;62:479–491. doi: 10.1016/j.metabol.2012.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paavola T, Kangas-Kontio T, Salonurmi T, Kuusisto S, Huusko T, Savolainen MJ, Kakko S. Plasma levels of antibodies against oxidized LDL are inherited but not associated with hdl-cholesterol level in families with early coronary heart disease. Atherosclerosis. 2012;224:123–128. doi: 10.1016/j.atherosclerosis.2012.06.056. [DOI] [PubMed] [Google Scholar]
- 33.Kyaw T, Tay C, Krishnamurthi S, Kanellakis P, Agrotis A, Tipping P, Bobik A, Toh BH. B1a b lymphocytes are atheroprotective by secreting natural igm that increases igm deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res. 2011;109:830–U834. doi: 10.1161/CIRCRESAHA.111.248542. [DOI] [PubMed] [Google Scholar]
- 34.Lewis MJ, Malik TH, Ehrenstein MR, Boyle JJ, Botto M, Haskard DO. Immunoglobulin m is required for protection against atherosclerosis in low-density lipoprotein receptor-deficient mice. Circulation. 2009;120:417–426. doi: 10.1161/CIRCULATIONAHA.109.868158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chou MY, Fogelstrand L, Hartvigsen K, Hansen LF, Woelkers D, Shaw PX, Choi J, Perkmann T, Backhed F, Miller YI, Horkko S, Corr M, Witztum JL, Binder CJ. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Res. 2009;119:1335–1349. doi: 10.1172/JCI36800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsimikas S, Duff GW, Berger PB, Rogus J, Huttner K, Clopton P, Brilakis E, Kornman KS, Witztum JL. Pro-inflammatory interleukin-1 genotypes potentiate the risk of coronary artery disease and cardiovascular events mediated by oxidized phospholipids and lipoprotein(a) J Am Coll Cardiol. 2014;63:1724–1734. doi: 10.1016/j.jacc.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Willeit P, Kiechl S, Kronenberg F, Witztum JL, Santer P, Mayr M, Xu Q, Mayr A, Willeit J, Tsimikas S. Discrimination and net reclassification of cardiovascular risk with lipoprotein(a): Prospective 15-year outcomes in the bruneck study. J Am Coll Cardiol. 2014;64:851–860. doi: 10.1016/j.jacc.2014.03.061. [DOI] [PubMed] [Google Scholar]
- 38.Leibundgut G, Witztum JL, Tsimikas S. Oxidation-specific epitopes and immunological responses: Translational biotheranostic implications for atherosclerosis. Curr Opin Pharmacol. 2013;13:168–179. doi: 10.1016/j.coph.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tsimikas S, Miyanohara A, Hartvigsen K, et al. Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. J Am Coll Cardiol. 2011;58:1715–1727. doi: 10.1016/j.jacc.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lichtman AH, Binder CJ, Tsimikas S, Witztum JL. Adaptive immunity in atherogenesis: New insights and therapeutic approaches. J Clin Invest. 2013;123:27–36. doi: 10.1172/JCI63108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Albers JJ, Koschinsky ML, Marcovina SM. Evidence mounts for a role of the kidney in lipoprotein(a) catabolism. Kidney Int. 2007;71:961–962. doi: 10.1038/sj.ki.5002240. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.