Abstract
Objective
In angiogenesis, circulating mononuclear cells are recruited to vascular lesions; however, the underlying mechanisms are poorly understood.
Approach and Results
Here, we characterize the functional role of protein tyrosine kinase 7 (PTK7)-expressing CD11b+ mononuclear cells in vitro and in vivo using a mouse model of angiogenesis. While the frequencies of PTK7+CD11b+ cells in the bone marrow remained similar after vascular endothelial growth factor (VEGF)-A induced neovascularization, we observed an 11-fold increase in the cornea. Importantly, VEGF-A–induced chemotaxis of PTK7+ cells was mediated by VEGF receptor (VEGFR) 2. In a co-culture with endothelial cells, PTK7+CD11b+ cells stabilized the vascular network for 2 weeks by expressing high levels of angiopoietin-1 (ANG-1). The enhanced vascular stability was abolished by knockdown of ANG-1 in PTK7+CD11b+ cells and could be restored by ANG-1 treatment.
Conclusions
We conclude that PTK7 expression in perivascular mononuclear cells induces VEGFR2 and ANG-1 expression, and thus contributes to vascular stabilization in angiogenesis.
Keywords: angiogenesis, mononuclear cells, PTK7, VEGFR2, angiopoietin-1
Introduction
Angiogenesis is a multifactorial process in which different cell types are involved, especially vascular endothelial cells (VEC), pericytes, fibroblasts, and bone marrow (BM)-derived cells.1–3 A variety of immune cells directly support angiogenesis, including mast cells, tumor-associated macrophages (TAMs), and Tie2-expressing macrophages (TEMs).4–6 Endothelial cells recruit inflammatory cells to the extravascular tissue due to their expression of different leukocyte adhesion molecules. In turn, immune cells produce soluble factors, such as chemokines, cytokines, and proteases, that bind to endothelial cells and influence their function and angiogenesis in a paracrine fashion.4 Other studies have shown that BM-derived cells have the ability to differentiate into endothelial cells, possibly by conversion first to endothelial progenitors.6, 7
Interestingly, several lines of evidence suggest the presence of other types of BM derived circulating progenitor cells that do not incorporate directly into vessel formation, but promote vascular stabilization.8, 9 These BM derived perivascular cells interact with and support endothelial cells in the blood vessels,10, 11 indicating the importance of endothelial progenitor cells (EPC) and supporting cells to vasculature stabilization. Because the exact cellular markers and functions of these supporting cells and how they are mobilized and recruited into the angiogenic area remain unknown,12–15 it is of great interest to characterize circulating progenitor cells and their capacity to support the formation of vascular networks.
The pseudokinase protein tyrosine kinase 7 (PTK7) is an atypical tyrosine kinase receptor that lacks catalytic activity in its kinase domain.16 PTK7 regulates cell migration polarity,17–19 T cell maturation, migration of BM-derived cells,20 and endothelial cell migration as well as proliferation during angiogenesis.21, 22 We have recently provided evidence for an interaction between PTK7 and VEGFR1 (Flt-1) leading to enhanced migration of VECs.22 PTK7 is expressed by circulating mononuclear and leukemic cells, and mediates their pro-migratory and anti-apoptotic effects.20, 23 Moreover, numerous EPC marker studies have highlighted that hematopoietic (VEGFR1+) cells interact with endothelial (VEGFR2+) BM-derived progenitor subsets to induce and progress angiogenesis.7, 24, 25 Although PTK7 is expressed by peripheral blood mononuclear cells (PBMCs) and it interacts with VEGFRs, the specific function of PTK7+ blood cells in angiogenesis has not been thoroughly investigated.
In this study, we examined the role of PTK7+ mononuclear cells in angiogenesis in vitro and in vivo using a VEGF-A micropellet implantation model. We show that BM-derived PTK7+ cells recruited into the cornea in response to VEGF-A are CD11b+ mononuclear cells. More importantly, PTK7+CD11b+ mononuclear cells express high levels of VEGFR2 and angiopoietin-1 and are involved not only in neovascularization, but also new vessel stabilization.
Materials and Methods
Materials and Methods are available in the online-only Data Supplement I and II.
Results
PTK7+ mononuclear cells are recruited to the site of new vessel formation
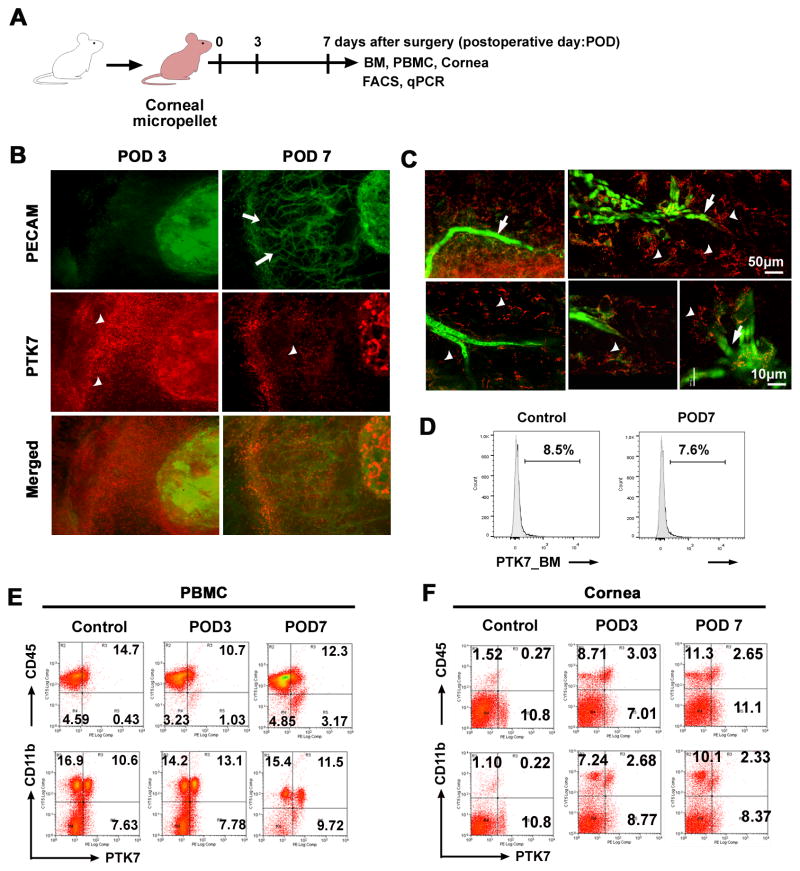
The main experiments of this study are schematically illustrated in Figure 1A. To investigate the ingress and localization of PTK7+cells in the cornea over time, we used an in vivo corneal micropocket angiogenesis model (Figure 1A). We found newly formed PECAM-1+ (also known as CD31+) blood vessels as early as postoperative day 3 (POD 3) after micropellet implantation surgery and a peak vessel growth at 7 days after VEGF-A micropellet implantation (white arrow in Figure 1B, upper panel). Interestingly, a number of PTK7+ cells (white arrowhead) localized near the angiogenic area (Figure 1B, middle panel). The population of PTK7+ cells peaked on day 2 in the cornea, was maintained until day 5, and then decreased (Supplement III). Using confocal microscopy we found that PTK7+ cells were scattered near the vascular branching area, as well as attached to new vessels (Figure 1C; white arrows indicate PECAM+ cells, white arrowheads indicate PTK7+ cells; and Supplement IV). However, most PTK7+ cells, located near the angiogenic area, did not express the VEC marker PECAM-1, and were not incorporated into new vessels (Figure 1B, C, and Supplement V, video clip).
Figure 1. PTK7+ cells recruit to the cornea after VEGF-A-induced neovascularization.
A, Schematic drawing illustrates the VEGF-A micropellet-induced corneal angiogenesis experiment. To induce corneal neovascularization VEGF-A (160 μg) micropellets were inserted into Balb/c mouse corneas (n=5). Corneas were harvested before micropellet implantation (day 0) and at postoperative day (POD) 3 and 7. B and C, Corneas were isolated at postoperative day (POD) 3 and 7, immunohistochemically stained for PECAM-1 (green) and PTK7 (red), and then examined by epifluorescence microscopy (× 200) (B) and confocal microscopy (C); white arrows indicate new vessels, arrowheads indicate PTK7+ cells. D, Representative flow cytometry histogram showing the percentage of PTK7+ cells in the bone marrow (BM) before (Control) and 7 days after micropellet implantation (POD7). E and F, Representative flow cytometry dot plots showing PTK7+CD45+ (upper row) and PTK7+CD11b+ (lower row) cells in peripheral blood mononuclear cells (PBMCs) (E) and cornea (F) before micropellet implantation (Control), and 3 (POD3) and 7 days (POD7) after implantation.
Next, we analyzed PTK7+ cells in the BM, peripheral blood, and cornea in VEGF-A micropellet-implanted mice using flow cytometry. In the BM and peripheral blood PTK7+ frequencies remained similar (Figure 1D and E; p=0.662 for BM and p=0.085 for PBMC, Students t-test) (Supplement VI). But, in the cornea PTK7+CD45+ and PTK7+CD11b+ cells were increased significantly 3 and 7 days after stimulation of angiogenesis (p<0.001, Students t-test; Figure 1F), indicating that the PTK7+ cells in angiogenic area are PTK7+ CD11b+ and PTK7+CD45+mononuclear cells.
VEGFR2 upregulation on PTK7+ cells promotes VEGF-A-mediated cell migration
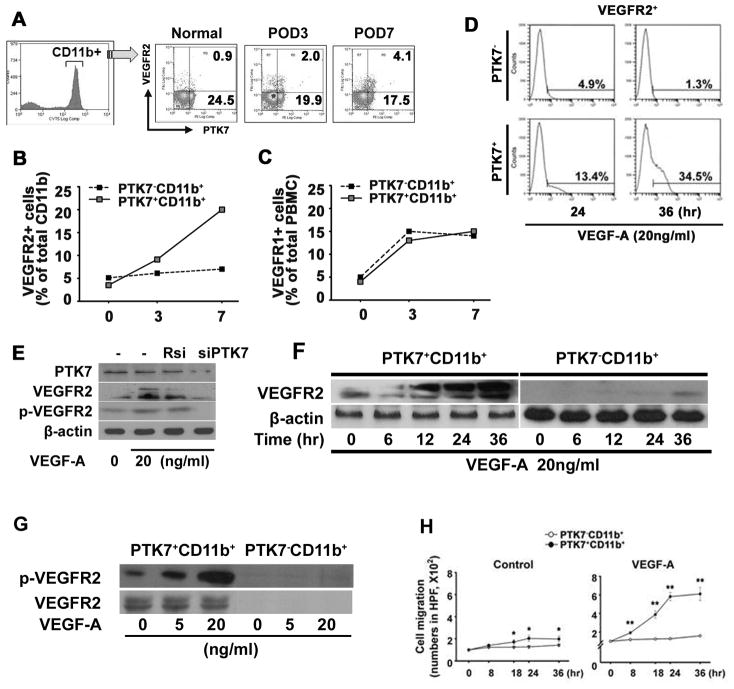
VEGFR2 plays a crucial role in vasculogenesis, angiogenesis, and hematopoiesis, and is considered as one of the most definitive EPC markers.26, 27 To address whether the recruitment of PTK7+ cells to the cornea is associated with angiogenesis, we investigated the expression of VEGFR2 on PTK7+ cells during VEGF-A-induced angiogenesis using the corneal micropellet implantation model. Before micropellet implantation (day 0), 0.9% of cells express VEGFR2 in PBMCs, but the frequencies of VEGFR2+PTK7+ CD11b+ cells increased ~4.6-fold 7 days after VEGF-A pellet implantation (0.9% to 4.1% on day 7) (Figure 2A). Interestingly, most of the VEGFR2+ cells were positive for PTK7+ (Figure 2A and B). Seven days after micropellet implantation (POD7), 20.8% of PTK7+CD11b+ cells expressed VEGFR2. But, only 6.1% of PTK7−CD11b+ cells were VEGFR2+ at 7 days after the implantation (Figure 2B). The frequencies of VEGFR1+ cells did not differ between PTK7+CD11b+ and PTK7−CD11b+ cells (Figure 2C).
Figure 2. VEGFR2 expression and migration of PTK7+ CD11b+ cells is induced by VEGF-A.
VEGF-A corneal pellets were implanted in 6-week-old Balb/c mice (n=7) and mice were analyzed before implantation (Control, day 0) and at postoperative days (POD) 3 and 7. Peripheral blood mononuclear cells (PBMCs) were stained with monoclonal anti-mouse PTK7-PE-, CD11b-FITC-, and VEGFR2-APC-conjugated antibodies. Frequencies of VEGFR2+PTK7+ were measured in CD11b+ cells on POD 0, 3, and 7 using flow cytometry (A). VEGFR2- (B) and VEGFR1- (C) expression was compared between PTK7+CD11b+ or PTK7−CD11b+ cells by flow cytometry at POD 0, 3, and 7. D, Representative flow analysis histogram shows VEGFR2+ cell frequencies on PTK7+CD11b+ and PTK7−CD11b+ cells (1.2 x 105 cells/well) 24 and 36 hours after VEGF-A stimulation (20ng/ml). VEGFR2 expression was measured by flow analysis. E, Expression of VEGFR2 by PTK7 siRNA (siPTK7) transfection was determined. After transfection of siPTK7 or random siRNA (Rsi), VEGF-A (20ng/ml) was treated for 24 hours then, the expression of VEGFR2 and phospho-VEGFR2 was measured by immunoblot. F, PTK7−CD11b+ and PTK7+CD11b+ cells were stimulated with VEGF-A (20 ng/ml) for 0 to 36 hours and the expression of VEGFR2 protein was measured by western blot. G. Phosphorylated VEGFR2 (Tyr951, 7H11) was compared between PTK7+CD11b+ and PTK7−CD11b+ cells by immunoblot at 1, 5, and 15 minutes after VEGF-A stimulation (20 ng/ml). H, PTK7−CD11b+ (white dot) and PTK7+CD11b+ (black dot) PBMCs were separated using flow cytometry and cell migration was determined in a time-dependent manner using a transwell assay. 8×104 PTK7+CD11b+ and PTK7−CD11b+ PBMCs were seeded in the upper chamber, with or without VEGF-A (20 ng/ml). The cells that migrated to the bottom of the lower chamber were stained with hematoxylin and counted using an inverted microscope. All experiments were repeated four times. Data are expressed as the mean ± standard deviation. *p < 0.05; **p < 0.01.
Next, we determined VEGFR2 expression of in vitro VEGF-A–stimulated PTK7+ and PTK7− CD11b+ PBMCs using flow cytometry and western blot. Similar to our in vivo data, VEGFR2 expression was increased only in PTK7+CD11b+ cells but not in PTK7−CD11b+ cells after VEGF-A stimulation (Figure 2D and E). Before in vitro treatment with VEGF-A, the mean frequencies of VEGFR2+ cells among PTK7−CD11b+ and PTK7+CD11b+ cells were 1.1% (0.3~2.1) and 2.8% (1.9~4.5%), respectively (p=0.014, data not shown). However, treatment of VEGF-A for 36 hours increased the VEGFR2 expression to 34.5% in PTK7+CD11b+ cells (Figure 2D, lower panel). In PTK7−CD11b+ cells VEGF-A treatment did not change the frequencies of VEGFR2 expression (Figure 2D, upper panel). To confirm the role of PTK7 in VEGFR2 induction, PTK7 siRNA was transfected into PTK7+CD11b+ cells, and the expression of VEGFR2 was measured after 24 hours of VEGF-A treatment. Interestingly, VEGF-A induced VEGFR2 expression in PTK7+CD11b+ cells was reduced when PTK7 was downregulated (Figure 2E). Additionally, we observed a VEGF-A dependent VEGFR2 increase in PTK7+ but not PTK7− cells using western blot (Figure 2E and 2F).
To analyze VEGFR2 activity, we measured VEGFR2 receptor phosphorylation in PTK7+CD11b+ cells. Treatment with VEGF-A significantly enhanced VEGFR2 phosphorylation in PTK7+CD11b+, but not PTK7−CD11b+ cells, in a dose-dependent manner (Figure 2G). In angiogenesis, VEGFR2 is critical for VEGF-A–mediated chemotaxis of EPCs into the area where the angiogenic process is initiated.26 Thus, we next analyzed whether VEGF-A treatment of PTK7+CD11b+ cells enhances their migratory potential using a transwell system. VEGF-A treatment significantly increased the migration of PTK7+CD11b+ cells, but not PTK7−CD11b+ cells. At 24 hours after treatment start, there was 8-fold increase of migration of PTK7+CD11b+ cells compared to PTK7−CD11b+ cells (Figure 2H).
Induction of VEGFR2 expression on PTK7+ cells depends on Nuclear Factor (NF)-κB activation by VEGF-A
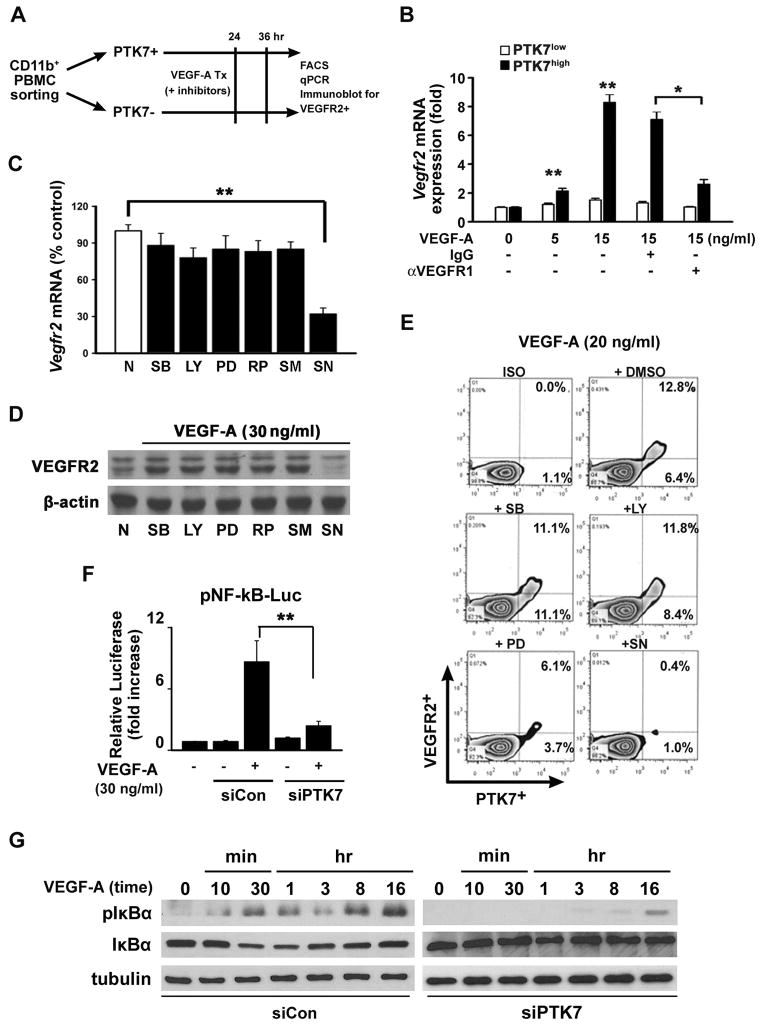
We next investigated which signals upregulate VEGFR2 in PTK7+ cells in vitro. We sorted CD11b+ PTK7+ and CD11b+ PTK7− cells from PBMCs, treated them with VEGF-A and then analyzed mRNA and protein expression of VEGFR2 (Figure 3A). Seven days after micropellet implantation, VEGFR2 mRNA expression was 8.9 times higher in PTK7+CD11b+ than in PTK7−CD11b+ cells upon in vitro stimulation with 15ng/ml VEGF-A (Figure 3B). Given that PTK7+ cells constitutively express VEGFR1 and regulate VEGFR1 signaling in mononuclear cells,22 we postulate that PTK7 expression affects VEGFR2 expression through VEGFR1 signaling. Indeed, increased VEGFR2 expression induced by VEGF-A could be inhibited using a VEGFR1 blocking antibody, implying that VEGF-A binding to VEGFR1 mediates VEGFR2 expression in PTK7+ cells (Figure 3B).
Figure 3. Enhanced VEGFR2 expression in PTK7+ cells is Nuclear Factor (NF)-κB dependent.
A, A schematic illustration showing how PTK7+ and PTK7− cells from peripheral blood mononuclear cells (PBMCs) were separated from VEGF-A pellet-implanted mice using FACS. B, Vegfr2 mRNA expression in PTK7+ and PTK7− cells was measured by real-time qPCR 12 hours after VEGF-A treatment in the absence or presence of 10μM anti-VEGFR1 neutralizing antibody or IgG isotype control antibody. C–E, PTK7+ and PTK7− cells were treated with various signal transduction inhibitors (30 μM; SB: SB203580, LY: LY294002, PD: PD98059, RP: Rapamycin, SM: SN50M, and SN: SN50) 30 minutes before VEGF-A (30 ng/ml) stimulation and then VEGFR2 mRNA (C), protein levels (D), and surface expression levels (E) were determined. F, PTK7 siRNA- or control siRNA-transfected PTK7+CD11b+ cells were cultured with VEGF-A. Two hours after incubation in 10% RPMI, the cells were transfected with NF-κB luciferase constructs for 12 hours. Following 30 minutes of VEGF-A treatment, cells were lysed, and luciferase activities were measured using a luminometer. All data are representative for four independent experiments and are expressed as the mean ± standard deviation. **p < 0.01. G, Control siRNA (siCon) or PTK7 siRNA (siPTK7)-transfected cells were treated with VEGF-A (20 ng/ml) as indicated, and western blot was performed using anti-phospho-IκB and anti-IκB antibodies.
To define the mechanisms underlying PTK7-mediated VEGFR2 upregulation, we used signal transduction inhibitors and evaluated their effect on vegfr2 mRNA expression in PTK7+CD11b+ cells. Only SN50, a well-known NF-κB inhibitor, but no other inhibitors, significantly suppressed vegfr2 mRNA expression (Figure 3C). Similarly, VEGFR2 protein expression was inhibited only by SN50 as analyzed via Western Blot (Figure 3D) and flow cytometry (Figure 3E). In addition, RAW-264.7 cells were transfected with PTK7 siRNA, and transcription regulator activities were determined in response to VEGF-A stimulation. Compared with control siRNA (siCON), PTK7 siRNA (siPTK7)-treated RAW-264.7 cells showed significantly decreased NF-κB activities (Figure 3F) and IκB phosphorylation (Figure 3G). These data indicate that VEGF-A activates NF-κB via VEGFR-1 and thus induces VEGFR-2 expression in PTK7+ cells.
PTK7+ mononuclear cells facilitate vessel stabilization in vitro
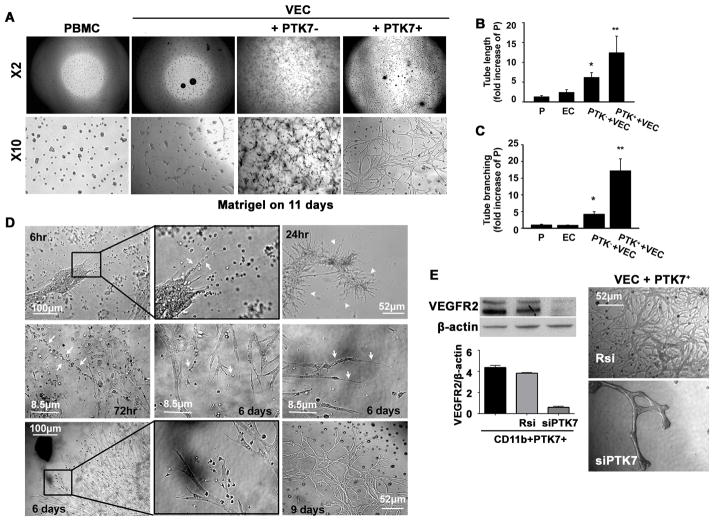
Our results showed that PTK7+ mononuclear cells express VEGFR2 and respond to VEGF-A, and thus may play a significant role in corneal angiogenesis. We used the matrigel assay to determine the exact role of PTK7+ cells in angiogenesis in vitro. When VECs were cultured alone, the tube-like structures formed at 3 hours and matured at 18 hours, after which the vascular networks began to disappear and simple Y- or T-shaped branch structures remained (Figure 4A). When PTK7−CD11b+ cells isolated from micropellet implanted mice were co-cultured with VECs, we did not find tube-like structures. Instead, we observed only small cell-to-cell contacts or branch-like structures (Figure 4A). Interestingly, VECs that were co-cultured with PTK7+CD11b+ cells from micropellet implanted mice developed tube-like structures, matured in a time-dependent manner, and were stable for 2 weeks (Figure 4A, B, and C).
Figure 4. PTK7+ mononuclear cells facilitate vessel growth and stability in vitro.
A, The tube formation assay was performed using a mouse vascular endothelial cell (VEC) line (MS1) and PTK7+ or PTK7− CD11b+ peripheral blood mononuclear cells (PBMCs;). Images were obtained using an inverted microscope at 11 days later (×2 and ×10: objective lens magnification). B and C, Five high-magnification (200×) pictures for each condition were taken, and the tube length (B), and branching (C) were measured using image analysis software. *p < 0.05 vs. PBMC, **p < 0.01 vs. PBMC. (VEC: vascular endothelial cell, PTK7−: PTK7−CD11b+, PTK7+: PTK7+CD11b+). The results are mean ± SD from three independent experiments. D, VECs were co-cultured with PTK7+ or PTK7− PBMCs and images were taken as early as 6 h and up to 9 days after starting the coculture. White arrows in the upper center panel (6 hours) indicate palisading PBMCs (black box, upper row). The white arrowheads in the upper right panel indicate new vessel buds growing from clusters of endothelial cells at 24 hours. Linear or palisading PBMCs were found near the growing vascular network (white arrows in the middle left panel; 72 hours). Cells with hair-like long processes were observed at the end of the blood vessels (white arrows in the middle center and middle right panel). Macrophage-like cells were linked to growing endothelial buds and the tips of the growing tubes (black box, lower row). A stable vascular network was observed up to 9 days after culture. E, PTK7+CD11b+ PBMCs were transfected with random siRNA (Rsi) or siVEGFR2 (siR2) and VEGFR2 expression levels was determined by immunoblot at 72 hours after transfection. Then, VEGFR2 knock down PTK7+CD11b+ cells were cultured with VECs for the tube formation assay. 14 days later we observed less tube formation in VECs when co-cultured with VEGFR2 knock down cells.
In Figure 4D we show VECs co-cultured with PTK7+CD11b+ cells from 6 hours for up to 9 days. Six hours after starting the assay we observed round PBMCs accumulating close to the VECs (Figure 4D, upper left panel, black box). At 24 hours round PBMCs (upper center panel, white arrow) gathered closer to VECs (upper right panel, white arrowhead). Next, network-like structures developed and matured within 72 hours. During the elongation of the vascular network, migratory myeloid cells were found near the vascular network (Figure 4D, middle row), which were attached to the growing tubes (Figure 4D, middle right panel, white arrows). These myeloid-like cells were sometimes linearly attached to each other, and were found near the blood vessels (middle left panel, white arrows, Figure 4D). This well-formed reticular vascular network was stable for up to 9 days. To determine whether VEGFR2 expression is able to facilitate tube formation and its stability, we knocked down VEGFR2 in a tube formation assay. After confirming a successful knock down of VEGFR2 in PTK7+CD11b+ cells by siVEGFR2 transfection (siR2) (Figure 4E), we co-cultured these cells with VECs as described above. VEC tube formation was significantly reduced when cocultured with siVEGFR2-transfected compared to random siRNA (Rsi)-transfected PTK7+CD11b+ cells (Figure 4E). This result confirms that VEGFR2 expression is crucial for vascular network formation.
PTK7+ mononuclear cells enhance vascular stability through angiopoietin-1 secretion
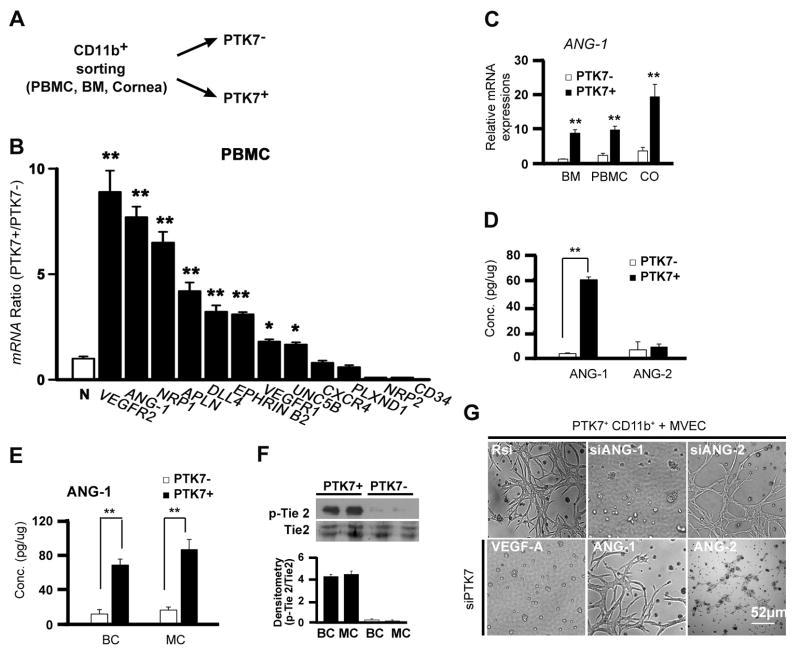
Based on our previous results, we hypothesized that PTK7+ cells have the potential to stabilize vessel formation. To clarify the specific angiogenic functions of PTK7+ mononuclear cells, we sorted CD11b+PTK7+ and CD11b+PTK7− cells from PBMCs, BM, or corneas 3 days after micropellet implantation (Figure 5A). We first compared mRNA expression of various angiogenic markers in sorted PTK7− and PTK7+ cells. mRNA expression of vegfr2, angiopoietin-1 (ANG-1), neurophilin 1 (NRP1), apelin (APLN), and delta-like 4 (DLL4) were significantly higher in PTK7+ than in PTK7− cells (Figure 5B). mRNA expression of ang-1 was significantly increased in PTK7+ compared with PTK7− cells isolated from BM, PBMC, and cornea (CO) (Figure 5C). Protein expression of ANG-1 was also significantly elevated in PTK7+ cells compared with PTK7− cells, whereas angiopoietin-2 (ANG-2) expression showed no significant difference between PTK7+ and PTK7− cells (Figure 5D). To analyze whether the ANG-1 production of PTK7+ cells needs direct VEC interaction or not, we used a PTK7+CD11b+/VEC mixed culture (MC) or the Boyden Chamber (BC), respectively. ANG-1 secretion from PTK7+ cells (Figure 5E) and subsequent phosphorylation of Tie2, a known receptor for ANG-1 (Figure 5F) was similar using the mixed culture and Boyden Chamber. These observations indicate that the presence of PTK7 itself is responsible for ANG-1 secretion in mononuclear cells. As ANG-1 is a well-known mediator secreted by pericytes to recruit VECs and promote vascular stability,12, 28 PTK7+ cells may stabilize vessel formation via ANG-1 secretion. Thus, we investigated the functional role of ANG-1 in PTK7+ cells. Indeed, PTK7+CD11b+ and VEC co-cultures treated with siRNA for ANG-1 (siANG-1) displayed less angiogenesis. Moreover, treatment with exogenous ANG-1 protein rescued vascular network formation in a co-culture condition with PTK7 knock-downed cells (Figure 5G). These data suggest that PTK7+ cells represent a subpopulation of BM-derived mononuclear cells that mediate vascular stabilization through ANG-1 production.
Figure 5. PTK7+ cells enhance vascular stability through angiopoietin-1.
A, A schematic drawing illustrates experiments for Figure 5A–E. B, Real-time qPCR analysis showing the expression of VEC markers in PTK7+CD11b+ and PTK7−CD11b+ cells. Comparison of the expression of N: GAPDH to the expression of empty pellets of vascular endothelial cell growth factor receptor (VEGFR) 2, Angiopoietin 1 (ANG-1), Neurophilin 1 (NRP1), Apelin (APLN), Delta-like 4 (DLL4), Ephrine B2 (EPHRIN B2), VEGFR1, unc-5-homolog B (UNC5B), CXCR4, plexin D1 (PLXND1), Neuropilin-2 (NRP-2), and CD34 was done. C, Three days after pellet insertion PTK7+CD11b+ and PTK7−CD11b+ cells were sorted by FACS from the bone marrow (BM), peripheral blood mononuclear cells (PBMC), and cornea. mRNA expression of ANG-1 was assessed using real-time qPCR. The fold increase compared to non-operated control mice was measured. D, Protein expression of ANG-1 and ANG-2 of in vitro cultured PTK7−CD11b+ and PTK7+CD11b+cells measured by ELISA after 24 hours of VEGF-A (40ng/ml) treatment. E and F, 5 × 104 PTK7+CD11b+ cells were cultured with 2 × 105 vascular endothelial cells (VECs) using a Boyden chamber (BC) or a mixed co-culture (MC). ANG-1 protein concentration (E) and phospho-Tie2 and total Tie2 (F) were measured by ELISA or immunoblot, respectively, 24 hours later. G, PTK7+CD11b+ cells were sorted and transfected with random siRNA (Rsi), siANG-1, siANG-2, or siPTK7. Then, cells were co-cultured with VECs in a matrigel for 5 days. As indicated 2 co-culture conditions of siPTK7 transfected cells and VECs were treated with 40ng/ml of ANG-1 or ANG-2 every 24 hrs. All experiments were repeated four times. Data are expressed as the mean ± standard deviation. *p < 0.05; **p < 0.01.
Discussion
In this study, we show for the first time that (i.) during early angiogenesis, increased frequencies of PTK7+ mononuclear cells are recruited to the angiogenic site, where they closely communicate with VECs and enhance vascular growing and stability; (ii.) VEGFR2 expression is significantly higher in PTK7+ mononuclear cells compared to PTK7− cells, and this expression is induced by VEGF-A through VEGFR1-mediated NF-κB activation; and (iii.) PTK7+ cells upregulate the expression of the angiogenic mediator ANG-1 and contribute to vascular stability. The main findings of this study are schematically illustrated in Figure 6.
Figure 6. Proposed mechanism for the role of PTK7+VEGFR2+ mononuclear cells in angiogenesis.
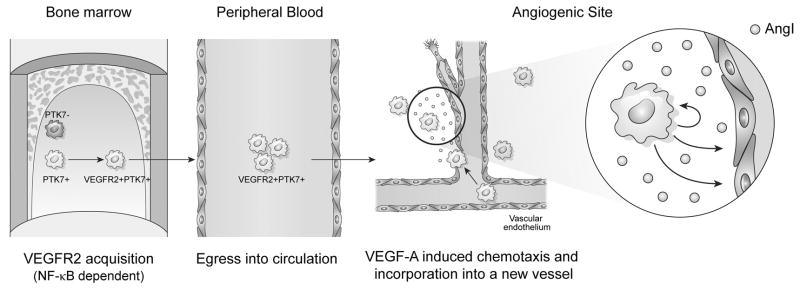
PTK7 expression by mononuclear cells is necessary for VEGFR2 acquisition in bone marrow and peripheral blood. These PTK7+VEGFR2+ mononuclear cells migrate to angiogenic sites where they promote angiogenesis by up-regulating ANG-1 secretion. Given their topographic localization and expression markers, they likely function in stabilizing blood vessel formation and/or have EPC potential.
Although the knowledge about EPCs has increased, the phenotypic and functional characterization of EPCs is hampered by the extreme rarity of these cells, controversial studies regarding surface marker expression, and the absence of standard in vitro or in vivo assays to characterize their function.29, 30 Nevertheless, EPCs are most commonly identified as CD133+CD34+VEGFR2+, CD45dimCD34+VEGFR2+, CD14+CD34low, and CD11b+VEGFR2+ cells.31 Because VEGFR2 is the only surface marker shared by all studies to identify EPCs, we focused on VEGFR2 expression by PTK7+ mononuclear cells in our study. Moreover, VEGFR2 contributes to angiogenesis and promotes endothelial cell migration and proliferation.
Here, we demonstrate that PTK7+ CD11b+ PBMCs express significantly higher amounts of VEGFR2 after angiogenic stimulus. Interestingly, not only CD11b+ cells, but also F4/80+ PBMCs showed higher PTK7 expression in VEGFR2+ cells, compared with VEGFR2−F4/80+ cells. (Supplement VII). PTK7 has previously been shown to interact with VEGFR1 in a VEGF-A-specific manner to enhance angiogenesis in VECs.22 Ohki and colleagues have reported that CD45+CD11b+VEGFR1+ cells egress from the BM after injury, and these cells display EPC characteristics.32 They also found that 50% of the CD11b population expresses VEGFR1, and that these cells show a 5-fold increased revascularization activity, compared to CD11b+ VEGFR1− cells. Interestingly, we found that most VEGFR2+CD45+ cells (96.4%) express VEGFR1. In addition, PTK7+CD11b+ cells express higher VEGFR2 levels than PTK7−CD11b+ cells, suggesting that VEGFR1 may be a prerequisite factor for VEGFR2 expression through a PTK7-mediated pathway.
While splenic PTK7+ and PTK7− cells express similar amounts of VEGFR2 mRNA, PTK7+ cells in the cornea or blood express significantly increased levels of VEGFR2 compared to PTK7− cells, which can be further increased by VEGF-A pellet insertion (Supplement VIII), indicating that VEGFR2 acquisition occurs in a site-specific manner, suggesting that other factors (e.g., neural, neighbor cells and matrix proteins) also affect the development of EPCs. A recent study demonstrated that VEGFR2 expression promotes EPC egression from the BM into peripheral blood.13 SDF-1/CXCR4 axis disruption induced CD11b+CD45+ BM cell mobilization. 33–35 However, Pitchford et al reported that VEGF-A activates EPC mobilization through VEGFR2, and not VEGFR1 or the SDF-1/CXCR4 axis,13 suggesting that VEGFR2 expression on PTK7 cells in BM EPCs may be critical for EPCs to egress from the BM into peripheral blood.
EPC-induced angiogenesis is a complex process that causes EPCs to migrate to the wound site. We propose that the PTK7+VEGFR2+ cells we characterized in our study are similar to “early-outgrowth cells” that are descendants of a monocyte-macrophage subset, and play a role in blood vessel homeostasis and angiogenesis initiation during wound healing and tissue ischemia.14, 36 These “early-outgrowth cells” express CD45 and other mononuclear cell markers (e.g., CD11b or CD14), fail to proliferate, and are defined as perivascular cells rather than lumen-lining cells. These cells act by secreting angiogenic factors and enzymes that can degrade the matrix.37–39 Similarly, PTK7+ cells primarily express mononuclear cell markers and do not display proliferative activity (data not shown). More importantly, PTK7+ cells are found in and around newly growing blood vessels in a scattered pattern, where they palisade along the growing vessels and aggregate into a blind-ended capillary-like structure. PTK7+ PBMCs express specific pericyte markers, including ANG-1, PDGF-β, and desmin (supplement IX), but not the tip cell marker CD34 (Figure 5). Since perivascular localized cells, which express ANG-1, PDGFR-β, and CD13 are considered as pericytes,10, 40 PTK7+ cells may function similar to classic pericytes, stabilizing vascular networks by modifying the environment and hence stabilizing tubular structures.
ANG-1 treatment may reduce vascular leakage, inhibit VEC apoptosis, and enhance vascular stability, branching, and remodeling of an immature vessel plexus into a more complex network.28, 41–43 ANG-1 expression from BM cells appears to have a critical role in angiogenesis, pericyte recruitment, and vascular stabilization.12, 28, 44 Our findings show that PTK7+ cells highly express ANG-1 and that the vascular stability of VECs is ANG-1 dependent (Figure 5), suggesting that PTK7+CD11b+ cells supply ANG-1 to the angiogenic area to promote vascularization at an early angiogenic time point.
In summary, this study highlights the relevance of PTK7 expression by mononuclear cells in promoting VEGF-A–mediated angiogenesis. Our data suggest that PTK7 amplifies the expression level of VEGFR2 on these cells, which is an important step in the initial phase of angiogenesis. Thus, targeting PTK7 in BM-derived mononuclear cells may provide an effective strategy in treating angiogenesis-related disorders.
Supplementary Material
Significance section.
In this study, we show for the first time that (i.) during early angiogenesis, increased frequencies of PTK7+ mononuclear cells are recruited to the angiogenic site, where they closely communicate with VECs and enhance vascular growing and stability; (ii.) VEGFR2 expression is significantly higher in PTK7+ mononuclear cells compared to PTK7− cells, and this expression is induced by VEGF-A through VEGFR1-mediated NF-κB activation; and (iii.) PTK7+ cells upregulate the expression of the angiogenic mediator ANG-1 contributing to vascular stability.
Acknowledgments
We thank Dr. Andrius Kazlauskas and Patricia D’Amore for providing materials for the in vitro angiogenesis studies, and we are grateful to Dong-Su Jang for his excellent support with the medical illustration. We also thank Dr. Susanne Eiglmeier for critical reading and her assistance in preparing the manuscript.
Sources of Funding
This work was supported by grants from the National Institutes of Health (NIH/NEI RO1-12963), and a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (HI13C0055).
Nonstandard Abbreviations and Acronyms
- PTK7
protein kinase 7
- ANG-1
angiopoietin-1
- EPC
endothelial progenitor cells
- VEC
vascular endothelial cell
- BM
bone marrow
- PBMC
peripheral blood mononuclear cells
Abbreviation
- PTK7
protein kinase 7
- ANG-1
angiopoietin-1
- EPC
endothelial progenitor cells
- VEC
vascular endothelial cell
- BM
bone marrow
- PBMC
peripheral blood mononuclear cells
- VEGFR
vascular endothelial growth factor receptor
- VEGF
vascular endothelial growth factor
- POD
postoperative day
- NF-kB
Nuclear factor-kappaB
- siCON
Control siRNA (si: small interfering)
- siR2
VEGFR2 siRNA
- ANG
angiopoietin
- NRP
neurophilin
- Dll4
Delta like 4
- SDF-1
stromal cell-derived factor 1 (SDF-1)
- UNC5B
unc-5-homolog B
- PLXND1
plexin D1
- BC
Boyden chamber
- MC
Mixed culture
Footnotes
Disclosures
None.
References
- 1.Dondossola E, Rangel R, Guzman-Rojas L, Barbu EM, Hosoya H, St John LS, Molldrem JJ, Corti A, Sidman RL, Arap W, Pasqualini R. Cd13-positive bone marrow-derived myeloid cells promote angiogenesis, tumor growth, and metastasis. Proc Natl Acad Sci U S A. 2013;110:20717–20722. doi: 10.1073/pnas.1321139110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hanahan D, Coussens LM. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Ahn GO, Brown JM. Role of endothelial progenitors and other bone marrow-derived cells in the development of the tumor vasculature. Angiogenesis. 2009;12:159–164. doi: 10.1007/s10456-009-9135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 5.De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: Regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28:519–524. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 6.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP. Evidence for circulating bone marrow-derived endothelial cells. Blood. 1998;92:362–367. [PubMed] [Google Scholar]
- 7.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–1201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 8.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human cd34+ac133+vegfr-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Zentilin L, Tafuro S, Zacchigna S, Arsic N, Pattarini L, Sinigaglia M, Giacca M. Bone marrow mononuclear cells are recruited to the sites of vegf-induced neovascularization but are not incorporated into the newly formed vessels. Blood. 2006;107:3546–3554. doi: 10.1182/blood-2005-08-3215. [DOI] [PubMed] [Google Scholar]
- 10.Armulik A, Genove G, Betsholtz C. Pericytes: Developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 11.Bagley RG, Weber W, Rouleau C, Teicher BA. Pericytes and endothelial precursor cells: Cellular interactions and contributions to malignancy. Cancer Res. 2005;65:9741–9750. doi: 10.1158/0008-5472.CAN-04-4337. [DOI] [PubMed] [Google Scholar]
- 12.Hattori K, Dias S, Heissig B, Hackett NR, Lyden D, Tateno M, Hicklin DJ, Zhu Z, Witte L, Crystal RG, Moore MA, Rafii S. Vascular endothelial growth factor and angiopoietin-1 stimulate postnatal hematopoiesis by recruitment of vasculogenic and hematopoietic stem cells. J Exp Med. 2001;193:1005–1014. doi: 10.1084/jem.193.9.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pitchford SC, Furze RC, Jones CP, Wengner AM, Rankin SM. Differential mobilization of subsets of progenitor cells from the bone marrow. Cell Stem Cell. 2009;4:62–72. doi: 10.1016/j.stem.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 14.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yamamoto S, Niida S, Azuma E, Yanagibashi T, Muramatsu M, Huang TT, Sagara H, Higaki S, Ikutani M, Nagai Y, Takatsu K, Miyazaki K, Hamashima T, Mori H, Matsuda N, Ishii Y, Sasahara M. Inflammation-induced endothelial cell-derived extracellular vesicles modulate the cellular status of pericytes. Scientific reports. 2015;5:8505. doi: 10.1038/srep08505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boudeau J, Miranda-Saavedra D, Barton GJ, Alessi DR. Emerging roles of pseudokinases. Trends Cell Biol. 2006;16:443–452. doi: 10.1016/j.tcb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Lu X, Borchers AG, Jolicoeur C, Rayburn H, Baker JC, Tessier-Lavigne M. Ptk7/cck-4 is a novel regulator of planar cell polarity in vertebrates. Nature. 2004;430:93–98. doi: 10.1038/nature02677. [DOI] [PubMed] [Google Scholar]
- 18.Shnitsar I, Borchers A. Ptk7 recruits dsh to regulate neural crest migration. Development. 2008;135:4015–4024. doi: 10.1242/dev.023556. [DOI] [PubMed] [Google Scholar]
- 19.Yen WW, Williams M, Periasamy A, Conaway M, Burdsal C, Keller R, Lu X, Sutherland A. Ptk7 is essential for polarized cell motility and convergent extension during mouse gastrulation. Development. 2009;136:2039–2048. doi: 10.1242/dev.030601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haines CJ, Giffon TD, Lu LS, Lu X, Tessier-Lavigne M, Ross DT, Lewis DB. Human cd4+ t cell recent thymic emigrants are identified by protein tyrosine kinase 7 and have reduced immune function. J Exp Med. 2009;206:275–285. doi: 10.1084/jem.20080996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin WS, Maeng YS, Jung JW, Min JK, Kwon YG, Lee ST. Soluble ptk7 inhibits tube formation, migration, and invasion of endothelial cells and angiogenesis. Biochem Biophys Res Commun. 2008;371:793–798. doi: 10.1016/j.bbrc.2008.04.168. [DOI] [PubMed] [Google Scholar]
- 22.Lee HK, Chauhan SK, Kay E, Dana R. Flt-1 regulates vascular endothelial cell migration via a protein tyrosine kinase-7-dependent pathway. Blood. 2011;117:5762–5771. doi: 10.1182/blood-2010-09-306928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Prebet T, Lhoumeau AC, Arnoulet C, Aulas A, Marchetto S, Audebert S, Puppo F, Chabannon C, Sainty D, Santoni MJ, Sebbagh M, Summerour V, Huon Y, Shin WS, Lee ST, Esterni B, Vey N, Borg JP. The cell polarity ptk7 receptor acts as a modulator of the chemotherapeutic response in acute myeloid leukaemia and impairs clinical outcome. Blood. 2010 doi: 10.1182/blood-2010-01-262352. [DOI] [PubMed] [Google Scholar]
- 24.Zubair AC, Malik S, Paulsen A, Ishikawa M, McCoy C, Adams PX, Amrani D, Costa M. Evaluation of mobilized peripheral blood cd34(+) cells from patients with severe coronary artery disease as a source of endothelial progenitor cells. Cytotherapy. 2010;12:178–189. doi: 10.3109/14653240903493409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. Vegfr1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Udi J, Wider D, Kleber M, Ihorst G, Muller A, Wasch R, Engelhardt M. Early and mature endothelial progenitors and vegfr2+-cells in multiple myeloma: Association with disease characteristics and variation in different cell compartments. Leuk Res. 2011;35:1265–1268. doi: 10.1016/j.leukres.2011.05.021. [DOI] [PubMed] [Google Scholar]
- 27.Kawasaki K, Watabe T, Sase H, Hirashima M, Koide H, Morishita Y, Yuki K, Sasaoka T, Suda T, Katsuki M, Miyazono K, Miyazawa K. Ras signaling directs endothelial specification of vegfr2+ vascular progenitor cells. J Cell Biol. 2008;181:131–141. doi: 10.1083/jcb.200709127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmad S, Cudmore MJ, Wang K, Hewett P, Potluri R, Fujisawa T, Ahmed A. Angiopoietin-1 induces migration of monocytes in a tie-2 and integrin-independent manner. Hypertension. 2010;56:477–483. doi: 10.1161/HYPERTENSIONAHA.110.155556. [DOI] [PubMed] [Google Scholar]
- 29.Schmid MC, Varner JA. Circulating endothelial progenitor cells. Methods Mol Biol. 2009;467:139–155. doi: 10.1007/978-1-59745-241-0_8. [DOI] [PubMed] [Google Scholar]
- 30.Yoder MC, Ingram DA. Endothelial progenitor cell: Ongoing controversy for defining these cells and their role in neoangiogenesis in the murine system. Curr Opin Hematol. 2009;16:269–273. doi: 10.1097/MOH.0b013e32832bbcab. [DOI] [PubMed] [Google Scholar]
- 31.Hristov M, Weber C. Endothelial progenitor cells in vascular repair and remodeling. Pharmacol Res. 2008;58:148–151. doi: 10.1016/j.phrs.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Ohki M, Ohki Y, Ishihara M, Nishida C, Tashiro Y, Akiyama H, Komiyama H, Lund LR, Nitta A, Yamada K, Zhu Z, Ogawa H, Yagita H, Okumura K, Nakauchi H, Werb Z, Heissig B, Hattori K. Tissue type plasminogen activator regulates myeloid-cell dependent neoangiogenesis during tissue regeneration. Blood. 2010;115:4302–4312. doi: 10.1182/blood-2009-08-236851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levesque JP, Liu F, Simmons PJ, Betsuyaku T, Senior RM, Pham C, Link DC. Characterization of hematopoietic progenitor mobilization in protease-deficient mice. Blood. 2004;104:65–72. doi: 10.1182/blood-2003-05-1589. [DOI] [PubMed] [Google Scholar]
- 34.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, Mericskay M, Gierschik P, Biessen EA, Weber C. Sdf-1alpha/cxcr4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–791. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 35.Levesque JP, Hendy J, Takamatsu Y, Simmons PJ, Bendall LJ. Disruption of the cxcr4/cxcl12 chemotactic interaction during hematopoietic stem cell mobilization induced by gcsf or cyclophosphamide. J Clin Invest. 2003;111:187–196. doi: 10.1172/JCI15994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 37.Ziegelhoeffer T, Fernandez B, Kostin S, Heil M, Voswinckel R, Helisch A, Schaper W. Bone marrow-derived cells do not incorporate into the adult growing vasculature. Circ Res. 2004;94:230–238. doi: 10.1161/01.RES.0000110419.50982.1C. [DOI] [PubMed] [Google Scholar]
- 38.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A. 2008;105:6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uemura A, Ogawa M, Hirashima M, Fujiwara T, Koyama S, Takagi H, Honda Y, Wiegand SJ, Yancopoulos GD, Nishikawa S. Recombinant angiopoietin-1 restores higher-order architecture of growing blood vessels in mice in the absence of mural cells. J Clin Invest. 2002;110:1619–1628. doi: 10.1172/JCI15621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Augustin HG, Koh GY, Thurston G, Alitalo K. Control of vascular morphogenesis and homeostasis through the angiopoietin-tie system. Nat Rev Mol Cell Biol. 2009;10:165–177. doi: 10.1038/nrm2639. [DOI] [PubMed] [Google Scholar]
- 42.Fuxe J, Tabruyn S, Colton K, Zaid H, Adams A, Baluk P, Lashnits E, Morisada T, Le T, O’Brien S, Epstein DM, Koh GY, McDonald DM. Pericyte requirement for anti-leak action of angiopoietin-1 and vascular remodeling in sustained inflammation. Am J Pathol. 2011;178:2897–2909. doi: 10.1016/j.ajpath.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thurston G, Wang Q, Baffert F, Rudge J, Papadopoulos N, Jean-Guillaume D, Wiegand S, Yancopoulos GD, McDonald DM. Angiopoietin 1 causes vessel enlargement, without angiogenic sprouting, during a critical developmental period. Development. 2005;132:3317–3326. doi: 10.1242/dev.01888. [DOI] [PubMed] [Google Scholar]
- 44.Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102:199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.