Abstract
Allogeneic hematopoietic cell transplantation (alloHCT) provides a potentially curative therapy for patients with high-risk or chemorefractory acute myeloid leukemia (AML). Historically, the applicability of alloHCT has been limited as only 30–35% of patients have human leukocyte antigen (HLA)-matched siblings and outcomes using other donor types have been markedly inferior due to excess toxicity, graft failure, graft-versus-host disease, and consequently non-relapse mortality. Advances in HLA typing, graft-versus-host disease prophylactic approaches, and other transplantation techniques have successfully addressed these historical challenges. Herein, we review recent alloHCT studies using volunteer unrelated donors, umbilical cord blood units, or HLA-haploidentical donors, specifically focusing on studies that compared outcomes between donor sources. Although none are randomized and most are retrospective, these analyses suggest that current outcomes for AML patients using most alternative donor types are comparable to those seen using HLA-matched-siblings.
Introduction
The curative potential of allogeneic hematopoietic cell transplantation (alloHCT) in treating patients with acute myeloid leukemia (AML) was first demonstrated over 50 years ago. Although cure was achievable in some patients with chemorefractory disease, alloHCT demonstrated a more dramatic benefit when used to treat AML patients earlier in their disease course.1 In fact, relapse was lower after alloHCT than after consolidation chemotherapy,2 suggesting that some patients with AML in first complete remission might benefit by proceeding directly to alloHCT.3, 4 Even so, many patients suffered non-relapse mortality (NRM),5 stemming from a number of challenges to the success of alloHCT. These included graft failure, graft-versus-host disease (GVHD) in both its acute and chronic forms, and post-grafting opportunistic infections related to long-lasting deficiencies in both humoral and cellular immunity.
Encouraging results with alloHCT were restricted to the minority of patients who had an HLA-matched-sibling donor (MSD). A suitable unrelated donor (whether HLA-matched-unrelated donor [MUD], one-locus HLA-mismatched-unrelated donor (mmUD), or umbilical cord blood [UCB] unit) can be found for most individuals.6 Furthermore, HLA-haploidentical (haplo) related donors are available for nearly all individuals. Nevertheless, outcomes were less favorable when alternative donors were used,7–11 establishing HLA-matched-sibling donors as the gold standard donor source. However, improvements in transplantation approaches over the past few decades have led to markedly improved outcomes after alternative donor alloHCT [Figure 1], now challenging whether MSD alloHCT still achieves superior outcomes. Herein, we review the expanding role of alternative donor alloHCT in the treatment of AML patients.
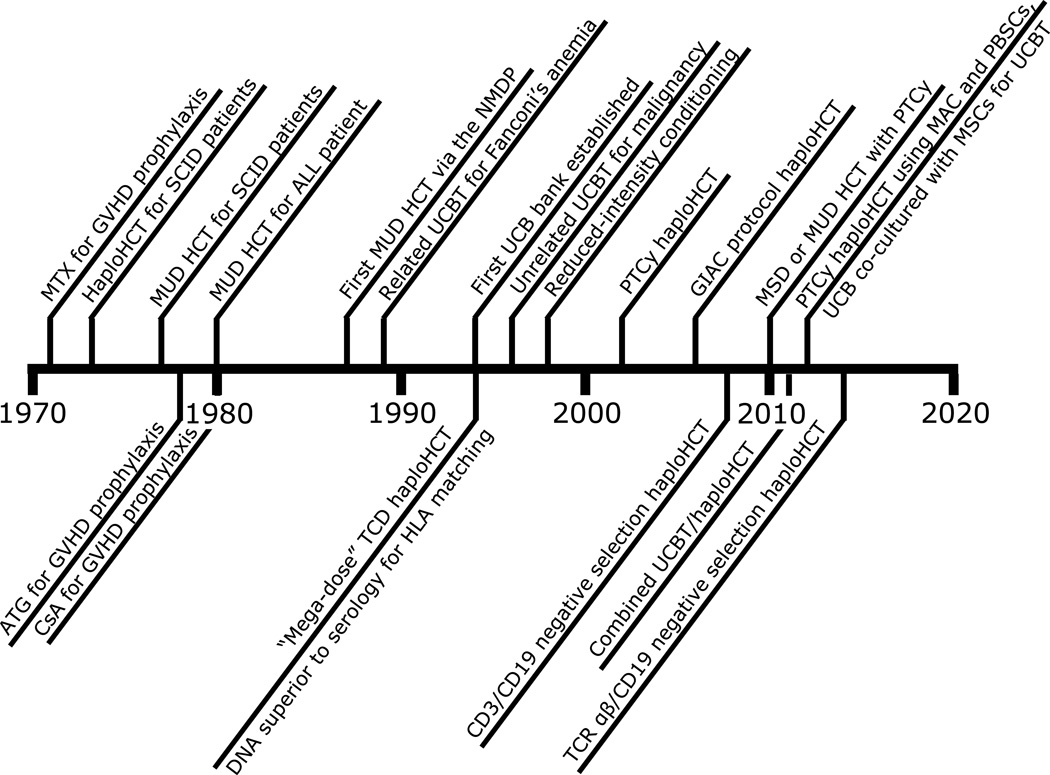
Figure 1. Timeline of important milestones in alternative donor allogeneic hematopoietic cell transplantation.
For all milestones, the year of manuscript publication was used. Early unsuccessful transplantations were not included. MTX=methotrexate, GVHD=graft-versus-host disease, HLA=human leukocyte antigen, HCT=allogeneic hematopoietic cell transplantation, HaploHCT=HLA-haploidentical HCT, SCID=severe combined immunodeficiency, MUD=HLA-matched-unrelated donor, ALL=acute lymphoblastic leukemia, ATG=anti-thymocyte globulin, CsA=cyclosporine-A, NMDP=National Marrow Donor Program, UCBT=umbilical cord blood transplantation, TCD=T-cell depletion, PTCy=post-transplantation cyclophosphamide, MSD=HLA-matched-sibling donor, TCR=T-cell receptor, MAC=myeloablative conditioning, PBSCs=peripheral blood stem cells, MSCs=mesenchymal stromal cells.
HLA-Matched-Unrelated Donors
Since HLA-matching has been prioritized in donor selection, a patient without an HLA-matched-related donor potentially could benefit from alloHCT using a volunteer HLA-matched-unrelated donor. Index cases from the early 1980s proved the feasibility of this approach for treating acute leukemia.12 Although initial studies suggested relative equivalence with MSD alloHCT,13 a large, prospective case-control, multi-institutional study showed inferior engraftment, higher rates of grade II-IV acute and extensive chronic GVHD, and worse survival for unrelated donor alloHCT compared with MSD alloHCT.10, 11
Given discrepancies in results between studies and the inclusion in some studies of HLA-mismatched- unrelated donors (mmUDs), large registry-based analyses were undertaken to evaluate the relative equivalence of MUD versus MSD alloHCT. The first compared alloHCT patients reported to the International Bone Marrow Transplant Registry between 1985 and 1991.14 Each of graft failure, grades II-IV and III-IV acute GVHD, chronic GVHD, and NRM (>50%) were higher for all alternative donors (MUDs, one locus mmUDs, or one- or two-locus HLA-mismatched related donors) when compared with MSDs. A later registry study from the Center for International Blood and Marrow Transplant Research (CIBMTR) reported on alloHCT for 4099 (941 8/8 MUDs and 3158 MSDs) adult patients with AML, ALL, or CML performed between 1995 and 2004.15 GVHD, particularly grade II-IV acute GVHD, was slightly more common in the MUD cohort. For AML patients, MUD allografting was associated with higher rates of both NRM and relapse, resulting in significantly lower DFS and questioning whether there indeed was a superior graft-versus-leukemia effect associated with MUD compared with MSD allografting.
Based on these and other studies, MUDs standardly have been considered a second-tier donor source. However, simultaneous to the second study above, advances in HLA typing were improving outcomes for MUD alloHCT. HLA typing originally had been performed by serologic methods for HLA-A, HLA-B, and HLA-DR only. The importance of HLA-C serologic matching was later recognized, although “permissive” mismatching in HLA-C may exist that do not deleteriously affect outcomes.16 By the mid-1990s, it was discovered that serologic typing was inferior to DNA-based typing.17, 18 In fact, one study published in 1998 performed HLA typing by both serologic and DNA methods and found that only 45% of patients who were serologically matched were in fact HLA-matched by DNA testing of HLA-A, -B, and DRB1.19 Furthermore, HLA-C and -DQ testing had not been performed serologically, and 41% of donor-recipient pairs were found to be incompatible at those loci by DNA testing.19
Therefore many patients from earlier studies of “MUD” alloHCT may not have in fact received 8/8 HLA-matched allografts. Even single HLA-mismatches in HLA-A, -B, -C, and – DRB1 were found to be associated with worse survival,20, 21 in addition to the negative effects of specific HLA-locus-mismatching on the incidences of graft failure, GVHD, and relapse.19–22 HLA matching at HLA-DQ, HLA-DP, and low expression HLA-DR loci also may impact outcomes, although effects of mismatching at these loci is much more prominent in alloHCT using 6/8 or 7/8 mmUDs.23, 24 Beyond better HLA typing, improved supportive care has played an important role in improving outcomes for unrelated donor alloHCT. Furthermore, the incorporation of anti-thymocyte globulin (ATG)25, 26 or more recently post-transplantation cyclophosphamide (PTCy)27, 28 into GVHD prophylactic approaches may alleviate some of the increased alloreactivity associated with MUD compared with MSD allografting.
Overall, with these advances in transplantation approaches, survival outcomes for AML patients treated with MUD alloHCT currently appear only slightly lower than those seen for patients receiving MSD alloHCT, and these small differences do not typically reach statistical significance [Table 1].28–36 While most studies do show higher rates of grade II-IV acute GVHD with MUDs, grade III-IV acute GVHD and NRM appear similar between the two donor types.28, 30, 32–34 Inconsistent effects on chronic GVHD have been reported; likely MUD allografting is associated with increased chronic GVHD, but this risk appears to be mitigated by the addition of ATG [Table 1].26 Interestingly, given the greater alloreactivity and associated GVHD that accompanies female-into-male allografting, male MUDs may be associated with nearly identical results as female MSD alloHCT.34
Table 1.
Selected recent comparative studies of MSD versus MUD T-cell-replete allogeneic cell transplantation for AML patients.
| Reference Number |
Years of Study |
# of Patients |
% AML Patients |
Patient Ages |
HLA Locus Matching |
% Given ATG |
Relapse And Survival Outcomes Reported At, Years |
Graft Failure, % |
Acute GVHD, II–IV, CI % |
Acute GVHD, III–IV, CI % |
Chronic GVHD, CI % |
NRM, CI % |
Relapse, CI % |
DFS, % | OS, % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSD | MUD | MSD | MUD | MSD | MUD | MSD | MUD | MSD | MUD | MSD | MUD | MSD | MUD | MSD | MUD | MSD | MUD | MSD | MUD | MSD | MUD | |||||
| 15a | 1995– 2004 |
3158 | 941 | 40 | 36 | 18– 60 |
8 | 1 | 5 | 5 | NR | NR | 34 | 52 | 16 | 21 | 42 | 49 | 24* 30 31* |
40* 39 44* |
15 22 46 |
22 21 43 |
61* 48 23* |
38* 41 13* |
NR | NR |
| 29 | 1995– 2004 |
168 | 51 | 100 | 100 | 50– 73 |
8 | 38 | 61 | 5 | NR | NR | 39 | 30 | 19 | 14 | 22 | 15 | ↔ | ↔ | ↔ | ↔ | 33 | 30 | ↔ | ↔ |
| 30b | 1995– 2006 |
226 | 254 | 100 | 100 | <1– 74 |
8 | 8 | 27 | 3 | NR | NR | 38* | 54* | 19* | 25* | 43* | 59* | 21 | 26 | 37 | 40 | 42 | 34 | 45 | 37 |
| 31c | 1998– 2004 |
62 | 89 | 100 | 100 | 19– 61 |
NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR | ↔ | ↔ | ↔ | ↔ | NR | NR | ↔ | ↔ |
| 32d | 1996– 2007 |
135 | 62 | 100 | 100 | 18– 69 |
10 | 6 | 13 | 5 | NR | NR | 64 | 77 | 21 | 16 | 50 | 50 | ↔ | ↔ | ~30 | ~25 | ~60 | ~55 | ~65 | ~58 |
| 33e | 2000– 2003 |
181 | 55 | NR | NR | <55 | 10 | 0 | 0 | 2 | 0 | 0 | 46* | 61* | 19 | 29 | NR | NR | 24 | 29 | 20 | 16 | 56 | 55 | 64 | 58 |
| 28f | 2004– 2011 |
78 | 60 | 100 | 100 | 18– 64 |
10 | 0 | 0 | 3 | 3 | 8 | 37* | 50* | 10 | 18 | 6 | 15 | 18 | 23 | 37 | 36 | 45 | 41 | 57 | 48 |
| 34g | 2004– 2011 |
1855 | 1360 | 100 | 100 | 18– 77 |
8 | 17 | 58 | 2 | 2 | 2 | 24* | 27* | 10 | 10 | 50 | 38 | 20 | 21 | 29 | 33 | 51 | 46 | 57 | 52 |
Indicates outcomes that are significantly different between MSD and MUD cohorts. Results indicate differences in univariate analysis unless multivariate analysis was performed and contradicted the univariate results.
↔ Indicates that the exact percentage was not reported but that there was no difference on testing between MSD and MUD alloHCT patients.
GVHD rates were not statistically compared. NRM, relapse, and DFS outcomes listed are for the AML cohort only. However, these results were not reported for the entire AML population but by three AML groups divided by disease status at alloHCT. The top line is AML in first complete remission, the second line is AML in second or subsequent complete remission, and the bottom
All patients had AML with unfavorable-risk cytogenetics in first complete remission.
This was a prospective study but donor selection was not randomized. Instead, donor selection was based on donor availability (biologic randomization).
Included patients with AML in first complete remission. Relapse and survival percentages were not reported explicitly but were shown in figures. Even so, statistical comparisons between MSD and MUD alloHCT were performed. NRM was not shown in a figure nor were percentages reported. However, there was no statistical difference between the two groups in regards to NRM with a hazard ratio of 0.95 (95% confidence interval, 0.32–2.78).
This was a prospective study designed to compare MSD versus MUD alloHCT. Although donor selection was not randomized, entry criteria, conditioning, and GVHD prophylaxis were standardized between the two cohorts. Seventy-eight percent of MSD alloHCT patients and 62% of MUD alloHCT patients had acute leukemia, but the percentage of AML patients was not specifically reported.
Data are shown for just AML patients included in this study of AML, ALL, and MDS patients. Of note, the difference in chronic GVHD was statistically significant for the entire cohort (8% for MSD versus 20% for MUD, p=0.02), but the difference within the AML subset was not significant (p=0.1).
This study compared MUD alloHCT using a male donor and MSD alloHCT using a female donor. Data from the AML cohort only are shown. Seventeen percent and 58% of MSD and MUD alloHCT patients, respectively, received anti-thymocyte globulin.
Abbreviations: MSD=HLA-matched-sibling donor, MUD=HLA-matched-unrelated donor, HLA=human leukocyte antigen, AML=acute myeloid leukemia, GVHD=graft-versus-host disease, CI=cumulative incidence, NRM=non-relapse mortality, DFS=disease-free survival, OS=overall survival, NR=not reported, alloHCT=allogeneic hematopoietic cell transplantation, ALL=acute lymphoblastic leukemia, MDS=myelodysplastic syndrome.
Ultimately, these studies are limited by most being retrospective with the few prospective studies not incorporating randomization of the donor source. Biologic randomization may alleviate some potential sources of bias but introduces others. For example, the time from diagnosis to transplant may be longer with MUD alloHCT or the threshold for MSD alloHCT may be lower than for MUD allografting due to perceived differences in the relative toxicities and outcomes between the two donor types. Even so, the 2–4 month delay in procuring a MUD allograft can make MUDs a non-viable donor option for many patients with aggressive AML in fragile remissions. Furthermore, it is much more challenging to re-access an unrelated donor for adoptive cell therapeutic strategies to prevent or treat relapse.
Umbilical Cord Blood
The potential of UCB to be used as a donor source for alloHCT was developed through the work of Broxmeyer and colleagues. In a study reported in 1989, these investigators examined over 100 human UCB units.37 Firstly, they determined that UCB-derived hematopoietic progenitor cells remained viable and functionally active for at least three days when stored at 4°C or even room temperature, allowing the routine transportation and cryopreservation of UCB to be performed. Secondly, the number of progenitor cells obtained from a single UCB unit was comparable to that reported necessary for successful engraftment of bone marrow alloHCT. These properties of UCB allowed the establishment of UCB banks that could provide HLA-matched or partially-HLA-mismatched-unrelated UCB parallel to that provided by the National Marrow Donor Program (NMDP) for volunteer unrelated donors. Although 75% of European-descent patients have an 8/8 MUD in the NMDP registry, such a donor is available for only 16–19% of African-Americans.6 Nevertheless, greater than 80% and 95% of adult and pediatric patients, respectively, of any ethnicity will have an available ≥ 4/6 HLA-matched (intermediate level at HLA-A and –B and high resolution at HLA-DRB1) UCB unit.6
Early studies demonstrated the feasibility of the UCBT approach.38 However, the ideal transplantation platform for UCBT has been debated. One major factor has been one- versus-two-unit UCBT for adults. The stem cell dose obtainable from many UCB units is often considered too low for engrafting larger adults. The use of double-unit UCBT (dUCBT) has demonstrated encouraging results, but it remains unclear whether an adequately-sized single-unit UCBT (sUCBT) is sufficient. Several studies have retrospectively39–42 or prospectively43 compared sUCBT versus dUCBT. Engraftment was similar in all studies. All but one43 report showed an increased risk of grade II-IV acute GVHD with dUCBT, but grade III-IV acute and chronic GVHD were less consistently higher after dUCBT. Three of the five studies showed lower relapse after dUCBT.39, 42, 43 NRM was similar between sUCBT and dUCBT in all studies, and only one report showed a survival advantage to dUCBT.39 The optimal conditioning intensity for UCBT also is uncertain. A recent retrospective study showed, as expected, higher NRM but lower relapse and thus similar overall survival (OS) after myeloablative conditioning compared with reduced-intensity conditioning (RIC) UCBT.44 Meanwhile, another retrospective study showed a survival advantage for AML patients receiving myeloablative conditioning.45
Regardless of disagreement as to the optimal UCBT platform, a number of analyses have compared outcomes between UCBT and alloHCT using MSDs or MUDs. An early registry study from the CIBMTR examined outcomes for children with acute leukemia undergoing bone marrow or single-unit UCB alloHCT from HLA-matched or HLA-mismatched donors.46 This study found similar survival for all groups, but the highest survival actually was seen with HLA-matched UCBT. However, an HLA-matched UCB unit is not available for most individuals,6 particularly adults who may require a large single-unit or double-unit UCBT. Within the last several years, a plethora of retrospective studies including AML patients have compared outcomes after unrelated (and generally HLA-mismatched) UCBT with outcomes after MSD and/or MUD alloHCT [Table 2].47–57 These studies consistently showed delayed neutrophil and platelet engraftment with higher incidences of non-engraftment in patients treated with UCBT. The effects on acute GVHD were less uniform with several showing reduced grade II–IV but not grade III–IV acute GVHD after UCBT. Chronic GVHD rates were consistently lower after UCBT, although these differences were not always statistically significant. However, these studies are complicated by differing percentages of patients within each cohort who received ATG, which was generally more frequently given to UCBT recipients. Almost all studies revealed higher NRM after UCBT, while the impact on relapse differed between studies. Due to the high NRM, most studies showed lower DFS and OS after UCBT, although in many cases the differences were not statistically significant. These studies conclude that UCBT is a reasonable alternative donor option when a MSD donor is not available. Whether unrelated UCB or MUD donors should be preferentially used when both are available is unclear at this time.
Table 2.
Selecteda recent comparative studies of unrelated UCBT versus T-cell-replete MSD or MUD alloHCT for AML patients.
| Ref. | Years of Study | # of Patients |
% AML Patients |
Patient Ages | # UCB Units | Conditioning Intensity | Acute GVHD, III–V, CI % |
Acute GVHD, IIII–V, CI % |
Chronic GVHD, CI % |
NRM, CI % |
Relapse, CI % |
PFS/DFS, % |
OS, % | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSD | MUD | UCB | MSD | MUD | UCB | MSD | MUD | UCB | MSD | MUD | UCB | MSD | MUD | UCB | MSD | MUD | UCB | MSD | MUD | UCB | MSD | MUD | UCB | MSD | MUD | UCB | |||||
| 47 | 2000–2005 | 311 | 173 | 100 | 100 | 16– 69 |
1 | MAC | 35 | 32 | NR | NR | 32 | 28 | 22* | 33* | 24 | 31 | 54* | 36* | 60* | 43* | |||||||||
| 48b | 2000– 2005 |
47 | 43 | 21 | 44 | ≥55 | 2– 88 % |
RIC | 42 | 49 | NR | NR | 40* | 17* | 23 | 28 | NR | NR | 30 | 34 | 43 | 34 | |||||||||
| 49c | 2000– 2009 |
313 | 161 | 94 | 80 | 21– 69 |
2 | RIC | 33* | 50* 33 |
14 | 17 18 |
56* | 34* 36 |
21* | 19 52* |
44 | 49 35 |
35* | 31 15* |
44* | 37 19* |
|||||||||
| 50d | 2000– 2010 |
82 | 35 | 80 | 100 | 100 | 100 | 50– 74 |
2 – 88 % |
RIC | 29 | 38 | 39 | 10 | 15 | 14 | 43 | 41 | 23 | 18 | 14 | 24 | 33* | 29* | 43* | 48 | 57 | 33 | 51 | 53 | 45 |
| 51 | 2001– 2008 |
204 | 152 | 128 | 48 | 33 | 53 | 10– 67 |
2 | MAC | 65 | 80* | 60* | 13 | 14 | 22 | 47* | 43* | 26* | 24* | 14 | 34* | 43* | 37* | 15* | 33 | 48 | 51 | NR | NR | NR |
| 52e | 2001–2009 | 38 | 60 | 68 | 73 | 55– 70 |
2– 95 % |
RIC | 38 | 45 | 26 | 21 | 61* | 33* | 25 | 25 | 34 | 47 | 34 | 22 | 37 | 31 | |||||||||
| 53f | 2002– 2006 |
964 | 165 | 59 | 46 | ≥16 | 1 | MAC | ↑* | ↓* | NR | NR | ↑* | ↓* | ↓* | ↑* | ↔ | ↔ | ↔ | ↔ | NR | NR | |||||||||
| 54g | 2004– 2008 |
221 | 64 | 33 | 36 | 19–73 | 2 | RIC | 20 | 14 | 7 | 3 | 54* | 22* | 10* | 27* | 50 | 43 | 40 | 30 | 50 | 46 | |||||||||
| 55h | 2007– 2010 |
30 | 20 | 39 | 42 | 33 | 22– 70 |
2 | RIC | 31 | 26 | 15 | 8 | 35 | 26 | 6* | 26 | 36 | 23 | 58 | 51 | 62 | 61 | ||||||||
| 56i | 2005– 2010 |
441 | 205 | 100 | 100 | 100 | 50– 75 |
2– 61 % |
Both | ↔ | ↔ | NR | NR | ↑* | ↓* | 27* | 35* | 35 | 35 | 39* | 28* | 43* | 30* | ||||||||
| 57j | 2005– 2011 |
36 | 13 | 32 | 100 | 100 | 100 | 18– 69 |
2–; 72 % |
RIC | 18* | 41* | 8 | 25 | 35 | 25 | 22 | 16 | 27* | 60* | 50* | 25* | 56 | 34 | |||||||
Indicates outcomes that are significantly different. Results indicate differences in univariate analysis unless multivariate analysis was performed and contradicted the univariate results.
All studies which began enrolling AML patients after 2000 and which separately reported outcomes for either MSD or MUD recipients (did not mix with partially-HLA-mismatched) were included.
ATG was given to 13% of MSD alloHCT recipients and 40% of UCBT recipients.
UCBT outcomes were split by those who received TCF conditioning and those who received other conditioning regimens. For outcomes, TCF UCBT is on the top line and UCBT using other conditioning is on the bottom line.
ATG was given to 30% of MSD, 86% of MUD, and 29% of UCB alloHCT recipients. DFS was significantly different on unadjusted analysis but was non-significant on adjusted analysis. The unadjusted DFS is shown as the adjusted percentages were not reported. The adjusted OS percentages were reported and are shown.
ATG was given to 57% of UCBT patients and 32% of MSD alloHCT patients.
ATG was given to 72% of UCBT patients, 28% of BM alloHCT patients, and 18% of PBSC alloHCT patients. Significant differences for acute GVHD were only seen between UCBT and PBSC alloHCT, but not between UCBT and BM alloHCT.
Includes 7/8 and 8/8 HLA-matched-unrelated donors. Ninety-one percent of donors were 8/8 HLA-matched with the recipient.
Two additional patients who received HLA-mismatched-unrelated alloHCT were included in the outcomes. ATG was given to all MSD and MUD recipients and only 6 (15%) of UCB recipients.
In vivo T-cell depletion was given to 39% of MUD recipients and 32% of UCB recipients. There were no significant differences in outcomes between UCBT patients receiving a single-unit or double-unit except for higher NRM after double-unit UCBT, with a trend towards lower relapse after double-unit UCBT.
All MSD and MUD recipients but no UCB recipients received ATG.
Abbreviations: UCB=umbilical cord blood, UCBT=umbilical cord blood transplantation, MSD=HLA-matched-sibling donor, MUD=HLA-matched-unrelated donor, HLA=human leukocyte antigen, alloHCT=allogeneic hematopoietic cell transplantation, Ref.=reference number, AML=acute myeloid leukemia, GVHD=graft-versus-host disease, CI=cumulative incidence, NRM=nonrelapse mortality, PFS=progression-free survival, DFS=disease-free survival, OS=overall survival, MAC=myeloablative conditioning, RIC=reduced-intensity conditioning, NR=not reported, ATG=anti-thymocyte globulin, TCF=Total body irradiation, Cyclophosphamide, and Fludarabine, BM=bone marrow, PBSC=peripheral blood stem cell.
Recent work in UCBT has focused on reducing the high NRM associated with the approach, particularly via improving the rapidity of engraftment and improving post-transplantation immune reconstitution. Co-culturing UCB cells with mesenchymal stromal cells prior to infusion resulted in nucleated cell expansion and reduced both graft failure and time to engraftment.58 An alternative strategy has involved infusing both an UCB unit and a haplo allograft.59–61 Although this approach resulted in rapid engraftment, two of the three published studies still showed high NRM of 28% and 35%. Overall, low chronic GVHD and potentially lower relapse with UCBT have made it a promising transplantation platform to continue to refine. Furthermore, this donor source is rapidly available to more than 80% of patients of any ethnicity without the need for further donor evaluation or matching.6
HLA-Haploidentical Donors
HLA-haploidentical (haplo) alloHCT initially generated poor outcomes, largely related to high rates of graft failure and GVHD.7–9 The Fred Hutchinson Cancer Research Center group compared alloHCT outcomes between 1975–1986 using either MSD or partially HLA-mismatched-related donors, with the latter group having higher incidences of graft failure (12%) and GVHD (>50% grade III-IV acute GVHD) as well as inferior survival (~10% for two- or three-locus HLA-mismatched alloHCT),8, 9 reflecting the intense bi-directional T-cell alloreactivity associated with HLA-mismatching.8, 9 Several investigative groups have since overcome these barriers to HLA-haploidentical alloHCT (haploHCT), among which three strategies have been the most developed.
The Memorial Sloan-Kettering and the University of Perugia groups pioneered the strategy of T-cell depletion (TCD), which was initially performed through negative selection by soybean agglutination and erythrocyte rosetting62, 63 and more recently has involved CD34-positive selection.64 TCD was accompanied by a “mega-dose” of CD34+ cells following intensive myeloablative and immunoablative conditioning, all designed to promote engraftment.62–64 Engraftment was 90–95% with this approach, and both acute and chronic GVHD were <10% despite no additional post-grafting GVHD prophylaxis. Relapse was not higher than would be expected for patients with high-risk or advanced hematologic malignancies undergoing T-cell-replete alloHCT, potentially related to natural killer cell alloreactivity.64 However, NRM was quite high at 37–53%, largely related to infection.62–64 Efforts to improve immune reconstitution after TCD haploHCT, including CD3/CD19-negative selection65 or reintroduction of lower levels of both conventional and regulatory T cells,66 have not thus far been successful in substantially reducing this high NRM. A European Blood and Marrow Transplant (EBMT) study of 173 AML patients treated with TCD haploHCT showed 2-year NRM rates of 36%, 54%, and 66% for patients in CR1, CR2, or advanced disease at the time of alloHCT.67 Relapse rates were 16%, 23%, and 32%, respectively, resulting in DFS rates of 48%, 21%, and 1%, respectively.
A second approach (called the GIAC protocol) was developed by the Peking University group in Beijing, China, and incorporated granulocyte colony-stimulating factor (GCSF) treatment of the donor, the use of both T-cell-replete PBSC and bone marrow allografts, and intensive pharmacologic immunosuppression including ATG.68–70 This strategy resulted in universal engraftment and favorable NRM and relapse rates. However, cumulative incidences of GVHD were quite high, particularly chronic GVHD which occurred in up to 74% of patients.69 A report by an Italian group of a modification of this approach through using only BM allografts and adding basiliximab was successful in reducing chronic GVHD to 17% but resulted in a 7% rate of graft failure and a NRM of 36%.71 Outcomes for AML patients treated with the GIAC protocol have appeared quite encouraging. One large report from the Peking group showed relapse rates for standard-risk and high-risk AML patients of 12% and 20%, respectively, resulting in DFS rates of 71% and 56%, respectively.70 Four reports from three Chinese groups have shown similar DFS after haploHCT using the GIAC protocol compared with outcomes using MSD alloHCT [Table 3].68, 72–74 In fact, one study of patients with very high-risk acute leukemia even showed better survival due to lower relapse after haploHCT.73 Grade II–IV acute GVHD occurred more frequently after haploHCT in all four studies. Chronic GVHD and NRM rates were similar between haploHCT and MSD alloHCT in three of the four studies.
Table 3.
Studies Comparing Haplo AlloHCT Using the GIAC Protocol Versus MSD AlloHCT for AML patients.
| Ref. | Years of Study |
# of Patients |
% AML Patients |
Patient Ages |
Relapse and Survival Outcomes Reported At, Years |
Acute GVHD, II–IV, CI% |
Acute GVHD, III–IV, CI % |
Chronic GVHD, CI % |
NRM, CI % |
Relapse, CI % |
DFS, % | OS, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSD | Haplo | MSD | Haplo | MSD | Haplo | MSD | Haplo | MSD | Haplo | MSD | Haplo | MSD | Haplo | MSD | Haplo | MSD | Haplo | ||||
| 68a | 2002– 2004 |
135 | 158 | 25 | 22 | 5–50 | 2 | 32* | 40* | 11 | 16 | 56 | 55 | 14 | 22 | 13 | 18 | 71 | 64 | 72 | 71 |
| 72b | 2003– 2005 |
51 | 56 | 31 | 25 | 4–59 | 2 | 14* | 27* | 4 | 7 | 31 | 23 | 8 | 12 | 17 | 22 | 76 | 68 | 80 | 70 |
| 73c | 2005– 2009 |
36 | 81 | 56 | 37 | 5–56 | 3 | 24* | 49* | 4 | 15 | 39 | 62 | 38 | 34 | 49* | 26* | 15* | 42* | 20* | 42* |
| 74d | 2008– 2013 |
90 | 99 | 47 | 29 | 9–56 | 5 | 16* | 42* | 6* | 17* | 24 | 41 | 5* | 30* | 34* | 14* | 64 | 58 | 77* | 61* |
Indicates outcomes that are significantly different.
The transplantation platform for haplo alloHCT used twice the dose of cytarabine, the inclusion of ATG, and a longer post-transplantation duration of MMF but was otherwise identical to that used for MSD alloHCT.
The conditioning used for MSD alloHCT was busulfan/cyclophosphamide or cyclophosphamide/total body irradiation without ATG. GVHD prophylaxis for haplo alloHCT added an additional dose of methotrexate on day +11, started CsA earlier during conditioning, and continued both CsA and MMF for a longer time period post-transplantation.
The transplantation platform for the haplo alloHCT included ATG, used twice the dose of cytarabine, omitted a dose of hydroxycarbamide on day −10, and extended the duration/tapering of MMF post-transplantation, but otherwise was identical to that used for MSD alloHCT.
MUD alloHCT also was included as a third comparator group in this study. All outcomes were similar between haplo alloHCT and MUD alloHCT recipients. The transplantation platform for HLA-matched alloHCT recipients was busulfan/cyclophosphamide except for those with central nervous system involvement or high white blood cell count at diagnosis in which case it was cytarabine, busulfan, cyclophosphamide, and semustine. Rabbit ATG also was given to MUD alloHCT recipients. The duration of GVHD prophylaxis with MTX, CsA, and MMF was longer for haplo alloHCT recipients.
Abbreviations: Haplo=HLA-haploidentical donor, MSD=HLA-matched-sibling donor, HLA=human leukocyte antigen, alloHCT=allogeneic hematopoietic cell transplantation, AML=acute myeloid leukemia, Ref.=reference number, GVHD=graft-versus-host disease, CI=cumulative incidence, NRM=non-relapse mortality, DFS=disease-free survival, OS=overall survival, ATG=anti-thymocyte globulin, CsA=cyclosporine A, MMF=mycophenolate mofetil, MUD=HLA-matched-unrelated donor.
A third approach was developed at Johns Hopkins Hospital and involved the administration of high-dose cyclophosphamide on days +3 and +4 after infusion of a T-cell-replete bone marrow allograft.75, 76 This PTCy strategy appears to work, at least in part, by eliminating proliferative, alloreactive T-cells, while preserving regulatory T-cells.76, 77 While graft failure was seen (2–13%),75, 78 given low intensity conditioning, most patients with graft failure quickly experienced autologous reconstitution.75 NRM, severe acute GVHD, and particularly chronic GVHD were quite low. Relapse was relatively high,75, 78 although studies by other centers using higher intensity conditioning seemed to provide more acceptable relapse rates while reducing graft failure and not significantly impacting NRM or GVHD.79, 80 No AML-specific outcomes have yet been published for PTCy haploHCT. However, the Hopkins experience using RIC has shown chronic GVHD of 16%, NRM of 10%, and OS of 52% at 4 years for AML patients in remission without measurable residual disease at haploHCT (Margaret Showel, personal communication).
Three retrospective studies including AML patients have compared outcomes between haploHCT using PTCy with MSD or MUD alloHCT not using PTCy and have shown that GVHD and survival outcomes were similar with haploHCT [Table 4].79–81 Recent unpublished registry data also have shown equivalence between outcomes using PTCy haploHCT and MUD alloHCT (Ciurea et al., American Society of Hematology, 2014). One of the published studies compared outcomes across five different donor types (also including mmUD and UCB) treated at the same institution over a seven year period.80 Those latter two groups had worse survival than the other three groups (MSD, MUD, haplo) with survival after UCBT being statistically lower than after MSD alloHCT. These differences in outcomes were largely reflective of slower immune reconstitution and higher NRM for MUD, mmUD, and UCB alloHCT. There was no statistical difference in relapse rates across treatment groups. Two parallel prospective studies of alternative donor transplantation, one using PTCy haploHCT and the other using UCBT, was recently reported.78 Eligibility criteria were standardized between the two protocols, and each used similar RIC-based transplantation platforms. Engraftment, acute and chronic GVHD, and NRM all were better after PTCy haploHCT. However, relapse rates were lower after UCBT, leading to statistically similar progression-free and overall survival rates between the two studies. These outcomes have spurred an ongoing randomized phase III study directly comparing UCBT versus PTCy haploHCT for the treatment of advanced hematologic malignancies.
Table 4.
Studies Comparing Haplo AlloHCT Using PTCy Versus MSD or MUD AlloHCT for AML patients.
| Ref. | Years of Study |
# of Patients |
% AML Patients |
Patient Ages |
Relapse and Survival Outcomes Reported At, Years |
Acute GVHD, II–IV, CI% |
Acute GVHD, III–IV, CI% |
Chronic GVHD, CI % |
NRM, CI% | Relapse, CI % | DFS, % | OS, % | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MSD | MUD | Haplo | MSD | MUD | Haplo | MSD | MUD | Haplo | MSD | MUD | Haplo | MSD | MUD | Haplo | MSD | MUD | Haplo | MSD | MUD | Haplo | MSD | MUD | Haplo | MSD | MUD | Haplo | MSD | MUD | Haplo | |||
| 79d | 2005– 2010 |
117 | 101 | 53 | 32 | 36 | 32 | NR | 2 | 27 | 39 | 30 | 8 | 11 | 11 | 54* 11* |
54* 12* |
38* 4* |
13 | 16 | 7 | 34 | 34 | 33 | 53 | 52 | 60 | 76 | 67 | 64 | ||
| 80e | 2006– 2012 |
176 | 43 | 92 | NR | NR | NR | 15–69 | 4 | 31* | 21 | 14* | 7 | 3 | 4 | 29* | 22 | 15* | 24 | 33 | 18 | 40 | 23 | 35 | 32 | 36 | 43 | 45 | 43 | 52 | ||
| 81f | 2005– 2012 |
87 | 108 | 32 | 67 | 66 | 69 | 20–76 | 3 | 31 | 29 | 29 | 11 | 6 | 0 | 43 | 30 | 19 | 20 | 35 | 24 | 28 | 23 | 33 | 36 | 27 | 30 | 56 | 66 | |||
Indicates outcomes that are significantly different.
Only patients with haplo alloHCT received PTCy. One-third of haplo alloHCT recipients received myeloablative conditioning, and 40% received PBSC allografts. Meanwhile, nearly all patients receiving MSD or MUD alloHCT received PBSCs. For chronic GVHD, the top line is all chronic GVHD and the bottom line is severe chronic GVHD.
This manuscript reported outcomes for five donor types and statistical comparisons took into account the presence of all five groups. For simplicity sake, outcomes for HLA-mismatched-unrelated donor and UCB alloHCT are not shown in this table. Of note, those two groups had the lowest survival with the overall survival for UCB alloHCT being significantly lower than MSD alloHCT. Only patients receiving haplo alloHCT received PTCy. MUD recipients received anti-thymocyte globulin. Percentage of patients with acute leukemia were reported (MSD 48%, MUD 43%, and haplo 43%), but the percentages of AML patients were not specified. Only moderate/severe chronic GVHD was reported; those percentages are included in the table.
All patients had either AML or myelodysplastic syndrome. All patients received fludarabine and melphalan conditioning, although haplo alloHCT recipients also received thiotepa. Only patients with haplo alloHCT received PTCy.
Abbreviations: Haplo=HLA-haploidentical donor, MSD=HLA-matched-sibling donor, MUD=HLA-matched-unrelated donor, HLA=human leukocyte antigen, alloHCT=allogeneic hematopoietic cell transplantation, PTCy=post-transplantation cyclophosphamide, AML=acute myeloid leukemia, Ref.=reference number, GVHD=graft-versus-host disease, CI=cumulative incidence, NRM=non-relapse mortality, DFS=disease-free survival, OS=overall survival, NR=not reported, PBSC=peripheral blood stem cell, UCB=umbilical cord blood.
Only one study published to date has compared different haploHCT approaches. An MD Anderson group examined their institutional outcomes from two successive clinical trials: one of TCD and the other of PTCy haploHCT. Although the patients were not treated concurrently and thus follow-up was shorter for the PTCy-treated patients, platelet engraftment, T-cell reconstitution, infectious complications, chronic GVHD, and particularly 1-year NRM (42% versus 16%) were significantly better for PTCy-treated patients. Progression was similar between the groups (36% versus 34%) leading to substantially better 1-year progression-free and overall survival for PTCy recipients (OS: 30% versus 64%). Different haploHCT approaches have not yet been compared prospectively, and all current approaches have their own challenges to address. Even so, haploHCT using any transplantation platform has the advantages of rapid donor availability for nearly all patients and the ready availability of the donor for further adoptive cell therapeutic strategies.
Conclusion
Given initial reports showing inferior outcomes with other donor sources, HLA-matched-sibling donors historically have been the preferred donor source. However, as only approximately one-third of patients have an HLA-matched-sibling donor, several alternative donor transplant strategies have been developed. Expanded registries both for volunteer unrelated donors and UCB units have enhanced access to alternative donor transplantation. Furthermore, advances over the past few decades in areas such as HLA typing, supportive care, and better prevention of GVHD have led to marked improvements in outcomes for alternative donor transplantation. Nevertheless, despite numerous retrospective studies showing relative equivalence between most alternative donor sources and MSDs, a MSD likely still will be used preferentially when available. This is particularly true as no randomized studies have been completed to directly compare MSDs with any alternative donor. However, the important prognostic impact of donor factors other than HLA-matching (e.g. age, sex, CMV serostatus, or natural killer cell alloreactivity) on post-transplantation outcomes is becoming increasingly recognized. Therefore, given the recent success of alternative donor transplantation, it is foreseeable that in the near future HLA-matching may no longer be the top priority in donor selection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES: The authors declare that they have no conflicts of interest or competing financial or personal relationships that could inappropriately influence the content of this article.
REFERENCES
- 1.Blume KG, Beutler E, Bross KJ, Chillar RK, Ellington OB, Fahey JL, et al. Bone-marrow ablation and allogeneic marrow transplantation in acute leukemia. N Engl J Med. 1980 May 8;302(19):1041–1046. doi: 10.1056/NEJM198005083021901. [DOI] [PubMed] [Google Scholar]
- 2.Champlin RE, Ho WG, Gale RP, Winston D, Selch M, Mitsuyasu R, et al. Treatment of acute myelogenous leukemia. A prospective controlled trial of bone marrow transplantation versus consolidation chemotherapy. Ann Intern Med. 1985 Mar;102(3):285–291. doi: 10.7326/0003-4819-102-3-285. [DOI] [PubMed] [Google Scholar]
- 3.Zittoun RA, Mandelli F, Willemze R, de Witte T, Labar B, Resegotti L, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med. 1995 Jan 26;332(4):217–223. doi: 10.1056/NEJM199501263320403. [DOI] [PubMed] [Google Scholar]
- 4.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A, et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood. 2000 Dec 15;96(13):4075–4083. [PubMed] [Google Scholar]
- 5.Thomas ED, Buckner CD, Banaji M, Clift RA, Fefer A, Flournoy N, et al. One hundred patients with acute leukemia treated by chemotherapy, total body irradiation, and allogeneic marrow transplantation. Blood. 1977 Apr;49(4):511–533. [PubMed] [Google Scholar]
- 6.Gragert L, Eapen M, Williams E, Freeman J, Spellman S, Baitty R, et al. HLA match likelihoods for hematopoietic stem-cell grafts in the U.S. registry. N Engl J Med. 2014 Jul 24;371(4):339–348. doi: 10.1056/NEJMsa1311707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Powles RL, Morgenstern GR, Kay HE, McElwain TJ, Clink HM, Dady PJ, et al. Mismatched family donors for bone-marrow transplantation as treatment for acute leukaemia. Lancet. 1983 Mar 19;1(8325):612–615. doi: 10.1016/s0140-6736(83)91793-2. [DOI] [PubMed] [Google Scholar]
- 8.Anasetti C, Amos D, Beatty PG, Appelbaum FR, Bensinger W, Buckner CD, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989 Jan 26;320(4):197–204. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 9.Anasetti C, Beatty PG, Storb R, Martin PJ, Mori M, Sanders JE, et al. Effect of HLA incompatibility on graft-versus-host disease, relapse, and survival after marrow transplantation for patients with leukemia or lymphoma. Hum Immunol. 1990 Oct;29(2):79–91. doi: 10.1016/0198-8859(90)90071-v. [DOI] [PubMed] [Google Scholar]
- 10.Hows J, Bradley BA, Gore S, Downie T, Howard M, Gluckman E. Prospective evaluation of unrelated donor bone marrow transplantation. The International Marrow Unrelated Search and Transplant (IMUST) Study. Bone Marrow Transplant. 1993 Oct;12(4):371–380. [PubMed] [Google Scholar]
- 11.Hows JM, Passweg JR, Tichelli A, Locasciulli A, Szydlo R, Bacigalupo A, et al. Comparison of long-term outcomes after allogeneic hematopoietic stem cell transplantation from matched sibling and unrelated donors. Bone Marrow Transplant. 2006 Dec;38(12):799–805. doi: 10.1038/sj.bmt.1705531. [DOI] [PubMed] [Google Scholar]
- 12.Hansen JA, Clift RA, Thomas ED, Buckner CD, Storb R, Giblett ER. Transplantation of marrow from an unrelated donor to a patient with acute leukemia. N Engl J Med. 1980 Sep 4;303(10):565–567. doi: 10.1056/NEJM198009043031007. [DOI] [PubMed] [Google Scholar]
- 13.Beatty PG, Hansen JA, Longton GM, Thomas ED, Sanders JE, Martin PJ, et al. Marrow transplantation from HLA-matched unrelated donors for treatment of hematologic malignancies. Transplantation. 1991 Feb;51(2):443–447. doi: 10.1097/00007890-199102000-00034. [DOI] [PubMed] [Google Scholar]
- 14.Szydlo R, Goldman JM, Klein JP, Gale RP, Ash RC, Bach FH, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997 May;15(5):1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 15.Ringden O, Pavletic SZ, Anasetti C, Barrett AJ, Wang T, Wang D, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009 Mar 26;113(13):3110–3118. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez-Vina MA, Wang T, Lee SJ, Haagenson M, Aljurf M, Askar M, et al. Identification of a permissible HLA mismatch in hematopoietic stem cell transplantation. Blood. 2014 Feb 20;123(8):1270–1278. doi: 10.1182/blood-2013-10-532671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santamaria P, Reinsmoen NL, Lindstrom AL, Boyce-Jacino MT, Barbosa JJ, Faras AJ, et al. Frequent HLA class I and DP sequence mismatches in serologically (HLA-A, HLA-B, HLA-DR) and molecularly (HLA-DRB1, HLA-DQA1, HLA-DQB1) HLA-identical unrelated bone marrow transplant pairs. Blood. 1994 Jan 1;83(1):280–287. [PubMed] [Google Scholar]
- 18.Petersdorf EW, Longton GM, Anasetti C, Martin PJ, Mickelson EM, Smith AG, et al. The significance of HLA-DRB1 matching on clinical outcome after HLA-A, B, DR identical unrelated donor marrow transplantation. Blood. 1995 Aug 15;86(4):1606–1613. [PubMed] [Google Scholar]
- 19.Sasazuki T, Juji T, Morishima Y, Kinukawa N, Kashiwabara H, Inoko H, et al. Effect of matching of class I HLA alleles on clinical outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. N Engl J Med. 1998 Oct 22;339(17):1177–1185. doi: 10.1056/NEJM199810223391701. [DOI] [PubMed] [Google Scholar]
- 20.Flomenberg N, Baxter-Lowe LA, Confer D, Fernandez-Vina M, Filipovich A, Horowitz M, et al. Impact of HLA class I and class II high-resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004 Oct 1;104(7):1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 21.Lee SJ, Klein J, Haagenson M, Baxter-Lowe LA, Confer DL, Eapen M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007 Dec 15;110(13):4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 22.Petersdorf EW, Gooley TA, Anasetti C, Martin PJ, Smith AG, Mickelson EM, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998 Nov 15;92(10):3515–3520. [PubMed] [Google Scholar]
- 23.Fernandez-Vina MA, Klein JP, Haagenson M, Spellman SR, Anasetti C, Noreen H, et al. Multiple mismatches at the low expression HLA loci DP, DQ, and DRB3/4/5 associate with adverse outcomes in hematopoietic stem cell transplantation. Blood. 2013 May 30;121(22):4603–4610. doi: 10.1182/blood-2013-02-481945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pidala J, Lee SJ, Ahn KW, Spellman S, Wang HL, Aljurf M, et al. Nonpermissive HLA-DPB1 mismatch increases mortality after myeloablative unrelated allogeneic hematopoietic cell transplantation. Blood. 2014 Oct 16;124(16):2596–2606. doi: 10.1182/blood-2014-05-576041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duggan P, Booth K, Chaudhry A, Stewart D, Ruether JD, Gluck S, et al. Unrelated donor BMT recipients given pretransplant low-dose antithymocyte globulin have outcomes equivalent to matched sibling BMT: a matched pair analysis. Bone Marrow Transplant. 2002 Nov;30(10):681–686. doi: 10.1038/sj.bmt.1703674. [DOI] [PubMed] [Google Scholar]
- 26.Socie G, Schmoor C, Bethge WA, Ottinger HD, Stelljes M, Zander AR, et al. Chronic graft-versus-host disease: long-term results from a randomized trial on graft-versus-host disease prophylaxis with or without anti-T-cell globulin ATG-Fresenius. Blood. 2011 Jun 9;117(23):6375–6382. doi: 10.1182/blood-2011-01-329821. [DOI] [PubMed] [Google Scholar]
- 27.Luznik L, Bolanos-Meade J, Zahurak M, Chen AR, Smith BD, Brodsky R, et al. High-dose cyclophosphamide as single-agent, short-course prophylaxis of graft-versus-host disease. Blood. 2010 Apr 22;115(16):3224–3230. doi: 10.1182/blood-2009-11-251595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanakry CG, Tsai HL, Bolanos-Meade J, Smith BD, Gojo I, Kanakry JA, et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood. 2014 Dec 11;124(25):3817–3827. doi: 10.1182/blood-2014-07-587477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schetelig J, Bornhauser M, Schmid C, Hertenstein B, Schwerdtfeger R, Martin H, et al. Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German Transplant Study Group. J Clin Oncol. 2008 Nov 10;26(32):5183–5191. doi: 10.1200/JCO.2007.15.5184. [DOI] [PubMed] [Google Scholar]
- 30.Gupta V, Tallman MS, He W, Logan BR, Copelan E, Gale RP, et al. Comparable survival after HLA-well-matched unrelated or matched sibling donor transplantation for acute myeloid leukemia in first remission with unfavorable cytogenetics at diagnosis. Blood. 2010 Sep 16;116(11):1839–1848. doi: 10.1182/blood-2010-04-278317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlenk RF, Dohner K, Mack S, Stoppel M, Kiraly F, Gotze K, et al. Prospective evaluation of allogeneic hematopoietic stem-cell transplantation from matched related and matched unrelated donors in younger adults with high-risk acute myeloid leukemia: German-Austrian trial AMLHD98A. J Clin Oncol. 2010 Oct 20;28(30):4642–4648. doi: 10.1200/JCO.2010.28.6856. [DOI] [PubMed] [Google Scholar]
- 32.Walter RB, Pagel JM, Gooley TA, Petersdorf EW, Sorror ML, Woolfrey AE, et al. Comparison of matched unrelated and matched related donor myeloablative hematopoietic cell transplantation for adults with acute myeloid leukemia in first remission. Leukemia. 2010 Jul;24(7):1276–1282. doi: 10.1038/leu.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yakoub-Agha I, Mesnil F, Kuentz M, Boiron JM, Ifrah N, Milpied N, et al. Allogeneic marrow stem-cell transplantation from human leukocyte antigen-identical siblings versus human leukocyte antigen-allelic-matched unrelated donors (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006 Dec 20;24(36):5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 34.Ringden O, Labopin M, Solders M, Beelen D, Arnold R, Ehninger G, et al. Who is the best hematopoietic stem-cell donor for a male patient with acute leukemia? Transplantation. 2014 Sep 15;98(5):569–577. doi: 10.1097/TP.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 35.Woolfrey A, Lee SJ, Gooley TA, Malkki M, Martin PJ, Pagel JM, et al. HLA-allele matched unrelated donors compared to HLA-matched sibling donors: role of cell source and disease risk category. Biol Blood Marrow Transplant. 2010 Oct;16(10):1382–1387. doi: 10.1016/j.bbmt.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bayraktar UD, de Lima M, Saliba RM, Maloy M, Castro-Malaspina HR, Chen J, et al. Ex vivo T cell-depleted versus unmodified allografts in patients with acute myeloid leukemia in first complete remission. Biol Blood Marrow Transplant. 2013 Jun;19(6):898–903. doi: 10.1016/j.bbmt.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Broxmeyer HE, Douglas GW, Hangoc G, Cooper S, Bard J, English D, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci U S A. 1989 May;86(10):3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rubinstein P, Carrier C, Scaradavou A, Kurtzberg J, Adamson J, Migliaccio AR, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998 Nov 26;339(22):1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 39.Labopin M, Ruggeri A, Gorin NC, Gluckman E, Blaise D, Mannone L, et al. Cost-effectiveness and clinical outcomes of double versus single cord blood transplantation in adults with acute leukemia in France. Haematologica. 2014 Mar;99(3):535–540. doi: 10.3324/haematol.2013.092254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruggeri A, Sanz G, Bittencourt H, Sanz J, Rambaldi A, Volt F, et al. Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimen, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia. 2014 Apr;28(4):779–786. doi: 10.1038/leu.2013.259. [DOI] [PubMed] [Google Scholar]
- 41.Scaradavou A, Brunstein CG, Eapen M, Le-Rademacher J, Barker JN, Chao N, et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013 Jan 31;121(5):752–758. doi: 10.1182/blood-2012-08-449108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verneris MR, Brunstein CG, Barker J, MacMillan ML, DeFor T, McKenna DH, et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009 Nov 5;114(19):4293–4299. doi: 10.1182/blood-2009-05-220525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kindwall-Keller TL, Hegerfeldt Y, Meyerson HJ, Margevicius S, Fu P, van Heeckeren W, et al. Prospective study of one- vs two-unit umbilical cord blood transplantation following reduced intensity conditioning in adults with hematological malignancies. Bone Marrow Transplant. 2012 Jul;47(7):924–933. doi: 10.1038/bmt.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mohty M, Labopin M, Volin L, Gratwohl A, Socie G, Esteve J, et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010 Nov 25;116(22):4439–4443. doi: 10.1182/blood-2010-02-266551. [DOI] [PubMed] [Google Scholar]
- 45.Oran B, Wagner JE, DeFor TE, Weisdorf DJ, Brunstein CG. Effect of conditioning regimen intensity on acute myeloid leukemia outcomes after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2011 Sep;17(9):1327–1334. doi: 10.1016/j.bbmt.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007 Jun 9;369(9577):1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 47.Atsuta Y, Suzuki R, Nagamura-Inoue T, Taniguchi S, Takahashi S, Kai S, et al. Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow transplantation in adult patients with acute leukemia. Blood. 2009 Feb 19;113(8):1631–1638. doi: 10.1182/blood-2008-03-147041. [DOI] [PubMed] [Google Scholar]
- 48.Majhail NS, Brunstein CG, Tomblyn M, Thomas AJ, Miller JS, Arora M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant. 2008 Mar;14(3):282–289. doi: 10.1016/j.bbmt.2007.12.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brunstein CG, Eapen M, Ahn KW, Appelbaum FR, Ballen KK, Champlin RE, et al. Reduced-intensity conditioning transplantation in acute leukemia: the effect of source of unrelated donor stem cells on outcomes. Blood. 2012 Jun 7;119(23):5591–5598. doi: 10.1182/blood-2011-12-400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peffault de Latour R, Brunstein CG, Porcher R, Chevallier P, Robin M, Warlick E, et al. Similar overall survival using sibling, unrelated donor, and cord blood grafts after reduced-intensity conditioning for older patients with acute myelogenous leukemia. Biol Blood Marrow Transplant. 2013 Sep;19(9):1355–1360. doi: 10.1016/j.bbmt.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010 Nov 25;116(22):4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Majhail NS, Brunstein CG, Shanley R, Sandhu K, McClune B, Oran B, et al. Reduced-intensity hematopoietic cell transplantation in older patients with AML/MDS: umbilical cord blood is a feasible option for patients without HLA-matched sibling donors. Bone Marrow Transplant. 2012 Apr;47(4):494–498. doi: 10.1038/bmt.2011.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010 Jul;11(7):653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen YB, Aldridge J, Kim HT, Ballen KK, Cutler C, Kao G, et al. Reduced-intensity conditioning stem cell transplantation: comparison of double umbilical cord blood and unrelated donor grafts. Biol Blood Marrow Transplant. 2012 May;18(5):805–812. doi: 10.1016/j.bbmt.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 55.Le Bourgeois A, Mohr C, Guillaume T, Delaunay J, Malard F, Loirat M, et al. Comparison of outcomes after two standards-of-care reduced-intensity conditioning regimens and two different graft sources for allogeneic stem cell transplantation in adults with hematologic diseases: a single-center analysis. Biol Blood Marrow Transplant. 2013 Jun;19(6):934–939. doi: 10.1016/j.bbmt.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 56.Weisdorf D, Eapen M, Ruggeri A, Zhang MJ, Zhong X, Brunstein C, et al. Alternative donor transplantation for older patients with acute myeloid leukemia in first complete remission: a center for international blood and marrow transplant research-eurocord analysis. Biol Blood Marrow Transplant. 2014 Jun;20(6):816–822. doi: 10.1016/j.bbmt.2014.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Devillier R, Harbi S, Furst S, Crocchiolo R, El-Cheikh J, Castagna L, et al. Poor outcome with nonmyeloablative conditioning regimen before cord blood transplantation for patients with high-risk acute myeloid leukemia compared with matched related or unrelated donor transplantation. Biol Blood Marrow Transplant. 2014 Oct;20(10):1560–1565. doi: 10.1016/j.bbmt.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 58.de Lima M, McNiece I, Robinson SN, Munsell M, Eapen M, Horowitz M, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012 Dec 13;367(24):2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen J, Wang RX, Chen F, Sun AN, Qiu HY, Jin ZM, et al. Combination of a haploidentical SCT with an unrelated cord blood unit: a single-arm prospective study. Bone Marrow Transplant. 2014 Feb;49(2):206–211. doi: 10.1038/bmt.2013.154. [DOI] [PubMed] [Google Scholar]
- 60.Kwon M, Bautista G, Balsalobre P, Sanchez-Ortega I, Serrano D, Anguita J, et al. Haplo-Cord Transplantation Using CD34 Cells from a Third-Party Donor to Speed Engraftment in High-Risk Patients with Hematologic Disorders. Biol Blood Marrow Transplant. 2014 Sep 22; doi: 10.1016/j.bbmt.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 61.Liu H, Rich ES, Godley L, Odenike O, Joseph L, Marino S, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011 Dec 8;118(24):6438–6445. doi: 10.1182/blood-2011-08-372508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Aversa F, Tabilio A, Terenzi A, Velardi A, Falzetti F, Giannoni C, et al. Successful engraftment of T-cell-depleted haploidentical "three-loci" incompatible transplants in leukemia patients by addition of recombinant human granulocyte colony-stimulating factor-mobilized peripheral blood progenitor cells to bone marrow inoculum. Blood. 1994 Dec 1;84(11):3948–3955. [PubMed] [Google Scholar]
- 63.Aversa F, Tabilio A, Velardi A, Cunningham I, Terenzi A, Falzetti F, et al. Treatment of high-risk acute leukemia with T-cell-depleted stem cells from related donors with one fully mismatched HLA haplotype. N Engl J Med. 1998 Oct 22;339(17):1186–1193. doi: 10.1056/NEJM199810223391702. [DOI] [PubMed] [Google Scholar]
- 64.Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, et al. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005 May 20;23(15):3447–3454. doi: 10.1200/JCO.2005.09.117. [DOI] [PubMed] [Google Scholar]
- 65.Bethge WA, Faul C, Bornhauser M, Stuhler G, Beelen DW, Lang P, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: an update. Blood Cells Mol Dis. 2008 Jan-Feb;40(1):13–19. doi: 10.1016/j.bcmd.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 66.Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, et al. HLA-haploidentical transplantation with regulatory and conventional T-cell adoptive immunotherapy prevents acute leukemia relapse. Blood. 2014 Jul 24;124(4):638–644. doi: 10.1182/blood-2014-03-564401. [DOI] [PubMed] [Google Scholar]
- 67.Ciceri F, Labopin M, Aversa F, Rowe JM, Bunjes D, Lewalle P, et al. A survey of fully haploidentical hematopoietic stem cell transplantation in adults with high-risk acute leukemia: a risk factor analysis of outcomes for patients in remission at transplantation. Blood. 2008 Nov 1;112(9):3574–3581. doi: 10.1182/blood-2008-02-140095. [DOI] [PubMed] [Google Scholar]
- 68.Lu DP, Dong L, Wu T, Huang XJ, Zhang MJ, Han W, et al. Conditioning including antithymocyte globulin followed by unmanipulated HLA-mismatched/haploidentical blood and marrow transplantation can achieve comparable outcomes with HLA-identical sibling transplantation. Blood. 2006 Apr 15;107(8):3065–3073. doi: 10.1182/blood-2005-05-2146. [DOI] [PubMed] [Google Scholar]
- 69.Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, et al. Haploidentical hematopoietic stem cell transplantation without in vitro T-cell depletion for the treatment of hematological malignancies. Bone Marrow Transplant. 2006 Aug;38(4):291–297. doi: 10.1038/sj.bmt.1705445. [DOI] [PubMed] [Google Scholar]
- 70.Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W, et al. Treatment of acute leukemia with unmanipulated HLA-mismatched/haploidentical blood and bone marrow transplantation. Biol Blood Marrow Transplant. 2009 Feb;15(2):257–265. doi: 10.1016/j.bbmt.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 71.Di Bartolomeo P, Santarone S, De Angelis G, Picardi A, Cudillo L, Cerretti R, et al. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013 Jan 31;121(5):849–857. doi: 10.1182/blood-2012-08-453399. [DOI] [PubMed] [Google Scholar]
- 72.Chen XH, Gao L, Zhang X, Zhang C, Kong PY, Liu H, et al. HLA-haploidentical blood and bone marrow transplantation with anti-thymocyte globulin: long-term comparison with HLA-identical sibling transplantation. Blood Cells Mol Dis. 2009 Jul-Aug;43(1):98–104. doi: 10.1016/j.bcmd.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 73.Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH, et al. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transplant. 2011 Jun;17(6):821–830. doi: 10.1016/j.bbmt.2010.08.023. [DOI] [PubMed] [Google Scholar]
- 74.Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, et al. T-cell-replete haploidentical HSCT with low-dose anti-T-lymphocyte globulin compared with matched sibling HSCT and unrelated HSCT. Blood. 2014 Oct 23;124(17):2735–2743. doi: 10.1182/blood-2014-04-571570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luznik L, O'Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, et al. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008 Jun;14(6):641–650. doi: 10.1016/j.bbmt.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luznik L, O'Donnell PV, Fuchs EJ. Post-transplantation cyclophosphamide for tolerance induction in HLA-haploidentical bone marrow transplantation. Semin Oncol. 2012 Dec;39(6):683–693. doi: 10.1053/j.seminoncol.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kanakry CG, Ganguly S, Zahurak M, Bolanos-Meade J, Thoburn C, Perkins B, et al. Aldehyde dehydrogenase expression drives human regulatory T cell resistance to posttransplantation cyclophosphamide. Sci Transl Med. 2013 Nov 13;5(211):211ra157. doi: 10.1126/scitranslmed.3006960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, et al. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011 Jul 14;118(2):282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, et al. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013 Apr 1;31(10):1310–1316. doi: 10.1200/JCO.2012.44.3523. [DOI] [PubMed] [Google Scholar]
- 80.Raiola AM, Dominietto A, di Grazia C, Lamparelli T, Gualandi F, Ibatici A, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant. 2014 Oct;20(10):1573–1579. doi: 10.1016/j.bbmt.2014.05.029. [DOI] [PubMed] [Google Scholar]
- 81.Di Stasi A, Milton DR, Poon LM, Hamdi A, Rondon G, Chen J, et al. Similar Transplantation Outcomes for Acute Myeloid Leukemia and Myelodysplastic Syndrome Patients with Haploidentical versus 10/10 Human Leukocyte Antigen-Matched Unrelated and Related Donors. Biol Blood Marrow Transplant. 2014 Dec;20(12):1975–1981. doi: 10.1016/j.bbmt.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]