Abstract
Background
Focal brain lesions (FBL) in HIV/AIDS frequently pose a diagnostic dilemma as the etiology varies from infective (tuberculoma, toxoplasmosis and tuberculous abscesses) to neoplastic lesions like lymphoma. For determining etiology, advanced neuroimaging techniques, serological and molecular biological tests have been evolved with varying sensitivities/specificities. Stereotactic biopsy (STB) of the lesions is reserved for lesions unresponsive to appropriate therapy.
Objective & Methods
In this study, the diagnostic yield of neuroimaging [Cranial CT (n=25), MRI (n=24), and Th 201/99 Tc SPECT scan (n=18)] is compared with histopathological diagnosis obtained by STB (n=21) or autopsy (n=4) in 25 HIV-1 subtype C seropositive individuals with FBL identified by neuroimaging with special reference to cerebral toxoplasmosis in an eighteen month study period (2006–2007).
Results & conclusion
Cerebral toxoplasmosis was the most frequent cause of FBL (21/25, 84%), followed by one case each of tuberculoma, progressive multifocal leukoencephalopathy (PML), primary central nervous system lymphoma (PCNSL) and measles inclusion body encephalitis (MIBE), the last two diagnosed at autopsy. Of the 21 cases of cerebral toxoplasmosis, definitive diagnosis with histopathological confirmation was available in 14/21 (66.6%), with indirect evidence suggesting probable toxoplasmosis in seven, all of whom responded to antitoxoplasma therapy. CT and MRI had comparable specificities (75%), while MRI had marginally higher sensitivity (85% versus 80.9%) in detecting multiple lesions. The positive predictive value of both CT and MRI were identical (94.4%), suggesting that CT maybe a cost effective screening tool in resource restricted settings, for evaluating FBL. Sensitivity of 99Tc SPECT scan for diagnosing inflammatory lesions was 75% but failed to differentiate PCNSL from toxoplasmosis. This study is the first of its kind from India analysing FBL with specific focus on cerebral toxoplasmosis in the setting of HIV-1 subtype C.
Keywords: Focal brain lesions, CT, MRI, stereotactic biopsy, toxoplasmosis, HIV
INTRODUCTION
Neurological complications develop in 40–80% of individuals with Human Immunodeficiency Virus (HIV) infection, particularly in the late stage of severe immunodeficiency (AIDS) [1]. Neurological involvement can result from opportunistic infections (OI), HIV encephalitis or neoplasms particularly primary CNS lymphomas. Focal brain lesions (FBL) constitute a common complication in HIV infected individuals, secondary to varied opportunistic infections, neoplasms or cerebrovascular diseases. Many opportunistic infections ranging from viruses, fungi and parasites are a common cause of mortality and morbidity in HIV infected individuals, especially in resource limited countries [2]. AIDS is not the same disease all over the world, but has distinct differences in biology dictated by the subgroup of HIV-1 virus. The spectrum of opportunistic infections that occur is governed by the endemicity of microorganisms prevalent in the environment [3,4]. In developing countries, cerebral toxoplasmosis and neurotuberculosis are more frequent [3–5] compared to viral infections like progressive multifocal leukoencephalopathy (PML) in contrast to high incidence of neoplastic lesions like CNS lymphoma in the West [6–7]. Etiological diagnosis is essential for patient management and institution of appropriate therapy. MRI and newer imaging techniques such as MR spectroscopy and SPECT, and serological and molecular biologic parameters have been employed with varying sensitivity and specificity as the immune suppression alters the clinical and imaging features, as well as laboratory parameters, making diagnosis a challenge.
The most common dilemma is differentiating cerebral toxoplasmosis from primary CNS lymphoma and tuberculoma with diverse therapeutic implications. In the presence of atypical clinical and neuroimaging features, establishing a definitive diagnosis becomes difficult particularly when serology and molecular biologic tests are also not specific. Noninvasive diagnostic modalities such as MR spectroscopy and SPECT imaging were evolved to distinguish primary CNS lymphoma from tuberculoma and avoid use of invasive techniques with varied results [8,9]. Empirical anti-toxoplasma therapy is recommended as the first line of treatment for most HIV infected patients with focal brain lesions and brain biopsy is reserved for only those patients who fail to respond clinically to empirical therapy for toxoplasmosis, serological tests for the protozoan infection is negative or the imaging reveals atypical features [10].
Studies comparing the diagnostic yield of these neuroimaging modalities vis a vis brain biopsies are very few in literature. Further, in India where HIV infection is predominantly caused by HIV-1 subgroup-C virus as confirmed by a subtype-specific PCR study [11] in contrast to HIV-1 Clade B in the West, the spectrum of FBL associated with AIDS is not recorded. To evaluate the diagnostic utility of CT and MRI, and SPECT scan in FBL with particular focus on cerebral toxoplasmosis, we reviewed all cases of FBL associated with HIV/AIDS over an eighteen month study period (2006–2007) from a tertiary care hospital for neurological disorders in South India and correlated the neuroimaging with neuropathological features of lesions obtained by stereotactic biopsy (STB) or following autopsy.
MATERIALS AND METHODS
All HIV-1 seropositive patients attending our outpatient or emergency services at a tertiary care specialty hospital for neurological disorders in South India, with single or multiple intracranial focal lesions identified by either CT or MRI were included in the study (July 2006 to December 2007) following informed consent. Institutional Ethics Committee approved the study. Children below the age of 15 years and pregnant women were excluded.
The patients were subjected to detailed neurological and systemic evaluation. Their demographic and clinical history was recorded in a pre-designed proforma.
All patients were investigated with complete hemogram including ESR, biochemical tests, serological test for HIV 1 and HIV 2, toxoplasma (IgG antibodies), CD4 count with CD4 / CD8 ratio, Chest X-ray, Cranial CT scan, (plain and contrast), MRI Brain -T1, T2, FLAIR sequences with contrast enhancement (performed on a 1.5 T or 3 T MRI machine). In addition, patients were subjected to Th201/99Tc-Sestamibi SPECT Scan. An index based on the ratio of thallium uptake in the lesion vs the contralateral scalp was calculated [12]. Lesions revealing enhanced uptake were considerd “non inflammatory” and suggestive of a neoplastic process like “lymphoma”. Lesions showing absence of uptake (other than normal uptake in nasopharynx) were interpreted as “inflammatory” pathology
Lumbar CSF obtained whenever the procedure was not contraindicated was analyzed serologically for antibodies to Toxoplasma gondii, Mycobacterium tuberculosis, Cryptococcal antigen and other common endemic bacterial infections in additional to routine tests. CT/MR guided Stereotactic biopsy (STB) of the lesion was performed following informed consent using a Leksell-Stereotactic frame by a trained neurosurgeon (SP) from the lesion taking care to include the contrast enhancing area on imaging and subjected to histological evaluation. In the presence of multiple lesions, the lesion in the least eloquent part of the brain was selected for biopsy and the deeper lesions were biopsied in preference to the surface lesions to avoid the risk of intracerebral hemorrhage. Biopsies were fixed in 10% buffered formalin and processed for paraffin embedding. Sections were stained with hematoxylin-eosin for routine evaluation and serial sections were immunostained by indirect immunoperoxidase for Toxoplasma gondii (polyclonal antibody to p30 antigen specific to the tachyzoite form of T gondii, 1:20 dilution, Novascastra Labs, UK) and JC virus (polyclonal antibody that recognizes viral capsid protein of JC virus, 1:200, DAKO, USA,).
Case definitions
Definite toxoplasmosis
Cases with histopathological evidence (either by biopsy or autopsy)of necrotizing non granulomatous inflammation along with tachyzoite forms of T gondii confirmed by immunohistochemistry were labeled as cases of “definite toxoplasmosis”.
Probable toxoplasmosis
Histological evidence of inflammatory pathology, endarteritic changes, necrosis with nuclear debris in the absence of immunohistochemical positivity for T gondii, with clinical/radiological response to antitoxoplasma regime within 4–6 weeks of therapy were considered “Probable” cases of Toxoplasma encephalitis.
Both “definite” and “probable” cases were considered as cerebral toxoplamsosis for calculating sensitivity and specificity of the serological tests and neuroimaging modalities.
Prior to stereotactic biopsies, all patients received only antiedema measures and short course of steroids. Five patients (cases 2,3,7,9 & 18) received four drug antituberculous therapy empirically from 3 to 8 months in view of endemicity in a primary care hospital. Four cases (cases 5,19,20, & 24) were on three drug HAART (highly active antiretroviral therapy) (two non-nucleoside reverse transcriptase inhibitors and one nucleoside-reverse transcriptase inhibitor) for 3 to 12 months period prior to being referred here. They were however irregular on therapy.
The neuroimaging findings were reviewed by a neuroradiologist (RS) blinded to the clinical features and other investigations and correlated with clinical, and histopathological features. Sensitivity and specificity of these imaging modalities in the evaluation of HIV/AIDS related focal brain lesions (FBL) were assessed with histopathology as gold standard for definitive diagnosis. Statistical analysis was carried out using SPSS version 15. Descriptive statistics, Chi Square test or Fisher’s exact probability test was used for analysis.
RESULTS
A total of 25 HIV seropositive patients (22 males and 3 females) were evaluated during the study period (July 2006 to December 2007) [age range: 16 to 58 years, (mean − 38.3 ± 9.6 years)]. All patients were symptomatic with the duration of illness ranging from 2 days to 60 days (mean 11.5 ± 13.4 days), and 92% of patients (23/25) presenting to the hospital within 30 days of onset of symptoms. Fifteen patients had symptoms of raised intracranial tension, and 17 had focal neurological deficits suggestive of FBL. Seven patients had seizures at presentation and one patient developed hemiballismus. CD4 counts from the antecubital venous blood ranged from 11 to 327/μL (mean 97.8 ± 76.7; median-72.5).
Based on the clinical findings, CSF and neuroimaging features, clinical diagnoses of space occupying lesion (n=17), chronic meningitis with vascular complications (n=5), progressive multifocal leukoencephalopathy (PML) (n=2) and focal idiopathic seizures (n=1) were considered.
Of the fourteen cases diagnosed to have definite cerebral toxoplasmosis, CT scan demonstrated single lesions in three, and multiple lesions in eleven cases. Contrast enhancement of the lesions was noted in seven (ring enhancing – 5, disc enhancement – 2) and non enhancing lesions in another seven cases. Basal ganglia was most frequent neuroanatomical site of involvement (11), followed by frontal lobe (9), thalamus (4), cerebellum (2) and one case each had lesions in brain stem and temporal region. MRI on the other hand highlighted multiple lesions in all 13 cases in which it was performed. Basal ganglia and frontal lobe were the most frequent locations (10), followed by involvement of parietal lobe (7), thalamus (5), cerebellum (4), occipital/temporal (3) and brain stem (1). Contrast enhancement was noted ten cases, with ring enhancement in nine, and ill-defined contrast enhancement in one case. Characteristic “eccentric target sign” was detected in only two cases. Seven cases demonstrated restricted diffusion within the lesion on diffusion weighted sequences. Interestingly, evidence of blooming reflecting hemorrhage was detected on gradient echo sequences in only two cases.
99 Tc SPECT scan performed in 18/25 patients, discriminated the cases as inflammatory (n=14) and noninflammatory/neoplastic (n=4), with one case of CNS lymphoma being misdiagnosed as an inflammatory pathology while three cases of toxoplasmosis were diagnosed as lymphoma.
Stereotactic biopsy was carried out in 21/25 cases. In addition, in four cases (cases 12, 13, 14 & 15, Table 1) who succumbed to their illness, the brain was examined at autopsy and evaluated histologically. Diagnosis of cerebral toxoplasmosis was established by detetcing Toxoplasma gondii tachyzoites by immunohistochemistry in 14 of 25 (Fig. 1A–D) (11 following stereotactic biopsy and three cases at autopsy), while non specific inflammatory pathology was noted in additional seven cases (Fig. 1E,F). All the seven (Cases 2, 4, 6, 7, 8, 10, 11, Table 1) were considered as “probable” Toxoplasma encephalitis as they showed significant clinical and radiological recovery following institution of antitoxoplasma regimen. One patient (case 5, Table 1) diagnosed as granulomatous lesion/ tuberculoma on CT, MRI as well as SPECT with negative toxoplasma serology and evidence of inflammatory pathology on stereotactic biopsy had clinical improvement following four drug anti tuberculous therapy (without additional antitoxoplasma regimen) suggesting probable mycobacterial pathology rather than toxoplasmosis. One case each of primary CNS lymphoma (Fig. 2A–D) and progressive multifocal leukoencephalopathy (PML) (Fig. 2E–H) were diagnosed by stereotactic biopsy. Four patients succumbed to the illness during the active phase within seven days of admission and autopsy confined to the examination of the brain was carried out 6–10 hours postmortem with informed written consent. One subject (Case 13, Table 1) diagnosed as granulomatous pathology on cranial CT scan and reversible posterior leukoencephalopathy syndrome on MRI, on histological evaluation of the brain at autopsy revealed measles inclusion body encephalitis (Fig. 2I–K) [13]. The sampling of the stereotatic biopsy, especially from the central necrotic area or the margin with chronic inflammation was most often responsible for non-diagnostic biopsies. The correlation of neuroimaging features and serology with biopsy diagnosis is provided in Table 1.
Table 1.
Correlation of Toxoplasma serology, neuroimaging features with pathological diagnosis
| No | Age (yrs) | CD4 /μl | Toxoplasma Serology | CT Diagnosis | MRI Diagnosis | SPECT | Pathological Diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | 41 | 57 | Positive | Toxoplasmosis | Toxoplasmosis | Granuloma | Toxoplasmosis |
| 2 | 29 | 87 | Positive | Toxoplasmosis | Toxoplasmosis | Not done | Inflammatory Probable TE |
| 3 | 38 | 42 | Positive | Toxoplasmosis | Toxoplasmosis | Not done | Toxoplasmosis |
| 4 | 45 | 121 | Positive | Toxoplasmosis | Toxoplasmosis | Granuloma | Inflammatory Probable TE |
| 5 | 41 | 251 | Negative | Tuberculoma | Tuberculoma | Granuloma | Encephalitis Probable Tuberculoma |
| 6 | 40 | 56 | Negative | Toxoplasmosis | Toxoplasmosis | Granuloma | Inflammatory Probable TE |
| 7 | 30 | 164 | Negative | Tuberculoma | Tuberculoma | Lymphoma | Inflammatory Probable TE |
| 8 | 26 | 327 | Negative | Toxoplasmosis | Demyelination | Granuloma | Inflammatory Probable TE |
| 9 | 52 | 14 | Negative | Toxoplasmosis | Toxoplasmosis | Lymphoma | Toxoplasmosis |
| 10 | 40 | 30 | Positive | Tuberculoma | Toxoplasmosis | Lymphoma | Inflammatory Probable TE |
| 11 | 47 | 79 | Negative | Toxoplasmosis | Toxoplasmosis | Lymphoma | Inflammatory Probable TE |
| 12 | 40 | 66 | Negative | Toxoplasmosis | Toxoplasmosis | Not done | Toxoplasmosis |
| 13 | 16 | 131 | Positive | Granuloma | *RPLE | Not done | *MIBE |
| 14 | 58 | NA | Positive | Toxoplasmosis | Not done | Not done | Toxoplasmosis |
| 15 | 47 | 63 | Positive | Toxoplasmosis | Toxoplasmosis | Not done | Toxoplasmosis |
| 16 | 28 | 161 | Positive | Tuberculoma | Toxoplasmosis | Granuloma | Toxoplasmosis |
| 17 | 44 | 105 | Positive | Tuberculoma | Tuberculoma | Granuloma | Toxoplasmosis |
| 18 | 38 | 11 | Positive | Toxoplasmosis | Toxoplasmosis | Granuloma | Toxoplasmosis |
| 19 | 41 | 64 | Positive | Toxoplasmosis | Toxoplasmosis | Granuloma | Toxoplasmosis |
| 20 | 31 | 33 | Negative | Toxoplasmosis | Toxoplasmosis | Granuloma | NHL-B cell type |
| 21 | 46 | 124 | Negative | PML | PML | Granuloma | PML |
| 22 | 54 | 18 | Negative | Toxoplasmosis | Toxoplasmosis | Granuloma | Toxoplasmosis |
| 23 | 32 | 44 | Negative | Toxoplasmosis | Toxoplasmosis | Granuloma | Toxoplasmosis |
| 24 | 35 | 137 | Positive | Toxoplasmosis | Toxoplasmosis | Granuloma | Toxoplasmosis |
| 25 | 45 | 162 | Positive | Toxoplasmosis | Toxoplasmosis | Not done | Toxoplasmosis |
RPLE : Reversible Posterior Leukoencephalopathy.
MIBE : Measles Inclusion Body Encephalitis.
PML : Progressive Multifocal Encephalopathy
Probable TE: Histological evidence of inflammatory pathology, endarteritic changes, necrosis with nuclear debris in the absence of immunohistochemical positivity for T gondii, with clinical/radiological response to antitoxoplasma regime were considered “Probable” Toxoplasma encephalitis.
Fig.1.
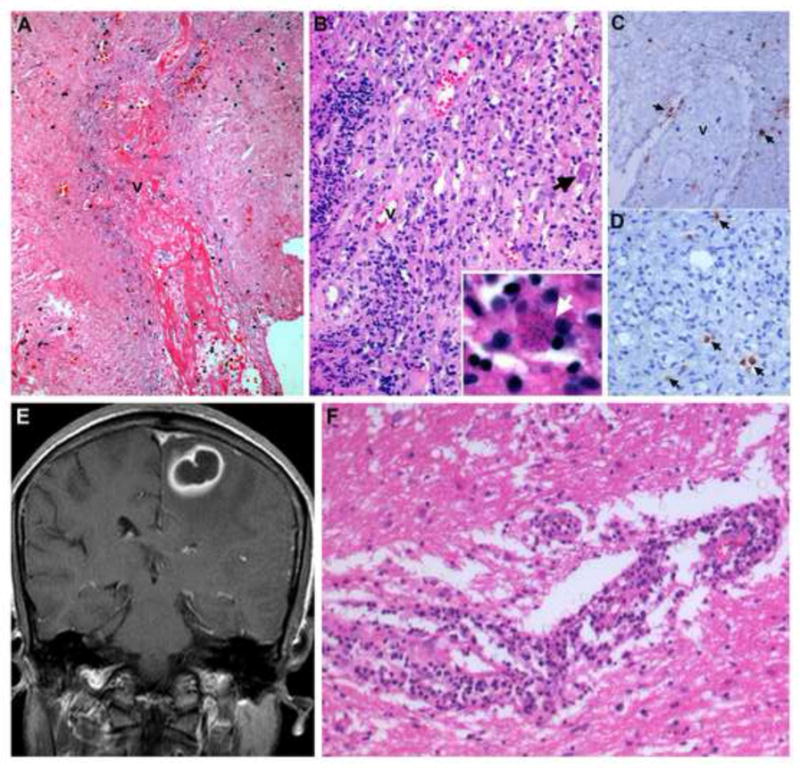
(A–D): STB findings in a case of definite toxoplasmosis
Stereotactic biopsy sampling the center of a Toxoplasma lesion, reveals thrombosed vessel (v) surrounded by fibrin rich necrosis (A). Dense lymphoplasmacytic infiltrate and thick inflamed vessels are seen in the wall of the lesion (B) with a single tissue cyst (B, inset, arrow). Immunostaining reveals randomly distributed tachyzoite of T gondii around endarteritic vessel (v) (C, arrows) and within the inflammatory infiltrates (D, arrow).
(E,F): STB findings in a case of Probable toxoplasmosis
Contrast enhanced T1weighted MR image in coronal plane shows a parasaggital lesion with characteristic “eccentric target sign” highly suggestive of toxoplasmosis (E). Stereotactic biopsy sampling the periphery of the lesion shows dense lymphoplasmacytic infiltrate in the white matter (F). No toxoplasma cysts could be identified on immunocytochemistry. The biopsy was considered to represent a non-specific inflammatory pathology but the patient recovered following antitoxoplasma therapeutic regime.
[A:HEx80; B:HEx160, B Inset:HEx800; C: Immunoperoxidase for T gondii x 200, D: Immunoperoxidase for T gondii x320, F:HEx240]
Fig. 2.
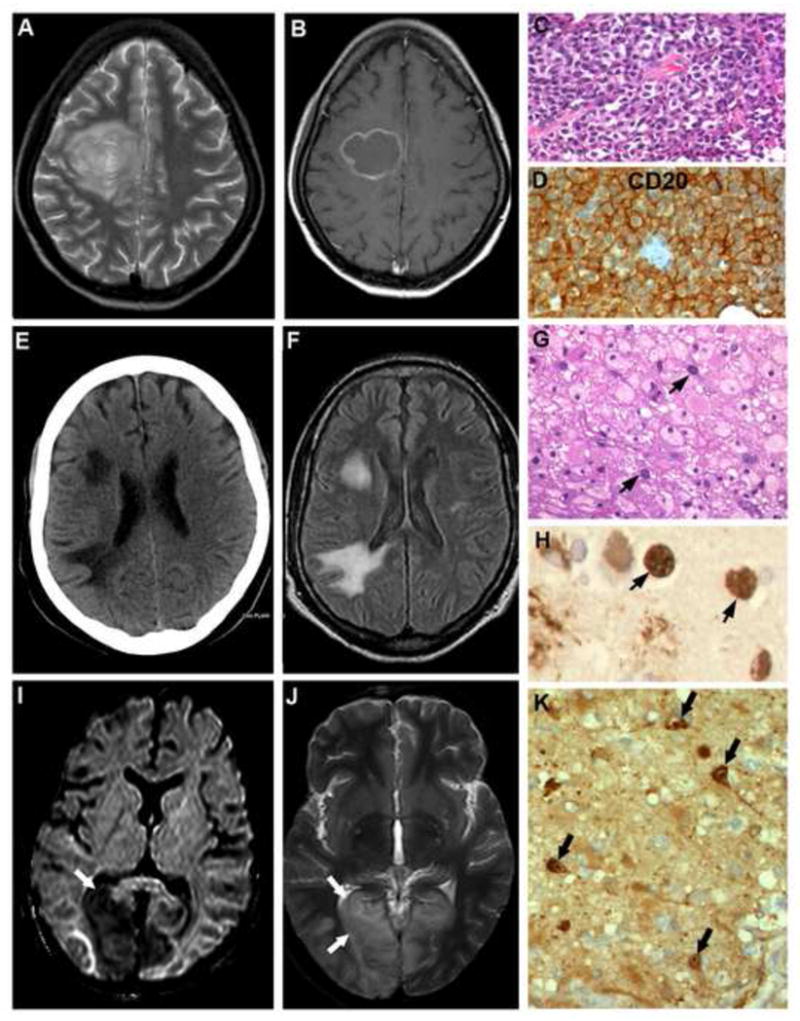
(A–D): Non Hodgkin’s lymphoma (case 20)
T2 weighted MR image shows concentric lamellar lesion (A) in posterior frontal white matter reaching the midline and enhancing post contrast (B) highly suggestive of Toxoplasmosis. On stereotactic biopsy, a densely cellular lesion was seen composed of CD20 positive neoplastic lymphoid cells, indictaive of B cell lymphoma (C,D).
[C: HEx320, D: Immunoperoxidase x600]
(E–H): Progressive multifocal leukoencephalopathy (case 21)
CT scan in axial plane shows two hypodense lesions in right frontal and anterior parietal white matter without mass effect or distortion of the ventricle (E) and diffuse cortical atrophy. Corresponding FLAIR sequence on MR imaging shows hyperintensities in frontal and parietal white matter (F).
G,H: Stereotactic biopsy revealed demyelination with florid foamy histiocytic reaction and occasional enlarged oligodendroglia (G, arrows) with intranuclear basophilic inclusions containing JC viral antigen diagnostic of PML (H, arrows).
[G: HEx240, H: Immunoperoxidase x 600]
(I–K): Measles inclusion body encephalitis (case No.13)
On MR imaging, lesions hypointense on T1 and hyperintense on T2 weighted images were seen involving both occipital poles (left more than right, arrows) (K,L). The occipital lesions at autopsy revealed panencephalitis with characteristic intranuclear inclusions containing measles viral antigen confirmed on immunohistochemistry (M).
[M: Immunoperoxidase for measles viral antigen x200]
Considering histopathology with immunohistochemical demonstration of T gondii organisms as the gold standard for definitive diagnosis, the sensitivity and specificity of various diagnostic modalities for focal brain lesions was evaluated with special reference to cerebral toxoplasmosis (Table 2). CT scan had sensitivity of 80.9% and specificity of 75% in diagnosing cerebral toxoplasmosis. Although MRI delineated multiple lesions compared to CT scans, sensitivity of MRI (85%) was only marginally higher than CT scans. Interestingly the positive predictive value of both CT scans and MRI was identical (94.4%). Sensitivity of Tc99 SPECT scan for detection of inflammatory lesions was 73.3% but failed to differentiate CNS lymphoma from Toxoplasma lesions (specificity 0%). Serological tests for toxoplasmosis had the lowest sensitivity of 61.9% whereas the specificity was 75%.
Table 2.
Sensitivity and specificity of diagnostic tests for cerebral toxoplasmosis
| Histopathology | |||||
|---|---|---|---|---|---|
| Confirmed Toxoplasmosis | Others* | Sensitivity | Specificity | ||
| Toxoplasma Serology (n=25) | Positive (n=14) | 13 | 1 | 61.9% PPV=92.9% |
75% NPV=27.3% |
| Negative (n=11) | 8 | 3 | |||
| Cranial CT Scan (n=25) | Suggestive of Toxoplasmosis (n=18) | 17 | 1 | 80.9% PPV=94.4% |
75% NPV=42.9% |
| Negative diagnosis (n=7) | 4 | 3 | |||
| Cranial MRI (n=24) | Suggestive of Toxoplasmosis (n=18) | 17 | 1 | 85% PPV=94.4% |
75% NPV=50% |
| Negative diagnosis (n=6) | 3 | 3 | |||
| SPECT (n=18) | Inflammatory (n=14) | 11 | 3 | 73.3% PPV=78.5% |
0% NPV=0% |
| Neoplastic (n=4) | 4 | 0 | |||
PPV – positive predictive value, NPV – negative predictive value
Others – includes tuberculosis, Non Hodgkin’s lymphoma, PML, and Measles inclusion body encephalitis
All the patients diagnosed as cerebral toxoplasmosis received treatment with a combination of sulfadiazine and pyrimethamine for 6 weeks followed by a maintenance dose, along with folinic acid supplementation following histopathological confirmation. A single patient developed idiosyncratic reaction of pancytopenia and bleeding necessitating change of regime to Clindamycin and Pyrimethamine. Three patients succumbed within one week of initiation of treatment. Fourteen cases of toxoplasmosis wherein follow up was available demonstrated clinical and radiological improvement within 3–4 weeks except for one case (case 5) in whom radiological persistence of lesions were observed despite clinical improvement. The single case of PML was started on HAART and referred to another hospital for further management.
DISCUSSION
Focal brain lesions (FBL) are one of the most common diagnostic problems in neurological practice in HIV related setting despite employing varied diagnostic modalities. Following immunosuppression (CD4<200/mm3), the most common causes of FBL are toxoplasmosis, primary CNS Lymphoma (PCNSL), and tuberculous granulomata or tuberculous abscesses [14]. Although brain biopsy remains the gold standard to establish the diagnosis, with increasing experience, standard management protocols have been developed based on imaging modalities without resorting to biopsy in the Western countries. Using appropriate serological tests and radiology it may be possible to differentiate the varied etiologies, yet conscious of the limitations. The knowledge of geographic-specific pathology and endemic infections, however, is essential in recognizing the offending organism, particularly in countries endemic for infections as the clinical and neuroimaging criteria maybe of limited value in determining the etiology of infective CNS mass lesions [15]. This is particulalry relevant in cases of HIV/AIDS, as differentiating toxoplasmosis from other CNS infections like neurotuberculosis, cysticercosis and fungal infection is essential to institute prompt and appropriate therapy. Even in areas where tuberculosis is endemic, the most common cause of FBL is found to be cerebral toxoplasmosis [14]. Though Thallium/ 99Tc sestamibi SPECT has been reported to be of use in differentiation, its specificity is uncertain [16].
The CT scan remains useful in the evaluation of focal brain lesions especially where MRI facility is not accessible in resource limited settings. In the present series, CT scan had sensitivity of 80.9% and specificity of 75% in diagnosing cerebral toxoplasmosis (Table 2). In six cases, CT diagnosis was erroneous. In four, CT was misdiagnosed as tuberculoma and patients had received antituberculous therapy for periods ranging from 3 to 8 months (cases 7,10,16,17). Stereotactic biopsy revealed toxoplasmosis leading to successful treatment. A single case of Non Hodgkins lymphoma was misdiagnosed as toxoplasmosis based on CT findings (case 20) and another considered as granulomatous pathology revealed measles inclusion body encephalitis at autopsy (case 13). Most lesions were multiple, and solitary lesions were recorded in 21.4% of cases, similar to the observation of Porter et al [17]. The neuroanatomical location of lesions in basal ganglia in addition to lobar involvement offered clue to the diagnosis of Toxoplasmosis. None of the earlier studies have evaluated the sensitivity and specificity of cranial CT scan as they used MRI as the minimum standard of investigation.
MRI delineated multiple lesions in constrast to single lesions on CT, demonstrating higher sensitivity of MRI in better delineation of the lesions. In the present study, the sensitivity of CT (80.9%) and MRI (85%) were comparable (Table 2), MRI missed the diagnosis in five cases [three cases of biopsy confirmed toxoplasmsosis were misdiagnosed as tuberculoma in two and demyelination in one while one case of NHL and measles inclusion body encephalitis were misdiagnosed as toxoplasma and reversible posterior leukoencephalopathy syndromes respectively].
The positive predictive value of both CT and MRI were identical (94.4%). Hence cranial imaging by CT maybe appropriate for screening of patients with FBL associated with HIV in resource limited setting. However in evaluating solitary FBL sparing the basal ganglionic regions, MRI is essential for better characterization and planning management strategy. Porter et al and Navia et al recorded contrast enhancement in 75% of cases of FBL caused by Toxoplasma gondii in contrast to the present series (50%) [17,18]. Whether this difference is due to association with HIV-1 Clade-C, which being a defective chemokine as demonstrated by in vitro studies, produces lower levels of cytokine activation and hence lesser contrast enhancement needs further evaluation [19].
The MRI is reported to be more sensitive than CT in detecting the eccentric target sign and lamellated appearance of the FBL [20]. The eccentric target sign was evident in only two instances in our confirmed cases of toxoplasmosis and its neuropathological correlate has recently been described by our group [21]. The concentric lamellated feature on MRI was however less specific being noted both in toxoplasmosis and non Hodgkin’s lymphoma. Similarly spontaneous haemorrhage recognized on gradient echo sequence can be found in focal brain lesions caused by toxoplasmosis as well as Non Hodgkins lymphoma, irrespective of prior steroid therapy [22]. This could be related to associated vascular invasion and tissue destruction.
The usefulness of 99Tc Sestamibi -SPECT in the evaluation of focal brain lesions appears to be limited as noted in our study. The sensitivity of this investigation for inflammatory lesions was 73.3% but failed to differentiate CNS lymphoma from Toxoplasma lesions in our study. Berry et al found that 4 of 5 patients with non Hodgkins lymphoma revealed enhanced uptake with Thallium/99-Tc sestamibi [12].
Similarly we found serological tests for toxoplasmosis to be useful for diagnosis with sensitivity of 61.9% and specificity of 75%. In an earlier study from our Centre, we found serological tests for toxoplasmosis to be valuable for diagnosis especially when lumbar puncture is contraindicated as a diagnostic procedure [23].
Stereotactic biopsy is extremely useful in the evaluation of HIV related focal brain lesions with good diagnostic sensitivity. The nonspecific inflammatory pathology noted in cases of “proable toxoplasmosis” has been attributed to sampling variation, prior antitoxoplasma therapy received or the chronicity masking the organisms within the fibrotic stroma. Interestingly in our study, all seven cases of probable toxoplasmosis on CT scan were non enhancing on contrast and on MRI, only one demonstrated blooming on gradient sequences suggesting bleed. These indicate that the lesions were in the organizing abscess stage of toxoplasmosis, wherein the lesion develops a non hemorrhagic necrotic centre with encapsulating reparative fibrous capsule, closely resembling tuberculoma leading to misdiagnosis on CT/MRI and a low yield of biopsy. In the present study, all except one patient (Case 21, Table 1), improved clinically and radiologically following antitoxoplasma therapy.
None of the patients developed biopsy related complications emphasizing relative safety of STB in diagnosing focal brain lesions. Other reports in literature that have evaluated the usefulness of stereotactic procedure in diagnosing FBL (Table 3) [24–26] found it to be particularly useful in cases of cerebral toxoplasmosis who failed to improve with emperical antitoxoplasma therapy. Chappell et al report that biopsy of enhancing rather than non enhancing lesions had higher yield [26].
Table 3.
Comparison of reported series of Focal Brain Lesions in HIV/AIDS
| Chappell et al, (1992) [21] | Feiden et al (1993) [22] | Yeo et al (2000) [23] | Present Study (2011) | |
|---|---|---|---|---|
| Type of Study | Retrospective review of 25 patients who underwent STB after failed treatment. | STB of 23 patients of FBL who failed to improve with antitoxoplasma treatment | Usefulness of STB in 10 AIDS patients, cross sectional study | Prospective study of 25 patients. Correlation of CT, MRI, SPECT with histopathology. |
| Diagnostic yield of STB | Contract enhancing lesions (87.5%) Non enhancing lesions (67%) |
22/25 (88%) | 9/10 (90%) | 21/21 cases (100%) |
| Nature of lesions |
Contrast enhancing: Lymphoma (9), Toxoplasmosis (2), Low grade glioma (1), Herpes cerebritis (1), Cryptococcoma (1), Non diagnostic (2). Non enhancing: PML (2), Non diagnostic (2) |
Lymphoma (9), Necrotising Toxoplasma encephalitis (7), PML (5), HIV encephalitis (1), Unspecified tissue changes (3) | Toxoplasmosis(1)?Tuberc ulosis (1), Lymphoma (5), Metastatic adenocarcinoma (1), Encephalitis (1), Abscess (1) | Toxoplasmosis (14), Inflammatory (8), NHL (1), PML (1), Measles inclusion Body Encephalitis (1). |
| Conclusions | Early biopsy useful in enhancing lesions whereas in non-enhancing lesions, usefulness of biopsy is not clear. | STB is a highly accurate diagnostic tool to ascertain the nature of focal intracerebral lesions in selected AIDS patients. | Useful in patients with FBL especially who fail antitoxoplasma treatment. | STB is extremely useful in the diagnosis of FBL associated with HIV. Should be planned early for effective proper management. |
All patients of cerebral toxoplasmosis received treatment with a combination of sulfadiazine and pyrimethamine for 6 weeks followed by a maintenance dose, along with folinic acid supplementation. We noted that antitoxoplasma treatment produced clinical improvement within four days, with radiological resolution beginning by two weeks and complete by 3–6 weeks. In our series, we had one rare case of measles inclusion body encephalitis, and one case of progressive multifocal leukoencephalopathy, manifesting as focal brain lesion [13]. This highlights the need to consider other pathological entities like PML and measles encephalitis when managing cases of focal brain lesions associated with HIV, for planning appropriate management.
Conclusion
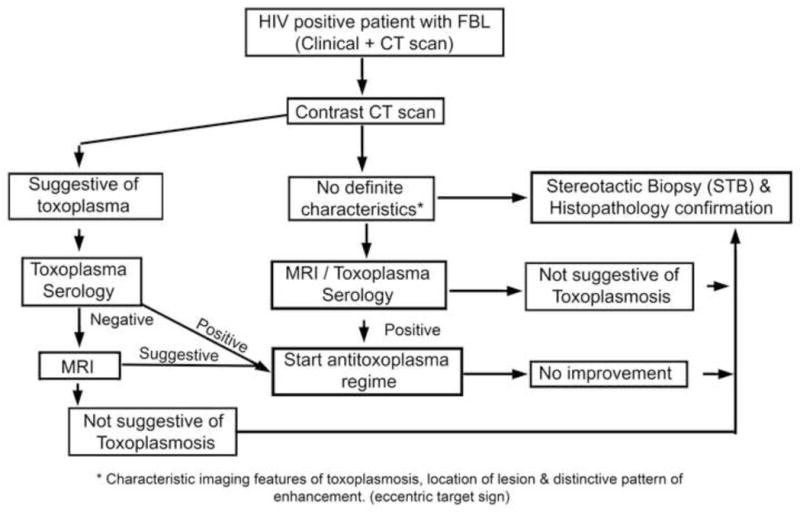
In the background of evaluating the sensitivity and specificity of various neuroimaging modalities with definitive diagnosis by histopathology, we propose an algorithm useful in the management of FBL associated with HIV-1 Clade C infection (Fig. 3). As we have stereotactically biopsied a single accessible lesion, the coexistence of multiple infections cannot be ruled out. This is the first study from India where retroviral infection is predominantly caused by HIV-1 Clade C virus with special biological features in contrast to Clade B described from the West. Further studies with a larger sample size utilizing multiple diagnostic modalities can enhance our insight into diagnosis and management of focal brain lesions.
Fig. 3.
Acknowledgments
This publication was partly supported by a subaward from The Johns Hopkins University, with funds provided from National Institute of Neurological Disorders and Stroke (NINDS) [Grant no: 1RO1NS055628-01A2]. Its contents of the study are solely the responsibility of the authors and do not represent the official view of NINDS or JHU. The authors also wish to acknowledge the technical assistance of Ms. Rajasakti V, Mr. Prasanna Kumar, Mr. Shivaji Rao and Mr. K. Manjunath, Human Brain Tissue Repository, Department of Neuropathology, National Institute of Mental Health & Neurosciences, Bangalore, India for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simpson D, Tagliati M. Neurologic manifestation of HIV infection. Ann Intern Med. 1994;121:769–85. doi: 10.7326/0003-4819-121-10-199411150-00008. [DOI] [PubMed] [Google Scholar]
- 2.Lane HC, Laughon BE, Falloon J, Kovacs JA, Davey RT, Jr, Polis MA, Masur H. NIH conference: Recent advances in the management of AIDS-related opportunistic infections. Ann Intern Med. 1994;120:945–55. doi: 10.7326/0003-4819-120-11-199406010-00007. [DOI] [PubMed] [Google Scholar]
- 3.Satishchandra P, Nalini A, Gourie-Devi M, Khanna N, Santosh V, Ravi V, Desai A, Chandramuki A, Jayakumar PN, Shankar SK. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, south India (1989–96) Indian J Med Re. 2000;111:14–23. [PubMed] [Google Scholar]
- 4.Shankar SK, Mahadevan A, Satishchandra P, Kumar RU, Yasha TC, Santosh V, Chandramuki A, Ravi V, Nath A. Neuropathology of HIV/AIDS with an overview of the Indian scene. Indian J Med Res. 2005;12:468–88. [PubMed] [Google Scholar]
- 5.Shankar SK, Satishchandra P, Mahadevan A, Yasha TC, Nagaraja D, Taly AB, Prabhakar S, Nath A. Low prevalence of progressive multifocal leukoencephalopathy in India and Africa: is there a biological explanation? J Neurovirol. 2003;9(Suppl 1):59–67. doi: 10.1080/13550280390195397. [DOI] [PubMed] [Google Scholar]
- 6.Grabar S, Lanoy E, Allavena C, Mary-Krause M, Bentata M, Fischer P, Mahamat A, Rabaud C, Costagliola D. Causes of the first AIDS-defining illness and subsequent survival before and after the advent of combined antiretroviral therapy. HIV Med. 2008;9:246–56. doi: 10.1111/j.1468-1293.2008.00554.x. [DOI] [PubMed] [Google Scholar]
- 7.Lewden C, May T, Rosenthal E, Burty C, Bonnet F, Costagliola D, Jougla E, Semaille C, Morlat P, Salmon D, Cacoub P, Chene G. Changes in causes of death among adults infected by HIV between 2000 and 2005: The “Mortalite 2000 and 2005” surveys (ANRS EN19 and Mortavic) J Acquir Immune Defic Syndr. 2008;48:590–8. doi: 10.1097/QAI.0b013e31817efb54. [DOI] [PubMed] [Google Scholar]
- 8.Berger JR, Kaszovitz B, Post MJ, Dickinson G. Progressive Multifocal leukoencephalopathy associated with human immunodeficiency virus infection. Ann Intern Med. 1987;107:78–87. doi: 10.7326/0003-4819-107-1-78. [DOI] [PubMed] [Google Scholar]
- 9.Haverkos HW. Assessment of therapy for toxoplasma encephalitis. The TE Study Group. Am J Med. 1987;82:907–14. doi: 10.1016/0002-9343(87)90151-3. [DOI] [PubMed] [Google Scholar]
- 10.Murray H. Toxoplasmosis. In: Dolin R, Masur H, Saag M, editors. AIDS Therapy. New York: Churchill Livingstone; 1999. pp. 307–27. [Google Scholar]
- 11.Siddappa NB, Dash PK, Mahadevan A, Jayasuryan N, Hu F, Dice B, Keefe R, Satish KS, Satish B, Sreekanthan K, Chatterjee R, Venu K, Satishchandra P, Ravi V, Shankar SK, Shankarappa R, Ranga U. identification of subtype C human immunodeficiency virus type 1 by subtype specific PCR and its use in the characterization of viruses circulating in the southern parts of India. J Clin Microbiol. 2004;42:2742–2751. doi: 10.1128/JCM.42.6.2742-2751.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry I, Gaillard JF, Guo Z, Cordoliani YS, Massip P, Manelfe C, Danet B. Cerebral lesions in AIDS; What can be expected from scintigraphy? Cerebral tomographic scintigraphy using thallium-201; a contribution to differential diagnosis of lymphomas and infectious lesions. J Neuroradiol. 1995;22:218–28. [PubMed] [Google Scholar]
- 13.Katrak SM, Mahadevan A, Taly AB, Sinha S, Shankar SK. A 16-year old male with cortical blindness and focal motor seizures. Ann Indian Acad Neurol. 2010;13:225–232. doi: 10.4103/0972-2327.70887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manji H, Miller R. The Neurology of HIV infection. J Neurol Neurosurg Psychiat. 2004;75(Suppl1):129–135. doi: 10.1136/jnnp.2003.034348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Santosh V, Mahadevan A, Chickabasaviah YT, Bharath RD, Krishna SS. Infectious lesions mimicking central nervous system neoplasms. Semin Diagn Pathol. 2010;27:122–35. doi: 10.1053/j.semdp.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 16.Miller RF, Hall-Craggs MA, Costa DC, Brink NS, Scaravilli F, Lucas SB, Wilkinson ID, Ell PJ, Kendall BE, Harrison MJ. Magnetic resonance imaging, thallium-201 SPET scanning, and laboratory analyses for discrimination of cerebral lymphoma and toxoplasmosis in AIDS. Sex Transm Inf. 1998;74:258–64. doi: 10.1136/sti.74.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immune deficiency syndrome. N Engl J Med. 1992;327:1643–48. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 18.Navia BA, Petito CK, Gold JWM, Cho ES, Jordan BD, Price RW. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann Neurol. 1986;19:224–38. doi: 10.1002/ana.410190303. [DOI] [PubMed] [Google Scholar]
- 19.Ranga U, Shankarappa R, Siddappa NB, Ramakrishna L, Nagendran R, Mahalingam M, Mahadevan A, Jayasuryan N, Satishchandra P, Shankar SK, Prasad VR. Tat protein of human immunodeficiency virus type 1 subtype C strains is a defective chemokine. J Virol. 2004;78:2586–590. doi: 10.1128/JVI.78.5.2586-2590.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karampekios S, Hesselink J. Cerebral Infections Eur Radiol. 2005;15:485–93. doi: 10.1007/s00330-004-2556-1. [DOI] [PubMed] [Google Scholar]
- 21.Kumar GG, Mahadevan A, Guruprasad AS, Kovoor JM, Satishchandra P, Nath A, Ranga U, Shankar SK. Eccentric target sign in cerebral toxoplasmosis: neuropathological correlate to the imaging feature. J Magn Reson Imaging. 2010;31:1469–472. doi: 10.1002/jmri.22192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chang L, Cornford ME, Chiang FL, Ernst TM, Sun NC, Miller BL. Radiologic- pathologic correlation. Cerebral toxoplasmosis and Lymphoma in AIDS. AJNR Am J Neuroradiology. 1995;16:1653–663. [PMC free article] [PubMed] [Google Scholar]
- 23.Adurthi S, Mahadevan A, Bantwal R, Satishchandra P, Ramprasad S, Sridhar H, Shankar SK, Nath A, Jayshree RS. Utility of molecular and serodiagnostic tools in cerebral toxoplasmosis with and without tuberculous meningitis in AIDS patients: A study from South India. Ann Indian Acad Neurol. 2010;13:263–70. doi: 10.4103/0972-2327.74197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chappell ET, Guthrie BL, Orenstein J. The role of stereotactic biopsy in the management of HlV-related focal brain lesions. Neurosurgery. 1992;30:825–29. doi: 10.1227/00006123-199206000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Feiden W, Bise K, Steude U, Pfister HW, Moller AA. The stereotactic biopsy diagnosis of focal intracerebral lesions in AIDS patients. Acta Neurol Scand. 1993;87:228–233. doi: 10.1111/j.1600-0404.1993.tb04107.x. [DOI] [PubMed] [Google Scholar]
- 26.Yeo KK, Yeo TT, Chan CY, Teo JS, Wong SY. Stereotactic brain biopsies in AIDS patients – early local experience. Singapore Med J. 2000;41:161–166. [PubMed] [Google Scholar]


