Abstract
CONTEXT
Active surveillance (AS) is an important strategy to reduce prostate cancer overtreatment. However, the optimal criteria for eligibility and predictors of progression while on AS are debated.
OBJECTIVE
To review primary data on markers, genetic factors and risk stratification for patient selection and predictors of progression during AS.
EVIDENCE ACQUISITION
Electronic searches were conducted in PubMed, Embase and CENTRAL from inception to April 2014 for original articles on biomarkers and risk stratification for AS.
EVIDENCE SYNTHESIS
Patient factors associated with AS outcomes in some studies include age, race, and family history. Multiple studies provide consistent evidence that lower percent free PSA, higher Prostate Health Index (phi), higher PSA density and greater biopsy core involvement at baseline predict a greater risk of progression. During follow-up, serial measurements of phi, PSA density, and repeat biopsy results predict later biopsy progression. While some studies have suggested a univariate relationship between urinary PCA3 and TMPRSS2:ERG with adverse biopsy features, these markers have not been consistently shown to independently predict AS outcomes. At this point, there is no conclusive data to support the use of genetic tests in AS Limitations of these studies include heterogeneous definitions of progression and limited follow-up.
CONCLUSIONS
There is a growing body of literature on patient characteristics, biopsy features, and biomarkers with potential utility in AS. More data are needed on practical applications such as combining these tests into multivariable clinical algorithms and long term outcomes, to further improve AS in the future.
PATIENT SUMMARY
Several PSA-based tests (free PSA, Prostate Health Index, PSA density) and the extent of cancer on biopsy can help to stratify the risk of progression during AS. Investigation into several other markers is underway.
INTRODUCTION
Prostate cancer (PCa) affects many men worldwide, with an estimated 899,000 diagnoses and 258 000 deaths in 2008.(1) Randomized trials have shown a positive effect of screening, with reductions in disease-specific mortality up to 21–30% (2, 3). Screening and early detection also lead to diagnosis of clinically insignificant disease (4), which may result in overtreatment and long-term effects on quality-of-life (4). Active surveillance (AS) is an important solution to reduce overtreatment (4). The underlying concept is to identify men with disease whose likelihood of progression is low without treatment and intervene only in those with disease progression during follow-up (5). The rationale is that most low risk PCa have an indolent course and the slow growth rate allows sufficient time during follow-up to detect cancers destined to become more aggressive during a window of curability (5). The long-term safety and effectiveness of AS depends on our ability to select appropriate patients and trigger delayed treatment when needed, while avoiding intervention in the remainder (6). Key questions are how to select patients for AS and how to detect disease progression and need for definitive treatment. Previously, van den Bergh et al (7) published an overview of 30 studies on clinical tools for AS patient selection and monitoring. To our knowledge, no comprehensive systematic review has yet been done examining patient factors, biopsy factors and markers that contribute to risk stratification in AS cohorts. In this systematic review, we provide that.
EVIDENCE ACQUISITION
We used PubMed, Embase and the Cochrane Central Register of Controlled Trials (CENTRAL) to perform electronic searches on biomarkers, genetics and risk stratification for patient selection and predicting progression on AS. Our search included any entries from inception to 4/2014 with no language restrictions and we followed the PRISMA methodology (see Appendix 1 for search strategy). All experimental and observational study designs containing primary data in AS populations were eligible for inclusion, including but not limited to controlled clinical trials, statistical modeling, case series, case-control, and cohort studies. Conference proceedings using these study designs were also included as per the Cochrane Handbook; whereas, we excluded comments, editorials, review articles, and all studies that were not performed in an AS population (e.g., studies in radical prostatectomy or watchful waiting populations). Articles evaluating different variables within the same institutional active surveillance program as another article were allowed if they provided unique data.
Results of the search & selection of studies
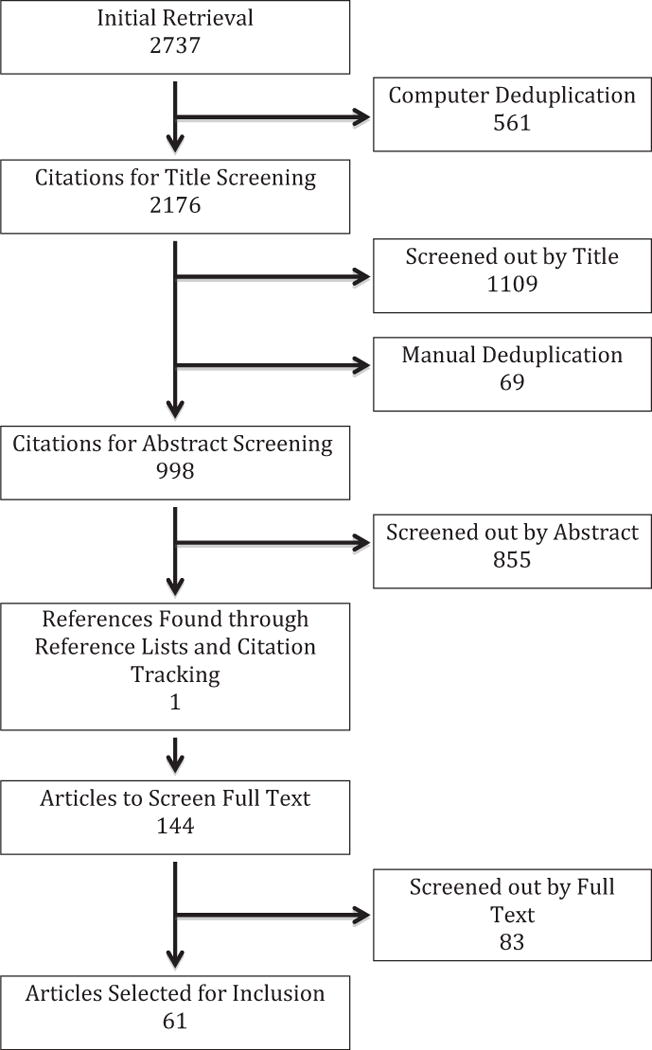
The initial search resulted in 2723 citations (Figure 1). After electronic removal of duplicates, 2176 citations remained. After initial title screening and manual de-duplication, 998 references remained for abstract review. Four authors (SL, SB, SC and MR) selected initial studies based on inclusion criteria by abstract screening. These studies were initially categorized into three categories: excluded, included, and possibly relevant. Included and possibly relevant studies were rescreened by three authors (SL, SB, MR) to confirm eligibility. 855 were removed for not meeting core inclusion criteria (not relevant to the topic not original research). All authors then participated in full-text screening for the remaining 143 citations identified by abstract review and an additional reference found by manual search of reference lists. Following full-text review, 61 citations were ultimately included in the evidence synthesis (Figure 1).
Fig. 1.
Data Extraction & Synthesis
Data were extracted by the research team using a standard form including the following themes: population, sample size, study design (prospective cohort, retrospective, etc.), aim of the study (selection of candidates, predictors of progression, or both), statistical methods (univariate, multivariate, etc.), type of marker tested, primary results, secondary results, limitations, and conclusions. We did not perform a formal assessment for bias or heterogeneity between studies for a complete systematic review.
After data extraction, data were synthesized by the research team. The primary outcomes of interest were baseline and longitudinal parameters to predict AS eligibility and progression (according to various definitions), respectively. For the evidence synthesis, studies were broadly grouped into those dealing with patient factors, clinical/biopsy factors, PSA derivatives, and genetics/genomics. For the purpose of this review, other types of tests such as MRI with potential use in AS and those that have not been tested in an AS population were not included.
EVIDENCE SYNTHESIS
Characteristics of the studies (appendix 2)
Of the 61 studies selected for inclusion, 53 were full-text published papers and the remaining 8 were conference abstracts. Overall, 14 were retrospective and 47 prospective. The eligibility criteria used for AS in published studies varied considerably, but many included PSA, clinical T-stage, Gleason score (GS), number of positive cores, and/or maximum cancer involvement. Table 1 provides an overview of the clinico-pathologic variables and biomarkers for risk stratification, organized by study/author and appendix 3 provides a summary of the statistical significance of these variables and biomarkers for risk stratification, organized by type of predictor.
Table 1.
Clinico-pathologic variables and biomarkers for risk stratification
| Authors and references | ‘Significant’ predictors/findings | ‘Not significant’ predictors | Outcome | Analysis | |
|---|---|---|---|---|---|
| Tseng et al (5) |
|
Age, PSA, PSAD (outcome: progression at initial biopsy) Age, PSA, percent fPSA (outcome: progression at second or subsequent biopsy) |
Biopsy progression (defined at surveillance biopsy as Gleason pattern 4 or 5, >2 biopsy cores with cancer or >50% involvement of any core with cancer) upon 1) initial surveillance biopsy and 2) second or subsequent surveillance biopsy |
Multivariate | |
| Abern et al (8) | Black race, PSA, household income | Age, insurance, number of biopsy cores at diagnosis | Discontinuation of AS for treatment (all cause and due to progression excluding preference) |
Multivariate | 3 models adjusting for socio economic and clinical parameters at the time of PC diagnosis |
| Iremashvili et al (9) | African American race, prostate volume, PSA density at diagnosis, 2 positive biopsy cores (vs. 1) | Age at diagnosis, Hispanic, metabolic syndrome components, family history, negative prostate biopsies before diagnosis, clinical stage, PSA | High grade cancer, more than 2 positive cores or greater than 20% involvement of any core on surveillance biopsy. | Multivariate | |
| Sundi et al (10) | AA race independent predictor of reclassification by grade | PSA, prostate size, BMI. AA race was not associated with reclassification by volume. | Reclassification on serial biopsy (by grade or volume, by grade only, and by volume only) |
Multivariate | |
| Cohn et al (11) | Model 1:PNI, BMI, race/ethnicity (African-American), PSA density. Model 2: PNI, Number of positive cores at diagnostic biopsy |
Maximum single core involvement, total tumor length | AS failure at confirmation biopsy (Gleason ≥7, >3 cores positive, single core with >50% involvement, and/or tumour volume >5% of total biopsy volume) | Multivariate | Model 1: PNI and non-biopsy variables significant p<0.2 in univariate. Model 2: Biopsy variables |
| Cullen et al (12) | Younger age, black race, comorbidity, higher T stage, higher Gleason (shorter time to secondary treatment Age, PSADT (mortality) |
Time to secondary treatments, all-cause mortality | Multivariate | ||
| Fleshner et al (13) | Higher age, family history, dutasteride administration, baseline PSA, baseline prostate volume | Race, PSA velocity, baseline DHT/T | Disease progression (any indication of prostate cancer treatment or pathological progression) | Multivariate | |
| Smith et al (14) | Initial cancer core length, PSAV (trend) |
Baseline PSA, baseline grade, stage, age, race | Positive second biopsy | Univariate | |
| Dall’ Era et al (15) | PSA density ≥0.15 at diagnosis, increase in GS on repeat biopsy | Age at diagnosis, race, relationship status, clinical risk group, PSA velocity >0.75 ng/ml/year | Active treatment | Multivariate | |
| Patel et al (16) | PSA velocity risk count of 2 increased reclassification risk in next year | Age at diagnosis, race, PSAD at diagnosis, overall PSAV during follow-up and cancer on first surveillance biopsy | Biopsy reclassification (>2 positive cores, >50% core involvement, or GS >6)) |
Multivariate | (Adjusted for age at diagnosis, race, PSAD at diagnosis, overall PSAV during followup and cancer on first surveillance biopsy.) |
| Shappley et al (17) | Outcome 1: age. clinical stage. Outcome 2: clinical stage. PSA, Gleason |
Outcome 1: Race, height, BMI, PSA at diagnosis, Gleason. Outcome 2: Age, deferred treatment at >12 months |
Outcome 1: Time to initiation of active treatment. Outcome 2: time to metastasis or death as a result of PCa. | Multivariate | |
| Lin et al (18) | Log PSA, abnormal DRE | PCA3, TMPRSS2:ER G, family history, race, age (N.B. subset analysis of men with negative DRE had similar results) |
Gleason ≥7 | Multivariate | |
| Bul et al (19) | Baseline variables: number of positive cores (2 vs 1 core), PSA density, age, baseline PSA value. Also PSA-DT at the time of repeat biopsy | Clinical T stage, number of biopsy cores | Reclassification (defined as >2 positive cores or Gleason >6 at repeat biopsy) |
Multivariate | Model 1: 1st repeat biopsy. Model 2: All repeat biopsies |
| Klotz et al (20) | Outcome 1: clinical stage >T2, GS >6 at baseline (predict definitive treatment). Outcome 2: PSADT <3 years (higher likelihood of BCR). Outcome 3: Age (higher risk of non-prostate cancer mortality than prostate cancer mortality) |
Outcome 1: PSA >10 at baseline. | Outcomes 1: Time to treatment, Outcome 2: PSA failure after radical treatment, Outcome 3: Mortality |
Univariate | |
| Sternberg et al (21) | Nomogram including Age. clinical stage. PSA, # positive cores at diagnosis. % positive cores at diagnosis, # negative and positive biopsies to date | Progression (defined as failure to meet the inclusion criteria during follow up) |
Multivariate | ||
| Whitson et al (22) | Lower prostate volume, older age (increased odds of progression) |
PSADT, time to repeat biopsy, stage (T2 vs T1c), biopsy source (UCSF vs other), Johns Hopkins criteria eligibility | Biopsy progression on repeat biopsy within 24 months of diagnosis (GS ≥7, >33% of cores positive or >50% of the maximum core positive) | Multivariate | Adjusted for age, prostate volume, clinical stage, biopsy source, whether the case met Johns Hopkins AS protocol criteria and time from diagnostic to repeat biopsy |
| Zhang et al (23) | High risk: Baseline PSA, time Low risk: Baseline PSA, age, GS, time Patients at high risk for progression had much shorter PSADT on average than patients at low risk. |
Average predicted evolution of serial PSA measurements over time estimated from a general linear mixed model of the natural log of PSA, by risk group, with high risk defined as an increase in Gleason grade to at least 7 (4+3) on repeat biopsy and low risk if not. | General linear mixed model | ||
| Berg et al (24) | Percentage of positive biopsies (PPB), ERG positivity (outcome 2) ERG positivity (outcome 4) |
Clinical T stage, diagnostic GS, age, PSA density, maximum tumour involvement (outcome 2) Clinical T stage, diagnostic GS, age, percentage of positive biopsies (PPB), PSA density, maximum tumour involvement (outcome 4) |
|
Multivariate | |
| Bul et al (25) | Model 1: PSA density and number of initial positive cores (two vs one). Model 2: PSA density and number of initial positive cores (two vs one), PSADT <3 yr | Age, PSA, clinical stage, and total number of biopsy cores | Reclassification at repeat biopsy (>2 positive cores or Gleason >6). | Multivariate | Models with (1) baseline characteristic s and (2) also with PSADT at repeat biopsy |
| Cary et al (26) | Negative confirmatory biopsy and lower PSA density predicted decreased risk of progression at 3 and 4 years. At 4 years, having negative confirmatory and year 3 biopsy | Age, clinical stage, percentage of diagnostic positive biopsy cores (3 & 4 years) Age, clinical tumor stage, percentage of diagnostic positive biopsy cores (4 yr) |
Upgrading to GS ≥4+3, >33% positive cores, and/or>50% of a single core at 3 year and 4 year timepoints | Multivariate | |
| Eggener et al (27) | Cancer on 2nd biopsy and higher # of cancerous cores on the 2 biopsies combined | Age, PSA, clinical stage, prostate volume, total number of biopsy cores sampled | Remaining on active surveillance | Univariate | |
| Hirama et al (28) |
|
Age, percentage of positive cores, maximum cancer involvement and %free PSA (base model). Prostate volume in model adjusted for %p2PSA and phi. |
Pathological reclassification (GS ≥7, >2 positive biopsy cores or more than 50 % cancer involvement of any biopsy core) upon the 1 year prostate biopsy |
Multivariate | |
| Klotz et al (29) | Decrease PSA free-to-total ratio correlated with a rapid PSADT. Short PSADT → more aggressive phenotype at radical prostatectomy (A short PSADT was also a criterion for active treatment) |
Grade, stage, baseline PSA and patient age (outcome 1) |
|
Univariate | |
| Makarov et al (30) | Serum marker: [−2]proPSA/% fPSA Tissue markers: [−5/−7]proPSA % area and [−5/−7]proPSA stain intensity in benign adjacent areas |
Age; tPSA; fPSA; %fPSA; [−2]proPSA (pg/mL); PSA density; prostate volume; number of positive cores; maximum % core involvement with cancer; cancer [−5/−7]proPSA % area, and stain intensity in tumor areas | Unfavorable biopsy conversion on annual surveillance biopsy (GS ≥7, ≥3 cores positive for cancer, >50% of any core involved with cancer) |
Univariate | |
| Soloway et al (31) | PSADT, clinical stage | Age, PSA level and Gleason score at diagnosis | “Progression to treatment” = treatment received yes/no (based on PSADT, GS ≥7, increased tumor volume, stage progression or patient preference) | Multivariate | |
| Venkitaraman et al (32) | PSA density and maximum tumor involvement of any core | PSA velocity (p=0.069), age, clinical T stage, GS score, initial PSA | Histological disease progression on repeat biopsy (defined as primary GS ≥4, >50% positive cores or a GS increase from 6 or less to 7 or greater) |
Multivariate | |
| Cornu et al (33) | PSA density, PCA3, TMPRSS2:ERG | Genotypes for rsl447295 and rs6983267 (both on 8q24), testosterone, age, positive family history | Gleason 4 | Multivariate | Model including both AS and men with elevated PSA |
| Isharwal et al (34) | Serum markers: −2proPSA, Prostate Health Index phi. Tissue markers: DNA content in benign adjacent and cancer tissue areas | Age, PSA, free PSA, %free PSA, PSAD, # cores with cancer, max percent core involved with cancer | Unfavourable biopsy conversion (GS≥7, Gleason pattern 4/5, ≥3 cores positive for cancer, >50% of any core involved with cancer) |
Multivariate | |
| van den Bergh (35) | # positive cores at initial biopsy | Age, clinical stage, PSA, PSADT, prostate volume, time to rebiopsy, # biopsy cores taken at diagnosis or rebiopsy, difference in # of biopsy cores, ratio between initial and repeat # of cores. No baseline factor associated with adverse pathology at delayed prostatectomy | Unfavorable repeat biopsy findings, delayed prostatectomy pathology | Univariate | |
| San Francisco et al (36) | Analysis 1: PSA density >0.08 ng/ml/cc, combination of PSAD and family history of PCa as risk score. Analysis 2: PSAV: PSA density, family history and risk score with all 3. Analysis 3: validated the PSAD cutoff of + 0.08 ng/ml/cc at first rebiopsy. |
Progression (defined as ≥3 positive cores, increased grade (Gleason ≥7) and/or >50% of any core involved with cancer) |
Multivariate | Analysis 1: clinical and pathologic variables at diagnosis biopsy Analysis 2: Considering changes between diagnosis biopsy and 1st rebiopsy. Analysis 3: For men with ≥2 repeat biopsies |
|
| Valeri et al (37) | Men in 40s with family history less likely to have insignificant prostate cancer vs general population | Insigificant PCa | Descriptive | ||
| Burton et al (38) | Smoking status (outcome 1). exercise, height (outcome 1,2) (N.B.: correlation PSA baseline and PSA growth) GS>7 (outcome 1,2) compared to <6 (outcome 1 and 2) |
Weight; BMI; waist circumference; hip circumference; inside leg length; alcohol; family history; occupational class | 1) PSA at age 50 and 2) PSA growth (yearly increase in log PSA) | Multivariate | Adjusted for age and GS (not in analysis that includes GS) |
| Goh et al (39) | Family history (FH) of PCa and single nucleotide polymorphisms (SNPs) not associated with adverse histology or time to treatment | +Recommendation for treatment due to PSAV >l +ng/ml/year or adverse histology (>50% of cores involved/any increase in +primary GS/increase in composite GS of +>=8) |
Univariate | ||
| Mukerji et al (40) | Number of positive cores, presence of high grade PIN, and Gleason score 4 | Failure of AS, switching to active treatment | Univariate | ||
| Van den Bergh et al (41) | Gleason 7 who met all other AS criteria better treatment free survival than those who did not. Gleason score 3+4 better than 4+3 | Treatment-free survival | Univariate | ||
| Venkitarama n et al (42) | PSA density | Clinical T stage, GS, initial PSA level, maximum percentage involvement of any core | PSA velocity before treatment (as an important predictor of prostate cancer mortality) |
Multivariate | |
| Jhavar et al (43) | Ki-67 (max Ki-67 labelling index). Gleason score. PSA density | Initial PSA | Time to radical treatment (recommended for PSAV >1 ng/ml/year, GS ≥4+3, or >50% cores involved) |
Multivariate | |
| Van As et al (44) | Free/total PSA ratio, clinical stage T2a (vs Tl) | Initial PSA level, Gleason score, PSA density, prostate volume, % positive cores, number of positive cores, and maximum involvement of any core | Time to radical treatment based on PSA velocity > 1 ng/ml/yr or histological progression (GS ≥4+3, or > 50% positive biopsy cores) | Multivariate | |
| Iremashvili et al (45) | Significant predictors combined into a nomogram: Number of positive cores on diagnostic and first surveillance biopsy, race, PSAD | Mean and max percent core involvement | Histologic progression during successive surveillance biopsies | Multivariate | |
| Iremashvili et al (46) | Number of positive cores (on diagnostic or 1st surveillance biopsy) and mean %core involvement (diagnostic biopsy). HGPIN significant for combination of the 2 biopsies | Number of cores taken | Biopsy progression (presence of Gleason 4/5 cancer, > two positive cores or >20% involvement of any core) | +Multivariate | Using data from diagnostic biopsy, first surveillance biopsy or both |
| Ng et al (47) | PSAD, PSAV, PSADT, max percentage of any core | Adverse histology on repeat biopsy defined as any of: primary Gleason grade ≥4,>50% cores positive, or initial Gleason score 3+3 upgraded to ≥3+4 | Multivariate | Adjusted for age, T stage, Gleason score, percentage of cores positive, baseline PSA level, maximum percentage of any core involved, prostate volume, PSA density, and free-total PSA ratio). | |
| Adamy et al (48) | Positive confirmatory biopsy (modified criteria without PSA) |
|
Progression defined as no longer meeting eligibility criteria
|
Multivariate | Adjusted for age, PSA, PSA density, prostate volume, number of positive cores, result of confirmatory biopsy (+/−) and initial biopsy extent (< 10 versus ≥ 10 cores) |
| Yee et al (49) | Initial biopsy extent (< 10 cores vs >10 cores) | Initial biopsy extent in relation to no longer meeting inclusion criteria on rebiopsy | Univariate | ||
| Fromont et al (50) | 1/3 no longer eligible on confirmatory bioposy | Concordance initial biopsy and confirmation biopsy (≥16 cores at ≤3 months after initiation to determine if patients still met the eligibility criteria) | Descriptive | ||
| Soloway et al (51) | Any tumor in the lst re-Bx | PSA doubling time, clinical stage | Treatment free survival (treatment indications: GS>3, increased tumor volume or # positive cores on rebiopsy, or preference) |
Univariate | |
| Umbehr et al (52) | PSA concentration at entry (but nonlinear pattern-unable to identify specific cutpoint) | Biopsy reclassification (GS ≥7, any Gleason pattern 4 or 5, ≥3 positive cores, or ≥50% cancer involvement/biopsy core) |
Multivariate | Adjusting for age, prostate volume, mean %f PSA and maximum percentage biopsy core involvement | |
| Barayan et al (53)’ | PSA density >0.15 | Disease progression, defined as the presence of >1 of the following criteria on repeat biopsies: >50% of cancer in any involved core, GS >4+3, and >3 positive biopsy cores | Multivariate | Adjusted for patients’ age, number of positive cores, maximum cancer percentage in any core, total number of cores and GS | |
| Welty et al (54) | PSA density, total number of biopsies, later year of diagnosis | Biopsy progression (upgrade GS ≥7 or increase in volume >33% cores) | Multivariate | ||
| Tosoian et al (55) | Baseline and longitudinal measures of %fPSA, %[−2]proPSA; [−2]proPSA/%fPSA, Prostate health index phi (both outcomes) | Baseline and longitudinal total PSA | 1) Risk of biopsy reclassification (Gleason score ≥7, >2 positive biopsy cores or>50% involvement of any core with cancer); 2) Upgrading only (GS ≥7) | Multivariate | Adjusted for age, date of diagnosis and PSA density |
| Komisarenk o et al (56) | Volume progression (>4 cores or>50% core involved) | Relation of increased volume of Gleason 6 PCa (4 cores or 50% of core involved) with the risk of later grade progression (>= 7) |
Univariate | ||
| Loblaw et al (57) | 13–86% of patients would have trigger for intervention based on PSA kinetics | Proportion of patients who had a trigger for treatment based on the various prostate specific antigen triggers: PSADT, PSAV, PSA threshold | Descriptive | ||
| Kakehi et al (58) | Only descriptives pathological findings, no formal analysis | No association between PSADT and aggressive findings on rebiopsy |
|
Descriptive | |
| Ross et al (59) | PSAV (calculated as PSA multiplied by the slope of a linear regression of log(PSA) | PSADT | Biopsy progression (Gleason score ≥7, or >2 positive cores, or >50% core involvement) | Univariate | |
| Khatami et al (60) | PSADT (Caveat: PSA predicting PSA & PSADT not known at time zero) |
PSA, ratio of free PSA and amount of cancer in biopsy | PSA relapse | Multivariate | |
| Iremashvili et al (61) | PSAV in men with at least 3 PSA’s over 18 months, and those with 4th and later biopsy | PSADT (calculated using the log-slope method) |
Biopsy progression (Gleason 4/5 cancer, more than two positive cores, or more than 20% involvement of any core) |
Univariate | |
| Krakowsky et al (62) | 2 men who died on AS initially met Epstein criteria. All 5 patients had a PSADT ≤1.6 years triggering radical therapy | Description of 5 PCa deaths in Toronto series | Descriptive | ||
| Pujara et al (63) | Median PSA doubling time higher in AS patients than in the control group Median PSA velocity lower in AS patients than in the control group |
AS patients compared to control group with at least 2 negative biopsies | Descriptive | ||
| Tosoian et al (66) | PCA3 | Progression on surveillance biopsy (defined as Gleason pattern 4 or 5, >2 positive biopsy cores or>50% involvement of any core with cancer) |
Multivariate | After adjustment for age at PCA3 measurement and date of diagnosis) | |
| Venkitamaran et al (67) | Urinary levels of either daidzein, genistein. enterolactone or equol (outcome l and 2) | Disease progression defined as: 1) adverse histology on repeat biopsy (primary Gleason grade >or= 4, or >50% positive cores) or 2) radical treatment for PSA velocity >1 ng/mL/year | Multivariate | ||
| Tausch et al (69) | NCCN risk group at entry (very low versus low risk) |
Multivariable | |||
| Van den Bergh (70) | PSADT using 1st 3 PSA’s indicative of PSADT using 5 measures (15% misclassification) | Correlation between PSADT using different number of PSA measurements | Correlations |
GS (Gleason score), PSA (prostate specific antigen), PSADT (prostate specific antigen doubling time), PSAV (prostate specific antigen velocity), PSAD (prostate specific antigen density), DRE (digital rectal exam)
Patient factors
In several papers, race was a risk factor for upgrading, biopsy reclassification and discontinuation of AS for treatment (8–12). The risk of progression was significantly increased in African American (AA) men. Using data from the prospective Johns Hopkins AS cohort (n=1801), Sundi et al (10) showed that AA men (n=256) with very low risk PCa were at significantly higher risk of grade reclassification compared to Caucasians. They concluded that, if the goal of AS is to monitor men with low grade disease, AA men may require alternative selection criteria. Cohn et al (11) similarly showed that AA race was a significant predictor of reclassification confirmation biopsy, along with perineural invasion, body mass index (BMI), PSA density and number of positive cores at diagnostic biopsy. However, in other studies race was not a significant predictor of surveillance biopsy outcome or conversion to active treatment (10, 13–18). For example, Smith and colleagues (14) investigated in a small study (n=71) the predictive value of race, baseline PSA, baseline grade, stage, age, and core length for positive second biopsy (cancer found), and the only significant variable was initial cancer core length.
Age has also been examined in many studies for predicting AS progression. Some showed a significant relationship between age with PSA evolution, biopsy reclassification, disease progression and risk of all-cause versus prostate cancer mortality (12, 13, 17, 19–23). Meanwhile, others found age not to be a significant contributor (5, 8, 9, 14–18, 24–35). Fleshner et al (13) reported that the relative risk of pathological progression (GS>6, >4 cores positive, or >50% core involvement) and disease progression (defined as treatment initiation) increased with higher age, higher baseline PSA, lower baseline prostate volume, and positive family history. Family history was also included by San Francisco et al. (36) in a risk score with PSA density to predict biopsy progression and Valeri et al. (37) reported that young men with a strong family history were less likely to have insignificant disease compared to the general population. On the contrary, Iremashvili and colleagues (9) aimed to identify clinical and demographic characteristics associated with an increased probability of progression and found no significant association for age, family history or baseline PSA. Several other studies also showed no relationship between family history with PSA growth (38), high grade disease (18), Gleason 4 (33), or time to treatment (39).
Only a few studies included BMI as a potential predictor of reclassification (10, 11), PSA growth (38), or time to active treatment (17), of which one found a significant association (11). Similarly, Iremashvili et al (9) did not find a significant relationship between metabolic syndrome components with increases in grade or extent of cancer on surveillance biopsy. In summary, age is an important factor in treatment selection and some studies suggest an increased risk of progression in AA men. There is conflicting data on the role of family history and BMI for AS risk stratification.
Biopsy factors
Several tumor and biopsy factors have been evaluated for an association with disease progression on AS: clinical stage, prostate volume, GS, number of biopsy cores, number of positive cores, maximum percentage of tumor involvement, and core length.
Many papers investigated the role of GS in relation to AS outcomes including PSA changes, disease progression, and time to treatment (12, 15, 17, 20, 23, 24, 29, 31, 32, 38, 40–44). A limited number of AS studies included men with GS >6, and Van den Bergh et al (41) reported that men with GS 7 PCa meeting all other AS criteria (PSA≤10, PSAD <0.2, T1c/T2 and ≤2 positive cores) had better 6-year treatment-free survival compared to GS 7 PCa not meeting the other AS criteria (100% versus 34%, respectively).
The extent of cancer on biopsy such as number of positive cores, cancer length and/or percentage of core involvement were also shown in the majority of studies to be important predictors of disease progression or the probability of remaining on AS (5, 9, 11, 14, 19, 21, 24, 25, 27, 32, 35, 40, 45–47). Bul et al (19) showed that the strongest predictors for short-term biopsy reclassification (> 2 positive cores or GS >6 at repeat biopsy) were PSA density and the number of positive cores (2 versus 1). One recent study by Sternberg et al (21) created a nomogram for progression on AS including age, clinical stage, PSA, number of positive cores at diagnosis, percent of positive cores at diagnosis, and number of positive and negative biopsies to date. Another study by Iremashvili et al (45) created a nomogram using race, PSA density and the total number of positive cores on diagnostic and first repeat biopsy to predict the probability of no progression on 2nd–4th repeat biopsies. Although nomograms may provide handy tools for multivariable risk stratification, external validation is necessary. Several other studies found no significant between the extent of cancer on biopsy and disease progression or the probability of remaining on AS (11, 24, 26, 28, 30, 34, 42, 44, 45, 48, 49).
Eggener et al (27) investigated the predictive value of age, PSA, clinical stage, prostate volume, and findings from diagnostic (pre-AS) and restaging biopsy in relation to the probability of remaining on AS. More positive cores on pre-AS biopsy, and the presence and extent of cancers on restaging biopsy, predicted a lower probability of remaining on AS (27). Many other studies have reported on the value of restaging biopsy. For example, Fromont et al. (50) reported that 1/3 of men were no longer eligible for AS on confirmatory biopsy. Other studies reported a positive confirmatory biopsy as a risk factor for subsequent progression (48, 51), while negative confirmatory biopsy is a favorable prognostic factor (26).
Finally, it is noteworthy that studies from most large surveillance programs worldwide have examined biopsy reclassification as a combination of upgrading and volume progression (9, 11, 19, 22, 28, 32, 36, 52–54), with few studies distinguishing the two (10, 55) Nevertheless, in 555 men from a prospective AS cohort, Komisarenko et al (56) found that patients with volume progression (>4 cores or >50% core involvement) were significantly more likely to have upgrading (GS ≥7) on subsequent AS biopsy versus those without volume progression (33.3% to 12.7%, P=0.003, respectively). However, only univariate analysis was reported. In summary, men with a greater extent of cancer on the initial biopsy are more likely to progress, and the presence and extent of prostate cancer on confirmatory biopsies also has strong prognostic significance.
PSA derivatives
Many papers provided more insight into PSA derivatives as markers in AS including: total PSA, %free PSA, PSA velocity (PSAV), PSA doubling time (PSADT), PSA density (PSAD), proPSA and the Prostate Health Index (phi).
Numerous papers examined the association between PSA and upgrading and/or increased tumor extent on biopsy with conflicting results (appendix 3). Many other studies evaluated PSA as a predictor of progression/conversion to active treatment.
There are also many studies on PSA kinetics during AS (12–16, 19, 20, 22, 23, 25, 29, 31, 32, 35, 36, 47, 51, 57–63). A major problem with many studies is the use of PSA as both an entry criterion (predictor) and also in the definition of progression/indications for intervention (outcome), creating a self-fulfilling prophecy. A patient with higher PSA at the time of treatment is more likely to have a higher PSA at the next measurement. Adamy et al (48) carried out an empirical demonstration of this circular reasoning and performed a study in which AS inclusion criteria was based on modified Epstein criteria, including a PSA ≤ 10ng/mL. When the authors defined progression as “no longer met inclusion criteria,” 61/238 patients would have been deemed to progress on AS, but excluding PSA from the definition, only half (n=32) would have progressed.
Khatami et al (60) evaluated the role of PSADT as a tool for selecting patients for AS. In a Cox model adjusted for PSA, ratio-free PSA and amount of cancer in biopsy, only the preoperative PSADT was a statistically significant predictor of PSA relapse after radical prostatectomy. However, the PSADT for men on AS was calculated using PSA at diagnosis and the latest PSA before active treatment or last follow-up. Thus, the Cox model included a predictor (PSADT) which was not known at baseline. Therefore, this study does not help inform patient selection since future PSA data are not known at the time of initial treatment decisions (64). Similarly, Soloway et al (31) reported that PSADT was a significant predictor for progression. However, the definition for progression in this study included both biopsy progression and PSA increases.
Many other studies have evaluated PSA kinetics during AS only using biopsy endpoints for progression. In men from the Johns Hopkins AS program, Ross et al (59) reported that 35% developed biopsy progression at 2.9 years median follow-up, and neither PSAV nor PSAD was a significant predictor on univariate analysis. Venkitaraman et al (32) reported that PSA density and maximum tumor involvement were predictors of histological disease progression (primary GS 4 or greater, >50% positive cores or GS increase from ≤6 to ≥7), but PSAV did not reach statistical significance on multivariate analysis (p=0.069). Whitson et al (22) revealed that PSADT was not significantly associated with risk of biopsy progression (grade and/or volume increase).
Iremashvili et al (61) showed that while PSADT was not associated with biopsy progression, PSAV significantly predicted tumor progression in certain subgroups, including men undergoing their fourth or later surveillance biopsy. However, in the overall population there was no significant increase in predictive accuracy compared to PSA alone. San Francisco et al (36) found that PSAV along with PSA density and family history highly predicted progression (≥3 positive cores, GS≥ 7 and/or >50% core involvement) (36). Finally, a recent study by Patel et al (16) examined PSAV risk count (number of times that PSAV exceeded a threshold of 0.4 ng/ml per year) as predictor of AS progression, which was shown to outperform standard PSA velocity. Overall, the 5-year probability of reclassification on biopsy (defined as GS> 6, ≥ 3 positive cores and >50% core involvement) was 9.7%, 18.7%, and 39.5% with risk counts of 0, 1, and 2 (p<0.01), and men with a risk count >1 (indicating at least 2 serial PSAV>0.4 ng/ml/year) had 4 times greater risk of reclassification on multivariable analysis. Meanwhile, the negative predictive value for reclassification in the next year was 91% for men with a risk count of 0–1, suggesting a potential means to reduce invasive biopsies if confirmed. It is important to note that risk count was only useful after the initial 2 years of AS on subset analysis.
In summary, the data on PSA kinetics for predicting AS progression are very mixed. While they may be used to prompt further diagnostic investigation, such as clinical evaluation, MRI and/or biopsy, their utility as a stand-alone trigger for intervention is questionable during the first few years. However, further study is warranted to evaluate a possible role for PSA velocity or risk count as a noninvasive predictor of underlying histologic progression for men who have been stable on AS for several years.
Conversely, several papers have demonstrated that PSAD predicts GS upgrading on serial biopsies during AS for apparent low-risk disease (5, 9, 11, 15, 19, 25, 26, 32, 33, 36, 42, 43, 45, 47, 53, 54). D’all Era et al (15) reported that PSA density >0.15 at diagnosis and increasing GS on repeat biopsy were significantly associated with receipt of secondary treatment. Further, Barayan et al (53) found that a PSAD >0.15ng/ml/cc is an important predictor for disease progression. San Francisco et al (36) concluded that PSAD>0.08ng/ml/cc at first rebiopsy was a significant predictor of subsequent progression. However, a drawback to using PSAD is the inaccuracy of assessing prostate volume by TRUS (65). In addition, PSAD was not a significant predictor of unfavorable biopsy in some studies including other new PSA-based measures which do not require prostate volume, such as proPSA and PSA velocity risk count (5, 16, 24, 30, 34, 44, 48).
Finally, several studies showed significant associations between unfavorable tumor pathology with %free PSA, [−2]proPSA, and the phi test combining total, free and [−2]proPSA (5, 28–30, 34, 36, 44, 55). Tseng et al (5) investigated the predictive value of age, PSA, PSAD, %fPSA, number of positive cores, maximum percentage core involvement, and diagnosis year for progression. Baseline variables that predicted progression on multivariate analysis were %fPSA<=15% and maximum percentage of core involvement >=35%. The authors concluded that initial %fPSA and maximum percentage of core involvement can risk stratify for AS biopsy outcome at 1 yr, suggesting that these baseline markers could also be used to confirm optimal eligibility. A prospective study of PSA isoforms was reported by Tosoian et al (55) in men from the Johns Hopkins AS program. Baseline and longitudinal %fPSA, %[2]proPSA, [2]proPSA/%fPSA and phi measurements were significantly associated with biopsy reclassification during 4.3 years median follow-up. Of all parameters, %[2]proPSA and phi provided the greatest predictive accuracy for reclassification to high grade cancer. For example, the C-index for biopsy reclassification was 0.79 using baseline phi and 0.82 using longitudinal phi measurements, suggesting utility in patient selection and predicting progression. Recently, the use of [−2]proPSA and phi were validated in a prospective Japanese AS population. Specifically, Hirama et al (28) showed that baseline [−2]proPSA and phi predicted pathological reclassification at 1 year.
Genetics/Genomics and Other Factors
Two urine markers that have recently been examined are PCA3 and TMPRSS2:ERG fusions (18, 33, 66). Lin et al (18) found that on univariate analysis, urinary PCA3 and TMPRSS2:ERG were significantly associated with higher volume disease and higher-grade disease; however, they were not significant on multivariate analysis. Furthermore, PCA3 and TMPRSS2:ERG together were not superior to PSA alone in predicting high-grade disease. Cornu et al (33) investigated the predictive value of urine PCA3, TMPRSS2:ERG, genotypes for rs1447295 and rs6983267 (on 8q24), testosterone and other clinical variables in relation to GS 4 on biopsy. Multivariable analysis showed that PCA3 was significantly associated with GS 4 as was PSAD and there was marginal significance for TMPRSS2:ERG. The 8q24 SNPs were not predictive of GS 4. They concluded that urine markers like PCA3 and TMPRSS2:ERG may help predict risk of more aggressive disease in certain subgroups. Tosoian et al (66) examined urinary PCA3 in men with very low risk cancer from the prospective Johns Hopkins cohort. PCA3 had poor discrimination (AUC 0.589), and was not significantly associated with short-term biopsy progression on multivariate analysis after accounting for age and diagnosis date (p=0.15). Overall, despite a univariate association of urinary PCA3 and TMPRSS2:ERG with higher risk features in some studies, there is a lack of definitive data showing incremental predictive accuracy compared to existing tools (18, 66). Whether there is any role for these markers in risk assessment during AS requires further study. A different set of urinary markers was evaluated by Venkitaraman et al. (67), who found no significant relationship between levels of phytoestrogens with biopsy progression.
Finally, a few studies have examined potential tissue markers. In a contemporary AS population, Berg et al (24) found that tissue overexpression of ERG measured by immunohistochemistry identifies AS patients with an increased risk of disease progression. The 2-year cumulative incidence of AS progression was 21.7% in ERG negative versus 58.6% in ERG positive patients, and ERG was a significant predictor of progression on multivariate analysis. Isharwal et al showed that the DNA content in the benign adjacent and cancer tissue areas was a significant predictor of AS biopsy reclassification on multivariate analysis, along with serum [−2]proPSA and phi (34) It was suggested that DNA content reflects upregulation of proliferation-related genes. A limitation of this study was a small number of men (n=71) with both serum and tissue specimens to evaluate both types of markers.
Limitations
A limitation of this synthesis is that definitions of progression vary widely in the literature, ranging from changes in PSA and/or DRE to increases in stage, grade and/or tumor volume on biopsy. However, early upgrading on repeat biopsy could imply initial sampling error (reclassification), whereas later upgrading may better reflect tumor dedifferentiation.(68) In addition, there have been changes to Gleason grading over time. Also, the current study did not address multiparametric MRI, which is emerging as a promising tool for AS selection and monitoring. Many other new markers such as 4K, Prolaris and Oncotype Dx prostate were not included in this systematic review, because they have only been evaluated in biopsy or radical prostatectomy cohorts, but not in AS cohorts. It is possible that a combination of markers may be used for AS in the future. However, additional data on incremental predictive value, cost and logistical considerations are also important. Finally, most studies have only short to intermediate follow-up for marker evaluation and additional follow-up is needed to examine their relationship to long-term disease-specific outcomes.
CONCLUSION
This review summarized patient and tumor factors as well as biomarkers to help select patients to AS and predict progression during AS. At the time of the initial decision to enroll in AS (baseline), many different factors have demonstrated utility at predicting future risk of progression. These include PSA density, percent free PSA, phi, and the extent of cancer on biopsy (number of positive cores or percentage core involvement). Other patient factors that can be considered for patient selection include age, race, and possibly family history. For patients undergoing AS, subsequent measurements of PSA density, phi, and restaging biopsies have all been shown to provide independent information on the risk of later progression. The literature on PSA kinetics during AS is limited by methodological flaws in many published studies. Based on currently available data, PSA kinetics seem to offer limited incremental predictive value for AS outcomes in the short-term. The PSAV risk count may be of aid in predicting late recurrence (after >2 years on AS). Multiple studies have failed to demonstrate independent predictive value of urinary PCA3 and TMPRSS2:ERG to predict progression on AS. Less has been published on tissue-based markers in AS populations, which is an important direction for future study. Finally, these data support a multivariable approach to patient selection and risk stratification for AS.
Supplementary Material
Acknowledgments
This paper has been developed in conjunction with the “Active Surveillance for low risk prostate cancer Conference (Chairs: C.H. Bangma, NL and J. Hugosson, SE – Co-Chair: L. Klotz, CAN – Honorary Chair: L.J. Denis, BE – ESO Prostate Programme Coordination: R. Valdagni, IT – Scientific Coordinators: M.J. Roobol, NL – S. Carlsson, SE/US.) The Conference has been organized in collaboration with EAU and endorsed by Europa Uomo”
References
- 1.Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol. 2012;61(6):1079–92. doi: 10.1016/j.eururo.2012.02.054. Epub 2012/03/20. [DOI] [PubMed] [Google Scholar]
- 2.Schroder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. The New England journal of medicine. 2012;366(11):981–90. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roobol MJ, Kerkhof M, Schroder FH, et al. Prostate cancer mortality reduction by prostate-specific antigen-based screening adjusted for nonattendance and contamination in the European Randomised Study of Screening for Prostate Cancer (ERSPC) Eur Urol. 2009;56(4):584–91. doi: 10.1016/j.eururo.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 4.Klotz L. Prostate cancer overdiagnosis and overtreatment. Current opinion in endocrinology, diabetes, and obesity. 2013;20(3):204–9. doi: 10.1097/MED.0b013e328360332a. [DOI] [PubMed] [Google Scholar]
- 5.Tseng KS, Landis P, Epstein JI, Trock BJ, Carter HB. Risk Stratification of Men Choosing Surveillance for Low Risk Prostate Cancer. Journal of Urology. 2010;183(5):1779–85. doi: 10.1016/j.juro.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lees K, Durve M, Parker C. Active surveillance in prostate cancer: patient selection and triggers for intervention. Current opinion in urology. 2012;22(3):210–5. doi: 10.1097/MOU.0b013e328351dc47. [DOI] [PubMed] [Google Scholar]
- 7.van den Bergh RC, Ahmed HU, Bangma CH, Cooperberg MR, Villers A, Parker CC. Novel tools to improve patient selection and monitoring on active surveillance for low-risk prostate cancer: a systematic review. Eur Urol. 2014;65(6):1023–31. doi: 10.1016/j.eururo.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Abern MR, Bassett MR, Tsivian M, et al. Race is associated with discontinuation of active surveillance of low-risk prostate cancer: results from the Duke Prostate Center. Prostate Cancer Prostatic Dis. 2013;16(1):85–90. doi: 10.1038/pcan.2012.38. Epub 2012/10/17. [DOI] [PubMed] [Google Scholar]
- 9.Iremashvili V, Soloway MS, Rosenberg DL, Manoharan M. Clinical and demographic characteristics associated with prostate cancer progression in patients on active surveillance. J Urol. 2012;187(5):1594–9. doi: 10.1016/j.juro.2011.12.082. Epub 2012/03/20. [DOI] [PubMed] [Google Scholar]
- 10.Sundi D, Kryvenko O, Epstein J, et al. Reclassification rates are higher among african american men than white men on active surveillance. Journal of Urology. 2014 doi: 10.1016/j.urology.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohn JA, Dangle PP, Wang CE, et al. The prognostic significance of perineural invasion and race in men considering active surveillance. BJU Int. 2013 doi: 10.1111/bju.12463. Epub 2013/10/11. [DOI] [PubMed] [Google Scholar]
- 12.Cullen J, Brassell S, Chen Y, Srivastava S, McLeod D. All-cause mortality among military health care beneficiaries with prostate cancer undergoing active surveillance. Journal of Urology. 2011 [Google Scholar]
- 13.Fleshner NE, Lucia MS, Egerdie B, et al. Effect of Baseline Characteristics on Relative Risk of Prostate Cancer Progression in the Reduction by Dutasteride of Clinical Progression Events in Expectant Management (Redem) Trial. European Urology Supplements. 2011;10(2):51–2. [Google Scholar]
- 14.Smith A, Coward M, Doak H, et al. Outcomes and implications of follow-up biopsies of men on active surveillance for low-risk prostate cancer. Journal of Urology. 2009 [Google Scholar]
- 15.Dall’Era MA, Konety BR, Cowan JE, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112(12):2664–70. doi: 10.1002/cncr.23502. Epub 2008/04/25. [DOI] [PubMed] [Google Scholar]
- 16.Patel HD, Feng Z, Landis P, Trock BJ, Epstein JI, Carter HB. Prostate specific antigen velocity risk count predicts biopsy reclassification for men with very low risk prostate cancer. J Urol. 2014;191(3):629–37. doi: 10.1016/j.juro.2013.09.029. Epub 2013/09/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shappley WV, 3rd, Kenfield SA, Kasperzyk JL, et al. Prospective study of determinants and outcomes of deferred treatment or watchful waiting among men with prostate cancer in a nationwide cohort. J Clin Oncol. 2009;27(30):4980–5. doi: 10.1200/JCO.2008.21.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin DW, Newcomb LF, Brown EC, et al. Urinary TMPRSS2:ERG and PCA3 in an active surveillance cohort: results from a baseline analysis in the Canary Prostate Active Surveillance Study. Clin Cancer Res. 2013;19(9):2442–50. doi: 10.1158/1078-0432.CCR-12-3283. Epub 2013/03/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bul M, Zhu X, Valdagni R, et al. Active surveillance for low-risk prostate cancer worldwide: the PRIAS study. Eur Urol. 2013;63(4):597–603. doi: 10.1016/j.eururo.2012.11.005. Epub 2012/11/20. [DOI] [PubMed] [Google Scholar]
- 20.Klotz L, Zhang LY, Lam A, Nam R, Mamedov A, Loblaw A. Clinical Results of Long-Term Follow-Up of a Large, Active Surveillance Cohort With Localized Prostate Cancer. Journal of Clinical Oncology. 2010;28(1):126–31. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 21.Sternberg I, Yu C, Paz G, et al. Predicting progression in patients followed with active surveillance for low-risk prostate cancer. Journal of Urology. 2014 [Google Scholar]
- 22.Whitson JM, Porten SP, Hilton JF, et al. The relationship between prostate specific antigen change and biopsy progression in patients on active surveillance for prostate cancer. J Urol. 2011;185(5):1656–60. doi: 10.1016/j.juro.2010.12.042. Epub 2011/03/23. [DOI] [PubMed] [Google Scholar]
- 23.Zhang LY, Loblaw A, Klotz L. Modeling prostate specific antigen kinetics in patients on active surveillance. Journal of Urology. 2006;176(4):1392–7. doi: 10.1016/j.juro.2006.06.103. [DOI] [PubMed] [Google Scholar]
- 24.Berg KD, Vainer B, Thomsen FB, et al. ERG Protein Expression in Diagnostic Specimens Is Associated with Increased Risk of Progression During Active Surveillance for Prostate Cancer. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.02.058. Epub 2014/03/19. [DOI] [PubMed] [Google Scholar]
- 25.Bul M, van den Bergh RC, Rannikko A, et al. Predictors of unfavourable repeat biopsy results in men participating in a prospective active surveillance program. Eur Urol. 2012;61(2):370–7. doi: 10.1016/j.eururo.2011.06.027. Epub 2011/06/28. [DOI] [PubMed] [Google Scholar]
- 26.Cary KC, Cowan JE, Sanford M, et al. Predictors of Pathologic Progression on Biopsy Among Men on Active Surveillance for Localized Prostate Cancer: The Value of the Pattern of Surveillance Biopsies. Eur Urol. 2013 doi: 10.1016/j.eururo.2013.08.060. Epub 2013/09/17. [DOI] [PubMed] [Google Scholar]
- 27.Eggener SE, Mueller A, Berglund RK, et al. A multi-institutional evaluation of active surveillance for low risk prostate cancer. J Urol. 2009;181(4):1635–41. doi: 10.1016/j.juro.2008.11.109. discussion 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirama H, Sugimoto M, Ito K, Shiraishi T, Kakehi Y. The impact of baseline [−2]proPSA-related indices on the prediction of pathological reclassification at 1 year during active surveillance for low-risk prostate cancer: the Japanese multicenter study cohort. J Cancer Res Clin Oncol. 2014;140(2):257–63. doi: 10.1007/s00432-013-1566-2. Epub 2013/12/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klotz L. Active surveillance with selective delayed intervention: using natural history to guide treatment in good risk prostate cancer. J Urol. 2004;172(5 Pt 2):S48–50. doi: 10.1097/01.ju.0000141712.79986.77. discussion S-1. [DOI] [PubMed] [Google Scholar]
- 30.Makarov DV, Isharwal S, Sokoll LJ, et al. Pro-prostate-specific antigen measurements in serum and tissue are associated with treatment necessity among men enrolled in expectant management for prostate cancer. Clin Cancer Res. 2009;15(23):7316–21. doi: 10.1158/1078-0432.CCR-09-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soloway MS, Soloway CT, Williams S, Ayyathurai R, Kava B, Manoharan M. Active surveillance; a reasonable management alternative for patients with prostate cancer: the Miami experience. BJU Int. 2008;101(2):165–9. doi: 10.1111/j.1464-410X.2007.07190.x. Epub 2007/09/14. [DOI] [PubMed] [Google Scholar]
- 32.Venkitaraman R, Norman A, Woode-Amissah R, et al. Predictors of histological disease progression in untreated, localized prostate cancer. Journal of Urology. 2007;178(3):833–7. doi: 10.1016/j.juro.2007.05.038. [DOI] [PubMed] [Google Scholar]
- 33.Cornu JN, Cancel-Tassin G, Egrot C, Gaffory C, Haab F, Cussenot O. Urine TMPRSS2:ERG fusion transcript integrated with PCA3 score, genotyping, and biological features are correlated to the results of prostatic biopsies in men at risk of prostate cancer. Prostate. 2013;73(3):242–9. doi: 10.1002/pros.22563. Epub 2012/07/24. [DOI] [PubMed] [Google Scholar]
- 34.Isharwal S, Makarov DV, Carter HB, et al. DNA content in the diagnostic biopsy for benign-adjacent and cancer-tissue areas predicts the need for treatment in men with T1c prostate cancer undergoing surveillance in an expectant management programme. BJU Int. 2010;105(3):329–33. doi: 10.1111/j.1464-410X.2009.08791.x. Epub 2009/08/15. [DOI] [PubMed] [Google Scholar]
- 35.van den Bergh RCN, Vasarainen H, van der Poel HG, et al. Short-term outcomes of the prospective multicentre ‘Prostate Cancer Research International: Active Surveillance’ study. Bju International. 2010;105(7):956–62. doi: 10.1111/j.1464-410X.2009.08887.x. [DOI] [PubMed] [Google Scholar]
- 36.San Francisco IF, Werner L, Regan MM, Garnick MB, Bubley G, DeWolf WC. Risk stratification and validation of prostate specific antigen density as independent predictor of progression in men with low risk prostate cancer during active surveillance. J Urol. 2011;185(2):471–6. doi: 10.1016/j.juro.2010.09.115. Epub 2010/12/21. [DOI] [PubMed] [Google Scholar]
- 37.Valeri A, Papin G, Moineau MP, et al. Is screen-detected prostate cancer in high risk families eligible for active surveillance? European Urology Supplements. 2010 [Google Scholar]
- 38.Burton AJ, Martin RM, Donovan JL, et al. Associations of lifestyle factors and anthropometric measures with repeat PSA levels during active surveillance/monitoring. Cancer Epidemiol Biomarkers Prev. 2012;21(10):1877–85. doi: 10.1158/1055-9965.EPI-12-0411. Epub 2012/08/04. [DOI] [PubMed] [Google Scholar]
- 39.Goh CL, Saunders EJ, Leongamornlert DA, et al. Clinical implications of family history of prostate cancer and genetic risk single nucleotide polymorphism (SNP) profiles in an active surveillance cohort. BJU Int. 2013;112(5):666–73. doi: 10.1111/j.1464-410X.2012.11648.x. Epub 2013/01/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mukerji GM, Nariculam J, Hellawell GO, Abel P, Winkler M. Outcome of patients on active surveillance for low-risk prostate cancer in a cohort of British men with low screening penetrance. BJU International. 2010 [Google Scholar]
- 41.van den Bergh RC, Roemeling S, Roobol MJ, et al. Gleason score 7 screen-detected prostate cancers initially managed expectantly: outcomes in 50 men. BJU Int. 2009;103(11):1472–7. doi: 10.1111/j.1464-410X.2008.08281.x. [DOI] [PubMed] [Google Scholar]
- 42.Venkitaraman R, Norman A, Woode-Amissah R, et al. Prostate-specific antigen velocity in untreated, localized prostate cancer. BJU Int. 2008;101(2):161–4. doi: 10.1111/j.1464-410X.2007.07175.x. Epub 2007/09/14. [DOI] [PubMed] [Google Scholar]
- 43.Jhavar S, Bartlett J, Kovacs G, et al. Biopsy tissue microarray study of Ki-67 expression in untreated, localized prostate cancer managed by active surveillance. Prostate Cancer Prostatic Dis. 2009;12(2):143–7. doi: 10.1038/pcan.2008.47. [DOI] [PubMed] [Google Scholar]
- 44.van As NJ, Norman AR, Thomas K, et al. Predicting the probability of deferred radical treatment for localised prostate cancer managed by active surveillance. Eur Urol. 2008;54(6):1297–305. doi: 10.1016/j.eururo.2008.02.039. Epub 2008/03/18. [DOI] [PubMed] [Google Scholar]
- 45.Iremashvili V, Burdick-Will J, Soloway MS. Improving risk stratification in patients with prostate cancer managed by active surveillance: a nomogram predicting the risk of biopsy progression. BJU Int. 2013;112(1):39–44. doi: 10.1111/bju.12112. Epub 2013/04/05. [DOI] [PubMed] [Google Scholar]
- 46.Iremashvili V, Manoharan M, Rosenberg DL, Soloway MS. Biopsy features associated with prostate cancer progression in active surveillance patients: comparison of three statistical models. BJU Int. 2013;111(4):574–9. doi: 10.1111/j.1464-410X.2012.11127.x. Epub 2012/05/09. [DOI] [PubMed] [Google Scholar]
- 47.Ng MK, Van As N, Thomas K, et al. Prostate-specific antigen (PSA) kinetics in untreated, localized prostate cancer: PSA velocity vs PSA doubling time. BJU Int. 2009;103(7):872–6. doi: 10.1111/j.1464-410X.2008.08116.x. [DOI] [PubMed] [Google Scholar]
- 48.Adamy A, Yee DS, Matsushita K, et al. Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J Urol. 2011;185(2):477–82. doi: 10.1016/j.juro.2010.09.095. Epub 2010/12/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yee DS, Adamy A, Pinochet R, et al. The rate of upgrading and upstaging on immediate repeat biopsy in patients eligible for active surveillance is not related to extent of first biopsy. Journal of Urology. 2010 [Google Scholar]
- 50.Fromont G, Irani J, Ruffion A, et al. Interest of early confirmatory biopsies and centralized pathological review to select prostate cancer patients for active surveillance. European Urology, Supplements. 2011 [Google Scholar]
- 51.Soloway MS, Soloway CT, Eldefrawy A, Acosta K, Kava B, Manoharan M. Careful Selection and Close Monitoring of Low-Risk Prostate Cancer Patients on Active Surveillance Minimizes the Need for Treatment. European Urology. 2010;58(6):831–5. doi: 10.1016/j.eururo.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 52.Umbehr MH, Platz EA, Peskoe SB, et al. Serum prostate-specific antigen (PSA) concentration is positively associated with rate of disease reclassification on subsequent active surveillance prostate biopsy in men with low PSA density. BJU Int. 2014;113(4):561–7. doi: 10.1111/bju.12131. Epub 2013/06/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Barayan GA, Brimo F, Begin LR, et al. Factors Influencing Disease Progression of Prostate Cancer under Active Surveillance: A McGill University Health Center Cohort. BJU Int. 2014 doi: 10.1111/bju.12754. Epub 2014/04/02. [DOI] [PubMed] [Google Scholar]
- 54.Welty C, Cowan J, Nguyen H, et al. Factors associated with biopsy progression on active surveillance. Journal of Urology. 2014 [Google Scholar]
- 55.Tosoian JJ, Loeb S, Feng Z, et al. Association of [−2]proPSA with biopsy reclassification during active surveillance for prostate cancer. J Urol. 2012;188(4):1131–6. doi: 10.1016/j.juro.2012.06.009. Epub 2012/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Komisarenko M, Wong L, Richard P, et al. Patients that demonstrate gleason 6 volume progression on active surveillance have a substantially greater risk of grade progression on further follow-up. Journal of Urology. 2014 [Google Scholar]
- 57.Loblaw A, Zhang L, Lam A, et al. Comparing prostate specific antigen triggers for intervention in men with stable prostate cancer on active surveillance. J Urol. 2010;184(5):1942–6. doi: 10.1016/j.juro.2010.06.101. Epub 2010/09/18. [DOI] [PubMed] [Google Scholar]
- 58.Kakehi Y, Kamoto T, Shiraishi T, et al. Prospective evaluation of selection criteria for active surveillance in Japanese patients with stage T1cN0M0 prostate cancer. Jpn J Clin Oncol. 2008;38(2):122–8. doi: 10.1093/jjco/hym161. Epub 2008/02/15. [DOI] [PubMed] [Google Scholar]
- 59.Ross AE, Loeb S, Landis P, et al. Prostate-specific antigen kinetics during follow-up are an unreliable trigger for intervention in a prostate cancer surveillance program. J Clin Oncol. 2010;28(17):2810–6. doi: 10.1200/JCO.2009.25.7311. Epub 2010/05/05. [DOI] [PubMed] [Google Scholar]
- 60.Khatami A, Aus G, Damber JE, Lilja H, Lodding P, Hugosson J. PSA doubling time predicts the outcome after active surveillance in screening-detected prostate cancer: results from the European randomized study of screening for prostate cancer, Sweden section. Int J Cancer. 2007;120(1):170–4. doi: 10.1002/ijc.22161. Epub 2006/10/03. [DOI] [PubMed] [Google Scholar]
- 61.Iremashvili V, Manoharan M, Lokeshwar SD, Rosenberg DL, Pan D, Soloway MS. Comprehensive analysis of post-diagnostic prostate-specific antigen kinetics as predictor of a prostate cancer progression in active surveillance patients. BJU Int. 2013;111(3):396–403. doi: 10.1111/j.1464-410X.2012.11295.x. Epub 2012/06/19. [DOI] [PubMed] [Google Scholar]
- 62.Krakowsky Y, Loblaw A, Klotz L. Prostate cancer death of men treated with initial active surveillance: clinical and biochemical characteristics. J Urol. 2010;184(1):131–5. doi: 10.1016/j.juro.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 63.Pujara AC, Stephenson AJ, Miocinovic R, Berglund RK, Jones JS. Prostate-specific antigen rises faster in patients with multiple negative biopsies compared to patients followed by active surveillance for low-risk prostate cancer. Journal of Urology. 2010 [Google Scholar]
- 64.Vickers A. In: Active surveillance for localized prostate cancer A new paradigm for clinical management. Kotz L, editor. Humana Press; 2012. [Google Scholar]
- 65.Loeb S, Han M, Roehl KA, Antenor JA, Catalona WJ. Accuracy of prostate weight estimation by digital rectal examination versus transrectal ultrasonography. J Urol. 2005;173(1):63–5. doi: 10.1097/01.ju.0000145883.01068.5f. Epub 2004/12/14. [DOI] [PubMed] [Google Scholar]
- 66.Tosoian JJ, Loeb S, Kettermann A, et al. Accuracy of PCA3 measurement in predicting short-term biopsy progression in an active surveillance program. J Urol. 2010;183(2):534–8. doi: 10.1016/j.juro.2009.10.003. Epub 2009/12/17. [DOI] [PubMed] [Google Scholar]
- 67.Venkitaraman R, Thomas K, Grace P, et al. Baseline urinary phytoestrogen levels and the natural history of untreated, localised prostate cancer in a British population. Int J Biol Markers. 2008;23(3):192–7. doi: 10.1177/172460080802300310. Epub 2008/10/25. [DOI] [PubMed] [Google Scholar]
- 68.Porten SP, Whitson JM, Cowan JE, et al. Changes in prostate cancer grade on serial biopsy in men undergoing active surveillance. J Clin Oncol. 2011;29(20):2795–800. doi: 10.1200/JCO.2010.33.0134. Epub 2011/06/03. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


