Abstract
Background
In the present study, different methods for preparation of platelet-rich plasma (PRP) are investigated in order to standardize the component in terms of growth factor content. The effects of concentration technique and storage duration are also analyzed.
Methods
PRP was collected from 40 donors by plateletpheresis as well as by the buffy coat and tube method. Concentration of growth factors was performed using double freeze thaw- and CaCl2-induced degranulation techniques. Growth factor estimation was performed using ELISA.
Results
The levels of growth factors were highest in PRP from buffy coat, moderately lower in plasma gained by plateletpheresis and lowest in that obtained by the tube method. Mean levels of platelet-derived growth factors (PDGF) AB and BB are significantly higher when CaCl2 was used for concentrating the growth factors. The mean levels of transforming growth factor β1 and insulin-like growth factor I were higher when applying the double freeze thaw technique. There was a substantial decline in the levels of growth factors during storage.
Conclusion
The buffy coat method is suitable as preparation method for PRP in most settings. The double freeze thaw technique is better suited as concentration technique as it causes lysis of both platelets and white blood cells for releasing growth factors and is easier to perform. Growth factors are not stable in plasma, thus PRP should be frozen immediately after preparation.
Key Words: Buffy coat, Growth factors, Platelet transfusion, Plateletpheresis
Introduction
In the current clinical practice, most of the platelet-containing preparations are used for systemic transfusion to patients with thrombocytopenia due to various medical reasons. However, there is steep growth of clinical interest in regenerative medicine with respect to the topical application of biomaterials containing platelets such as platelet gel or platelet-rich plasma (PRP). These platelet preparations have been used for accelerating wound healing and bone regeneration as they contain a host of powerful mitogenic and chemotactic growth factors [1,2]. Because of these growth factors stored in the α-granules of the platelet, platelets are a promising tool as topical biomaterial for wound healing. The most prominent growth factors in this respect are: platelet-derived growth factor (PDGF), transforming growth factor (TGF) and insulin-like growth factors I (IGF-1) [1,3,4]. These molecules stimulate various cells such as fibroblasts, smooth muscles and osteoblasts, are chemo-attractants for cells involved in wound repair, and also are effective against infectious agents [5,6].
There is uncertainty regarding clinical efficacy of PRP because of the limited knowledge on the effect of contaminating cells and the poor characterization of these components with respect to growth factor concentrations. PRP preparation lacks standardization, and the method used for preparation might have a potentially significant impact on the levels of platelet recovery and activation. Platelet activation during preparation of the platelet concentrate can result in early granule release and loss of growth factors during the collection process. The protocols for biological processing of growth factors also differ widely [7,8]. It is therefore critical to recognize that each PRP preparation method may differ in regard to platelet number, platelet activation rates, and growth factor profiles [9].
In blood banks, PRP components are prepared by various methods including whole blood donation, apheresis as well as the tube method. There is also heterogeneity in the protocols for preparing clinical-grade solutions for final application. Researchers have employed techniques such as single or repeated freeze thaw cycles, addition of CaCl2 or a combination of CaCl2 and thrombin to obtain platelet lysates containing varying amount of growth factors. Additionally, the cellular composition of PRP as well as the storage duration may be significant factors determining the final concentration of growth factors and thus, its biological responsiveness.
The objective of the present study was to provide data on growth factor concentrations in standardized platelet concentrates obtained from identical source material, prepared by different blood bank methods, concentrated by two different techniques, and stored for various times. The effect of platelet and WBC count on the levels of growth factors was also determined. In order to investigate the in vitro effect of various PRP preparations on cellular functions, a fibroblast proliferation assay was performed.
Material and Methods
The study was conducted in a tertiary care hospital and research center after taking approval from the ethical committee of the institute. 40 healthy donors passing the criteria for plateletpheresis were randomly included in the study after taking informed consent. EDTAanticoagulated blood samples were collected prior to plateletpheresis procedure, and cell counts were done using an automated cell counter (Sysmex KX-21, Cobe, Japan).
PRP Collection
PRP was collected by apheresis using the discontinuous cell separation method (MCS 3p, Haemonetics, Munich, Germany). The cell separator was programmed to collect 3 × 1011 platelets in 250 ml plasma (PA). On the basis of entered donor data and the ratio of whole blood to acid citrate dextrose (ACD), the on-board processor calculated the whole blood flow rate and volume to be processed. From the final unit, approximately 10 ml of PRP was transferred in a satellite platelet storage bag attached with main collection bag using a sterile connecting device (Composeal; Fresenius, Bad Homburg, Germany) for sampling.
One unit of whole blood was collected from the same donor 48-72 h after plateletpheresis in a quadruple bag (Terumo Penpol, Thiruvananthapuram, India). PRP was prepared by the buffy coat method (PB). Additional 20 ml blood was collected simultaneously in a single blood collection bag containing CPDA (citrate/phosphate/dextrose/adenine) as an anticoagulant in 1:7 ratio, followed by transfer of anticoagulated blood to polystyrene tubes and centrifugation at 1,000 rpm (447 × g) for 10 min. Red cells were discarded, and remaining plasma was again centrifuged at 3,000 rpm for 10 min. The supernatant was removed leaving 2 ml of plasma in which pellet was re-suspended. It was termed as PRP by tube method (PT).
Sampling
The platelet count was determined in all PRP samples by an automated cell counter, and residual WBC count was carried out microscopically by Nageotte's chamber. On the day of preparation, 2 ml sample was taken aseptically from each PRP and divided in two halves. One was subjected to double freeze thaw and another to CaCl2 treatment. The sampling was done on days 3 and 5 from PA and PB. These were subjected to double freeze thaw.
Final Concentration
Double freeze thaw: The sample was frozen at −80 °C. It was thawed at room temperature after 48 h and refrozen immediately thereafter. Just before analysis, it was again thawed and centrifuged for 10 min at 10,000 rpm in a microcentrifuge.
CaCl2treatment: Pharmaceutical grade CaCl2 was added to 1 ml PRP at a final concentration of 0.04 mol/l. This was followed by incubation at 37 °C for at least 1 h (or until complete clot retraction). The PRP was then centrifuged for 10 min at 10,000 rpm in a microcentrifuge; the supernatant was separated and stored at −80 °C until analysis.
Quantitative Analysis of Growth Factors
Commercial ELISA kits (R&D Systems, Minneapolis, MN, USA) were used to quantify the concentrations of PDGF-AB, PDGF-BB, TGF-β1, and IGF-I in these specimens. ELISAs were performed according to the manufacturer's instructions as described below.
Fibroblast Proliferation Assay
A normal adult human skin sample was obtained. The skin was cut into small pieces, which were incubated overnight at 37 °C in a collagenase type II solution 4 mg/ml for 4 h at 37 °C. Fibroblasts were grown from explants in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 2 mmol/l glutamine, penicillin (100 U/ml) and streptomycin (100 µg/ml) and plated in a 60-mm culture dish at 37 °C in a humidified CO2 incubator. Fibroblasts were then seeded in 96-well plates in 0.5 ml of the DMEM medium with 2% FBS. When cells reached 30% of confluent growth, the medium was washed off and cells were incubated for 24 h in DMEM without serum. The medium was refreshed before adding PRP. Eight different growth conditions were tested with PRP in the DMEM medium: PA double freeze thaw, PA CaCl2, PB double freeze thaw, PB CaCl2, PT double freeze thaw, PT CaCl2, DMEM medium (negative control) and 2% FBS (positive control). After 5 days of incubation, the proliferation of fibroblasts was measured with an MTT assay (Sigma-Aldrich Chemie, GmbH, Taufkirchen, Germany), a colorimetric assay that measures the chemical reduction of MTT (3-(4.5-Dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide) into formazan, which is directly proportional to the number of viable cells in the tested culture. Cultures were incubated for 2-3 h in a culture medium with 0.5 mg/ml of MTT. The resulting formazan was then eluted with acidified isopropanol (0.04 N HCl in isopropanol), and the optical density was measured at 570 nm.
Statistical Analysis
Results are presented as mean ± standard deviation (SD). Two-way analysis of variance was used to determine significant differences between means. Tukey's post hoc test was performed for all pair-wise comparisons and significance was set at p < 0.05. Student's t-test was applied to compare means of growth factor levels by two concentration techniques. Effects of cellular concentration on levels of growth factors were examined by multiple regression analysis using the least-squares method.
Results
The study population comprised of 32 males and 8 females, all within an age range of 20-52 years. Mean platelet count of the donors' blood was 231.80 ± 54.39 × 106/ml.
Preparation Methods of PRP
In PA, the mean platelet and WBC concentration was 1.19 × 109/ml (range 0.98-1.63 × 109/ml), and 1.40 × 106/ ml (range 0.2-3.0 × 106/ml), respectively. In PB, the mean platelet and WBC concentration was 1.06 × 109/ ml (range 0.82-1.31 × 109/ml) and 2.04 × 106/ ml (range 0.6-3.2 × 106/ml), respectivley. In PT, the mean platelet and WBC concentration was 0.80 × 109/ ml (range 0.62-1.10 × 109/ml) and 22.98 × 106/ ml (range 16.4-27.3 × 106/ml), respectively.
The mean levels of growth factors in PRP prepared by different methods are shown in table 1. Growth factor levels in PRP were analyzed after freezing and thawing twice, thus reflecting the total intracellular and extracellular growth factor content. The levels were highest in PB, lower in PA and lowest in PT.
Table 1.
Properties of PRP prepared by different methods on the day of preparation
| PA | PB | PT | |
|---|---|---|---|
| Platelet concentration × 109/ ml | 1.19 ± 0.15* | 1.06 ± 0.15* | 0.80 ± 0.12*† |
| WBC concentration × 106/ ml | 1.40 ± 0.82†‡ | 2.04 ± 0.81*‡ | 22.98 ± 8.92*† |
| PDGF-AB, ng/ml | 158.17 ± 27.50†‡ | 173.63 ± 22.63*‡ | 128.52 ± 27.21*† |
| PDGF-BB, ng/ml | 24.14 ± 4.98* | 33.32 ± 4.40*‡ | 21.58 ± 5.98† |
| TGF-β1, ng/ml | 130.35 ± 14.98†‡ | 156.03 ± 21.42*‡ | 89.31 ± 18.56*† |
| IGF-1, ng/ml | 80.78 ± 9.71* | 87.36 ± 10.18* | 78.49 ± 13.32*† |
p < 0.05 in comparison to PA.
p < 0.05 in comparison to PB.
p < 0.05 in comparison to PT.
Final Concentration Technique and Growth Factor Levels
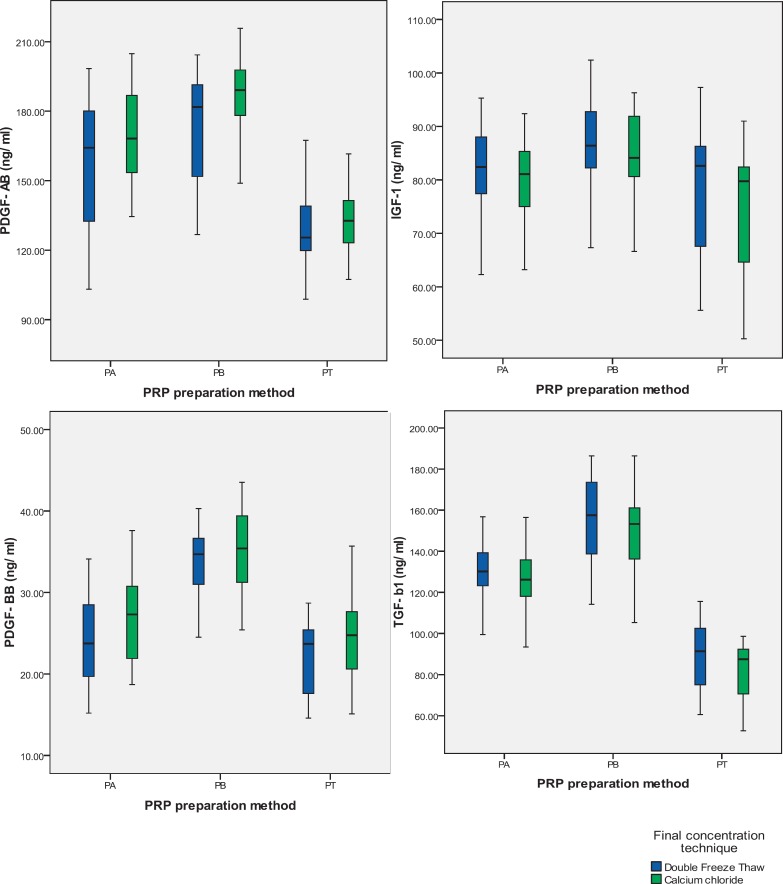
Figure 1 shows the comparison of the levels of growth factors obtained by double freeze thaw- or CaCl2-induced degranulation technique in PRP obtained by all three preparation methods. It is evident that in most cases, mean levels of PDGF-AB and PDGF-BB were significantly higher with CaCl2 technique. On the other hand, the mean levels of TGF-β1 and IGF-1 were not significantly different when comparing both methods of preparation although there was a trend (nonsignificant) to higher levels with double freeze thaw technique.
Fig. 1.
Effect of final concentration technique on the quantity of growth factors. In double freeze thaw technique, sample was frozen and thawed twice, whereas in CaCl2 treatment pharmaceutical grade CaCl2 was added to PRP followed by incubation at 37 °C for 1 h. *p < 0.05 (Student's t-test) versus double freeze thaw technique.
Storage and Growth Factor Levels
We have assessed the influence of storage on the growth factor content of plateletpheresis- and buffy coat-derived PRP stored at 22 °C for 3 or 5 days. The results of our measurements are presented in table 2. On 3-day storage period, the mean levels of growth factors declined compared to their levels on the day of preparation. At the end of storage i.e. day 5, the total growth factor levels had decreased substantially.
Table 2.
Effect of storage on the quantity of growth factors
| PA |
PB |
|||||
|---|---|---|---|---|---|---|
| day 0 | day 3 | day 5 | day 0 | day 3 | day 5 | |
| PDGF-AB, ng/ml | 158.17 ± 27.50†‡ | 121.85 ± 21.20*‡ | 103.95 ± 18.51*† | 173.63 ± 22.63†‡ | 135.65 ± 21.74*‡ | 109.70 ± 17.57*† |
| PDGF-BB, ng/ml | 24.14 ± 4.98†‡ | 19.52 ± 3.57* | 17.77 ± 3.04* | 33.32 ± 4.40†‡ | 28.19 ± 4.58‡ | 19.94 ± 3.96*† |
| TGF-β1, ng/ml | 130.35 ± 14.98†‡ | 114.03 ± 12.75*‡ | 99.34 ± 12.78*† | 156.03 ± 21.42†‡ | 131.70 ± 18.29*‡ | 102.77 ± 12.07*† |
| IGF-1, ng/ml | 80.78 ± 9.71†‡ | 62.85 ± 8.95*‡ | 53.56 ± 7.98*† | 87.36 ± 10.18†‡ | 72.31 ± 11.12*‡ | 60.28 ± 11.18*† |
p < 0.05 in comparison to day 0.
dagger: p < 0.05 in comparison to day 3.
Dagger: p < 0.05 in comparison to day 5.
Regression Analysis to Determine the Variation of Growth Factor Levels in Relation to the Cellular Content of PRP
For the whole series of measurements, this analysis revealed a correlation between the platelet concentration on the one hand and the PDGF-AB and PDGF-BB levels on the other (p < 0.05) for PA and PB (table 3). We found no statistically significant correlation between other growth factor (TGF-β1 and IGF-1) levels and platelet concentration. In case of PT, there were no statistically significant correlations between growth factor levels and platelet concentration. However, the proportion of variance that was attributable to the WBC concentration was 47.8% (r2 = 0.478) for PDGF-AB and 38.7% (r2 = 0.387) for PDGF-BB, which was statistically significant (p < 0.05).
Table 3.
Correlation of quantity of growth factors and concentrations of WBC & platelets in PRP
| PA |
PB |
PT |
||||
|---|---|---|---|---|---|---|
| WBC r2 (R) | platelets (R) | WBC (R) | platelets (R) | WBC (R) | platelets (R) | |
| PDGF-AB | 0.184 (18.4) | 0.335* (33.5) | 0.295 (29.5) | 0.309* (30.9) | 0.478* (47.8) | 0.223 (22.3) |
| PDGF-BB | 0.166 (16.6) | 0.404* (40.4) | 0.203 (20.3) | 0.396*(39.6) | 0.387* (38.7) | 0.217 (21.7) |
| TGF-ß1 | 0.173 (17.3) | 0.271 (27.1) | 0.177 (17.7) | 0.298 (29.8) | 0.260 (26.0) | 0.181 (18.1) |
| IGF-1 | 0.022 (2.2) | 0.073 (7.3) | 0.041 (4.1) | 0.066 (6.6) | 0.083 (8.3) | 0.054 (5.4) |
r2 = Pearson's correlation coefficient. R = proportion of variance.
p < 0.05.
Fibroblast Proliferation Assay
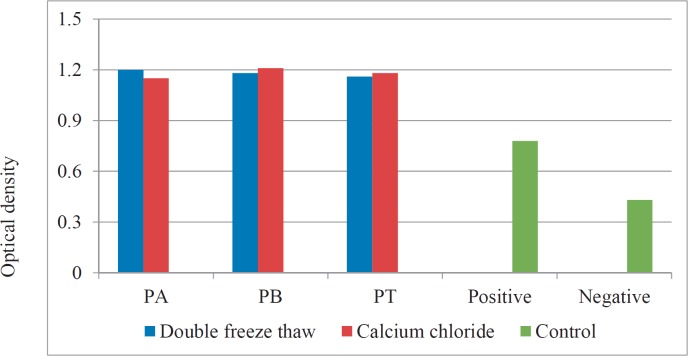
PRP prepared by all three methods and concentrated by different techniques has demonstrated to induce fibroblast proliferation in vitro. However, there was no significant difference in the optical density of the medium containing various PRP preparations (fig. 2).
Fig. 2.
Proliferation of fibroblast cell culture in the presence of various PRP preparations.
Discussion
PRP has newly emerged as topical surgical adjunct. Platelet components have recently been used as part of tissue engineering strategies in various surgical interventions. The specific cell regenerative properties which influence the immediate tissue environment are conferred by growth factors contained in platelet granules. These platelet preparations have been applied in diverse clinical fields. They have been used locally by ophthalmologists as an adjuvant to the ophthalmologic treatment of macular holes [10,11]; they have been used in cases of bone grafting showing to promote bone formation [12,13]; and they have proved effective in healing of chronic venous ulcers in cases of vascular surgery and dermatology [14,15]. Platelet concentrates has also been found to enhance healing of various kinds of wounds [16,17]. There are a number of variables which contribute to the reported inconsistencies in clinical and experimental outcomes making it difficult to standardize PRP as a therapeutic modality. These factors include preparation method [18], activation status and methods [19,20], platelet concentration [21], leukocyte concentration [22], and patient-specific effects [23]. Each of these variables has the potential to influence the properties of the resultant PRP. In the present study, three preparation methods have been investigated to characterize standardized blood bank component and final growth factor concentration techniques and to identify that resulting in the highest growth factor yield. Growth factor content of PRP before, during and after storage has been analyzed to determine the optimal time of component preparation before application.
When comparing the preparation methods, there was no significant difference between PA and PB with respect to their platelet concentration. Platelet concentration was significantly lower in PT-derived PRP. On the other hand, WBC concentration of PT-derived PRP was found to be remarkably higher. The growth factor levels of these preparations were assessed taking double freeze thaw as index concentration technique. The levels of all growth factors were significantly higher in PRP obtained by PB as compared to those gained by PA and PT. Zimmermann et al. [24] also reported highest levels of growth factors to be contained in buffy coat platelets. However, their platelet samples were highly concentrated by an additional centrifugation step and the resultant samples showed growth factor levels more than 10 times as high as those in original samples.
All three methods are capable of delivering therapeutic levels of growth factors in the clinical setting. PA and PB both have the advantage to be closed systems allowing for sterile preparations with nearly no risk of bacterial contamination. The PT technique as an open system has been suggested to be an alternative system for perioperative platelet preparation that force the maintenance of a maximal 6-hour interval between platelet preparation and use [24]. PB offers the best potential in terms of growth factor concentration, cost effectiveness as well as the simplicity of collection, even for autologous use in the patients.
One important factor which influences the final quantity of growth factors is the method employed to release them. Double freeze thaw of the PRP causes membrane disruption due to temperature shock, whereas CaCl2 could mediate an increase in endogenous synthesis of PDGF isoforms in thrombocytes and subsequent degranulation by promoting the formation of native thrombin [25]. In the present study, the CaCl2-induced degranulation technique has yielded significantly higher amounts of PDGF-AB and PDGF-BB, whereas double freeze thaw has yielded higher amounts of IGF-1 and TGF-β in most samples (fig. 1). In a similar study, Durante et al. [26] concluded that even if additional scientific efforts are required for full characterization of physical approach to release growth factors, temperature shock could represent a simple and clinically compliant approach to control the relative availability of selected growth factors in the final solution. In a recent study it has been found that neither platelet activation during clot formation nor platelet disruption by freezing leads to a complete release of all growth factors from intracellular granules into the surrounding liquid. This could possibly be explained by platelet-derived microparticle formation containing growth factors upon activation or shear stress [27,28]. However, growth factors that remain within microparticles may be undetectable in immunoassays. Zimmermann et al. [29] identified one technique that is more appropriate for the release of growth factors from platelets compared to others, that is to say lysis using the detergent Triton-X-100.
To evaluate the effect of storing the PRP in the blood bank before clinical application, we analyzed PA- and PB-derived PRP on days 3 and 5 after preparation applying the double freeze thaw technique for the growth factor release. We have observed a steep fall in growth factor levels by the end of storage. Sekido et al. [30] reported that PDGF activity of platelet concentrates in a bioassay was at the same level during preservation for up to 5 days. However, many previous studies reported that platelets lose a significant proportion of their PDGF during storage and thus became less efficient in their growth-promoting activity [31,32,33]. Zimmermann et al. [24] found a synchronous decrease in the total supernatant plus intracellular growth factor content and therefore concluded that growth factors are delivered from platelets during storage and are not stable in plasma at 22 °C.
Regression analysis showed that platelet concentration was not the sole determinant for the levels of growth factors. The contaminating WBCs also contributed considerably to the variation of growth factor levels in PRP. It has well been proved that WBCs themselves contain and produce growth factors [34]. In PA- and PB-derived PRP, there was a high concentration of platelets and a low concentration of WBCs. Therefore, the variance in these components was mainly caused by platelet concentration. On the other hand in PT-derived PRP, there was lowest concentration of platelets and highest concentration of WBCs when comparing the three preparation methods. Hence, there was a significant relationship between the growth factor concentration and the WBC contamination which had caused nearly one third to one half of the variance in PDGF-AB and PDGF-BB concentration. The levels of TGF-β1 was only partially influenced by WBC concentration. The levels of IGF-1 did not show any significant variance in dependence of platelet or WBC count.
There is also a high inter-individual variation in cellular production and storage of cytokines [35]. Moreover, platelet activation prior to application is also a determinant of the growth factor levels. We could not assess this effect due to resource constraints which remains a lacunae in our study. There are variations in the activation of platelets due to production method. If more platelets become activated by the preparation before a final centrifugation step, a significant amount of growth factors may be lost with a discarded supernatant [36].
We have applied PRP prepared and concentrated by various methods in the culture media for fibroblast cells in order to assess its potential for inducing the proliferation. There was a marked enhancement in the proliferation of fibroblasts which has also been observed previously in other studies [37,38,39]. We could not discern any statistically significant difference in the proliferation-inducing potential of PRP prepared by different methods and concentrated by different techniques. In a study done by Valeri et al. [40], no significant differences were observed in the fibroblast cell count between the releasate from untreated PRP and the releasates from PRP after the treatments. Proliferation of fibroblasts in tissue culture was found to be similar to the releasates from PRP treated with the different agonists. The authors suggested that the non-platelet derived TGF-β1, but not PDGF-AA, PDGF-AB, and PDGF-BB, was the growth factor that stimulates the proliferation of fibroblasts in tissue culture whose levels have been found to be similar in PRP and platelet-poor plasma. In the present study, we also could not find any statistically significant difference in the levels of TGF-β1 in different PRP preparations which may explain the similarity in their potential to induce proliferation in fibroblast culture.
To summarize, clinical efficacy of PRP remains unpredictable, mainly due to poor characterization of the components. The study aimed to compare different preparation methods, concentration techniques, and storage durations in order to optimize the yield of growth factors. The buffy coat method may be suitable as preparation method for PRP in a resource-poor country like ours. Double freeze thaw is better suited as concentration technique as it causes lysis of both platelets and WBCs for releasing growth factors and as it can comparatively easily be performed. Growth factors are not stable in plasma at room temperature; thus PRP should be frozen immediately after preparation. Additional comprehensive studies need to be conducted for determining the the various factors causing variability in growth factor levels of various components.
Disclosure Statement
Conflict of Interest: None.
Acknowledgement
We are grateful to Dr Vikas Agarwal, Additional Professor, Department of Clinical Immunology, and to Sanjay Gandhi, Postgraduate, Institute of Medical Sciences, Lucknow, India.
References
- 1.Greenhalgh DG. The role of growth factors in wound healing. J Trauma. 1996;41:159–167. doi: 10.1097/00005373-199607000-00029. [DOI] [PubMed] [Google Scholar]
- 2.Hollinger JO, Buck DC, Bruder SP. Biology of bone healing: its impact on clinical therapy. In: Lynch SE, Genco RJ, Marx RE, editors. Tissue Engineering: Applications in Maxillofacial Surgery and Periodontics. Carol Stream: Quintessence Publishing; 1999. pp. 17–53. [Google Scholar]
- 3.Ross R, Raines EW, Bowen-Pope DF. The biology of platelet-derived growth factor. Cell. 1986;46:155–169. doi: 10.1016/0092-8674(86)90733-6. [DOI] [PubMed] [Google Scholar]
- 4.Miyazono K, Takaku F. Platelet-derived growth factors. Blood Rev. 1989;3:269–276. doi: 10.1016/0268-960x(89)90034-9. [DOI] [PubMed] [Google Scholar]
- 5.Abdennagy B, Hott M, Marie PJ. Effects of platelet-derived growth factor on human and mouse osteoblastic cells isolated from the trabecular bone surface. Cell Biol Int Rep. 1992;16:235–247. doi: 10.1016/s0309-1651(06)80125-6. [DOI] [PubMed] [Google Scholar]
- 6.Gruber R, Varga F, Fischer MB, Watzek G. Platelets stimulate proliferation of bone cells: involvement of platelet-derived growth factor, microparticles and membranes. Clin Oral Implants Res. 2002;13:529–535. doi: 10.1034/j.1600-0501.2002.130513.x. [DOI] [PubMed] [Google Scholar]
- 7.Weibrich G Kleis WK, Hitzler WE, Hafner G. Comparison of the platelet concentrate collection system with the plasma rich in growth factors kit to produce platelet rich plasma: a technical report. Int J Oral Maxillofac Implants. 2005;20:118–123. [PubMed] [Google Scholar]
- 8.Sanchez AR, Sheridan PJ, Kupp LI. Is platelet rich plasma the perfect enhancement factor? A current review. In J Oral Maxillofac Implants. 2003;18:93–103. [PubMed] [Google Scholar]
- 9.Eppley BL, Woodell JE, Higgins J. Platelet quantification and growth factor analysis from platelet-rich plasma: implications for wound healing. Plast Reconstr Surg. 2004;114:1502–1508. doi: 10.1097/01.prs.0000138251.07040.51. [DOI] [PubMed] [Google Scholar]
- 10.Paques M, Chastang C, Mathis A, et al. Effect of autologous platelet concentrate in surgery for idiopathic macular hole: results of a multicenter, double-masked, randomized trial. Ophthalmology. 1999;106:932–938. doi: 10.1016/s0161-6420(99)00512-6. [DOI] [PubMed] [Google Scholar]
- 11.Mulhern MG, Cullinane A, Cleary PE. Visual and anatomical success with short-term macular tamponade and autologous platelet concentrate. Graefes Arch Clin Exp Ophthalmol. 2000;238:577–583. doi: 10.1007/s004170000154. [DOI] [PubMed] [Google Scholar]
- 12.Kims SG, Chung CH, Kim YK, Park JC, Lim SC. Use of particulate dentin-plaster of Paris combination with/without platelet-rich plasma in the treatment of bone defects around implants. Int J Oral Maxillofac Implants. 2002;17:86–94. [PubMed] [Google Scholar]
- 13.Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:638–646. doi: 10.1016/s1079-2104(98)90029-4. [DOI] [PubMed] [Google Scholar]
- 14.Borzini P, Mazzucco L, Panizza R, et al. Regarding ‘randomized trial and local biological effect of autologous platelets used as adjuvant therapy for chronic venous leg ulcers'. J Vasc Surg. 2004;39:1146–1147. doi: 10.1016/j.jvs.2004.01.047. [DOI] [PubMed] [Google Scholar]
- 15.Ficarelli E, Bernuzzi G, Tognetti E, et al. Treatment of chronic venous leg ulcers by platelet gel. Dermatol Ther. 2008;21(suppl 1):S13–17. doi: 10.1111/j.1529-8019.2008.00196.x. [DOI] [PubMed] [Google Scholar]
- 16.Mazzucco L, Medici D, Serra M, et al. The use of autologous platelet gel to treat difficult to heal wounds: a pilot study. Transfusion. 2004;44:1013–1018. doi: 10.1111/j.1537-2995.2004.03366.x. [DOI] [PubMed] [Google Scholar]
- 17.Saad Setta H, Elshahat A, Elsherbiny K, et al. Platelet-rich plasma versus platelet-poor plasma in the management of chronic diabetic foot ulcers: a comparative study. Int Wound J. 2011;8:307–312. doi: 10.1111/j.1742-481X.2011.00797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Everts PA, Brown Mahoney C, Hoffmann JJ, Schonberger JP, Box HA, van Zundert A, Knape JT. Platelet-rich plasma preparation using three devices: implications for platelet activation and platelet growth factor release. Growth Factors. 2006;24:165–171. doi: 10.1080/08977190600821327. [DOI] [PubMed] [Google Scholar]
- 19.Martineau I, Lacoste E, Gagnon G. Effects of calcium and thrombin on growth factor release from platelet concentrates: kinetics and regulation of endothelial cell proliferation. Biomaterials. 2004;25:4489–4502. doi: 10.1016/j.biomaterials.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 20.Virchenko O, Grenegard M, Aspenberg P. Independent and additive stimulation of tendon repair by thrombin and platelets. Acta Orthop. 2006;77:960–966. doi: 10.1080/17453670610013295. [DOI] [PubMed] [Google Scholar]
- 21.Anitua E, Sanchez M, Zalduendo MM, de la Fuente M, Prado R, Orive G, Andia I. Fibroblastic response to treatment with different preparations rich in growth factors. Cell Prolif. 2009;42:162–170. doi: 10.1111/j.1365-2184.2009.00583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundman EA, Cole BJ, Fortier LA. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am J Sports Med. 2011;39:2135–2140. doi: 10.1177/0363546511417792. [DOI] [PubMed] [Google Scholar]
- 23.Mazzocca AD, McCarthy MB, Chowaniec DM, Cote MP, Romeo AA, Bradley JP, Arciero RA, Beitzel K. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94:308–316. doi: 10.2106/JBJS.K.00430. [DOI] [PubMed] [Google Scholar]
- 24.Zimmermann R, Jakubietz R, Jakubietz M, Strasser E, Schlegel A, Wiltfang J, Eckstein R. Different preparation methods to obtain platelet components as a source of growth factors for local application. Transfusion. 2001;41:1217–1224. doi: 10.1046/j.1537-2995.2001.41101217.x. [DOI] [PubMed] [Google Scholar]
- 25.Bahou WF. Platelet systems biology using integrated genetic and proteomic platforms. Thromb Res. 2012;129(suppl 1):S38–S45. doi: 10.1016/S0049-3848(12)70014-2. [DOI] [PubMed] [Google Scholar]
- 26.Durante C, Agostini F, Abbruzzese L, Toffola RT, Zanolin S, Suine C, Mazzucato M. Growth factor release from platelet concentrates: analytic quantification and characterization for clinical applications. Vox Sang. 2013;105:129–136. doi: 10.1111/vox.12039. [DOI] [PubMed] [Google Scholar]
- 27.Baj-Krzyworzeka M, Majka M, Pratico D, Ratajczak J, Vilaire G, Kijowski J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp Hematol. 2002;30:450–459. doi: 10.1016/s0301-472x(02)00791-9. [DOI] [PubMed] [Google Scholar]
- 28.Barry OP, FitzGerald GA. Mechanisms of cellular activation by platelet microparticles. Thromb Haemost. 1999;82:794–800. [PubMed] [Google Scholar]
- 29.Zimmermann R, Arnold D, Strasser E, Ringwald J, Schlegel A, Wiltfang J, Eckstein R. Sample preparation technique and white cell content influence the detectable levels of growth factors in platelet concentrates. Vox Sang. 2003;85:283–289. doi: 10.1111/j.0042-9007.2003.00361.x. [DOI] [PubMed] [Google Scholar]
- 30.Sekido Y, Morishima Y, Ohya K. Activity of platelet-derived growth factor (PDGF) in platelet concentrates and cryopreserved platelets determined by PDGF bioassay. Vox Sang. 1987;52:27–30. doi: 10.1111/j.1423-0410.1987.tb02984.x. [DOI] [PubMed] [Google Scholar]
- 31.Ledent E, Wasteson A, Berlin G. Growth factor release during preparation and storage of platelet concentrates. Vox Sang. 1995;68:205–209. doi: 10.1111/j.1423-0410.1995.tb02573.x. [DOI] [PubMed] [Google Scholar]
- 32.Wadhwa M, Seghatchian MJ, Lubenko A, Contreras M, Dilger P, Bird C, Thorpe R. Cytokine levels in platelet concentrates: quantitation by bioassays and immunoassays. Br J Haematol. 1996;93:225–234. doi: 10.1046/j.1365-2141.1996.4611002.x. [DOI] [PubMed] [Google Scholar]
- 33.Fujihara M, Ikebuchi K, Wakamoto S, Sekiguchi S. Effects of filtration and gamma radiation on the accumulation of RANTES and transforming growth factor-β1 in apheresis platelet concentrates during storage. Transfusion. 1999;39:498–505. doi: 10.1046/j.1537-2995.1999.39050498.x. [DOI] [PubMed] [Google Scholar]
- 34.Hughes AD, Clunn GF, Refson J, Demoliou-Mason C. Platelet-derived growth factor (PDGF): actions and mechanisms in vascular smooth muscle. Gen Pharmacol. 1996;27:1079–1089. doi: 10.1016/s0306-3623(96)00060-2. [DOI] [PubMed] [Google Scholar]
- 35.Weibrich G, Kleis WK, Hafner G, Hitzler WE. Growth factor levels in platelet-rich plasma and correlations with donor age, sex, and platelet count. J Craniomaxillofac Surg. 2002;30:97–102. doi: 10.1054/jcms.2002.0285. [DOI] [PubMed] [Google Scholar]
- 36.Lozano M, Estebanell E, Cid J, Diaz-Ricart M, Mazzara R, Ordinas A, Escolar G. Platelet concentrates prepared and stored under currently optimal conditions: minor impact on platelet adhesive and cohesive functions after storage. Transfusion. 1999;39:951–959. doi: 10.1046/j.1537-2995.1999.39090951.x. [DOI] [PubMed] [Google Scholar]
- 37.Setiawati EM. Natural growth factor: platelet rich plasma stimulates proliferation of fibroblast cell culture. Indonesian J Trop Infect Dis. 2010;1:102–104. [Google Scholar]
- 38.Krasna M, Domanovic D, Tomsic A, Svajger U, Jeras M. Platelet gel stimulates proliferation of human dermal fibroblasts in vitro. Acta Dermatovenerol Alp Pannonica Adriat. 2007;16:105–110. [PubMed] [Google Scholar]
- 39.Kakudo N, Minakata T, Mitsui T, Kushida S, Notodihardjo FZ, Kusumoto K. Proliferation-promoting effect of platelet-rich plasma on human adipose-derived stem cells and human dermal fibroblasts. Plast Reconstr Surg. 2008;122:1352–1360. doi: 10.1097/PRS.0b013e3181882046. [DOI] [PubMed] [Google Scholar]
- 40.Valeri CR, Saleem B, Ragno G. Release of platelet-derived growth factors and proliferation of fibroblasts in the releasates from platelets stored in the liquid state at 22°C after stimulation with agonists. Transfusion. 2006;46:225–229. doi: 10.1111/j.1537-2995.2006.00705.x. [DOI] [PubMed] [Google Scholar]