Abstract
X chromosome-linked severe combined immunodeficiency disease (SCIDX1) is characterized by the absence of T-cell and natural killer cell development and results from molecular mutations of the interleukin 2 receptor (IL-2R) gamma chain. The IL-2R gamma chain is a common component of the IL-2, IL-4, and IL-7 receptor systems, which may explain the severe immunophenotype in SCIDX1. We have previously described an atypical SCIDX1 syndrome demonstrating poorly functioning peripheral T cells, which we hypothesized to represent a variant allele at the SCIDX1 locus. We now demonstrate that a splice site mutation in the IL-2R gamma gene is responsible for this atypical SCIDX1. Aberrant RNA splicing resulted in the generation of two IL-2R gamma transcripts: an abundant, nonfunctional isoform containing a small intronic insertion and a second functional isoform with a single amino acid substitution present in limited amounts. Radiolabeled IL-2 binding studies revealed a 5-fold decreased level of expression of functional high-affinity IL-2Rs, which correlated with the quantity of full-length IL-2R gamma transcripts. Further analysis of the T-cell antigen receptor beta-chain repertoire of the patient's T cells demonstrated oligoclonality in multiple V beta families, thus strongly suggesting that the defect in the IL-2R gamma chain generated a limited number of peripheral T-cell clones. This atypical SCIDX1 patient demonstrates that certain IL-2R gamma chain abnormalities can also result in partial immunodeficiency phenotypes, potentially through differential effects on the IL-2, IL-4, or IL-7 receptor systems.
Full text
PDF
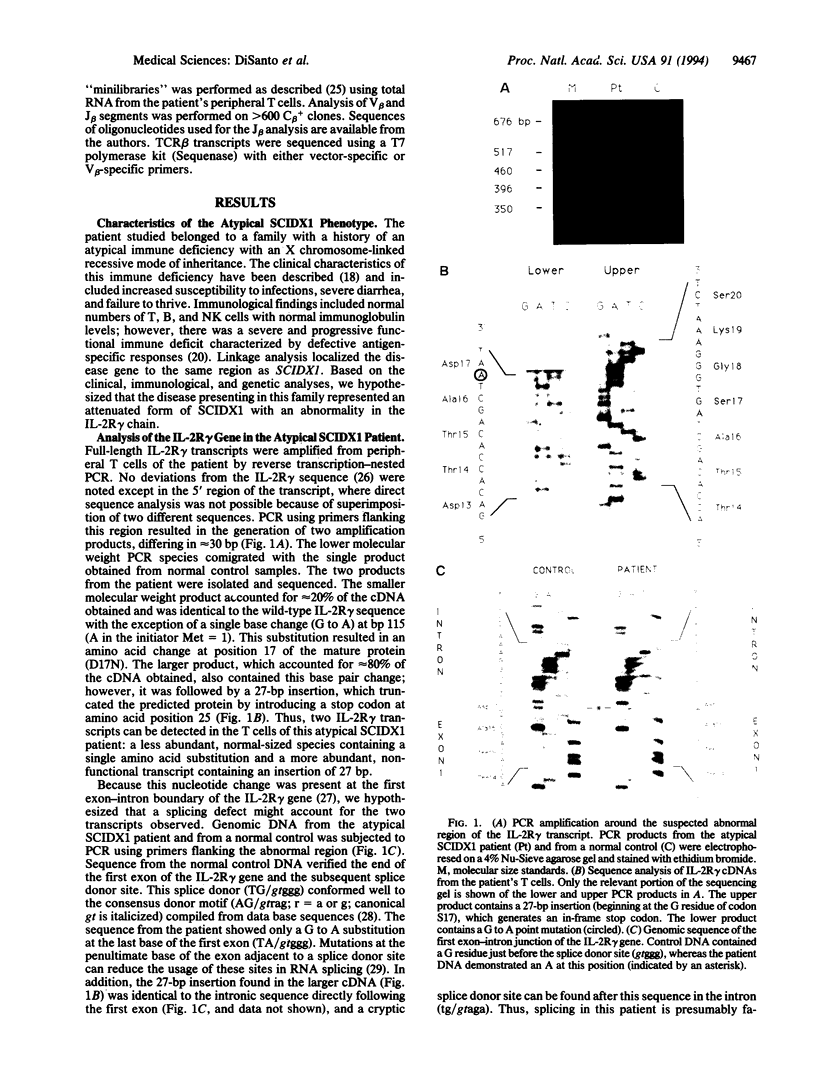
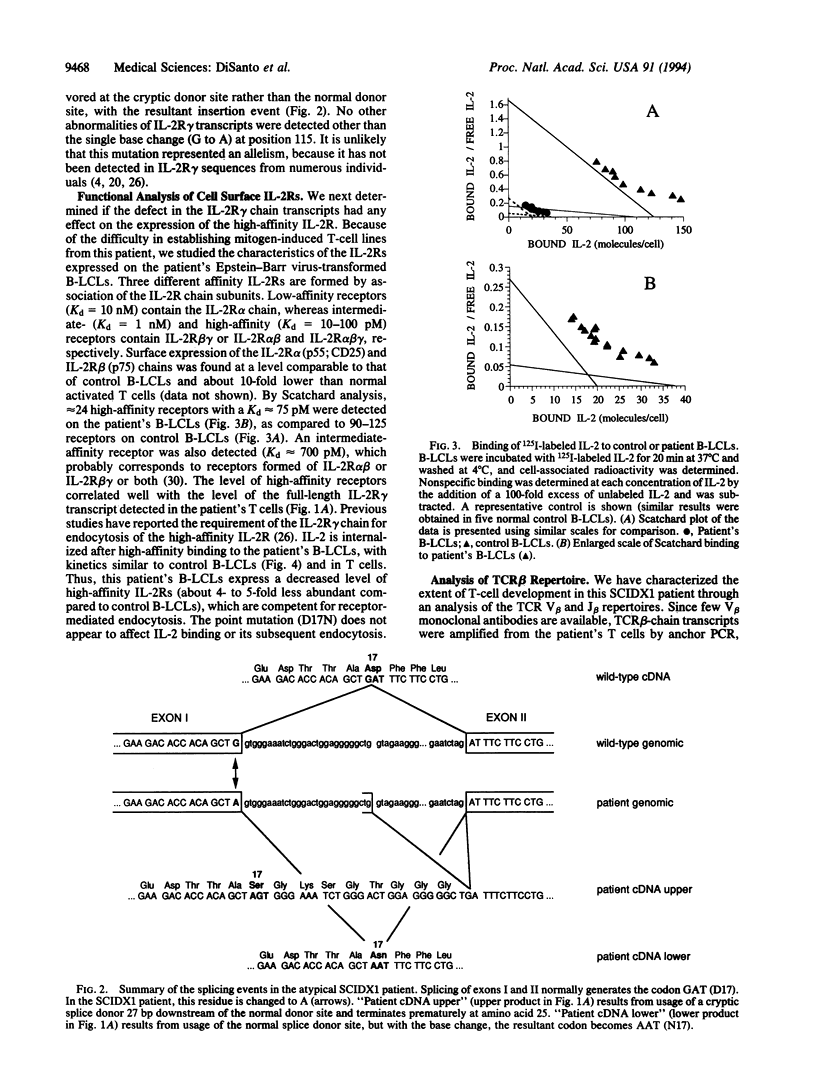
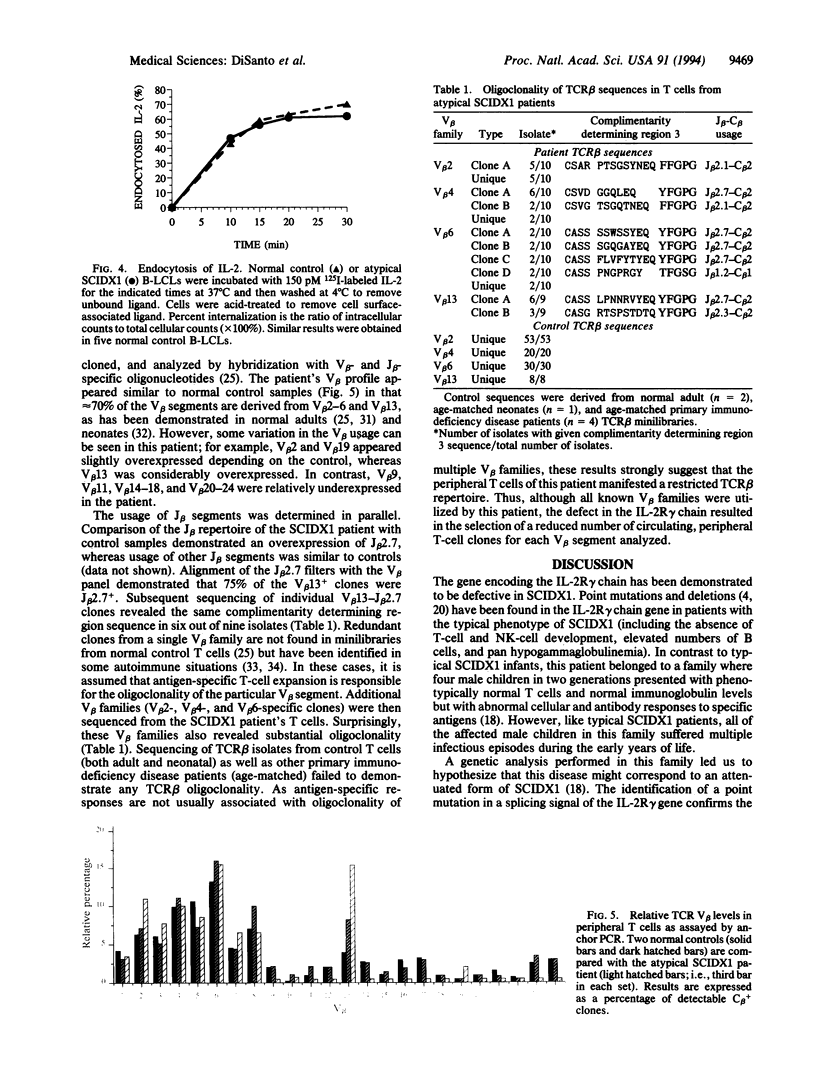

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Hornig H., Padgett R. A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986 Nov 21;47(4):555–565. doi: 10.1016/0092-8674(86)90620-3. [DOI] [PubMed] [Google Scholar]
- Asao H., Takeshita T., Ishii N., Kumaki S., Nakamura M., Sugamura K. Reconstitution of functional interleukin 2 receptor complexes on fibroblastoid cells: involvement of the cytoplasmic domain of the gamma chain in two distinct signaling pathways. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4127–4131. doi: 10.1073/pnas.90.9.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahadoran P., Rieux-Laucat F., Le Deist F., Blanche S., Fischer A., de Villartay J. P. Lack of selective V beta deletion in peripheral CD4+ T cells of human immunodeficiency virus-infected infants. Eur J Immunol. 1993 Aug;23(8):2041–2044. doi: 10.1002/eji.1830230850. [DOI] [PubMed] [Google Scholar]
- DiSanto J. P., Dautry-Varsat A., Certain S., Fischer A., de Saint Basile G. Interleukin-2 (IL-2) receptor gamma chain mutations in X-linked severe combined immunodeficiency disease result in the loss of high-affinity IL-2 receptor binding. Eur J Immunol. 1994 Feb;24(2):475–479. doi: 10.1002/eji.1830240232. [DOI] [PubMed] [Google Scholar]
- DiSanto J. P., Keever C. A., Small T. N., Nicols G. L., O'Reilly R. J., Flomenberg N. Absence of interleukin 2 production in a severe combined immunodeficiency disease syndrome with T cells. J Exp Med. 1990 May 1;171(5):1697–1704. doi: 10.1084/jem.171.5.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duprez V., Cornet V., Dautry-Varsat A. Down-regulation of high affinity interleukin 2 receptors in a human tumor T cell line. Interleukin 2 increases the rate of surface receptor decay. J Biol Chem. 1988 Sep 15;263(26):12860–12865. [PubMed] [Google Scholar]
- Duprez V., Dautry-Varsat A. Receptor-mediated endocytosis of interleukin 2 in a human tumor T cell line. Degradation of interleukin 2 and evidence for the absence of recycling of interleukin receptors. J Biol Chem. 1986 Nov 25;261(33):15450–15454. [PubMed] [Google Scholar]
- Grabstein K. H., Waldschmidt T. J., Finkelman F. D., Hess B. W., Alpert A. R., Boiani N. E., Namen A. E., Morrissey P. J. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med. 1993 Jul 1;178(1):257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson E. J., Kingston R., Owen J. J. Importance of IL-2 receptors in intra-thymic generation of cells expressing T-cell receptors. Nature. 1987 Sep 10;329(6135):160–162. doi: 10.1038/329160a0. [DOI] [PubMed] [Google Scholar]
- Kondo M., Takeshita T., Higuchi M., Nakamura M., Sudo T., Nishikawa S., Sugamura K. Functional participation of the IL-2 receptor gamma chain in IL-7 receptor complexes. Science. 1994 Mar 11;263(5152):1453–1454. doi: 10.1126/science.8128231. [DOI] [PubMed] [Google Scholar]
- Kondo M., Takeshita T., Ishii N., Nakamura M., Watanabe S., Arai K., Sugamura K. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science. 1993 Dec 17;262(5141):1874–1877. doi: 10.1126/science.8266076. [DOI] [PubMed] [Google Scholar]
- Kuijpers K. C., van Dongen J. J., van der Burg P., Roos M. T., Vonk J., de Abreu R., de Korte D., van Noesel C. J., Weening R. S., van Lier R. A. A combined immunodeficiency with oligoclonal CD8+, V beta 3-expressing, cytotoxic T lymphocytes in the peripheral blood. J Immunol. 1992 Nov 15;149(10):3403–3410. [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Uchiyama T., Smith K. A., Waldmann T. A., Greene W. C. A monoclonal antibody that appears to recognize the receptor for human T-cell growth factor; partial characterization of the receptor. Nature. 1982 Nov 18;300(5889):267–269. doi: 10.1038/300267a0. [DOI] [PubMed] [Google Scholar]
- Lowenthal J. W., Howe R. C., Ceredig R., MacDonald H. R. Functional status of interleukin 2 receptors expressed by immature (Lyt-2-/L3T4-) thymocytes. J Immunol. 1986 Oct 15;137(8):2579–2584. [PubMed] [Google Scholar]
- Minami Y., Kono T., Miyazaki T., Taniguchi T. The IL-2 receptor complex: its structure, function, and target genes. Annu Rev Immunol. 1993;11:245–268. doi: 10.1146/annurev.iy.11.040193.001333. [DOI] [PubMed] [Google Scholar]
- Noguchi M., Adelstein S., Cao X., Leonard W. J. Characterization of the human interleukin-2 receptor gamma chain gene. J Biol Chem. 1993 Jun 25;268(18):13601–13608. [PubMed] [Google Scholar]
- Noguchi M., Nakamura Y., Russell S. M., Ziegler S. F., Tsang M., Cao X., Leonard W. J. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science. 1993 Dec 17;262(5141):1877–1880. doi: 10.1126/science.8266077. [DOI] [PubMed] [Google Scholar]
- Noguchi M., Yi H., Rosenblatt H. M., Filipovich A. H., Adelstein S., Modi W. S., McBride O. W., Leonard W. J. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993 Apr 9;73(1):147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- Puck J. M., Nussbaum R. L., Conley M. E. Carrier detection in X-linked severe combined immunodeficiency based on patterns of X chromosome inactivation. J Clin Invest. 1987 May;79(5):1395–1400. doi: 10.1172/JCI112967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raulet D. H. Expression and function of interleukin-2 receptors on immature thymocytes. Nature. 1985 Mar 7;314(6006):101–103. doi: 10.1038/314101a0. [DOI] [PubMed] [Google Scholar]
- Rieux-Laucat F., Le Deist F., Selz F., Fischer A., de Villartay J. P. Normal T cell receptor V beta usage in a primary immunodeficiency associated with HLA class II deficiency. Eur J Immunol. 1993 Apr;23(4):928–934. doi: 10.1002/eji.1830230425. [DOI] [PubMed] [Google Scholar]
- Robb R. J., Munck A., Smith K. A. T cell growth factor receptors. Quantitation, specificity, and biological relevance. J Exp Med. 1981 Nov 1;154(5):1455–1474. doi: 10.1084/jem.154.5.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg W. M., Moss P. A., Bell J. I. Variation in human T cell receptor V beta and J beta repertoire: analysis using anchor polymerase chain reaction. Eur J Immunol. 1992 Feb;22(2):541–549. doi: 10.1002/eji.1830220237. [DOI] [PubMed] [Google Scholar]
- Russell S. M., Keegan A. D., Harada N., Nakamura Y., Noguchi M., Leland P., Friedmann M. C., Miyajima A., Puri R. K., Paul W. E. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science. 1993 Dec 17;262(5141):1880–1883. doi: 10.1126/science.8266078. [DOI] [PubMed] [Google Scholar]
- Sadlack B., Kühn R., Schorle H., Rajewsky K., Müller W., Horak I. Development and proliferation of lymphocytes in mice deficient for both interleukins-2 and -4. Eur J Immunol. 1994 Jan;24(1):281–284. doi: 10.1002/eji.1830240144. [DOI] [PubMed] [Google Scholar]
- Schorle H., Holtschke T., Hünig T., Schimpl A., Horak I. Development and function of T cells in mice rendered interleukin-2 deficient by gene targeting. Nature. 1991 Aug 15;352(6336):621–624. doi: 10.1038/352621a0. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshita T., Asao H., Ohtani K., Ishii N., Kumaki S., Tanaka N., Munakata H., Nakamura M., Sugamura K. Cloning of the gamma chain of the human IL-2 receptor. Science. 1992 Jul 17;257(5068):379–382. doi: 10.1126/science.1631559. [DOI] [PubMed] [Google Scholar]
- Weinberg K., Parkman R. Severe combined immunodeficiency due to a specific defect in the production of interleukin-2. N Engl J Med. 1990 Jun 14;322(24):1718–1723. doi: 10.1056/NEJM199006143222406. [DOI] [PubMed] [Google Scholar]
- Wengler G. S., Allen R. C., Parolini O., Smith H., Conley M. E. Nonrandom X chromosome inactivation in natural killer cells from obligate carriers of X-linked severe combined immunodeficiency. J Immunol. 1993 Jan 15;150(2):700–704. [PubMed] [Google Scholar]
- de Saint Basile G., Arveiler B., Oberlé I., Malcolm S., Levinsky R. J., Lau Y. L., Hofker M., Debre M., Fischer A., Griscelli C. Close linkage of the locus for X chromosome-linked severe combined immunodeficiency to polymorphic DNA markers in Xq11-q13. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7576–7579. doi: 10.1073/pnas.84.21.7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint-Basile G., Le Deist F., Caniglia M., Lebranchu Y., Griscelli C., Fischer A. Genetic study of a new X-linked recessive immunodeficiency syndrome. J Clin Invest. 1992 Mar;89(3):861–866. doi: 10.1172/JCI115665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Saint-Basile G., Le Deist F., de Villartay J. P., Cerf-Bensussan N., Journet O., Brousse N., Griscelli C., Fischer A. Restricted heterogeneity of T lymphocytes in combined immunodeficiency with hypereosinophilia (Omenn's syndrome). J Clin Invest. 1991 Apr;87(4):1352–1359. doi: 10.1172/JCI115139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Boehmer H., Crisanti A., Kisielow P., Haas W. Absence of growth by most receptor-expressing fetal thymocytes in the presence of interleukin-2. Nature. 1985 Apr 11;314(6011):539–540. doi: 10.1038/314539a0. [DOI] [PubMed] [Google Scholar]