Abstract
Purpose
Retinal ganglion cell (RGC) death is the final event leading to visual impairment in glaucoma; therefore, identification of neuroprotective strategies able to slow down or prevent the process is one of the main challenges for glaucoma research. The purpose of this study was to evaluate the neuroprotective potential of RGC death induced by the in vivo transient increase in intraocular pressure (IOP) of a combined treatment with forskolin, homotaurine, and L-carnosine. Forskolin (7beta-acetoxy-8, 13-epoxy-1a, 6β, 9a-trihydroxy-labd-14-en-11-one) is an activator of adenylate cyclase that decreases IOP by reducing aqueous humor production and functions as a neuroprotector due to its neurotrophin-stimulating activity. Homotaurine is a natural aminosulfonate compound endowed with neuromodulatory effects, while the dipeptide L-carnosine is known for its antioxidant properties.
Methods
Retinal ischemia was induced in the right eye of adult male Wistar rats by acutely increasing the IOP. Forskolin, homotaurine, and L-carnosine were intravitreally injected and RGC survival evaluated following retrograde labeling with FluoroGold. Total and phosphorylated Akt and glycogen synthase kinase-3β (GSK-3β) protein levels, as well as calpain activity, were analyzed with western blot. Protein kinase A (PKA) was inhibited by intravitreal injection of H89.
Results
A synergic neuroprotective effect on RGC survival was observed following the combined treatment with forskolin, homotaurine, and L-carnosine compared to forskolin alone. The observed neuroprotection was associated with reduced calpain activity, upregulation of phosphoinositide 3-kinase (PI3K)/Akt pathway, and inhibition of GSK-3β but was independent from PKA activation and distinct from the hypotensive effects of forskolin.
Conclusions
A multidrug/multitarget approach, by interfering with several pathways involved in RGC degeneration, may be promising to achieve glaucoma neuroprotection.
Introduction
Glaucoma is one of the major ocular neurodegenerative diseases leading to loss of visual function and impaired quality of life [1]. Elevated intraocular pressure (IOP) is considered the main risk factor, and although it is no longer used to diagnose the disease, IOP is still the only target for glaucoma therapy [2]. Pharmacological therapies aiming at lowering IOP, including drugs that increase aqueous humor outflow or suppress aqueous humor production, are currently available [3]. However, lowering IOP does not always prevent the progression of the disease. Optic atrophy can occur in the presence of IOP values that fall within the normal range (i.e., normal tension glaucoma), and clinical studies have documented that even when IOP is pharmacologically controlled, optic nerve damage can still progress in a significant number of patients [4,5]. Apoptotic retinal ganglion cell (RGC) death is the final event leading to visual loss in glaucoma [6], and therefore, strategies aimed at preventing or attenuating RGC degeneration might fulfill the need for a better glaucoma treatment.
Efforts have been made to identify drugs endowed with neuroprotective effects and able to preserve visual functioning. However, the recent unexpected failure of a clinical trial on patients with glaucoma testing the efficacy of memantine, an uncompetitive N-methyl D-aspartate (NMDA) receptor antagonist prescribed in Alzheimer disease, raised several doubts regarding the strategies to achieve neuroprotection in glaucoma [7]. It is conceivable that a single drug that hits one target might have limited efficacy in preventing the progression of a disease that has a multifactorial pathogenesis [8]. In fact, RGC death occurs through a complex series of pathological events and involves several pathways. Changes in neurotrophin signaling, oxidative stress, excitotoxicity, mitochondrial dysfunction, protein misfolding, hypoxic and ischemic phenomena, and autoimmunity, have all been identified as contributing factors to glaucoma-associated RGC death [9]. Therefore, the use of a combination of drugs acting simultaneously on different mechanisms may offer a more powerful tool for preventing RGC degeneration.
The diterpenoid forskolin (7beta-acetoxy-8, 13-epoxy-1α, 6β, 9α-trihydroxy-labd-14-en-11-one) is an adenylate cyclase activator [10] that has been shown to decrease IOP by reducing aqueous humor production in animals [11-14] and humans [15-17] suggesting potential use for glaucoma treatment. Evidence has also been reported suggesting that forskolin promotes neuronal survival by stimulating neurotrophin activity in models of RGC death [18,19].
L-carnosine, a dipeptide composed of β-alanine and L-histidine, exerts several biologic effects including antioxidant action, pH buffering, and heavy metal chelating activities [20-22]. The neuroprotective effects of L-carnosine have been shown in cerebellar granule neurons exposed to β-amyloid [23] and in animal models of brain ischemia [24]. Furthermore, the recently published results of two clinical trials reported positive effects of L-carnosine treatment in patients with chronic discirculatory encephalopathy and Parkinson disease [25,26].
Homotaurine (3-amino-1-propane sulfonic acid, tramiprosate) is a natural aminosulfonate compound endowed with neuromodulatory effects. A recent study reported its neuroprotective effect following ischemic stroke in rats [27] and the post-hoc analysis of a failed phase III clinical trial with tramiprosate demonstrated significant positive effects on secondary endpoints in patients with Alzheimer’s [28]. Here, using a well-established animal model of acute angle-closure glaucoma, we tested the effect of the combination of forskolin, L-carnosine, and homotaurine on RGC survival and provide evidence on their synergic neuroprotective effect.
Methods
Animals
Male Wistar rats (280–330 g) were purchased from Charles River (Lecco, Italy) and housed with a 12 h:12 h light-dark cycle with ad libitum access to food and water. Animal care and experimental procedures were performed in accordance with the guidelines of the Italian Ministry of Health for Animal Care (DM 116/1992) and the ARVO Statement for the Use of Animals in Ophtalmic and Vision Research. The protocol was approved by the Italian Minister of Health (Protocol Number 110000351). All surgical procedures were performed under deep anesthesia, and all efforts were made to minimize suffering.
Retinal ischemia
Retinal ischemia was induced in adult rats with a transient increase in IOP according to the method previously reported [29]. Animals were deeply anesthetized with intraperitoneal (i.p.) injection of chloral hydrate (400 mg/kg) and laid on a heating pad to maintain the body temperature at 37 °C. Topical anesthesia was induced by 0.4% oxibuprocaine eye drops (Novesina, Novatis, Italy). A 27-gauge infusion needle, connected to a 500 ml bottle of sterile saline, was inserted in the anterior chamber of the right eye, and the saline container was elevated to rise IOP above 80 mmHg for 50 min. Retinal ischemia was confirmed by whitening of the fundus. For each animal, the left eye was used as the non-ischemic control.
Body temperature was monitored before and after ischemia, and animals with values lower than 35.5 °C were excluded. Animals were sacrificed by cervical dislocation at 1 h or 7 days following the 50 min of ischemia. Retinas were quickly dissected, snap frozen in liquid nitrogen, and stored at −80 °C until use.
Intraocular pressure recording
IOP was monitored at the beginning and at the end of the ischemia, and following 1 h reperfusion using a tonometer (Icare Lab/Tonolab, Italy). Corneal analgesia was achieved using topical oxibuprocaine 0.4% drops (Novesina, Novartis Farma, Italy), the eyelids were gently retracted, and a tonometer probe was pointed on the central cornea. Each recorded value was the average of three repeated measurements.
Intravitreal administration
Forskolin (Sigma-Aldrich, Milan, Italy) was dissolved in sterile dimethyl sulfoxide (DMSO) at the concentration of 10 mM. A stock solution of homotaurine and L-carnosine (Truffini e Reggè Farmaceutici S.r.l., Milan, Italy) were prepared in sterile water at 1 mM and 100 mM concentrations, respectively. Stock solution (10 mM) of the PKA inhibitor H89 (Sigma-Aldrich) was prepared in sterile DMSO. Stocks were subsequently diluted to the final concentration using sterile PBS (1X; 10 mM NA2HPO4, 2 mM KH2PO4, 2.7 mM KCl and 137 mM NaCl, pH 7.4).
Intravitreal injection was performed by puncturing the eye with a 23-gauge needle at the cornea-sclera junction, and the drugs administered with a 5 µl Hamilton syringe. Administration of forskolin (0.6–6 nmol/eye, corresponding at about 10–100 μM as the final concentration in the vitreous), homotaurine (0.059 μmol/eye), L-carnosine (0.036 μmol/eye) alone or in combination, or control solution (2.5–5% DMSO), was performed 1 h before and following the 50 min of ischemia. Where indicated, a single intravitreal injection of H89 (2 mM; 3 μl/eye; corresponding to about 100 μM as the final concentration in the vitreous) was performed 75 min before the ischemia was induced.
The duration of the injection (3 μl/eye) was 3 min in all instances. At the indicated time points, animals were sacrificed, and subjects with visible lens damage or vitreous hemorrhage were excluded from the analysis.
Retrograde labeling of RGCs
To evaluate cell loss, RGCs were retrogradely labeled by stereotaxically injecting the fluorescent tracer FluoroGold (Fluka, Sigma-Aldrich) into the superior colliculus. The tracer is taken up by the axon terminals of the RGCs in the superior colliculus and transported retrogradely to the soma in the retina [30]. Briefly, 4 days after the ischemic insult, the rats were anaesthetized and immobilized in a stereotaxic device (Kopf 900, Analytical Control, Milan, Italy); positions of superior colliculi were identified with the Paxinos and Watson atlas (1998). The skull was exposed, and 2 µl of 5% Fluoro-Gold solution (Fluka, Sigma-Aldrich) was injected on both sides of the skull 6 mm posterior to the bregma, 1.2 mm lateral to the sagittal suture, and 4 mm deep from the bone surface using a Hamilton syringe with a 33 gauge needle (Reno, NV). The skin was then sutured, and a 0.3% tobramycin ointment was applied (Alcon, Milan, Italy). Seven days after ischemia (3 days following the FluoroGold injection), the animals were killed and eyeballs enucleated and fixed for 30 min in paraformaldehyde 4% (PFA). The anterior segment of the eye was removed and the posterior eye cup additionally fixed for 1 h. The isolated retinas were divided into four quadrants (nasal, temporal, upper, and lower) and mounted on the slide using Vectashield medium (Vector Laboratories, DBA, Milan, Italy). Twenty images per retina (two from the peripheral, two from the middle, and one from the central retina for each quadrant) were acquired using a deconvolution microscope (Leica Microsystems CMS EL6000, GBH, Mannheim, Germany) at 40X magnification (size of the field: 320.4×239.3 μm) and subjected to cell count by a blind investigator. The total number of labeled cells in the ischemic eye was compared with that of the contralateral eye and expressed as a percentage of RGC loss.
Immunoblot analysis
Retinas were lysed in ice-cold radioimmunoprecipitation assay (RIPA) buffer (50 mM Tris-HCl (pH 8), 150 mM NaCl, 1 mM EDTA, 0.1% sodium dodecylsulfate (SDS), 1% IGEPAL, 0.5% sodium deoxicholate) containing protease inhibitor cocktail (cod. P8349; Sigma-Aldrich). For the analysis of the phosphorylated proteins, the retinas were homogenized in 130 µl of ice-cold lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 2 mM EDTA, 2 mM EGTA, 1% Triton, 1 nM okadaic acid, protease (cod. P8349, Sigma-Aldrich), and phosphatase inhibitor cocktail (cod. 524,625, Calbiochem, La Jolla, CA). Lysates were centrifuged for 15 min at 10,000 ×g at 4 °C and supernatants assayed for protein content with the Bio-Rad DC Protein Assay Kit (Bio-Rad Laboratories, Milan, Italy).
Equal amounts of the total proteins were separated with SDS–polyacrylamide gel electrophoresis (SDS–PAGE), transferred onto polyvinylidene fluoride (PVDF) membranes (Immobilon-P, Sigma-Aldrich), and blocked with 5% non-fat milk in Tris-buffered saline containing 0.05% Tween-20 for 1 h at room temperature. Primary antibodies were incubated overnight at 4 °C followed by a horseradish peroxidase conjugated secondary antibody for 1 h at room temperature. Protein bands were visualized with the enhanced chemiluminescence (ECL) Western Blotting Detection kit (ECL, Amersham Biosciences, GE Healthcare, Milan, Italy) and the chemiluminescence signal detected using X-ray films (Hyperfilm ECL, Amersham Bioscience). Autoradiographic films were scanned, digitalized at 300 dpi, and band quantification was performed using ImageJ software (NIH, Bethesda, MD).
The following primary antibodies and dilutions were used: anti-Akt 1:2,000 dilution rabbit polyclonal (Cell Signaling Technology, Beverly, MA); anti-phospho-Akt (Ser473) 1:1,000 dilution rabbit polyclonal antibody (Cell Signaling Technology); anti-GSK-3β 1:2,000 dilution (Cell Signaling Technology) rabbit polyclonal; anti-phospho-GSK-3β (Ser9) 1:1,000 dilution (Cell Signaling Technology); anti-spectrin (non-erythroid) monoclonal antibody (MAB 1622) 1:3,000 (Chemicon International Inc., Temecula, CA); anti-actin 1:1,000 (clone AC-40, Sigma-Aldrich); anti-β-tubulin 1: 20,000 (clone B-5–1-2, Sigma-Aldrich); anti-GAPDH 1:30,000 (Applied Biosystem, Carlsbad, CA). Species-specific horseradish peroxidase conjugated goat immunoglobulin G (IgG; Pierce Biotechnology, Rockford, IL) were used as secondary antibodies.
Statistical analysis
Data are expressed as the mean ± SEM of three to six independent experiments and evaluated statistically for difference by ANOVA followed by the Tukey-Kramer test for multiple comparisons. Where indicated, the Student t test was used to evaluate differences between two means. A value of p less than 0.05 was considered to be significant.
Results
Forskolin prevents RGC loss induced by ischemia/reperfusion
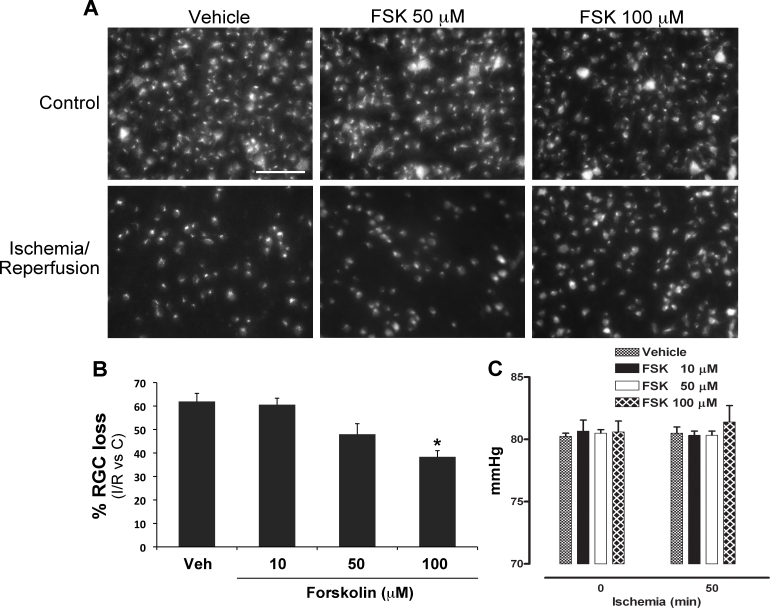
Previous work from our and other groups showed a significant decrease in RGC survival following retinal ischemia induced by a transient IOP increase [31]. In agreement with those results, a significant loss of FluoroGold (FG)-labeled RGCs was observed, 7 days following the insult, in the retina undergone ischemia/reperfusion compared to the contralateral, non-ischemic retina (Figure 1A-B). To evaluate the effect of forskolin on RGC survival, a double intravitreal injection (10–100 μM; 1 h before starting the ischemia and at the end of the 50 min of ischemia) was performed. Significant neuroprotection was reported following treatment with 100 μM forskolin compared to the vehicle-treated retina. A trend toward a decrease in RGC loss was evident in 50 μM forskolin treated samples, whereas a lower concentration (10 μM) was ineffective (Figure 1A–B).
Figure 1.
Intravitreal treatment with forskolin reduces RGC death induced by retinal ischemia. A: Representative fluorescent photomicrograph of whole-mount retinas showing the effect of forskolin intravitreal treatment (50–100 μM) on retinal ganglion cell (RGC) survival at 7 days following retinal ischemia. Reduced RGC loss was evident in the retina treated with 100 μM FSK compared to vehicle-treated samples. Images are representative of four independent experiments. Scale bar=75 μm. B: Histogram showing the dose-dependent reduction of RGC death in forskolin-treated retinas (10–100 μM) compared to the vehicle-treated samples. Significant neuroprotection was reported following treatment with the highest dose tested (100 μM). Twenty images per retina were acquired, and FluoroGold (FG)-labeled cells were counted. The total number of labeled cells in the ischemic eye was compared with the contralateral eye and expressed as a percentage of RGC loss. Results are reported as mean ± SEM of four independent experiments (*p<0.05 versus vehicle-treated ischemic retinas; ANOVA followed by Tukey-Kramer multiple comparisons test). C: Histogram showing the intraocular pressure (IOP) values recorded right after the increase in IOP and before the procedure was ended. No significant changes in IOP values were reported in the forskolin-treated eyes compared to the vehicle-treated eyes. Results are reported as mean ± SEM of four independent experiments (FSK: forskolin; C: control non-ischemic retina; I/R: ischemic retina).
It has been previously shown that forskolin is able to decrease aqueous humor production and therefore reduce IOP in rabbits, monkeys, and humans [11]. To exclude that the observed neuroprotection was due to the hypotensive effect of forskolin, IOP was recorded during the procedure. No significant changes in IOP values were reported right after and before the end of IOP rise in the forskolin-treated eyes compared to vehicle treated (Figure 1C); similarly, no differences were reported following 1 h reperfusion (vehicle: 12.16±0.44 mmHg versus 100 μM forskolin: 11.83±0.16 mmHg; n=3 per group), thus suggesting that the effects on RGC survival were IOP-independent.
Homotaurine and L-carnosine potentiate forskolin neuroprotection in experimental glaucoma
To evaluate if the combination with homotaurine and L-carnosine was able to improve the neuroprotective effect observed following forskolin treatment, the three compounds were administered together. The doses of homotaurine and L-carnosine to be used in combination with the effective concentration of forskolin (100 μM) were extrapolated from the ratio between the components present in a food supplement commercially available in Italy and indicated as a dietary supplement to support retinal health.
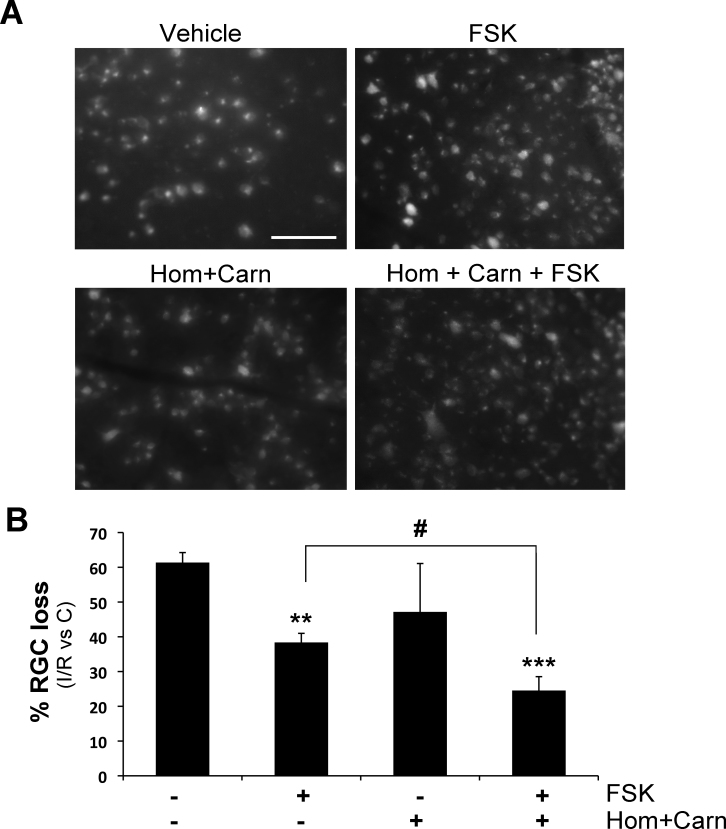
At the chosen doses, the intravitreal administration of homotaurine (0.059 μmol/eye) combined with L-carnosine (0.036 μmol/eye) did not afford neuroprotection following retinal ischemia. However, when the two compounds were administered in combination with forskolin the percentage of RGC loss was significantly reduced compared to forskolin alone (Figure 2).
Figure 2.
Intravitreal application of homotaurine and L-carnosine potentiates the neuroprotection afforded by forskolin in retinas subjected to ischemia/reperfusion. Intravitreal treatment with homotaurine (0.059 μmol/eye) and L-carnosine (0.036 μmol/eye) did not afford neuroprotection following retinal ischemia; administration of the two compounds in combination with forskolin (100 μM) significantly potentiated the survival of retinal ganglion cells (RGCs; evaluated 7 days following retinal ischemia) compared to forskolin alone. A: Representative photomicrographs of FluoroGold (FG)-labeled RGCs in whole-mount retinas are shown. Scale bar=75 μm. B: Histogram shows the results of the FG-labeled RGC count expressed as mean ± SEM of four independent experiments (**p<0.01, ***p<0.001 versus vehicle-treated ischemic retinas; #p<0.05; ANOVA followed by Tukey-Kramer multiple comparisons test). (FSK: forskolin; Hom: homotaurine; Carn: L-carnosine; C: control non-ischemic retina; I/R: ischemic retina).
Forskolin/homotaurine/L-carnosine association reduces calpain activation following ischemia/reperfusion
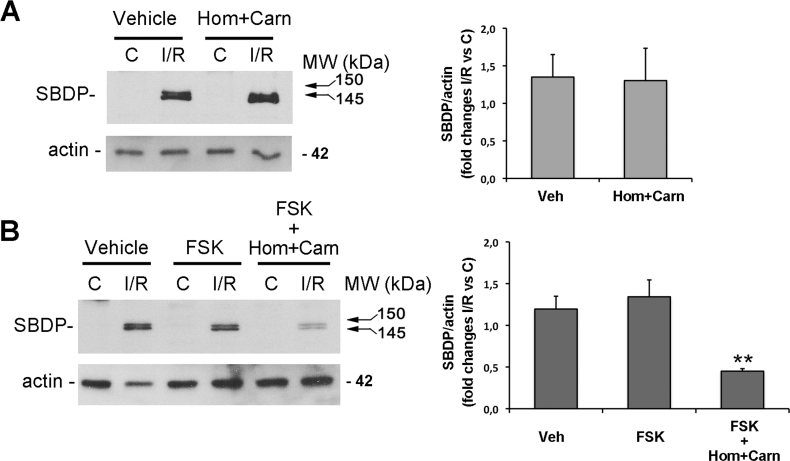
Excitotoxicity significantly contributes to RGC death following retinal ischemia/reperfusion [32]. The calcium overload, consequent to NMDA glutamate receptors overstimulation, leads to the activation of calcium-dependent cysteine proteases, calpains, an event mainly related to the necrotic death occurring during the early stages of reperfusion [33,34]. We investigated calpain activation by monitoring the accumulation of 150/145 kDa α-spectrin breakdown products (SBDPs) generated by calpains following 1 h reperfusion [33,35].
Neither treatment with homotaurine/L-carnosine association, nor with forskolin was able to reduce the buildup of the typical 150/145 kDa doublet observed after 1 h reperfusion (Figure 3). However, the association between forskolin, homotaurine, and L-carnosine strongly reduced the accumulation of 150/145 kDa SBDPs, supporting the previously observed potentiated neuroprotective effects of the combined drugs.
Figure 3.
Treatment with forskolin/homotaurine/L-carnosine prevents calpain activation following 1 h reperfusion. Immunoblotting shows the absence of effect of intravitreal treatment with (A) homotaurine and L-carnosine (Hom+Carn) or (B) forskolin alone on the typical increase of calpain-specific 150/145 kDa alfa-spectrin break down products (SBDPs) observed in the ischemic retina after 1 h of reperfusion. In contrast, intravitreal treatment with forskolin/homotaurine/L-carnosine association (B) significantly reduced calpain activation induced by retina ischemia/reperfusion after 1 h of reperfusion as shown by the reduced intensity of the 150/145 kDa SBDP bands. A representative immunoblot from three independent experiments is shown. Histograms show the results (expressed as mean ± SEM of three experiments) of the densitometric analysis of the autoradiographic bands relative to 150/145 SBDPs normalized to the value of actin and compared to the contralateral eye. (**p<0.01; ANOVA followed by Tukey-Kramer multiple comparisons test; C: control non-ischemic retina; I/R: ischemic retina; Hom: homotaurine; Carn: L-carnosine; FSK: forskolin; MW: molecular weight).
Intravitreal treatment with forskolin/homotaurine/L-carnosine increases Akt activation and GSK-3β phosphorylation in the retina subjected to ischemia/reperfusion
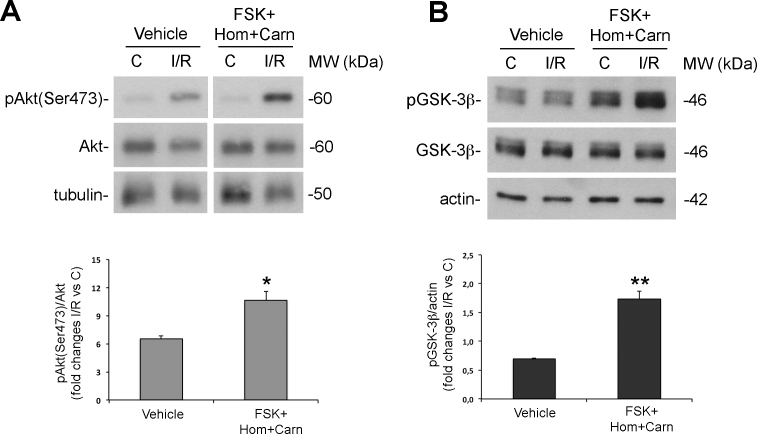
Akt (also known as protein kinase B) is a downstream component of the phosphoinositide 3-kinase (PI-3K) signaling that exerts prosurvival and antiapoptotic effects. We have previously reported activation of the PI3K/Akt pathway in RGCs in response to retinal ischemia [29]. In particular, we have shown an upregulation of the phosphorylated active form of Akt (pAkt) following 1 h reperfusion. Here, to elucidate the mechanisms underlying the neuroprotection afforded by the proposed association of drugs, we tested its effect on Akt activation by checking on the levels of the phosphorylated form.
As shown in Figure 4A, treatment with forskolin/homotaurine/L-carnosine significantly increases pAkt levels compared with either contralateral or vehicle-treated retinas (Figure 4A).
Figure 4.
Intravitreal treatment with forskolin/homotaurine/L-carnosine increases Akt activation and glycogen synthase kinase-3β (GSK-3β) phosphorylation in the retina subjected to ischemia/reperfusion. Intravitreal treatment with the tested association of drugs (100 μM forskolin + 0.058 μM/eye homotaurine + 0.039 μM/eye L-carnosine) significantly enhanced Akt phosphorylation on Ser473 typically reported after 1 h of reperfusion (A) and induced GSK-3β phosphorylation on Ser9 (B). Histograms show the results of densitometric analysis normalized on the loading control from five independent experiments (mean ± SEM; *p<0.05, **p<0.01 versus vehicle; Student’s test; C: control non-ischemic retina; I/R: ischemic retina; Hom: homotaurine; Carn: L-carnosine; FSK: forskolin; MW: molecular weight).
Glycogen synthase kinase-3β (GSK-3β) is a downstream substrate of Akt [36,37]. Retinas treated with forskolin/homotaurine/L-carnosine solution and subjected to ischemia followed by 1 h reperfusion showed a significant increase of pGSK-3β (Ser9) compared to the contralateral or vehicle-treated retinas (Figure 4B) confirming that the observed changes in pAkt levels correlates with increased activity of the enzyme.
RGC neuroprotection afforded by treatment with forskolin/homotaurine/L-carnosine is PKA-independent
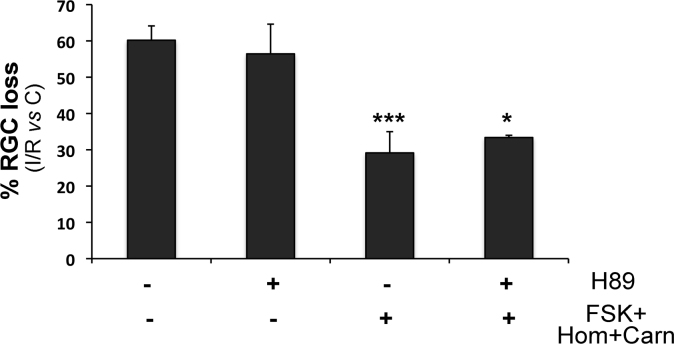
Forskolin increases intracellular level of cyclic adenosine monophosphate (cAMP), which, in turn, activates protein kinase A (PKA) [10]. To investigate the involvement of PKA in the neuroprotection observed with forskolin plus homotaurine/L-carnosine, we tested the effect of a PKA inhibitor, H89 (100 μM; given intravitreally 75 min before ischemia). Figure 5 shows that treatment with the PKA inhibitor did not revert the effect on RGC survival afforded by the combination of drugs, suggesting that this is a PKA-independent effect.
Figure 5.
Treatment with a PKA inhibitor did not prevent the neuroprotective effect observed in the ischemic retina following treatment with forskolin/homotaurine/L-carnosine. Protein kinase A (PKA) inhibitor H89 was given intravitreally 75 min before ischemia (100 μM; 15 min before administration of the association of drugs). PKA inhibition did not revert the neuroprotection observed following treatment with the combination of drugs. The histogram shows the results of FluoroGold (FG)-labeled retinal ganglion cell (RGC) count, performed at 7 days following retinal ischemia, expressed as a percentage of RGC loss compared to control non-ischemic retina (C). Results are reported as mean ± SEM of three independent experiments (***p<0.001, **p<0.01 versus vehicle-treated ischemic retinas; ANOVA followed by Tukey-Kramer multiple comparisons test; C: control non-ischemic retina; I/R: ischemic retina; Hom: homotaurine; Carn: L-carnosine; FSK: forskolin).
Discussion
RGC death is the final event leading to visual impairment in glaucoma; therefore, identification of neuroprotective strategies able to slow down or prevent the process is one of the main challenges for glaucoma research. The purpose of our study was to investigate the neuroprotective potential of a combined treatment with three natural substances, each endowed with neuroprotective properties, in a well-established animal model of acute glaucoma induced by a transient increase in IOP [29,31]. In this model, the ocular hypertension leads to a transitory ischemia that mimics the hypoxic stress that RGCs may undergo in glaucoma [38].
Our results demonstrate that the adenylate cyclase activating agent forskolin partially prevents RGC death following retinal ischemia, and this effect is potentiated by the simultaneous administration of homotaurine and L-carnosine. The observed potentiated neuroprotection is associated with reduced calpain activity and upregulation of the PI3K/Akt pathway, while it is insensitive to PKA inhibition and distinct from the reported hypotensive action of forskolin [39,40].
Forskolin increases cAMP, and this reduces aqueous inflow leading to the reported intraocular hypotensive effect [11,15]. Under the present experimental conditions, the latter effect might not be seen since the IOP was maintained constant by the application of an external pressure.
Prior data have reported the neuroprotective effects of forskolin on RGCs when applied in combination with neurotrophins. Indeed, addition of forskolin to brain-derived neurotrophic factor (BDNF), ciliary derived neurotrophic factor (CTNF), and insulin-like growth factor-1 (IGF-I) in culture medium promotes RGC survival [19,41]. Similarly, when added to a combined treatment with BDNF and CTNF, forskolin significantly improves the survival of axotomized RGCs in the cat retina [18,42].
We showed in vivo evidence of a dose-dependent neuroprotective effect of forskolin on RGCs that was independent from exogenous neurotrophines. More importantly, for the purpose of our study, RGC survival significantly increased when homotaurine and L-carnosine were combined with forskolin, although these were ineffective when administered alone.
In our experimental setting, the decrease in RGC loss observed at 7 days following the insult was preceded by an increased activation of calpain during the 1 h of reperfusion. Calpains are a superfamily of calcium-dependent cysteine proteases whose activation has been associated with neuronal degeneration and cell death in different systems including the retina [43,44].
Calpains are activated following ocular hypertension [44,45] and ischemia-reperfusion [33,46], and their pharmacological inhibition reduces cell loss in the ganglion cell layer [46,47]. Therefore, the decrease in calpain activity observed here following treatment with forskolin/homotaurine/L-carnosine could be interpreted as a biochemical indicator of reduced retinal damage. Furthermore, since activation of calpain occurs early during reperfusion [33], we can hypothesize that the neuroprotection afforded by the combined treatment is mediated, at least in part, by events occurring in the initial phase of reperfusion.
Although more than one mechanism might account for the synergic neuroprotection afforded by the present combination of drugs, in the attempt to dissect the pathways involved, we observed a significant upregulation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway in the retinas treated with the combined drugs. Full activation of Akt requires phosphorylation on Ser473 [48] and occurs following stimulation of transmembrane receptors by growth factors and hormones [49]. This kinase plays a central role in multiple cellular processes and exerts prosurvival and antiapoptotic effects [50]. We and others have recently shown that activation of Akt is an important component of the endogenous neuroprotective response of RGCs to retinal ischemia [29,51]; treatment with a PI3K inhibitor significantly reduced the number of RGCs surviving to the insult [29]. Furthermore, it has been shown that activation of the PI3K/Akt pathway is often induced by substances able to prevent RGC death [52,53]. Accordingly, here we suggest that the observed increase of Akt phosphorylation is involved in the neuroprotection afforded by the tested association of drugs.
GSK-3β is one of the Akt downstream substrates, and its activity is inversely correlated to PI3K/Akt pathway activation [36]. GSK-3β plays a critical role in the promotion of apoptosis in neurons [54] and increased kinase activity has been associated with neuronal degeneration [55]. However, pharmacological inhibition of GSK-3β has been proposed as an effective strategy for reducing neuronal death in several in vivo and in vitro models of neuronal injuries [56,57].
Increased survival observed in neuronal cultures exposed to cAMP elevating agents, including forskolin, has been associated with upregulation of GSK-3β phosphorylation [58,59]. Therefore, it may be conceivable that in our experimental conditions increased phosphorylation of GSK-3β following treatment with forskolin/homotaurine/L-carnosine is implicated in the observed neuroprotection.
To explain the reported synergic neuroprotection, we can speculate that, in our system, the antioxidant properties of homotaurine and L-carnosine [60-62] per se might not be sufficient to protect RGCs, though they might buffer the burst of oxidative stress that occurs during the initial phase of reperfusion [63]. This might in turn generate a more permissive environment for the neuroprotective effects exerted by forskolin [64]. One hypothesis that may be considered is that, in the presence of homotaurine and L-carnosine, forskolin increases the responsiveness of RGCs to endogenous trophic molecules, i.e., BDNF and CTNF, that are transiently and early upregulated as part of the retinal defense responses [65-67]. This mechanism would explain the upregulation of Akt phosphorylation observed following the treatment.
It cannot be excluded that the neuroprotection afforded by forskolin may unmask or stabilize the neuroprotective effect of homotaurine and L-carnosine, which has been observed in other models of neurodegeneration [22,27,68,69]. We reported consistent neuroprotection following treatment with forskolin/homotaurine/L-carnosine, while we observed higher variability in the RGC death outcome following treatment with homotaurine combined with L-carnosine (Figure 2).
In conclusion, we showed that a combined treatment with forskolin, homotaurine, and L-carnosine affords neuroprotection in retinal ischemia. Further experiments are needed to clarify the mechanisms responsible for the observed neuroprotective effect, although our data suggest the involvement of the PI3K/Akt/GSK3-β pathway. The data reported in this study also suggest that the use of a combination of drugs may represent an interesting strategy to be investigated to achieve neuroprotection in glaucoma.
Acknowledgments
We gratefully acknowledge the financial support of SOOFT Italia S.p.A. We also thank Mr. Guido Fico for skillful technical support.
References
- 1.Gupta N, Ang LC, Noel de Tilly L, Bidaisee L, Yucel YH. Human glaucoma and neural degeneration in intracranial optic nerve, lateral geniculate nucleus, and visual cortex. Br J Ophthalmol. 2006;90:674–8. doi: 10.1136/bjo.2005.086769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kwon YH, Fingert JH, Kuehn MH, Alward WL. Primary open-angle glaucoma. N Engl J Med. 2009;360:1113–24. doi: 10.1056/NEJMra0804630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bucolo C, Salomone S, Drago F, Reibaldi M, Longo A, Uva MG. Pharmacological management of ocular hypertension: current approaches and future prospective. Curr Opin Pharmacol. 2013;13:50–5. doi: 10.1016/j.coph.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 4.Quigley HA. Glaucoma. Lancet. 2011;377:1367–77. doi: 10.1016/S0140-6736(10)61423-7. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz B, Takamoto T, Martin J. Increased rate of visual field loss associated with larger initial visual field threshold values on follow-up of open-angle glaucoma. J Glaucoma. 2004;13:120–9. doi: 10.1097/00061198-200404000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Qu J, Wang D, Grosskreutz CL. Mechanisms of retinal ganglion cell injury and defense in glaucoma. Exp Eye Res. 2010;91:48–53. doi: 10.1016/j.exer.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osborne NN. Recent clinical findings with memantine should not mean that the idea of neuroprotection in glaucoma is abandoned. Acta Ophthalmol (Copenh) 2009;87:450–4. doi: 10.1111/j.1755-3768.2008.01459.x. [DOI] [PubMed] [Google Scholar]
- 8.Youdim MB, Buccafusco JJ. Multi-functional drugs for various CNS targets in the treatment of neurodegenerative disorders. Trends Pharmacol Sci. 2005;26:27–35. doi: 10.1016/j.tips.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Baltmr A, Duggan J, Nizari S, Salt TE, Cordeiro MF. Neuroprotection in glaucoma - Is there a future role? Exp Eye Res. 2010;91:554–66. doi: 10.1016/j.exer.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Metzger H, Lindner E. The positive inotropic-acting forskolin, a potent adenylate cyclase activator. Arzneimittelforschung. 1981;31:1248–50. [PubMed] [Google Scholar]
- 11.Caprioli J, Sears M. Combined effect of forskolin and acetazolamide on intraocular pressure and aqueous flow in rabbit eyes. Exp Eye Res. 1984;39:47–50. doi: 10.1016/0014-4835(84)90113-1. [DOI] [PubMed] [Google Scholar]
- 12.Caprioli J, Sears M. Forskolin lowers intraocular pressure in rabbits, monkeys, and man. Lancet. 1983;1:958–60. doi: 10.1016/s0140-6736(83)92084-6. [DOI] [PubMed] [Google Scholar]
- 13.Caprioli J, Sears M, Bausher L, Gregory D, Mead A. Forskolin lowers intraocular pressure by reducing aqueous inflow. Invest Ophthalmol Vis Sci. 1984;25:268–77. [PubMed] [Google Scholar]
- 14.Zeng S, Shen B, Wen L, Hu B, Peng D, Chen X, Zhou W. Experimental studies of the effect of Forskolin on the lowering of intraocular pressure. Yan Ke Xue Bao. 1995;11:173–6. [PubMed] [Google Scholar]
- 15.Burstein NL, Sears ML, Mead A. Aqueous flow in human eyes is reduced by forskolin, a potent adenylate cyclase activator. Exp Eye Res. 1984;39:745–9. doi: 10.1016/0014-4835(84)90073-3. [DOI] [PubMed] [Google Scholar]
- 16.Seto C, Eguchi S, Araie M, Matsumoto S, Takase M. Acute effects of topical forskolin on aqueous humor dynamics in man. Jpn J Ophthalmol. 1986;30:238–44. [PubMed] [Google Scholar]
- 17.Meyer BH, Stulting AA, Muller FO, Luus HG, Badian M. The effects of forskolin eye drops on intra-ocular pressure. S Afr Med J. 1987;71:570–1. [PubMed] [Google Scholar]
- 18.Watanabe M, Tokita Y, Kato M, Fukuda Y. Intravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retina. Neuroscience. 2003;116:733–42. doi: 10.1016/s0306-4522(02)00562-6. [DOI] [PubMed] [Google Scholar]
- 19.Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–19. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- 20.Guiotto A, Calderan A, Ruzza P, Borin G. Carnosine and carnosine-related antioxidants: a review. Curr Med Chem. 2005;12:2293–315. doi: 10.2174/0929867054864796. [DOI] [PubMed] [Google Scholar]
- 21.Boldyrev AA, Stvolinsky SL, Fedorova TN, Suslina ZA. Carnosine as a natural antioxidant and geroprotector: from molecular mechanisms to clinical trials. Rejuvenation Res. 2010;13:156–8. doi: 10.1089/rej.2009.0923. [DOI] [PubMed] [Google Scholar]
- 22.Bellia F, Vecchio G, Cuzzocrea S, Calabrese V, Rizzarelli E. Neuroprotective features of carnosine in oxidative driven diseases. Mol Aspects Med. 2011;32:258–66. doi: 10.1016/j.mam.2011.10.009. [DOI] [PubMed] [Google Scholar]
- 23.Boldyrev A, Bulygina E, Leinsoo T, Petrushanko I, Tsubone S, Abe H. Protection of neuronal cells against reactive oxygen species by carnosine and related compounds. Comp Biochem Physiol B Biochem Mol Biol. 2004;137:81–8. doi: 10.1016/j.cbpc.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Bae ON, Majid A. Role of histidine/histamine in carnosine-induced neuroprotection during ischemic brain damage. Brain Res. 2013;1527:246–54. doi: 10.1016/j.brainres.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Boldyrev A, Fedorova T, Stepanova M, Dobrotvorskaya I, Kozlova E, Boldanova N, Bagyeva G, Ivanova-Smolenskaya I, Illarioshkin S. Carnosine [corrected] increases efficiency of DOPA therapy of Parkinson's disease: a pilot study. Rejuvenation Res. 2008;11:821–7. doi: 10.1089/rej.2008.0716. [DOI] [PubMed] [Google Scholar]
- 26.Makletsova MG, Fedorova TN, Maksimova MY, Boldyrev AA. Effects of carnosine on polyamine levels in red blood cells of patients with hypertonic discirculatory encephalopathy. Bull Exp Biol Med. 2012;154:210–2. doi: 10.1007/s10517-012-1914-2. [DOI] [PubMed] [Google Scholar]
- 27.Wu S, Yue Y, Tian H, Tao L, Wang Y, Xiang J, Wang S, Ding H. Tramiprosate protects neurons against ischemic stroke by disrupting the interaction between PSD95 and nNOS. Neuropharmacology. 2014;83:107–17. doi: 10.1016/j.neuropharm.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 28.Caltagirone C, Ferrannini L, Marchionni N, Nappi G, Scapagnini G, Trabucchi M. The potential protective effect of tramiprosate (homotaurine) against Alzheimer's disease: a review. Aging Clin Exp Res. 2012;24:580–7. doi: 10.3275/8585. [DOI] [PubMed] [Google Scholar]
- 29.Russo R, Cavaliere F, Berliocchi L, Nucci C, Gliozzi M, Mazzei C, Tassorelli C, Corasaniti MT, Rotiroti D, Bagetta G, Morrone LA. Modulation of pro-survival and death-associated pathways under retinal ischemia/reperfusion: effects of NMDA receptor blockade. J Neurochem. 2008;107:1347–57. doi: 10.1111/j.1471-4159.2008.05694.x. [DOI] [PubMed] [Google Scholar]
- 30.Sellés-Navarro I, Villegas-Perez MP, Salvador-Silva M, Ruiz-Gomez JM, Vidal-Sanz M. Retinal ganglion cell death after different transient periods of pressure-induced ischemia and survival intervals. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996;37:2002–14. [PubMed] [Google Scholar]
- 31.Nucci C, Tartaglione R, Rombola L, Morrone LA, Fazzi E, Bagetta G. Neurochemical evidence to implicate elevated glutamate in the mechanisms of high intraocular pressure (IOP)-induced retinal ganglion cell death in rat. Neurotoxicology. 2005;26:935–41. doi: 10.1016/j.neuro.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Russo R, Rotiroti D, Tassorelli C, Nucci C, Bagetta G, Bucci MG, Corasaniti MT, Morrone LA. Identification of novel pharmacological targets to minimize excitotoxic retinal damage. Int Rev Neurobiol. 2009;85:407–23. doi: 10.1016/S0074-7742(09)85028-9. [DOI] [PubMed] [Google Scholar]
- 33.Russo R, Berliocchi L, Adornetto A, Varano GP, Cavaliere F, Nucci C, Rotiroti D, Morrone LA, Bagetta G, Corasaniti MT. Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis. 2011;2:e144. doi: 10.1038/cddis.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujita R, Ueda M, Fujiwara K, Ueda H. Prothymosin-alpha plays a defensive role in retinal ischemia through necrosis and apoptosis inhibition. Cell Death Differ. 2009;16:349–58. doi: 10.1038/cdd.2008.159. [DOI] [PubMed] [Google Scholar]
- 35.Wang KK. Calpain and caspase: can you tell the difference? by kevin K.W. WangVol. 23, pp. 20–26. Trends Neurosci. 2000;23:59. doi: 10.1016/s0166-2236(99)01536-2. [DOI] [PubMed] [Google Scholar]
- 36.Cross DA, Alessi DR, Cohen P, Andjelkovich M, Hemmings BA. Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature. 1995;378:785–9. doi: 10.1038/378785a0. [DOI] [PubMed] [Google Scholar]
- 37.van Weeren PC, de Bruyn KM, de Vries-Smits AM, van Lint J, Burgering BM. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation. Characterization of dominant-negative mutant of PKB. J Biol Chem. 1998;273:13150–6. doi: 10.1074/jbc.273.21.13150. [DOI] [PubMed] [Google Scholar]
- 38.Kaur C, Foulds WS, Ling EA. Hypoxia-ischemia and retinal ganglion cell damage. Clin Ophthalmol. 2008;2:879–89. doi: 10.2147/opth.s3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vetrugno M, Uva MG, Russo V, Iester M, Ciancaglini M, Brusini P, Centofanti M, Rossetti LM. Oral administration of forskolin and rutin contributes to intraocular pressure control in primary open angle glaucoma patients under maximum tolerated medical therapy. J Ocul Pharmacol Ther. 2012;28:536–41. doi: 10.1089/jop.2012.0021. [DOI] [PubMed] [Google Scholar]
- 40.Pescosolido N, Librando A. Oral administration of an association of forskolin, rutin and vitamins B1 and B2 potentiates the hypotonising effects of pharmacological treatments in POAG patients. Clin Ter. 2010;161:e81–5. [PubMed] [Google Scholar]
- 41.Shen S, Wiemelt AP, McMorris FA, Barres BA. Retinal ganglion cells lose trophic responsiveness after axotomy. Neuron. 1999;23:285–95. doi: 10.1016/s0896-6273(00)80780-1. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe M, Fukuda Y. Survival and axonal regeneration of retinal ganglion cells in adult cats. Prog Retin Eye Res. 2002;21:529–53. doi: 10.1016/s1350-9462(02)00037-x. [DOI] [PubMed] [Google Scholar]
- 43.Wang KK. Calpain and caspase: can you tell the difference? Trends Neurosci. 2000;23:20–6. doi: 10.1016/s0166-2236(99)01479-4. [DOI] [PubMed] [Google Scholar]
- 44.Azuma M, Shearer TR. The role of calcium-activated protease calpain in experimental retinal pathology. Surv Ophthalmol. 2008;53:150–63. doi: 10.1016/j.survophthal.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang W, Fileta J, Rawe I, Qu J, Grosskreutz CL. Calpain activation in experimental glaucoma. Invest Ophthalmol Vis Sci. 2010;51:3049–54. doi: 10.1167/iovs.09-4364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sakamoto YR, Nakajima TR, Fukiage CR, Sakai OR, Yoshida YR, Azuma MR, Shearer TR. Involvement of calpain isoforms in ischemia-reperfusion injury in rat retina. Curr Eye Res. 2000;21:571–80. [PubMed] [Google Scholar]
- 47.Oka T, Walkup RD, Tamada Y, Nakajima E, Tochigi A, Shearer TR, Azuma M. Amelioration of retinal degeneration and proteolysis in acute ocular hypertensive rats by calpain inhibitor ((1S)-1-((((1S)-1-benzyl-3-cyclopropylamino-2,3-di-oxopropyl)amino)carbonyl)-3-methylbutyl)carbamic acid 5-methoxy-3-oxapentyl ester. Neuroscience. 2006;141:2139–45. doi: 10.1016/j.neuroscience.2006.05.060. [DOI] [PubMed] [Google Scholar]
- 48.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–51. [PMC free article] [PubMed] [Google Scholar]
- 49.Brazil DP, Hemmings BA. Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci. 2001;26:657–64. doi: 10.1016/s0968-0004(01)01958-2. [DOI] [PubMed] [Google Scholar]
- 50.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–98. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 51.Nakazawa T, Shimura M, Tomita H, Akiyama H, Yoshioka Y, Kudou H, Tamai M. Intrinsic activation of PI3K/Akt signaling pathway and its neuroprotective effect against retinal injury. Curr Eye Res. 2003;26:55–63. doi: 10.1076/ceyr.26.1.55.14254. [DOI] [PubMed] [Google Scholar]
- 52.Qi Y, Chen L, Zhang L, Liu WB, Chen XY, Yang XG. Crocin prevents retinal ischaemia/reperfusion injury-induced apoptosis in retinal ganglion cells through the PI3K/AKT signalling pathway. Exp Eye Res. 2013;107:44–51. doi: 10.1016/j.exer.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 53.Weishaupt JH, Rohde G, Polking E, Siren AL, Ehrenreich H, Bahr M. Effect of erythropoietin axotomy-induced apoptosis in rat retinal ganglion cells. Invest Ophthalmol Vis Sci. 2004;45:1514–22. doi: 10.1167/iovs.03-1039. [DOI] [PubMed] [Google Scholar]
- 54.Hetman M, Xia Z. Signaling pathways mediating anti-apoptotic action of neurotrophins. Acta Neurobiol Exp (Warsz) 2000;60:531–45. doi: 10.55782/ane-2000-1374. [DOI] [PubMed] [Google Scholar]
- 55.Beurel E, Jope RS. The paradoxical pro- and anti-apoptotic actions of GSK3 in the intrinsic and extrinsic apoptosis signaling pathways. Prog Neurobiol. 2006;79:173–89. doi: 10.1016/j.pneurobio.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang MH, Chuang DM. Regulation and function of glycogen synthase kinase-3 isoforms in neuronal survival. J Biol Chem. 2007;282:3904–17. doi: 10.1074/jbc.M605178200. [DOI] [PubMed] [Google Scholar]
- 57.Kelly S, Zhao H, Hua Sun G, Cheng D, Qiao Y, Luo J, Martin K, Steinberg GK, Harrison SD, Yenari MA. Glycogen synthase kinase 3beta inhibitor Chir025 reduces neuronal death resulting from oxygen-glucose deprivation, glutamate excitotoxicity, and cerebral ischemia. Exp Neurol. 2004;188:378–86. doi: 10.1016/j.expneurol.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 58.Chin PC, Majdzadeh N, D'Mello SR. Inhibition of GSK3beta is a common event in neuroprotection by different survival factors. Brain Res Mol Brain Res. 2005;137:193–201. doi: 10.1016/j.molbrainres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 59.Li M, Wang X, Meintzer MK, Laessig T, Birnbaum MJ, Heidenreich KA. Cyclic AMP promotes neuronal survival by phosphorylation of glycogen synthase kinase 3beta. Mol Cell Biol. 2000;20:9356–63. doi: 10.1128/mcb.20.24.9356-9363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gossai D, Lau-Cam CA. The effects of taurine, taurine homologs and hypotaurine on cell and membrane antioxidative system alterations caused by type 2 diabetes in rat erythrocytes. Adv Exp Med Biol. 2009;643:359–68. doi: 10.1007/978-0-387-75681-3_37. [DOI] [PubMed] [Google Scholar]
- 61.Cheng J, Wang F, Yu DF, Wu PF, Chen JG. The cytotoxic mechanism of malondialdehyde and protective effect of carnosine via protein cross-linking/mitochondrial dysfunction/reactive oxygen species/MAPK pathway in neurons. Eur J Pharmacol. 2011;650:184–94. doi: 10.1016/j.ejphar.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 62.Shi Q, Yan H, Li MY, Harding JJ. Effect of a combination of carnosine and aspirin eye drops on streptozotocin–induced diabetic cataract in rats. Mol Vis. 2009;15:2129–38. [PMC free article] [PubMed] [Google Scholar]
- 63.Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 64.Santos RC, Araujo EG. Cyclic AMP increases the survival of ganglion cells in mixed retinal cell cultures in the absence of exogenous neurotrophic molecules, an effect that involves cholinergic activity. Braz J Med Biol Res. 2001;34:1585–93. doi: 10.1590/s0100-879x2001001200011. [DOI] [PubMed] [Google Scholar]
- 65.Lönngren U, Näpänkangas U, Lafuente M, Mayor S, Lindqvist N, Vidal-Sanz M, Hallböök F. The growth factor response in ischemic rat retina and superior colliculus after brimonidine pre-treatment. Brain Res Bull. 2006;71:208–18. doi: 10.1016/j.brainresbull.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 66.Guo XJ, Tian XS, Ruan Z, Chen YT, Wu L, Gong Q, Wang W, Zhang HY. Dysregulation of neurotrophic and inflammatory systems accompanied by decreased CREB signaling in ischemic rat retina. Exp Eye Res. 2014;125:156–63. doi: 10.1016/j.exer.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 67.Rudzinski M, Wong TP, Saragovi HU. Changes in retinal expression of neurotrophins and neurotrophin receptors induced by ocular hypertension. J Neurobiol. 2004;58:341–54. doi: 10.1002/neu.10293. [DOI] [PubMed] [Google Scholar]
- 68.Baek SH, Noh AR, Kim KA, Akram M, Shin YJ, Kim ES, Yu SW, Majid A, Bae ON. Modulation of mitochondrial function and autophagy mediates carnosine neuroprotection against ischemic brain damage. Stroke. 2014;45:2438–43. doi: 10.1161/STROKEAHA.114.005183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ji YS, Park JW, Heo H, Park JS, Park SW. The neuroprotective effect of carnosine (beta-alanyl-L-histidine) on retinal ganglion cell following ischemia-reperfusion injury. Curr Eye Res. 2014;39:634–41. doi: 10.3109/02713683.2013.855235. [DOI] [PubMed] [Google Scholar]