Abstract
Background
The association between diabetes mellitus (DM) and low secretory immunoglobulin A (s-IgA) secretion rates is one mechanism suspected of influencing susceptibility to infections among DM patients. However, several studies have shown contradictory results. We examined these two factors to seek evidence of an association among older people.
Methods
We analyzed a prospective cohort of 2306 subjects (1209 men and 1097 women) around 64 years old from the New Integrated Suburban Seniority Investigation (NISSIN) Project in Nisshin, Japan. DM statuses were ascertained from levels of fasting plasma glucose and HbA1c, and s-IgA secretion rates were obtained from 5-min saliva samples. We used an analysis of covariance adjusted for possible confounders to compare s-IgA secretion rates according to DM status.
Results
s-IgA secretion rates in DM participants were lower than in those classified as normal (18.6 µg/min vs 15.0 µg/min, P = 0.03), even after elimination of the effects of possible confounders.
Conclusions
DM was associated with lower s-IgA secretion rates. This suggests that lower s-IgA levels may be a mechanism of susceptibility to infection in individuals with DM.
Key words: diabetes mellitus, secretory immunoglobulin A, saliva
INTRODUCTION
Secretory immunoglobulin A (s-IgA), the predominant immunoglobulin in secretions of the mucosal immune system, plays an important primary role in antigen excretion.1 It is found in saliva, intestinal secretions, bronchoalveolar lavage fluid, urine, tears, and other mucosal fluids. It inhibits attachment and replication of pathogenic microorganisms, thus preventing colonization of these pathogens. It is also capable of neutralizing toxins and viruses.1,2 A study of humans with mucosal humoral immunodeficiencies suggests that the absence of s-IgA leads to an increase in mucosal infections and respiratory tract infections.3 Moreover, suppression of s-IgA is associated with increased incidence of upper respiratory tract infection (URTI) in elite athletes4,5 and healthy adults.6
Diabetes mellitus (DM) is associated with an increased risk of morbidity from infectious diseases, such as pneumonia and urinary tract infections.7,8 Several previous studies have reported that DM patients have impaired chemotaxis of the immune cells, defective phagocytosis of macrophages, and increased production of free radicals.9,10 Hyperglycemia can cause microangiopathy, which can lead to ulceration, secondary infection,11 and neuropathy. Microangiopathy may also result in impaired bladder emptying, which increases susceptibility to urinary tract infections.12 Several mechanisms seem to cause susceptibility to infections in patients with diabetes. A low s-IgA secretion rate is suspected to be one of these mechanisms; however, previous studies have shown contradictory results.13,14 Branco-de-Almeida et al reported that DM patients had lower mean levels of s-IgA than non-DM patients,13 while Yavuzyilmaz et al reported that DM patients had higher mean levels of s-IgA than healthy controls.14 These studies13,14 used small and limited community samples and failed to control for factors such as age, educational background, smoking, physical activity, or periodontitis, which are now known to be related to both s-IgA secretion and DM.15–17
We investigated the association between DM and s-IgA secretion rates using data from the New Integrated Suburban Seniority Investigation (NISSIN) Project, a large community sample of older people.
MATERIALS AND METHODS
Study population
This cross-sectional study was conducted using baseline data from the NISSIN Project, an age-specific prospective cohort study in Nisshin City, which is located near Nagoya City, Japan. Using the basic resident registry of Nisshin City, residents aged 64 years were invited to attend a free health check-up every April from 1996 through 2005; ultimately, 3073 subjects were recruited into this cohort (43.9% of the eligible population). Details of the NISSIN Project have been described elsewhere.18 Subjects were given a comprehensive medical and dental check-up in the early morning after an overnight fast. Participants in the present study were limited to those recruited from 1998 until 2005, as saliva samples were collected during this period.
Of the 2579 subjects that we approached, 2557 agreed to participate. From these, 2492 saliva samples (from 1275 men and 1217 women) were obtained. We excluded 183 participants because too little saliva was collected for assay. A further three participants were excluded due to missing fasting plasma glucose (FPG) and glycated hemoglobin (HbA1c) data. Thus, a total of 2306 subjects (1209 men and 1097 women) were analyzed.
Until 2001, oral consent was obtained using an opt-out approach, and written consent by an opt-in approach was used thereafter. We followed the checklist of the STROBE (STrengthening the Reporting of OBservational studies in Epidemiology) guidelines.19 This study was approved by the Ethics Committees of Nagoya University Graduate School of Medicine, the National Center for Geriatrics and Gerontology of Japan, the Aichi Medical University School of Medicine, and the Hokkaido University Graduate School of Medicine.
Sample collection
The medical check-up consisted of clinical and laboratory blood testing, including measurement of FPG and HbA1c. All clinical tests were performed in a single laboratory. A self-administered questionnaire was used to assess demographic and lifestyle factors. Demographic factors included gender and educational background. Lifestyle factors included smoking status and physical activity.
Dentists performed a dental check-up, and community periodontal index (CPI) codes were recorded as an estimate of periodontitis. CPI codes of 0–4 were assigned: 0 for healthy cases; 1 for bleeding on probing; 2 for presence of calculus; 3 for a probing pocket depth of 4–5 mm, and 4 for a probing pocket depth of ≥6 mm. The highest CPI code recorded for any of each participant’s teeth was applied to that participant.
Definition of diabetes mellitus status
Levels of HbA1c were determined via the Japan Diabetes Society (JDS) method. JDS HbA1c (%) was converted to the internationally used HbA1c (%) measurement as defined by the National Glycohemoglobin Standardization Program (NGSP), using the following formula: NGSP (%) = 1.02 × JDS (%) + 0.25 (%). DM was then classified as:
Normal: FPG <6.1 mmol/l and HbA1c (NGSP) <5.6% (38 mmol/mol)20
Pre-DM: 6.1 mmol/l ≤ FPG <7.0 mmol/l; or 5.6% (38 mmol/mol) ≤ HbA1c (NGSP) <6.5% (48 mmol/mol)20
DM: FPG ≥7.0 mmol/l or HbA1c (NGSP) ≥6.5% (48 mmol/mol),20 or use of medication for DM
Use of medication for DM was obtained via the question: “Are you taking any oral medications for DM?”. We did not include a question about subcutaneous injection of insulin.
Salivary s-IgA analysis
Salivary s-IgA analysis was performed using the same method as previously reported.21 Briefly, to determine the volume and concentration of saliva, samples of unstimulated saliva were collected with two cotton swabs (Salivettes; Sarstedt Ltd., Leicester, UK) prior to the dental check-up. The swabs were placed under the tongue of each participant and removed after 5 minutes, and the saliva was extracted from the cotton by centrifugation. Then we stored the saliva samples in tubes at −20°C until analysis. An enzyme-linked immunoabsorbent assay (ELISA) was performed to measure the s-IgA concentration in the saliva using an IgA test (MBL Inc., Santa Clara, CA, USA). ELISA was performed once a year from 1998 until 2005, and the laboratory personnel conducting the tests did not have knowledge of the diabetes status of any samples.
Statistical method
The s-IgA secretion rates (µg/min) were calculated as the product of saliva volume (mL/min) and s-IgA concentration (µg/mL). Because data did not fall into a normal distribution, the s-IgA secretion rate was subjected to log transformation before analysis. This strategy has been adopted in other studies.22,23
To compare s-IgA secretion rates according to DM status, we used an analysis of covariance and adjusted for variables such as gender, assay batch (attended year), educational background (under junior high school, high school, college or higher, and unknown), smoking status (current smoker, ex-smoker, never smoker, and unknown), physical activity (almost none, more than once a week, and unknown), periodontitis (yes: CPI = 4; no: CPI < 4), and intake of any medication (yes or no). We considered these variables as confounding factors based on previous reports.15–17,22–25 Since the assay batch variable reflected inter-assay variation, it could have been a potential confounder. Tests for linear trends were conducted to assess associations between the original continuous variables of HbA1c and s-IgA secretion rate. All P values were two-tailed and considered statistically significant when P < 0.05. All statistical analyses were performed using JMP Pro 10.0.2 software (SAS Institute Inc., Cary, NC, USA).
RESULTS
Of 1209 men and 1097 women, 176 men (14.6%) and 77 women (7.0%) were categorized as having DM. Characteristics of those with DM did not differ between subjects who were eligible for participation and who were excluded for producing too little saliva to assay (data not shown). Table shows the distribution of participants according to DM status. The proportion of patients with DM was low among those with relatively high educational background. Never having smoked tended to be associated with normal DM status. Physical activity and CPI had no association with DM status in this study. Medication intake was positively associated with DM status.
Table. Distribution of participants according to DM statusa (n = 2306).
| Normal (n = 1262) | Pre-DM (n = 791) | DM (n = 253) | |
| Gender | |||
| Male | 635 (50.3) | 398 (50.3) | 176 (69.6) |
| Female | 627 (49.7) | 393 (49.7) | 77 (30.4) |
| Educational background | |||
| Junior high school or lower | 395 (31.3) | 235 (29.7) | 75 (29.6) |
| High school | 537 (42.5) | 368 (46.5) | 110 (43.5) |
| College or higher | 316 (25.0) | 184 (23.3) | 65 (25.7) |
| Smoking status | |||
| Never smoker | 712 (56.4) | 425 (53.7) | 101 (39.9) |
| Ex-smoker | 336 (26.6) | 222 (28.1) | 104 (41.1) |
| Current smoker | 214 (17.0) | 143 (18.1) | 48 (19.0) |
| Physical activity | |||
| Almost none | 612 (48.5) | 393 (49.7) | 109 (43.1) |
| More than once a week | 648 (51.4) | 397 (50.2) | 144 (56.9) |
| Community periodontal index | |||
| <4 | 1181, 93.58 | 742 (93.8) | 238 (94.1) |
| 4 | 81, 6.42 | 49 (6.2) | 15 (5.9) |
| Receiving medication | |||
| No | 540 (42.8) | 314 (39.7) | 61 (24.1) |
| Yes | 722 (57.2) | 477 (60.3) | 192 (75.9) |
DM, diabetes mellitus; FPG, fasting plasma glucose.
Values are expressed as numbers (%). Some factors have missing values, so the totals are not always 100%.
aDM status was classified as, Normal: FPG < 6.1 mmol/l and HbA1c < 5.6% (38 mmol/mol); pre-DM: 6.1 mmol/l ≤ FPG < 7.0 mmol/l, or 5.6% (38 mmol/mol) ≤ HbA1c < 6.5% (48 mmol/mol); DM: FPG ≥ 7.0 mmol/l or HbA1c ≥ 6.5% (48 mmol/mol).
The median s-IgA secretion rate was 35.7 µg/min (min: 0.0310 µg/min, max: 568 µg/min). Mean saliva volume did not differ significantly between normal, pre-DM, and DM participants (data not shown).
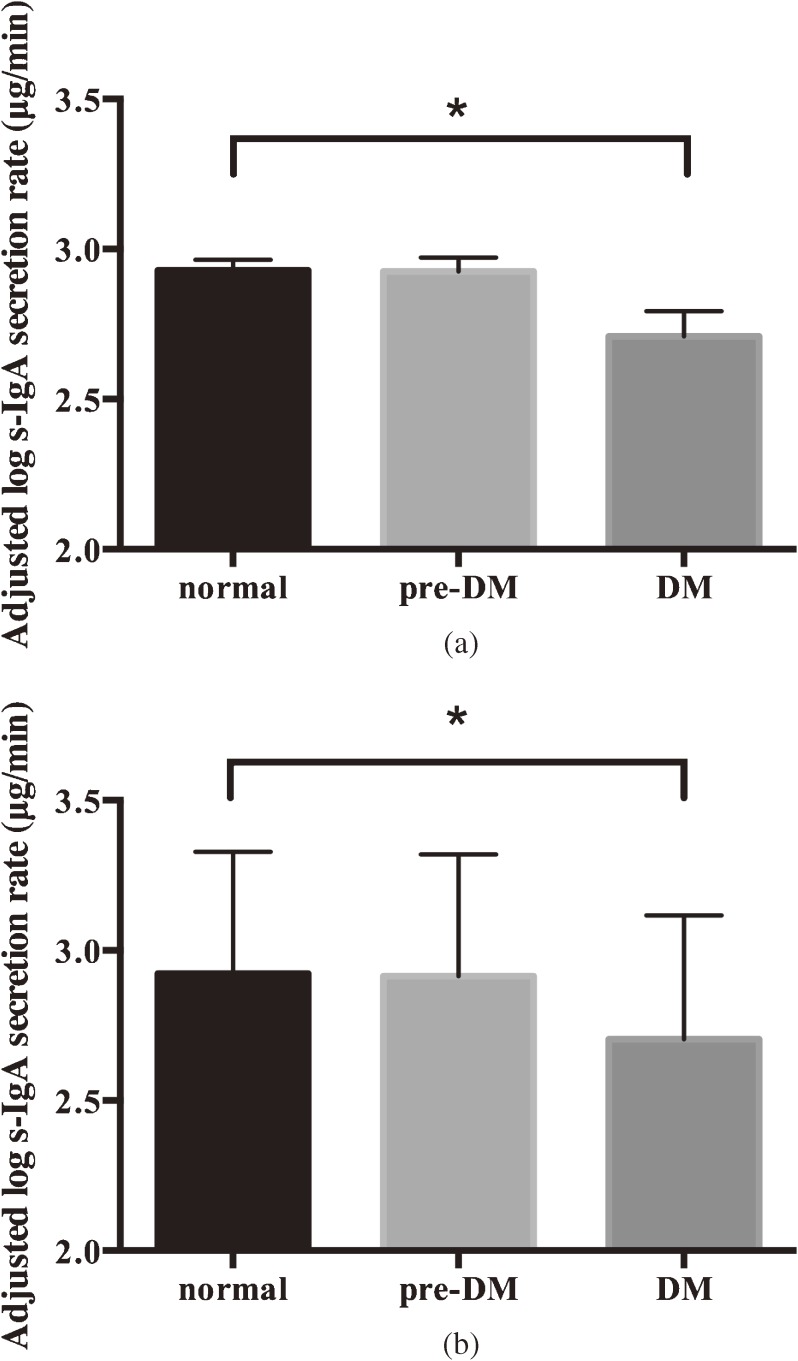
Figure 1a shows the mean log s-IgA secretion rates according to DM status after adjusting for minor confounders, such as gender and assay batch. s-IgA secretion rates were lower among participants with DM than those classified as normal (18.6 µg/min vs 15.0 µg/min, P = 0.03), even after elimination of the effects of all possible confounders, such as assay batch, gender, educational background, smoking status, physical activity, CPI, and medication intake (Figure 1b). Low s-IgA secretion rate also had a linear relationship (P for trend = 0.03) with high HbA1c.
Figure 1. Mean (SD) log s-IgA secretion rate according to diabetes mellitus (DM) status. Analyses shown in Figure 1a were controlled for gender and assay batch. Analyses in Figure 1b were controlled for gender, assay batch, educational background, smoking status, community periodontal index, medication, and physical activity. *P < 0.05.
DISCUSSION
The present study is the first to show an association between DM and low s-IgA secretion rates in a large sample of older people. The findings are important because of the increasing number of DM patients and deaths from pneumonia in older Japanese people.26,27
This cross-sectional study was developed from a previous case-control study that showed that DM patients presented with lower s-IgA levels than non-DM patients.13 Moreover, our study assessed a large community sample of old people of almost the same age controlled for possible confounders. Potential mechanisms involved in the association between DM and low s-IgA secretion rate are not clear. However, a recent study revealed a link between low levels of peritoneal B-1a cells and low production of IgM in DM mice.28 Experiments in vitro have confirmed the causal relationship of high concentrations of glucose on reduced secretion of total IgM.28 Similar mechanisms may work to induce lower s-IgA secretion rates in human DM patients’ salivary glands. More female participants (n = 121) were excluded because too little saliva was collected for assay than male participants (n = 62). It is possible that more women had low salivary flow and suffered from xerostomia than men.25,29
Although the differences in s-IgA secretion rates between normal status participants and those with DM status were small, they were consistent and remained significant following adjustment for several variables known to have some influence on the s-IgA secretion rate. They are also of the same order of magnitude reported previously for s-IgA secretion rates and stress.22,23 Nevertheless, whether associations of this magnitude are of clinical significance remains unclear. A previous cohort study, although based on a younger and smaller sample of athletes than the present study, has shown that subjects prone to developing URTI had significantly lower saliva s-IgA secretion rates than URTI-free subjects.5 This suggests that our findings might have some clinical importance. Further prospective studies are needed to clarify the nature of the associations between s-IgA secretion rates and the development of other infectious diseases such as URTI, pneumonia, and urinary tract infections.
The present study has several limitations. First, it is cross-sectional and thus could not show a causal relationship between hyperglycemia and low s-IgA secretion rates. Second, we did not obtain information about the varieties or frequencies of medications taken by participants. Many drugs, such as diuretics, antihistamines, and anti-hypertension drugs, are known to reduce saliva volume as an adverse side effect.23,30 Subjects with DM status might take more medications, which may itself result in reduced saliva volume. However, in a restricted analysis of participants who took no medication, DM was still associated with low s-IgA secretion rate (data not shown). Therefore, the lack of detailed information on medications taken might not have affected our results. Third, the proportion of participants strongly suspected to be DM patients (HbA1c ≥6.1% or receiving medication for DM) in our study was 13.7% (17.0% of men and 10.1% of women), which is close to the national estimate in a similar age group in 2006 (13.6%; 14.7% in men and 12.8% in women).31 However, the proportion of participants who might be DM patients (HbA1c 5.6%–6.1%, without receiving medication for DM) was 21.2% (20.7% in men and 21.9% in women) in our study, which is slightly higher than the national estimate (15.4%; 14.4% in men and 16.1% in women). Of note, however: differences in the prevalence of diabetes or pre-diabetes would not affect the association between DM and s-IgA secretion rate.
In conclusion, DM was associated with low s-IgA secretion rate in the present study. Given the prominent role of s-IgA in immune defense on mucosal surfaces and the frequency with which infections are initiated on these surfaces, s-IgA affords a pathway by which higher plasma glucose levels might increase susceptibility to infectious diseases in DM individuals. This result suggests that low s-IgA levels may be a mechanism explaining susceptibility to infection in DM individuals.
ACKNOWLEDGMENTS
The authors express their sincere appreciation to the Health Center and Hygiene Department of Nisshin City for their generous cooperation. We also gratefully acknowledge the special efforts of the Nisshin Medical and Dental Associations. We thank Dr. Mariko Naito for her advice on our analysis. This study was supported by a Grant-in-Aid for Scientific Research (B) from the Japanese Ministry of Education, Culture, Sports, Science and Technology (MEXT; no. 15390197). The authors report no potential conflicts of interest relevant to this article.
J.O. conceived, analyzed, and wrote the manuscript. S.U. helped with the analysis and reviewed the manuscript. H.O. supervised saliva sample collection and IgA measurements. K.W. and M.A. collected the data and coordinated the study. T.K. designed and supervised the study, collected the data and obtained funding. A.H. reviewed the manuscript. A.T. designed and supervised the study, collected the data, reviewed the manuscript, and obtained funding.
Conflicts of interest: None declared.
REFERENCES
- 1.Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010;8:656–67. 10.1038/nrmicro2384 [DOI] [PubMed] [Google Scholar]
- 2.Woof JM, Mestecky J. Mucosal immunoglobulins. Immunol Rev. 2005;206:64–82. 10.1111/j.0105-2896.2005.00290.x [DOI] [PubMed] [Google Scholar]
- 3.Aghamohammadi A, Cheraghi T, Gharagozlou M, Movahedi M, Rezaei N, Yeganeh M, et al. IgA deficiency: correlation between clinical and immunological phenotypes. J Clin Immunol. 2009;29:130–6. 10.1007/s10875-008-9229-9 [DOI] [PubMed] [Google Scholar]
- 4.Nieman DC, Henson DA, Dumke CL, Lind RH, Shooter LR, Gross SJ. Relationship between salivary IgA secretion and upper respiratory tract infection following a 160-km race. J Sports Med Phys Fitness. 2006;46:158–62. [PubMed] [Google Scholar]
- 5.Gleeson M, Bishop N, Oliveira M, McCauley T, Tauler P, Muhamad AS. Respiratory infection risk in athletes: association with antigen-stimulated IL-10 production and salivary IgA secretion. Scand J Med Sci Sports. 2012;22:410–7. 10.1111/j.1600-0838.2010.01272.x [DOI] [PubMed] [Google Scholar]
- 6.Klentrou P, Cieslak T, MacNeil M, Vintinner A, Plyley M. Effect of moderate exercise on salivary immunoglobulin A and infection risk in humans. Eur J Appl Physiol. 2002;87:153–8. 10.1007/s00421-002-0609-1 [DOI] [PubMed] [Google Scholar]
- 7.Benfield T, Jensen JS, Nordestgaard BG. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia. 2007;50:549–54. 10.1007/s00125-006-0570-3 [DOI] [PubMed] [Google Scholar]
- 8.Leegaard A, Riis A, Kornum JB, Prahl JB, Thomsen VØ, Sørensen HT, et al. Diabetes, glycemic control, and risk of tuberculosis: a population-based case-control study. Diabetes Care. 2011;34:2530–5. 10.2337/dc11-0902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delamaire M, Maugendre D, Moreno M, Le Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14:29–34. [DOI] [PubMed] [Google Scholar]
- 10.Sato N, Shimizu H, Suwa K, Shimomura Y, Kobayashi I, Mori M. MPO activity and generation of active O2 species in leukocytes from poorly controlled diabetic patients. Diabetes Care. 1992;15:1050–2. 10.2337/diacare.15.8.1050 [DOI] [PubMed] [Google Scholar]
- 11.Ngo BT, Hayes KD, DiMiao DJ, Srinivasan SK, Huerter CJ, Rendell MS. Manifestations of cutaneous diabetic microangiopathy. Am J Clin Dermatol. 2005;6:225–37. 10.2165/00128071-200506040-00003 [DOI] [PubMed] [Google Scholar]
- 12.Boyko EJ, Fihn SD, Scholes D, Abraham L, Monsey B. Risk of urinary tract infection and asymptomatic bacteriuria among diabetic and nondiabetic postmenopausal women. Am J Epidemiol. 2005;161:557–64. 10.1093/oxfordjournals.aje.a000181 [DOI] [PubMed] [Google Scholar]
- 13.Branco-de-Almeida LS, Alves CM, Lopes FF, Pereira Ade F, Guerra RN, Pereira AL. Salivary IgA and periodontal treatment needs in diabetic patients. Braz Oral Res. 2011;25:550–5. 10.1590/S1806-83242011000600013 [DOI] [PubMed] [Google Scholar]
- 14.Yavuzyilmaz E, Yumak O, Akdoğanli T, Yamalik N, Ozer N, Ersoy F, et al. The alterations of whole saliva constituents in patients with diabetes mellitus. Aust Dent J. 1996;41:193–7. 10.1111/j.1834-7819.1996.tb04855.x [DOI] [PubMed] [Google Scholar]
- 15.Evans P, Der G, Ford G, Hucklebridge F, Hunt K, Lambert S. Social class, sex, and age differences in mucosal immunity in a large community sample. Brain Behav Immun. 2000;14:41–8. 10.1006/brbi.1999.0571 [DOI] [PubMed] [Google Scholar]
- 16.Waszkiewicz N, Zalewska A, Szajda SD, Waszkiewicz M, Szulc A, Kepka A, et al. The effect of chronic alcohol intoxication and smoking on the output of salivary immunoglobulin A. Folia Histochem Cytobiol. 2012;50:605–8. 10.5603/FHC.2012.0085 [DOI] [PubMed] [Google Scholar]
- 17.Akimoto T, Kumai Y, Akama T, Hayashi E, Murakami H, Soma R, et al. Effects of 12 months of exercise training on salivary secretory IgA levels in elderly subjects. Br J Sports Med. 2003;37:76–9. 10.1136/bjsm.37.1.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitamura T, Kawamura T, Tamakoshi A, Wakai K, Ando M, Ohno Y. Rationale, design, and profiles of the New Integrated Suburban Seniority Investigation (NISSIN) Project: a study of an age-specific, community-based cohort of Japanese elderly. J Epidemiol. 2009;19:237–43. 10.2188/jea.JE20081026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 20.American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36 Suppl 1:S67–74. 10.2337/dc13-S067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimura K, Ozeki M, Juneja LR, Ohira H. L-Theanine reduces psychological and physiological stress responses. Biol Psychol. 2007;74:39–45. 10.1016/j.biopsycho.2006.06.006 [DOI] [PubMed] [Google Scholar]
- 22.Phillips AC, Carroll D, Evans P, Bosch JA, Clow A, Hucklebridge F, et al. Stressful life events are associated with low secretion rates of immunoglobulin A in saliva in the middle aged and elderly. Brain Behav Immun. 2006;20:191–7. 10.1016/j.bbi.2005.06.006 [DOI] [PubMed] [Google Scholar]
- 23.Gallagher S, Phillips AC, Evans P, Der G, Hunt K, Carroll D. Caregiving is associated with low secretion rates of immunoglobulin A in saliva. Brain Behav Immun. 2008;22:565–72. 10.1016/j.bbi.2007.11.007 [DOI] [PubMed] [Google Scholar]
- 24.Sloan CA, Engels HJ, Fahlman MM, Yarandi HE, Davis JE. Effects of exercise on S-IGA and URS in postmenopausal women. Int J Sports Med. 2013;34:81–6. [DOI] [PubMed] [Google Scholar]
- 25.Eliasson L, Birkhed D, Osterberg T, Carlén A. Minor salivary gland secretion rates and immunoglobulin A in adults and the elderly. Eur J Oral Sci. 2006;114:494–9. 10.1111/j.1600-0722.2006.00413.x [DOI] [PubMed] [Google Scholar]
- 26.Japan Ministry of Health, Labour and Welfare [Internet]. Tokyo: Vital statistics in Japan. [Updated 2013 July 19; cited 2014 Jun 23]. Available from: http://www.mhlw.go.jp/toukei/saikin/hw/jinkou/geppo/nengai12/dl/gaikyou24.pdf (in Japanese).
- 27.Japan Ministry of Health, Labour and Welfare [Internet]. Tokyo: National Health and Nutrition Survey. [Updated 2011 Jul 14; cited 2014 Jun 23]. Available from: http://www.mhlw.go.jp/bunya/kenkou/eiyou09/dl/01-kekka-01.pdf (in Japanese).
- 28.Jennbacken K, Ståhlman S, Grahnemo L, Wiklund O, Fogelstrand L. Glucose impairs B-1 cell function in diabetes. Clin Exp Immunol. 2013;174:129–38. 10.1111/cei.12148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nederfors T, Isaksson R, Mörnstad H, Dahlöf C. Prevalence of perceived symptoms of dry mouth in an adult Swedish population—relation to age, sex and pharmacotherapy. Community Dent Oral Epidemiol. 1997. Jun;25:211–6. 10.1111/j.1600-0528.1997.tb00928.x [DOI] [PubMed] [Google Scholar]
- 30.Sreebny LM, Schwartz SS. A reference guide to drugs and dry mouth—2nd edition. Gerodontology. 1997;14:33–47. 10.1111/j.1741-2358.1997.00033.x [DOI] [PubMed] [Google Scholar]
- 31.Japan Ministry of Health, Labour and Welfare [Internet]. Tokyo: Vital statistics in Japan. [Updated 2010 July 23; cited 2014 Jun 23]. Available from: http://www.mhlw.go.jp/houdou/2008/04/h0430-2.html (in Japanese).