Abstract
[Purpose] This study aimed to examine the expression of transforming growth factor β1 (TGF-β1) and type I collagen by applying high voltage pulsed current stimulation (HVPCS) with a visible contraction intensity to white rats with induced wounds. [Subjects] Thirty-six white rats were used for this study. HVPCS with a non-visible contraction intensity was applied to experimental group I, and HVPCS with a visible contraction intensity was applied to experimental group II. Placebo stimulation was applied to the control group. [Methods] After wounds were triggered, the intervention appropriate for each group was applied. Changes in the size of their wounds and expression of TGF- β1 and type I collagen were measured on the third, fifth, and seventh days. [Results] Comparison of the sizes of the wounds among the groups showed that the most significant decreases were found in experimental group II on the fifth and seventh days. TGF-β1 expression comparison revealed that experimental group II had the most expression on the fifth day. [Conclusion] HVPCS with a visible contraction intensity was effective in promoting wound healing by increasing expression of TGF-β1 and synthesis of type I collagen.
Key words: High voltage pulsed current stimulation, Transforming growth factor β1, Wound
INTRODUCTION
A wound refers to a case where the continuity of a normal skin structure is destroyed by physical damage to the skin, and the process of recovering such discontinuity is referred to as wound healing1). Wound healing consists of three phases—inflammation, proliferation, and remodeling—and wound healing progresses by cells playing their unique roles in each phase2).
Thus far, 13 types of collagens acting importantly in the wound healing process have been discovered, and among them, type I accounts for 85% of the wound healing process3). Collagens engage in normal or pathological bioprocesses such as cell combination, cell differentiation, wound healing, all kinds of fibroses and inflammatory responses, and cancer transformation4).
Collagen synthesis in fibroblasts is influenced by diverse cytokines including transforming growth factor β1 (TGF-β1), insulin-like growth factor 1, interleukin 1, and platelet-derived growth factor, and in particular, TGF-β1 is known to play a major in vivo role5). TGF-β1, which is secreted from fibroblasts, macrophages, and platelets, triggers chemotaxis of fibroblasts, macrophages, and monocytes. Stimulation of fibroblasts increases synthesis and secretion of collagen fibers and other substrate components and inhibits secretion of fiber decomposition enzymes. TGF-β1 also stimulates endothelial blood vessel cells, thereby promoting formation of blood vessels6, 7). Therefore, TGF-β1 plays the most vital role in the proliferation and remodeling phases8).
Kloth noted that electrical stimulation applied to wounded skin tissues increased angiogenesis and perfusion, which raised the activity of granulation tissues and fibroblasts, thereby promoting wound healing speed9). Physical therapy approaches to wound healing include HVPCS, microcurrents, low-intensity laser, and ultrasound10,11,12,13). HVPCS has a monophasic twin peak pulse wave form and very short pulse duration and therefore can safely stimulate deep tissues without damage14). The effects of HVPCS related to wound healing are a decrease in chronic decubitus ulcer, inhibition of staphylococcus aureus growth, an increase in perfusion around ischemic wounds, and improved microcirculation15,16,17,18). Doran et al. observed that HVPCS with the cathode as an active electrode decreased the size of the inflammatory area19). Lee et al. applied HVPCS to the wound areas of rats for seven days and observed that the experimental group’s wound healing rate and fibroblast and collagen density were significantly higher than those of the control group10). Jeong and Song applied HVPCS to normal skin of rats for seven days and noted that expression of collagen I and TGF-β1 was promoted in the dermis layer20).
However, most previous studies have concerned HVPCS with a non-visible contraction intensity, and research on the effects of HVPCS with a visible contraction intensity on wound healing is lacking. Accordingly, the aim of this study was to compare the expressions of TGF-β1 and collagen I at each time point by applying HVPCS with a non-visible contraction intensity to wound areas of white rats, thereby presenting a theoretical groundwork for its clinical practice.
SUBJECTS AND METHODS
Thirty-six white adult male Sprague-Dawley rats (NTacSam:SD, Samtako Bio Korea, Osan, Republic of Korea), which were six weeks old and weighed from 200 to 220 g, were used and were equally and randomly assigned to experimental group I, experimental group II, and the control group (n = 12 per group). HVPCS with a non-visible contraction intensity was applied to experimental group I, HVPCS with a visible contraction intensity was applied to experimental group II, and placebo stimulation was applied to the control group under the same condition as used for the experimental groups after wounds were triggered. For histological sections, four rats from each group were sacrificed on the third, fifth, and seventh days. During the experimental period, whether the rats had any trauma or disease was checked, and stress factors were minimized by reducing environmental changes. The temperature and humidity of the breeding room were maintained at 22 ± 2 °C and 55 ± 10%, respectively. The light-dark cycle was set at 12 hours to maintain the constant conditions in the breeding room during the experimental period. The rats were allowed to freely take water and solid feed (SamYang Co., Republic of Korea). The Institutional Animal Care and Use Committee at DongShin University approved the protocols used in this study, and the animals were cared for in accordance with the Guidelines for Animal Experiments (2010).
The rats were subjected to general anesthesia using a respiratory anesthetic, sevoflurane, and the hair on their back area was removed using a depilatory cream (Veet depilatory cream, Reckitt Benckiser, France). After removing the hair, the skin was disinfected with 75% alcohol so that no depilatory cream remained on the skin. A wound with a diameter of 10 mm was induced in the left and right sides using a No. 11 surgical blade at locations more than 15 mm laterally distant from the center. The panniculus carnosus was exposed to induce wounds in a circular form, and the wound areas were dressed with sterilized gauze. The rats rested for 24 hours after induction of the wounds without any manipulation in order to minimize the stress and physiological response resulting from the wounds21). The skin tissue of a rat has a panniculus carnosus layer, and therefore induction of a circular wound only may induce a wound healing speed similar to that of humans22, 23). Accordingly, this study induced circular wounds in the rats.
The wounds were covered with sterilized gauze soaked with normal saline. An active electrode (2×2 cm) was fixed on them, and an inactive electrode of the same size was fixed on the abdomen and connected to an HVPCS device (Endomed 482, Enraf- Nonius, Rotterdam, Netherlands). The same pulse frequency and pulse duration were established for application of HVPCS to each group. A non-visible contraction intensity of 25 to 65 V was applied to the experimental group I, and a visible contraction intensity of 40 to 85 V was applied to the experimental group II thirty minutes per day, for six days. Placebo stimulation was applied to the control group under the same condition as the experimental groups. All the wound areas of the groups were disinfected after application of HVPCS. For the first three days, the cathode was applied as the active electrode, and from the fourth day, the anode was applied as the active electrode20). Fibroblasts move to the cathode due to their electrotropism24), and when the active electrode for HVPCS is the cathode, cellular responses of fibroblasts are activated25).
The diameters of wounds were measured right after their induction, on the third day, on the fifth day, and on the seventh day with the naked eyes using calipers (Mitutoyo Corporation, Kawasaki, Japan) capable of measuring length in 0.05 mm increments. In order to compare the sizes of wounds, the sizes of the left and right wounds were added and divided by two (Fig. 1).
Fig. 1.
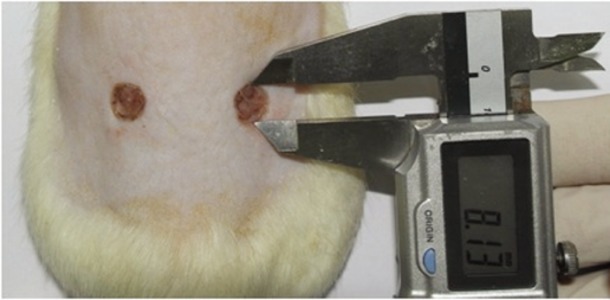
Wound diameter measured with calipers
Western blotting was conducted in order to examine the expression of TGF-β1 after application of HVPCS. The wound tissues treated according to each manipulation condition were washed three times with phosphate-buffered saline at 4 °C cut finely Lysis buffer (300 mM NaCl, 50 mM Tris–Cl, pH 7.6) with a volume three times larger than that of the tissues, 0.5% Triton X100, 2 mM phenylmethylsulfonyl fluoride, 2 µl/Ml aprotinin, and 2 µl/Ml leupeptin were added at 4 °C to react for 40 minutes. Thereafter, they were centrifuged at 4 °C and 14,000 rpm for 15 minutes to extract supernatant fluids. Protein quantification was performed with the supernatant fluids, and the extracted proteins were distributed to 7.5–12% sodium dodecyl sulfate (SDS) polyacrylamide gel for electrophoresis26). Then blotting was performed on a polyvinylidene difluoride membrane (Amersham, 0.2 µm, pore size, USA)27). The membrane was reacted for one hour at 4 °C in 5% (w/v) nonfat dried milk containing triethanolamine-buffered saline (TBS, phosphate-buffered saline containing 0.01% (v/v) Tween 20) and washed with TBS. TGF-β1 (sc-146, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was reacted for 24 hours at 4 °C, and to verify whether there was a specific reaction and the degree of the reaction, a goat anti-rabbit IgG antibody (ab6721, Abcam, Cambridge, MA, USA) was reacted as the secondary antibody for one hour; an enhanced chemical luminescence kit (RPN 2106, Amersham Life Science, Inc., USA) was used to determine the results. To examine whether the same amount of protein was loaded, monoclonal anti-β-actin (A-5316, 1:5,000, Sigma-Aldrich, St, Louis, MO, USA) and goat anti-mouse IgG (BD Bioscience, Franklin Lakes, NJ, USA) antibodies were used for comparison.
After application of HVPCS, immunohistochemical assessment was conducted in order to look at expression of type I collagen. After the paraffin removal and hydration processes for the produced slide, 30% Tris-ethylenediaminetetraacetic acid (EDTA) was reacted for 40 minutes at 90 °C and cooled for 20 minutes at a room temperature in order to remove nonspecific reactions for immunohistochemistry. Then as a preprocessing step, 3% hydrogen peroxidase was used to block the activity of endogenous peroxidase. Thereafter, a PBS solution to which blocking serum had been added was used so that the primary antibody solution would be well absorbed into the tissue inside. The tissue was then cleaned with 0.01 M PBS several times, and the primary antibody for type 1 collagen (Sigma-Aldrich, St. Louis, MO, USA) was diluted 1:100, 1:200, 1:500, and 1:2000 with PBS. It was reacted over night at 4 °C and then washed with PBS. Then, the universal antibody was diluted with blocking serum in PBS and reacted for 60 minutes at a room temperature. Again, the tissue was washed with 0.01 M PBS three times, for five minutes each time and reacted with streptavidin for 30 minutes at room temperature. After washing with PBS, color development was performed with DAB (3,3′-Diaminobenzidine, D3939, Sigma-Aldrich, St. Louis, MO, USA) for 20 seconds. Then, it was washed with PBS once for three minutes, counterstained with hematoxylin, washed twice for three minutes in flowing water, and dehydrated with 70%, 80%, 90%, and 100% ethanol for two minutes for each. The tissue was treated with 100% xylene twice for two minutes to render it transparent and then sealed with Canada balsam (Sigma-Aldrich, St. Louis, MO, USA) to produce permanent samples.
For statistical analysis of this study, PASW Statistics, ver 18.0, was used. In order to verify the significance of each group’s wound size and expression of TGF-β1 according to intensity of HVPCS, one-way analysis of variance (ANOVA) was conducted to examine differences among the groups at each measured time point, and Duncan’s test was performed as a post hoc test. A paired t-test was carried out in order to look at each group’s differences in measured variables at each measured time point. The significance level was set at α=0.05. Immunochemical assessment of type I collagen was performed in a semiquantitative manner and classified as follows: negative reaction, mild negative reaction when stainability was weak, moderate positive reaction when stainability was moderate, and strong positive reaction when stainability was strong28).
RESULTS
According to the results of comparing changes in wound size at each time point, the control group’s wound size was significantly decreased on the fifth and seventh days (p<0.01), while the wound sizes of experimental groups I and II were significantly decreased at all time points (p<0.05). According to the results of one-way ANOVA for comparison among the groups, there was significant difference on the fifth and seventh days, and therefore, Duncan’s post hoc test was carried out. The wound size of experimental group II was the most significantly decreased on the fifth and seventh days (p<0.01) (Table 1).
Table 1. Comparison of changes in wound size (Unit: mm).
| Day 0 | Day 3 | Day 5†† | Day 7†† | |
|---|---|---|---|---|
| Control group (n=12) | 10.5 ± 0.3 | 9.9 ± 1.0 | 8.6 ± 0.3** | 6.78 ±1.0** |
| Experimental group I (n=12) | 10.2 ± 0.1 | 8.9 ± 0.2** | 7.9 ± 0.3** | 5.72 ± 0.2** |
| Experimental group II (n=12) | 10.5 ± 0.2 | 8.5 ± 0.7* | 5.7 ± 1.0** | 3.74 ± 0.3** |
| F | 2.1 | 4.0 | 19.4 | 22.0 |
| Post hoc | a, b>c | a>b>c |
Values are presented as the mean±SD. Tested by one-way ANOVA (†p<0.05; ††p<0.01) and Duncan’s multiple range test. Tested by paired t-test (*p<0.05; **p<0.01). a. Control group: sham group (wound induced). b. Experimental group I: 25–65V, high voltage pulsed current (HVPCS). c. Experimental group II: 40–85V, HVPCS.
According to the results of comparing the expressions of TGF-β1 at each time point, the expression of TGF-β1 in the experimental groups was significantly increased on the fifth day (p<0.05). According to the results of one-way ANOVA to compare the expression of TGF-β1 among the groups, there were significant differences on the fifth day, and therefore, Duncan’s post hoc test was conducted. The expression of TGF-β1 in experimental group II was highest the fifth day (p<0.01) (Table 2).
Table 2. Comparison of changes in TGF β-1 expression (Unit: %).
| Day 3 | Day 5†† | Day 7 | |
|---|---|---|---|
| Control group (n=12) | 99.2±8.1 | 99.0±5.7 | 99.5±9.7 |
| Experimental group I (n=12) | 105.7±8.1 | 130.2±12.1 | 101.2±8.8 |
| Experimental group II (n=12) | 105.7±7.9 | 146.6±11.4* | 117.0±10.7 |
| F | 0.8 | 22.5 | 3.8 |
| Post hoc | c>b>a |
Values are presented as the mean±SD. Tested by one-way ANOVA (†p<0.05; †† p<0.01) and Duncan's multiple range test. Tested by paired t-test (*p<0.05; **p<0.01). a. Control group: sham group (wound induced). b. Experimental group I: 25–65V, high voltage pulsed current (HVPCS). c. Experimental group II: 40–85V, HVPCS.
According to the results of observation of the immunoreactivity to type I collagen, which is known to be a component making up most of the internal structure of a wound, the control group and experimental group I showed a mild positive reaction (+) on the fifth day, and experimental group II showed a strong positive reaction (+++) in the dermis on the fifth day and a moderate positive reaction (++) on the seventh day (Table 3 and Figs. 2, 3, 4).
Table 3. Immunohistochemical reaction of type I collagen of the skin.
| Group | Period | ||
|---|---|---|---|
| 3 days | 5 days | 7 days | |
| Control group | - | + | - |
| Experimental group I | - | + | - |
| Experimental group II | - | +++ | ++ |
Fig. 2.
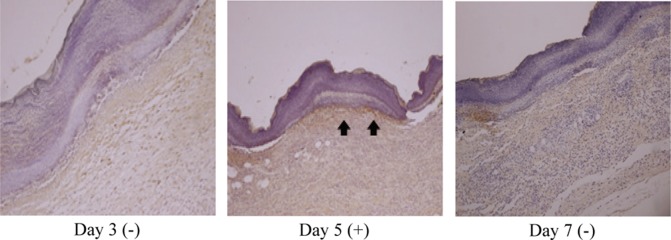
Immunohistochemical findings for the type I collagen reaction in skin from the control group (immunohistochemical stain, ×100)
Fig. 3.
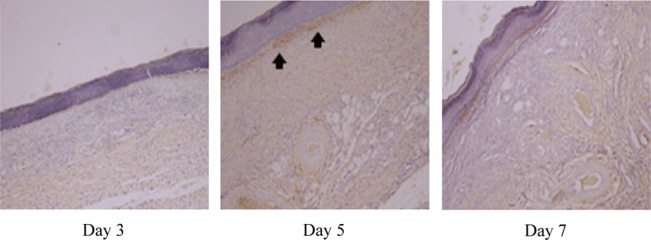
Immunohistochemical findings for the type I collagen reaction in skin from experimental group I (immunohistochemical stain, ×100)
Fig. 4.
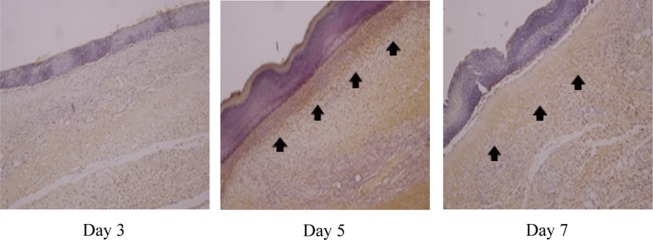
Immunohistochemical findings for the type I collagen reaction in skin from experimental group II (immunohistochemical stain, ×100)
DISCUSSION
HVPCS has a monophasic twin peak pulse wave form, low voltage of 150 V or higher, low total current (1.5 mA), and very short pulse duration29). The effects of HVPCS include a decrease in chronic decubitus ulcer, inhibition of staphylococcus aureus growth, an increase in perfusion around ischemic wounds, and improved microcirculation15,16,17,18).
This study aimed to examine expression of type I collagen and TGF-β1 by applying HVPCS with a visible contraction intensity to wound-induced white rats. The same pulse frequency and duration were set for application of HVPCS to each group. A non-visual contraction intensity of 25 to 65 V was applied to experimental group I, and a visual contraction intensity of 40 to 85 V was applied to experimental group II; both groups received HVPCS for thirty minutes per day for six days. According to the results of comparing changes in wound size at each time point, the control group saw a significant decrease in its wound size on the fifth and seventh days (p<0.01), while experimental groups I and II saw a significant decrease in their wound sizes at all time points (p<0.05). Wound size was compared among the groups, and that of the experimental group II was more significantly decreased than the other groups on the fifth and seventh days (p<0.01).
TGF-β1 is an important factor that regulates inflammation and fibroses30). Li and Huard noted that TGF-β1 was most expressed on the third day31). Jeong and Song applied HVPCS with a non-visible contraction intensity (140 µs, 120 pps, and 30–50 V) to normal white rats 30 minutes per day for seven days and reported that expression of TGF-β1 in the dermis significantly increased relative to the control group20). However, Western blotting was conducted in the present study to compare the expression of TGF-β1 in each group at each time point. The expression of TGF- β1 in experimental group II was significantly increased on the fifth day (p<0.05), which was more or less different from previous study results. According to the results of comparing the expression of TGF-β1 among the groups at each time point, the expression of TGF-β1 in experimental group II was found to be the highest on the fifth day (p<0.05). This result is considered to have been caused by the relatively high stimulation intensity, which triggers contraction of nerve roots as a result of the pumping action of the muscles and increased local vasodilatation and oxygen supply to tissues32, 33), thereby stimulating endothelial blood vessel cells and promoting formation of blood vessels, which in turns induces stimulation of macrophages and platelets, resulting in increased expression of TGF-β17).
According to the results of the immunohistochemical reaction performed to examine the expression of type I collagen, the control group and experimental group I showed a mild positive reaction (+) on the fifth day, and experimental group II showed a strong positive reaction (+++) in the dermis on the fifth day and a moderate positive reaction (++) on the seventh day. Richard et al. observed that wounds were in the inflammatory phase on the third day after injury and that they were in the remodeling phase on the sixth day, with the inflammatory stage almost over and granulation tissue starting to form34). This result shows that synthesis of type I collagen is most active on the fifth day and that synthesis of type I collagen is promoted by HVPCS with a visible contraction intensity. Kobayasi et al. reported that TGF-β1 promoted fibroblast expression35). Lee et al. also observed that fibroblast expression induced collagen synthesis10). In the present study as well, it is judged that TGF-β1 increased by HVPCS with a visible contraction intensity promoted type I collagen synthesis.
Comparison of the sizes of the wounds among the groups showed that the most significant decrease in wound size was found in experimental group II on the fifth and seventh days. According to the result of comparison of the expression of TGF-β1 in each group at each time point, the expression of TGF-β1 in experimental group II was significantly increased on the fifth day. According to the results of the immunohistochemical reaction performed to examine the expression of type I collagen, the control group and experimental group I showed a mild positive reaction (+) on the fifth day, and experimental group II showed a strong positive reaction (+++) in the dermis on the fifth day and a moderate positive reaction (++) on the seventh day. The above results suggest that HVPCS with a visible contraction intensity increased expression of TGF-β1 and synthesis of type I collagen, promoting wound healing.
REFERENCES
- 1.Probst CW: Wound healing and specific tissue regeneration. In: Textbook of small animal surgery, 2nd ed. Philadelphia: WB Saunders, 1993, pp 53–63. [Google Scholar]
- 2.Coulombe PA: Wound epithelialization: accelerating the pace of discovery. J Invest Dermatol, 2003, 121: 219–230. [DOI] [PubMed] [Google Scholar]
- 3.Uitto J, Olsen DR, Fazio MJ: Extracellular matrix of the skin: 50 years of progress. J Invest Dermatol, 1989, 92: 61S–77S. [DOI] [PubMed] [Google Scholar]
- 4.Pogulis RF, Freytag SV: Constribution of specific-action elements to activity of the mouse pro-α2(I)collagen enhancer. J Biochem, 1993, 268: 2493–2499. [PubMed] [Google Scholar]
- 5.Moulin V: Growth factors in skin wound healing. Eur J Cell Biol, 1995, 68: 1–7. [PubMed] [Google Scholar]
- 6.Sporn MB, Roberts AB: Transforming growth factor-β: recent progress and new challenges. J Cell Biol, 1992, 119: 1017–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Kane S, Ferguson MW: Transforming growth factor β s and wound healing. Int J Biochem Cell Biol, 1997, 29: 63–78. [DOI] [PubMed] [Google Scholar]
- 8.Kang KY, Kim H: Low dose treatment of RhTGF-1 restore the wound healing defect in dexamethasone-treated rats. J Korean Phys Anthropol, 2010, 23: 207–215. [Google Scholar]
- 9.Kloth LC: How to use electrical stimulation for wound healing. Nursing, 2002, 32: 17. [DOI] [PubMed] [Google Scholar]
- 10.Lee JH, Song IY, Kim JG: Acceleration of wound healing and collagen deposition in rat skin by high voltage pulsed current stimulation. J Korean Soc Phys Ther, 2003, 15: 1–12. [Google Scholar]
- 11.Lee JW, Yoon SW, Kim TH, et al. : The effects of microcurrents on inflammatory reaction induced by ultraviolet irradiation. J Phys Ther Sci, 2011, 23: 693–696. [Google Scholar]
- 12.Kim SH, Jeon JS: The study on wound healing in rabbit skins by low-intensity laser Irradiation. Korean J Biomed Lab Scienc, 2000, 6: 119–129. [Google Scholar]
- 13.Fisher BD, Hiller CM, Rennie SG: A comparison of continuous ultrasound and pulsed ultrasound on soft tissue injury markers in the rat. J Phys Ther Sci, 2003, 15: 65–70. [Google Scholar]
- 14.Bergstrom N: Treatment of Pressure Ulcers. Clinical Practice Guideline No. 15. U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research, AHCPR Publication, No. 95-0652, 1994.
- 15.Herminawaty D, Defi IR, Probowo T, et al. : Promising treatment for pressure ulcers using high voltage pulsed current stimulation. Gerontechnology (Valkenswaard), 2014, 13: 202. [Google Scholar]
- 16.Merriman HL, Hegyi CA, Albright-Overton CR, et al. : A comparison of four electrical stimulation types on Staphylococcus aureus growth in vitro. J Rehabil Res Dev, 2004, 41: 139–146. [DOI] [PubMed] [Google Scholar]
- 17.Goldman R, Brewley B, Zhou L, et al. : Electrotherapy reverses inframalleolar ischemia: a retrospective, observational study. Adv Skin Wound Care, 2003, 16: 79–89. [DOI] [PubMed] [Google Scholar]
- 18.Goldman R, Rosen M, Brewley B, et al. : Electrotherapy promotes healing and microcirculation of infrapopliteal ischemic wounds: a prospective pilot study. Adv Skin Wound Care, 2004, 17: 284–294. [DOI] [PubMed] [Google Scholar]
- 19.Dolan MG, Mychaskiw AM, Mendel FC: Cool-water immersion and high-voltage electric stimulation curb edema formation in rats. J Athl Train, 2003, 38: 225–230. [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong MA, Song IY: Effects of electrical stimulation on type I Collagen mRNA and TGF-β1 mRNA expression in the dermis. J Kor Soc Cosm, 2008, 14: 992–1005. [Google Scholar]
- 21.Cha MS, Lee HJ, Bae JH, et al. : Effects of sevoflurane on wound healing process. Korean J Anesthesiol, 2009, 57: 78–83. [DOI] [PubMed] [Google Scholar]
- 22.Montandon D, D’andiran G, Gabbiani G: The mechanism of wound contraction and epithelialization: clinical and experimental studies. Clin Plast Surg, 1977, 4: 325–346. [PubMed] [Google Scholar]
- 23.Mogford JE, Mustoe TA: Experimental Models of Wound Healing. Cutaneous wound healing. London: Martin Dunitz, 2001, pp 109–122. [Google Scholar]
- 24.Brown MJ, Loew LM: Electric field-directed fibroblast locomotion involves cell surface molecular reorganization and is calcium independent. J Cell Biol, 1994, 127: 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JH, Jekal SJ, Park RJ: Enhance of migration and proliferation of cells from tendon biopsies by high voltage pulsed current stimulation. J Korean Soc Phy Ther, 2002, 14: 162–171. [Google Scholar]
- 26.Bradford MM: A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem, 1976, 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 27.DiFiglia M, Sapp E, Chase K, et al. : Huntingtin is a cytoplasmic protein associated with vesicles in human and rat brain neurons. Neuron, 1995, 14: 1075–1081. [DOI] [PubMed] [Google Scholar]
- 28.Kim GY, Na SY, Kim KY, et al. : The histological observation of the effects of pulsed ultrasound on wound healing of rats. Phys Ther Korea, 2005, 12: 80–90. [Google Scholar]
- 29.Sandoval MC, Ramirez C, Camargo DM, et al. : Effect of high-voltage pulsed current plus conventional treatment on acute ankle sprain. Rev Bras Fisioter, 2010, 14: 193–199. [DOI] [PubMed] [Google Scholar]
- 30.Shi Y, Massagué J: Mechanisms of TGF-β signaling from cell membrane to the nucleus. Cell, 2003, 113: 685–700. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Huard J: Differentiation of muscle-derived cells into myofibroblasts in injured skeletal muscle. Am J Pathol, 2002, 161: 895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dodgen PW, Johnson BW, Baker LL, et al. : The Effect of electrical stimulation on cutaneous oxygen supply in diabetic older adult. Phys Ther, 1987, 67: 793–793. [Google Scholar]
- 33.Faghri PD, Votto JJ, Hovorka CF: Venous hemodynamics of the lower extremities in response to electrical stimulation. Arch Phys Med Rehabil, 1998, 79: 842–848. [DOI] [PubMed] [Google Scholar]
- 34.Richard AF, Clark MD, Denver CD: Cutaneuos tissue repair: basic biologic consideration. Am Acad Dermatol, 1985, 13: 701. [DOI] [PubMed] [Google Scholar]
- 35.Kobayashi T, Kim H, Liu X, et al. : Matrix metalloproteinase-9 activates TGF-β and stimulates fibroblast contraction of collagen gels. Am J Physiol Lung Cell Mol Physiol, 2014, 306: L1006–L1015. [DOI] [PMC free article] [PubMed] [Google Scholar]


