Abstract
The microbiota that populate the mammalian intestine are critical for proper host physiology, yet simultaneously pose a potential danger. Intestinal antigen-presenting cells, namely macrophages and dendritic cells (DCs), are integral components of the mucosal innate immune system that maintain co-existence with the microbiota in face of this constant threat. Intestinal macrophages and DCs integrate signals from the microenvironment to orchestrate innate and adaptive immune responses that ultimately lead to durable tolerance of the microbiota. Tolerance is not a default response, however, because macrophages and DCs remain poised to vigorously respond to pathogens that breach the epithelial barrier. In this review, we summarize the salient features of macrophages and DCs in the healthy and inflamed intestine and discuss how signals from the microbiota can influence their function.
From birth, the mammalian intestine is colonized with a complex microbiota leading to a lifelong mutualistic relationship.1 This diverse microbial population confers several evolutionary advantages to the host while simultaneously introducing a robust antigenic challenge that has the potential to initiate intestinal inflammation. Despite this threat, the host manages to maintain intestinal homeostasis via a sophisticated immune cell network that promotes tolerance to the microbiota while permitting responsiveness to invading pathogens.2,3 Central to this discrimination process are intestinal antigen-presenting cells (APCs), predominantly composed of macrophages and dendritic cells (DCs), that are separated from the microbiota by a single layer of epithelial cells. Together, intestinal macrophages and DCs integrate cues from epithelial, immune, and stromal cells to direct innate and adaptive immunity.4–10 Inappropriate responses to these signals can lead to a breakdown of tolerance toward the microbiota and culminate in uncontrolled inflammation, such as that observed in Crohn disease and ulcerative colitis.11 This review will focus on the role of intestinal macrophages and DCs in the steady state and during inflammation, as well as how these cells interface with the microbiota.
Development and Phenotypic Characterization of Intestinal Macrophages and DCs
Intestinal Macrophage and DC Development
The tissue microenvironment plays a key role in regulating the differentiation of macrophages and DCs from myeloid progenitor cells. In the intestine, the local milieu is shaped by the microbiota, enteric antigens, and immune cells that collectively contribute to the developmental outcome of macrophage and DC precursors entering the intestine. Intestinal macrophages, for example, are maintained and replenished by Ly6C+ monocytes that continually enter the intestine during the steady state and inflammation, a process referred to as the monocyte waterfall. These Ly6C+ monocytes subsequently differentiate into resident intestinal macrophages through a series of intermediary stages.12–15
The monocytes that produce intestinal macrophages are originally derived from macrophage-DC progenitors, which are the same bone marrow progenitors that can produce intestinal DCs.16 The ultimate fate of macrophage-DC progenitors in the intestine is, thus, determined by specific cytokines and growth factors in the tissue microenvironment that dictate different developmental programs. The maturation of monocytes that produce intestinal macrophages is under the control of the colony-stimulating factor 1 (Csf1) receptor and its stimulation by Csf1. Accordingly, the number of intestinal macrophages is significantly reduced in Csf1 receptor–deficient mice17 and in mice treated with anti-Csf1 receptor antibody.18 Csf1op/op mice, which have a mutation in the gene encoding Csf1, also have markedly reduced numbers of intestinal macrophages.19
Macrophage-DC progenitors can alternatively differentiate into common DC progenitors that are the precursors of conventional DCs and plasmacytoid DCs. Common DC progenitors can produce pre-DCs that develop into peripheral DCs, including intestinal CD103+ DCs, in a FMS-like tyrosine kinase 3 (Flt3)–dependent manner.17 Thus, intestinal CD103+ DCs expand in vivo in response to Flt3L20 and are substantially decreased in mice deficient for Flt3 or Flt3L.17 Other growth factors can further influence the homeostasis of different subsets of DCs, as highlighted by data demonstrating that CD103+CD11b+ intestinal DCs require Csf2 receptor stimulation via Csf2 (formerly granulocyte-macrophage colony-stimulating factor) for development in the steady state; however, this factor is dispensable for the differentiation of inflammatory DCs.17 The future identification of additional mediators that control macrophage and DC development may further our understanding of their ontogeny.
Phenotype of Intestinal Macrophages and DCs
Studies investigating intestinal macrophage and DC development have gained support from recent advancements in the phenotypic characterization of these cells. Analyses of cell morphology and surface markers have allowed for the clear distinction of intestinal macrophages and DCs from one another as well as the definition of different subsets of each population. When examining cellular structure, macrophages can typically be identified by the presence of large phagocytic vacuoles in the cytoplasm, whereas DCs exhibit dendrite-like projections.5
In addition to microscopy, multicolor flow cytometry has been instrumental in distinguishing intestinal macrophage and DC populations from each other, as well as from additional cell types. Clear identification of APCs from collagenase-digested intestinal cells can be achieved by inclusion of the two core markers: CD45, to select for leukocytes, and major histocompatibility complex (MHC) II, to mark cells with exogenous antigen-presenting ability. Additional markers can then be used to define populations of macrophages and DCs.
Initial work investigating cell surface markers expressed by intestinal APCs relied on the presence of F4/80 and the alpha X integrin, CD11c. F4/80 has long-standing use as a macrophage-specific marker and, when used in combination with the core APC markers, CD45 and MHCII, can discern macrophages from DCs in the healthy intestine.21 On the other hand, the utility of CD11c as a DC-specific marker is limited because of the fact that intestinal macrophages and DCs both express moderate to high levels of this antigen, precluding clear delineation of DCs from macrophages in the intestine.4,5,7,22,23
A similarly complex issue exists with regard to CD11b because it is expressed by nearly all macrophages, but also a subset of intestinal DCs as well as eosinophils and neutrophils.7 To ensure exclusion of these cells, the eosinophil-specific marker, Siglec-F, and the neutrophil-specific marker, Ly6G, can be used during analysis of intestinal macrophages and DCs. Another marker that has gained particular attention on intestinal APCs is CX3 chemokine receptor 1 (CR1), which is involved in the extension of transepithelial dendrites into the intestinal lumen during bacterial infection.24 Although CX3CR1 is highly expressed on resident intestinal macrophages25,26 that are located in the lamina propria, as well as the smooth muscle layer of the intestine,17 it can also be expressed at intermediate levels by some DCs during inflammation.13,27 The high-affinity IgG receptor, CD64, has also been used to specifically identify intestinal macrophages.7,14 Beyond F4/80, CD11b, CX3CR1, and CD64, intestinal macrophages can also be further identified by the differential expression of CD14, CD68, and Toll-like receptor (TLR)2.7
Resident intestinal DCs can be distinguished from macrophages primarily by their expression of CD103 and lack of CX3CR1.22,26 CD103+CX3CR1− DCs can further be divided into CD11b+ and CD11b− subsets, both of which express CCR7 and can migrate to mesenteric lymph nodes (mLNs) and imprint gut-homing markers on naïve T cells.26 Therefore, at the steady state, intestinal DCs can be defined among APCs (CD45+MHCII+) as CD11b+/−CD11c+F4/80−CD103+CX3CR1−CD64− cells and may be contrasted from macrophages, which are CD11b+CD11c+/−F4/80+CD103−CX3CR1+CD64+ cells (Figure 1).13,22 In addition to this panel, intestinal DCs can also be further identified by CD272 and CD26 expression.28 Collectively, these markers can also be used in complex scenarios, such as inflammation; however, certain cell surface antigens can change expression in the presence of inflammatory stimuli. Continued advancements in cell surface marker characterization will aid in elucidating the biological functions of intestinal macrophages and DCs.
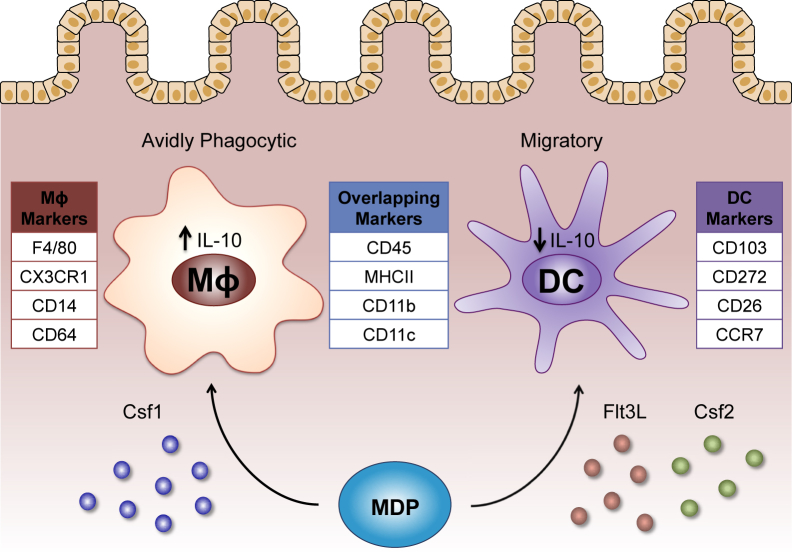
Figure 1.
Distinguishing characteristics of mouse intestinal macrophages (MΦ) and dendritic cells (DCs). Colony-stimulating factor 1 (Csf1) favors the differentiation of intestinal macrophages from macrophages and DC progenitors (MDPs), whereas FMS-like tyrosine kinase 3 ligand (Flt3L) and colony-stimulating factor 2 (Csf2) enhance the differentiation of MDPs into the DC lineage. After populating the intestine, macrophages and DCs can be identified by various cell surface markers. Antigens expressed predominantly by intestinal macrophages include F4/80, CX3CR1, CD14, and CD64, whereas intestinal DCs express CD103, CD272, CD26, and CCR7. Additional markers, including CD45, major histocompatibility complex (MHC) II, CD11b, and CD11c, overlap across both cell types. Macrophages and DCs in the intestine also exhibit a functional dichotomy. Intestinal macrophages are avidly phagocytic and constitutively produce IL-10, in contrast to intestinal DCs, which efficiently migrate to mesenteric lymph nodes (mLNs) and produce lower levels of IL-10.
Intestinal Macrophages and DCs in the Steady State
Homeostatic Functions of Intestinal Macrophages
During the steady state, intestinal macrophages maintain tolerance toward food antigens and the intestinal microbiota without compromising their ability to react to microbes that breach the epithelial barrier. To control bacteria that translocate past the epithelium, intestinal macrophages are highly phagocytic and have robust bactericidal activity.29 On uptake of bacteria, however, intestinal macrophages do not produce a strong respiratory burst or synthesize nitric oxide, two potentially damaging processes.7 Resident intestinal macrophages also express low levels of TLRs and associated signaling machinery, and do not produce inflammatory cytokines, such as IL-1, IL-6, IL-12, IL-23, or tumor necrosis factor (TNF) after exposure to bacterial signals.5,10,23,29,30 In mice, this state of inflammatory anergy is largely attributable to IL-10 that is constitutively expressed by intestinal macrophages. When IL-10 or IL-10 receptors (IL-10Rs) are blocked, intestinal macrophages become highly responsive to TLR ligands.23,30,31 These in vitro data provide evidence that the ability of macrophages to produce and/or respond to IL-10 are both involved in regulating their proinflammatory responsiveness. Recent in vivo data have clarified this issue by illustrating the requirement for IL-10R signaling in macrophages in restraining inflammation. In these studies, specific deletion of IL-10R, but not IL-10 itself, in CX3CR1+ resident macrophages led to the development of spontaneous colitis.32 Functional analyses found that IL-10R–deficient macrophages displayed exaggerated proinflammatory responses with little IL-10 production.33 In addition, the transfer of wild-type intestinal macrophages, but not IL-10R–deficient macrophages, prevented colitis in the T-cell transfer model of colitis. These changes in IL-10R–deficient macrophages were also observed in humans with IL-10R deficiencies who develop early-onset inflammatory bowel disease (IBD).33 Collectively, these data strongly support the concept that intestinal macrophage-mediated tolerance of the microbiota is maintained by responsiveness to IL-10 produced by nonmacrophage cells. Likely sources of IL-10 in the intestine are CD4+Foxp3+ regulatory T cells (Treg cells)34 and type 1 regulatory cells.35 In addition, other factors, such as transforming growth factor (TGF)-β and peroxisome proliferator-activated receptor γ, may help to regulate the hyporesponsiveness of intestinal macrophages toward luminal antigens.9,29 This may be particularly relevant for human intestinal macrophages, which exhibit inflammatory anergy yet do not spontaneously secrete IL-10.9,29
Despite their hyporesponsiveness to inflammatory stimuli, intestinal macrophages actively promote tolerogenic immune responses during the steady state. One way intestinal macrophages do this is by inducing Foxp3+ Treg cells, which are essential in suppressing inflammation and establishing oral tolerance. In the presence of TGF-β, production of IL-10 by intestinal macrophages can lead to the induction,23 maintenance,36 and expansion37 of Foxp3+ Treg cells in vitro and in vivo. Indeed, initial studies found that intestinal macrophages co-cultured with naïve CD4+ T cells could strongly induce the differentiation of Treg cells in an IL-10–dependent manner.23 Intestinal macrophage-derived IL-10 is important for Treg cell induction in vivo, as illustrated by the fact that CX3CR1-deficient mice display a loss of oral tolerance coinciding with abolished IL-10 production and blunted Treg cell proliferation in the lamina propria.37 Whether human intestinal macrophages can similarly influence Treg cell function is currently unknown. Interestingly, human intestinal macrophages express chemokine ligand 20 (macrophage inflammatory protein 3a), the ligand for CCR6, which is expressed on IL-10–producing induced Treg cells, and this axis may lead to close interactions between macrophages and Treg cells in the intestine.38
The ability of intestinal macrophages to modulate Treg cell abundance in vitro and in vivo may directly or indirectly inhibit the differentiation of proinflammatory CD4+ T cells. For example, loss of intestinal macrophages because of CX3CR1 or CX3CL1 deficiency resulted in enhanced type 17 helper T-cell (Th17)–driven colitis, which was reversed by the adoptive transfer of CX3CR1+ macrophages.25 Deficiency of CD11b results in defective oral tolerance and enhanced Th17 responses, effects that may be associated with reduced intestinal macrophages.39 The ability of macrophages to suppress Th17 responses in the intestine may result from inhibiting the Th17-promoting functions of CD103+CD11b+ DCs.23 Notably, resident macrophages do not readily migrate to mLNs in the presence of intestinal microbiota40 and, therefore, must exert these regulatory functions locally in the lamina propria.37 Overall, intestinal macrophages can regulate themselves and neighboring immune cells through a variety of innate and adaptive immune mechanisms that ultimately aid in the prevention of pathological inflammation.
Contributions of DCs to Intestinal Homeostasis
In addition to macrophages, DCs are also found in the intestine, where they drive tolerogenic responses through their communication with the adaptive immune system.41–43 It is now well appreciated that many DCs in the intestine express CD103 and can be further subdivided into CD11b+ and CD11b− subsets. These CD103+ DCs express high levels of CCR7, which allows for constitutive migration to mLNs at the steady state.44 Examination of oral tolerance to soluble food antigens illustrated that this migratory ability of intestinal DCs plays an influential role in establishing immune tolerance. Indeed, removal of lymph nodes or deletion of CCR7 interfered with the proper establishment of oral tolerance.44
A fundamental mechanism by which intestinal DCs appear to promote oral tolerance is through the generation of Foxp3+ Treg cells. Seminal studies revealed that CD103+ DCs isolated from either the small intestinal lamina propria or mLNs were able to induce the differentiation of Treg cells from naïve CD4+ T cells in the presence of TGF-β and retinoic acid.45–47 Interestingly, retinoic acid production by DCs is involved in the up-regulation of the gut-homing markers, α4β7 and CCR9, on T cells.48,49 A loss of this homing ability abrogates oral tolerance, thus demonstrating the importance of DC imprinting on T cells for immune tolerance.
In addition to inducing Treg cells, intestinal DCs can also influence Th17 responses. In particular, CD103+CD11b+ DCs are able to drive Th17 differentiation in the lamina propria of mice in a process that is dependent on the transcription factor interferon regulatory factor 4 and production of IL-6 and IL-23.50,51 Depletion of DCs or loss of interferon regulatory factor 4 function correlates with a significant decrease in Th17 cell numbers. CD1c+CD11b+ DCs, the human equivalent of mouse CD103+CD11b+ DCs, also expressed interferon regulatory factor 4 and are similarly capable of promoting Th17 responses.51 CD103+CD11b+ DCs also appear to be an obligate source of IL-23 that is required for survival after infection with the attaching-and-effacing pathogen Citrobacter rodentium.52
Given the divergent roles of intestinal DCs in promoting Treg and Th17/22 responses, it is important to consider how the same subsets of intestinal DCs can affect opposing T-cell responses in vivo. Intestinal DCs exhibit plasticity in influencing adaptive responses on the basis of the specific microenvironment they encounter.2,53 Consistent with this notion, the density of CD103+CD11b+ DCs throughout the intestine correlates with the number of Th17 cells, with both being abundant in the small intestine and rare in the colon. In contrast, DCs and macrophages that preferentially promote Foxp3+ Treg cells are most abundant in the colon, where a higher abundance of Treg cells can be found.22
The ability of intestinal DCs to stimulate Th17 responses is also dependent on the presence of unique microbiota, specifically segmented filamentous bacteria (SFB).22,54 Although not completely understood, other less prominent subsets of DCs in the intestine can also influence adaptive immune responses. Intestinal CD103−CD11b+ cells are a heterogeneous population of both macrophages and DCs.28 CCR2+ DCs from this CD103−CD11b+ population constitutively express IL-12/IL-23p40 and harbor the ability to drive IL-17A production by T cells in vitro.28
The involvement of intestinal macrophages and DCs in promoting distinct adaptive immune responses has led to many new intriguing questions regarding their involvement in antigen acquisition and presentation. With the close proximity of the microbiota to the lamina propria, it has been proposed that macrophages and DCs can directly sample luminal contents.
CX3CR1+ lamina propria cells, most likely macrophages, can extend dendrite-like processes into the intestinal lumen and capture bacteria.24 Although the physiological importance of this activity remains unclear, it may be involved in defending against invasive pathogens.24 Interestingly, CX3CR1+ cells, which do not migrate to mLNs, preferentially take up antigen compared with migratory CD103+ cells, which are inefficient at sampling and acquiring antigen from the intestinal lumen.55
The conundrum of how intestinal DCs acquire antigen when macrophages are the main phagocytic cells in the steady-state intestine was recently clarified. Mazzini et al56 elegantly demonstrated that CX3CR1+ macrophages can efficiently uptake luminal antigen and transfer it to CD103+ DCs via a mechanism mediated through direct cell-to-cell gaps junctions. Deletion of connexin 43, a protein component of gap junctions, specific to CD11c+ cells prevented this antigen transfer and diminished the ability of CD103+ DCs to present antigen and induce Treg cell differentiation in vitro and prevented the establishment of oral tolerance in vivo.56
Macrophages are not the only cells that can take part in antigen transfer to intestinal CD103+ DCs. Small-intestine goblet cells have also been reported to function as passages delivering low-molecular-weight soluble antigens from the intestinal lumen to underlying CD103+ DC cells,57 and intestinal CD103+ DCs can directly sample bacterial antigens on migration into the epithelium.55 The relative contribution and functional importance of these antigen acquisition pathways remain to be elucidated.
Functions of Intestinal APCs during Inflammation
Macrophages and Intestinal Inflammation
The onset of intestinal inflammation in humans and animals is typically associated with disruptions in the epithelial barrier and the consequent penetration of luminal bacteria into the lamina propria. Innate immune recognition of these translocating bacteria can trigger extensive cellular infiltration and activation with the induction of proinflammatory cascades that can drastically alter the network of intestinal APCs.11 Remarkably, amid the influx of intestinal antigen in response to epithelial barrier damage, resident lamina propria macrophages exhibit inflammatory anergy, remain hyporesponsive to TLR agonists, and secrete high levels of IL-10.13,15 Despite the anergy of resident intestinal macrophages, they can be rapidly overwhelmed by a massive influx of inflammatory macrophages that arise from circulating Ly6C+ monocytes into the inflamed intestine. These Ly6C+ monocytes express CCR2, the receptor for chemokine ligand 2 (alias monocyte chemoattractant protein-1), which is involved in trafficking these cells to sites of inflammation.15,58,59
Once in the inflamed intestine, Ly6C+ monocytes differentiate under the control of local inflammatory cues and up-regulate TLR2, nucleotide-binding oligomerization domain-containing protein 2 (NOD2), triggering receptor expressed by myeloid cells (TREM)-1, and other inflammatory markers.15 Unlike resident intestinal macrophages that remain refractory to inflammatory stimuli, inflammatory monocytes/macrophages become highly responsive to microbial stimulation and produce large amounts of proinflammatory cytokines, including IL-1, IL-6, IL-23, and TNF.15,27,58 These inflammatory mediators subsequently initiate downstream effects that contribute to inflammation and damage in the intestine through the up-regulation of adhesion molecules on the vascular endothelium, increasing epithelial permeability and enhancing recruitment of additional mononuclear and granulocytic cells. The mediators secreted by proinflammatory macrophages also promote DC activation and the differentiation of Th1 and Th17 cells (Figure 2).
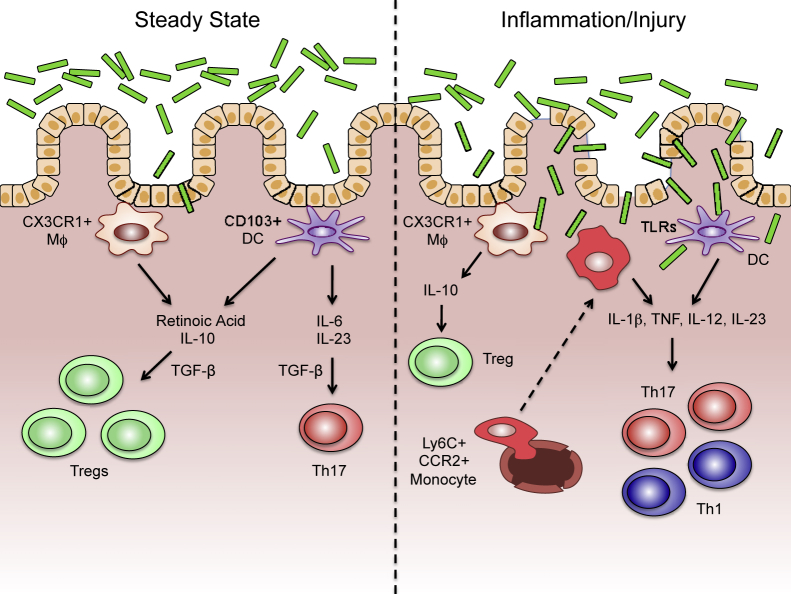
Figure 2.
The function of intestinal macrophages (MΦ) and dendritic cells (DCs) in the steady state and during inflammation/injury. In the steady state, resident CX3CR1+ macrophages and CD103+ DCs maintain tolerance toward the intestinal microbiota via the production of retinoic acid and IL-10, which, in combination with transforming growth factor (TGF)-β, induce regulatory T cells (Treg) cells. On encountering certain bacteria, CD103+ DCs can also produce IL-6 and IL-23, which drive type 17 helper T-cell (Th17) differentiation in a TGF-β–dependent manner. During inflammation/injury, Ly6C+CCR2+ monocytes are recruited into the intestine, where they, along with resident DCs, react to translocating bacteria through innate signaling pathways [eg, Toll-like receptor (TLR)]. These signals drive proinflammatory cytokine production, including IL-1β, tumor necrosis factor (TNF), IL-12, and IL-23, which can promote pathogenic Th1 and Th17 responses.
In addition to Ly6C and CCR2, inflammatory macrophages in the intestine also express lower levels of CX3CR1 and MHCII, making them easily distinguishable from resident intestinal macrophages. Interestingly, both resident and inflammatory macrophages are believed to derive from the same Ly6C+ monocyte precursors, highlighting the role of the local inflammatory milieu in intestinal macrophage fate determination.27 Furthermore, the presence of functionally distinct macrophage populations in the intestine may help direct therapeutic strategies focused on ameliorating pathogenic inflammation. For example, mice deficient for CCR2 are resistant to acute and chronic models of colitis,15,58 and administration of CCR2-neutralizing antibodies to mice can prevent the influx of inflammatory macrophages and colonic inflammation.15 Another target that may also be useful in limiting pathology induced by inflammatory macrophages is peroxisome proliferator-activated receptor-γ, which is expressed by resident intestinal macrophages. Peroxisome proliferator-activated receptor-γ stimulation can inhibit proinflammatory cytokine secretion, restrict CCR2-mediated migration of proinflammatory monocytes, and ameliorate experimental colitis.60
Another area of importance because of its potential for therapeutic applications is the ability of macrophages to participate in the resolution of intestinal inflammation and to promote wound healing. One way intestinal macrophages can contribute to these processes is through the expression of TREM2. By using an in vivo acute injury model generated by taking a biopsy of the colonic mucosa, TREM2-expressing macrophages were shown to contribute to epithelial proliferation, suppression of proinflammatory cytokine production, and closure of the wound bed. Intestinal macrophages expressing TREM2 were also shown to produce IL-4 and IL-13, which can function to activate Stat6 and arginase expression.61 Arginase derived from intestinal macrophages may shift l-arginine use toward polyamine production, resulting in epithelial proliferation, which is important for wound healing.62 Intestinal macrophages have also been reported to significantly reduce the severity of experimental colitis63 by increasing collagen deposition and secreting IL-10.64 In agreement with these findings, mice with defective TGF-β signaling specific to mature macrophages produce less IL-10 and are unable to resolve dextran sulfate sodium–induced colitis.65 Intestinal macrophages also express the enzyme cyclooxygenase 2 and produce prostaglandin E2, a lipid mediator that can aid in wound healing via its direct effects on the epithelial stem cell niche.66 Collectively, these data support the concept that intestinal macrophages help to not only enforce tolerance in the steady state, but to also participate in wound healing and repair processes.
DCs during Intestinal Inflammation
Similar to inflammatory macrophages, DCs display heightened levels of activation and increased proinflammatory cytokine secretion during intestinal inflammation.67 Recent evidence has demonstrated that intestinal CD103+ DCs migrate to and accumulate within the mLNs during experimental colitis, where they express low levels of TGF-β and retinaldehyde dehydrogenase enzymes that are required for the generation of retinoic acid from vitamin A. Thus, instead of efficiently priming Foxp3+ Treg cells, inflammatory DCs preferentially induce Th1 and Th17 responses during colitis.53
Thymic stromal lymphopoietin production by CD103+ DCs, which restrains Th17 responses during the steady state, is also down-regulated during experimental colitis.68 These changes in DC function may be linked to the findings that intestinal DCs are poised to rapidly respond to bacterial components, such as flagellin, that breach the epithelial barrier during intestinal inflammation.
CD103+CD11b+ small-intestine lamina propria DCs can express TLR5 and respond to flagellin by promoting Th1 and Th17 cells as well as the differentiation of IgA-producing plasma cells.69 Consistently, chronic colitis can lead to an increased number of CD103+CD11b+ DCs and Th17 cells in the colon.22 Flagellin-mediated stimulation of TLR5 on CD103+CD11b+ lamina propria DCs was also observed to rapidly and transiently increase the production of IL-23, which, in turn, induced IL-22–mediated expression of the antimicrobial peptide, RegIIIγ.70 Thus, activation of intestinal DCs can potentiate inflammation and promote tissue homeostasis.
Interactions of Intestinal Macrophages and DCs with the Microbiota
Microbiota-Induced APC Recruitment
Collectively, the microbiota outnumbers the total number of human cells by >10-fold. The highest abundance of these bacteria can be found in the intestine, with the colon harboring the highest density of approximately 1012 organisms/mL of luminal contents. These bacteria are in close proximity to the intestinal mucosa, and unique bacterial species are adept at penetrating the thick mucus layer and can directly interact with intestinal epithelial cells and underlying macrophages and DCs.
Because there exists a close temporal relationship between the acquisition of microbiota and development of the immune system during ontogeny, it is likely that this relationship influences intestinal macrophage and/or DC homeostasis. Interestingly, an initial wave of macrophages can be detected in the intestinal mucosa before birth, when the intestine has yet to be colonized by bacteria.12 However, after this initial seeding of the intestine, circulating Ly6C+ monocytes are responsible for expanding the pool of intestinal macrophages in a process that is dependent on the intestinal microbiota.12
In contrast to macrophages, the microbiota appears to be less involved in recruiting DCs into the intestine because germ-free and conventionalized mice harbor similar numbers of lamina propria DCs.71 It is possible that an original wave of DCs enter the intestine before microbial colonization, which is required to stimulate the development of lymphoid tissue in the intestine on colonization with microbiota.72 Although germ-free and conventionalized mice have similar numbers of intestinal DCs, conventionalization of germ-free mice leads to an increase in the number of CD11c+ DCs in Peyer patches, lymphoid follicles, and mLNs.71 Thus, the intestinal microbiota may contribute to the recruitment and/or expansion of DCs in the gastrointestinal-associated lymphoid tissue, whereas the major effects of the microbiota on lamina propria DCs may be to alter functional responses.
Sensing of the Microbiota
Sensing of luminal microbes and their components by macrophages and DCs is also important for promoting intestinal homeostasis. For example, the presence of the microbiota is required to maintain resident CX3CR1+ macrophages in the intestinal lamina propria. In mice treated with antibiotics or mice with MyD88 deficiency, CX3CR1+ macrophages up-regulate CCR7 and migrate to mLNs, where they can present antigens to induce T-cell responses and the differentiation of IgA-producing B cells.40 This study highlighted an interesting dual role of CX3CR1+ macrophages, especially because these cells are important for restraining inflammation in the steady state. In addition, bacteria sensing may be required to promote the anti-inflammatory program of resident intestinal macrophages and influence inflammatory anergy.73 Evidence for this comes from experiments showing that colonic macrophages from MyD88-deficient or germ-free mice have reduced expression of IL-10 and IL-10–inducible genes and enhanced proinflammatory cytokine responses.74
MyD88-dependent sensing of the microbiota by intestinal macrophages can also stimulate the production of Csf2 by innate lymphoid cells (ILCs) in a process involving IL-1β production.75,76 Csf2 derived from type 3 ILCs (ILC3) appears to be important for the maintenance of intestinal tolerance because its loss coincides with decreased intestinal macrophage and DC numbers, reduced expression of TGF-β, IL-10, and retinaldehyde dehydrogenase enzymes, and decreases in Foxp3+ Treg cells in the intestine.76 Similarly, ILC3-derived IL-22 can play a beneficial role by enhancing barrier function and aiding in mucosal healing.75
Investigations of bacterial sensing in the intestine have established that the responsiveness of macrophages and DCs can be controlled by unique bacteria-derived signals (Figure 3). Much of the evidence in this area stems from studies using germ-free mice colonized with different bacteria or bacterial by-products. For example, colonization of germ-free mice with Bacteroides fragilis77 or a collection of Clostridium strains from clusters IV and XIVa78 can preferentially promote the induction and function of Treg cells in the colon. In the case of B. fragilis, expression of polysaccharide A can activate plasmacytoid DCs in a TLR2-depedent manner to induce the expansion of intestinal Treg cells.77,79,80
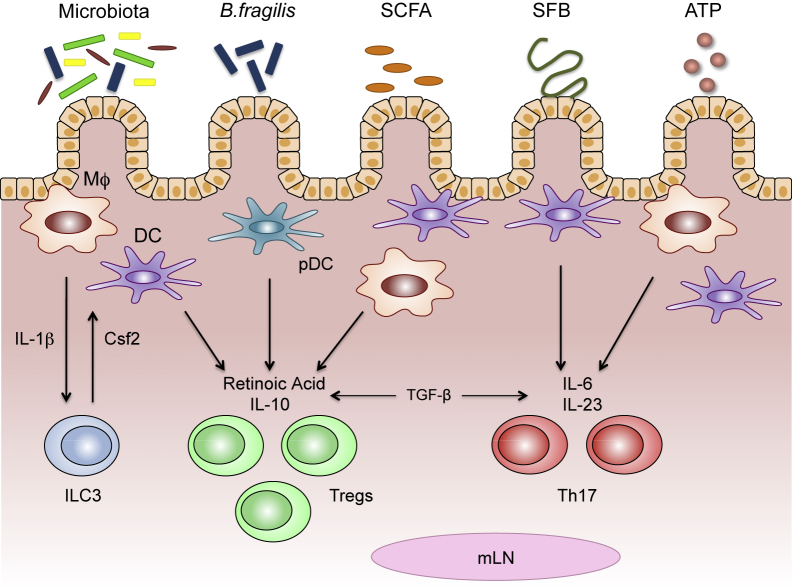
Figure 3.
Microbial factors condition intestinal macrophages (MΦ) and dendritic cells (DCs) to promote unique T-cell responses. Different members of the microbiota and their components can stimulate intestinal macrophages and/or DCs to induce regulatory T cells (Tregs) or type 17 helper T cells (Th17). Macrophages secrete IL-1β in response to commensal bacteria, prompting the production of colony-stimulating factor 2 (Csf2) from type 3 innate lymphoid cells (ILC3s). Csf2 can then engage macrophages and DCs to produce regulatory molecules (eg, retinoic acid and IL-10) involved in the induction of Treg cells. Polysaccharide A (PSA), expressed by Bacteroides fragilis and commensal-derived short-chain fatty acids (SCFAs), can also act on intestinal macrophages and DCs to stimulate retinoic acid and IL-10 production, and induce Treg cell differentiation. Segmented filamentous bacteria (SFB) can gain close contact with the intestinal epithelium, initiating signaling programs that drive the secretion of IL-6 and IL-23 from macrophages and DCs, leading to Th17 differentiation. ATP derived from commensal bacteria can bind receptors on intestinal macrophages and/or DCs, leading to enhanced IL-6 and IL-23 expression and the induction of Th17 cells. Both Treg and Th17 differentiation also require transforming growth factor (TGF)-β, which is constitutively expressed in the intestine. mLN, mesenteric lymph node; pDC, plasmacytoid dendritic cell.
In addition, specific members of the gut microbiota are able to produce short-chain fatty acids (acetate, butyrate, and propionate) through the fermentation of dietary fiber. Sensing of butyrate by intestinal macrophages and DCs via the niacin receptor, GPR109a, can lead to increased production of IL-10, the up-regulation of retinaldehyde dehydrogenase enzymes, and the induction of Treg cell differentiation.81
More important, not all members of the microbiota induce Treg responses. SFB, for example, is now well appreciated to potently induce intestinal Th17 responses in mice.54 It has been demonstrated that intestinal lamina propria macrophages and DCs from mice raised in the absence of SFB preferentially stimulate CD4+ cells to become Treg cells, whereas mice raised with microbiota containing SFB preferentially stimulate Th17 cells.22 SFB is unique among other members of the microbiota in that it generates an intestinal milieu that can induce antigen-specific Th17 differentiation against food and/or bacterial antigens directly in the intestinal lamina propria and not gastrointestinal-associated lymphoid tissue.82–84 Th17 differentiation has also been linked to the ability of SFB to stimulate serum amyloid A production in the intestine, which then acts on CD103+CD11b+ DCs to induce IL-6 and IL-23.54
Finally, a subset of CD70+CD11c(low) APCs in the colon have been reported to induce Th17 cells in response to bacteria-derived ATP. ATP can signal through the P2X and P2Y ATP receptors expressed by CD70+ APCs to induce the production of IL-6 and IL-23, which augments Th17 differentiation. Although the exact sources of ATP remain unknown, germ-free mice have significantly reduced levels of fecal ATP, suggesting that components of the microbiota are major producers.85 These findings further underline the importance of the microbiota and the local intestinal milieu in modulating macrophage and DC function.
In addition to macrophages and DCs, ILCs are also capable of presenting antigen in the intestine. ILCs, which can be divided into three different subsets on the basis of the cytokines they produce, have recently been found by several different groups to express MHCII.83,86,87 The ability of ILCs to present antigen through MHCII is important for maintaining intestinal homoeostasis, especially in terms of regulating host-microbiota reactions. Not only is antigen presentation by intestinal ILCs important for driving the expulsion of parasitic helminths,87 but this process in ILCs is also important for limiting commensal bacteria-specific CD4+ T-cell responses and restraining intestinal inflammation.86 Indeed, the loss of MHCII expression in ILCs leads to dysfunctional T-cell responses to the microbiota and spontaneous intestinal inflammation.86
Human Intestinal Macrophages and DCs in IBD
Although experimental mouse systems have led to major new insights into the development and function of intestinal macrophages and DCs, human macrophages and DCs do not share some of the markers and functions of their mouse counterparts. For example, human intestinal macrophages do not express CD11b, CD11c, or CX3CR1, which are all highly expressed on mouse intestinal macrophages. Instead, human intestinal macrophages can be identified by the expression of human leukocyte antigen-D related, CD68, and CD13.
Examination of human intestinal biopsy specimens has also identified a unique population of inflammatory macrophages expressing CD14 that may contribute to the pathogenesis of Crohn disease.88 These CD14+ cells, which are derived from blood monocytes, exhibit antigen-presenting ability and are significantly increased in inflamed intestinal tissue.88 Interestingly, monocyte chemoattractant protein-1, the ligand for CCR2, is also significantly up-regulated in mucosal biopsy specimens from inflamed sites and may be responsible for the recruitment of CD14+ mononuclear cells.9 CD14+ macrophages isolated from the inflamed intestine produce high levels of IL-23 and TNF, induce interferon-γ secretion by mononuclear cells, and can promote Th1 and Th17 differentiation.88,89 The ability of these intestinal macrophages to influence T-cell responses may also occur through the secretion of TNF-like ligand 1A (encoded by TNFSF15) that works in cooperation with IL-23.90,91
The study of host genetics has also offered additional insight into the role of intestinal macrophages and DCs in human IBD. Genome-wide association studies have identified numerous susceptibility loci for human Crohn disease and ulcerative colitis. These studies have implicated several high-risk genes that may be involved in intestinal macrophage and DC functions. Of particular interest is the gene encoding the intracellular pattern recognition receptor NOD2. Loss-of-function mutations in NOD2 are currently the strongest genetic link to human IBD development and have been found in approximately 30% of Crohn disease patients with small-intestinal inflammation.92
NOD2, which recognizes the bacterial cell wall component muramyl dipeptide, is expressed in both macrophages and DCs, and impaired signaling in these cells may be involved in the initiation of inflammatory hyperresponsiveness. Chronic stimulation of NOD2 in macrophages leads to down-regulation of TLRs, induction of inflammatory anergy, and increased bactericidal activity. Accordingly, monocyte-derived macrophages from humans harboring loss-of-function NOD2 mutations demonstrate increased reactivity to TLR agonists and decreased bacterial killing.93,94
Mutations in NOD2 have also been linked to aberrant function in intestinal DCs. Intact NOD2 signaling in human DCs is required for the induction of miR-29 that can down-regulate IL-23 production and prevent Th17 cell differentiation. DCs isolated from Crohn disease patients harboring NOD2 polymorphisms fail to induce miR-29 and display increased IL-23 secretion when exposed to bacteria.95
In addition to NOD2, genome-wide association studies have identified several autophagy-related genes, including autophagy-related 16-like 1 (Atg16L1), and the immunity-related GTPase family M (IRGM) that confer susceptibility to Crohn disease.92,96 Atg16L1 and IRGM are expressed by intestinal macrophages and DCs, and dysfunction in these proteins can affect the normal autophagy process.97–100
Autophagy is an important function of macrophages and DCs, and its disruption can lead to improper bacterial trafficking and antigen presentation, which can jeopardize intestinal homeostasis.97,100 Dysfunctional autophagy in these cells is also related to increased proinflammatory cytokine production.98,100 Interestingly, loss of NOD2 leads to dysfunctional autophagy,97 suggesting a direct link between these pathways, which may contribute to human IBD development. Overall, the identification of these risk genes may provide further insight into how to manipulate resident and inflammatory macrophage and DC functions during IBD.11,96
Conclusions
APCs found in the intestine are an integral part of the mucosal immune system in both health and disease. Intestinal macrophages and DCs act in concert to perform a variety of immunoregulatory functions that ultimately help forge a tolerogenic relationship with the microbiota and promote intestinal homeostasis. During inflammation, however, macrophages and DCs can react to microbial components and contribute to intestinal pathology. The decision of tolerance versus overt reactivity is, thus, influenced by how macrophages and DCs integrate signals from the microbiota and immune and nonimmune cells in the local environment. Although animal studies have greatly expanded our knowledge of intestinal APCs, future efforts aimed at understanding intestinal macrophage and DC function in humans are warranted. Continued advancements in the identification and characterization of steady-state and inflammatory APCs in animals and humans will help to clarify how these cells orchestrate mucosal immune responses and afford the opportunity to manipulate these cells for therapeutic purposes in intestinal diseases, such as IBD.
Footnotes
Supported by NIH grants 1R01DK097256 (T.L.D.) and 1F30DK097904-01 (D.G.).
Disclosures: None declared.
The American Society for Investigative Pathology (ASIP) Cotran Early Career Investigator Award recognizes early career investigators with demonstrated excellence as an investigator with recently established or emerging independence and with a research focus leading to an improved understanding of the conceptual basis of disease. Timothy L. Denning, recipient of the ASIP 2014 Cotran Early Career Investigator Award, delivered a lecture entitled “Intestinal Antigen Presenting Cells During Homeostasis and Inflammation” on April 29, 2014, at the annual meeting of the ASIP in San Diego, CA.
References
- 1.Backhed F., Ley R.E., Sonnenburg J.L., Peterson D.A., Gordon J.I. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 2.Maloy K.J., Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 3.Mowat A.M., Agace W.W. Regional specialization within the intestinal immune system. Nat Rev Immunol. 2014;14:667–685. doi: 10.1038/nri3738. [DOI] [PubMed] [Google Scholar]
- 4.Bain C.C., Mowat A.M. Macrophages in intestinal homeostasis and inflammation. Immunol Rev. 2014;260:102–117. doi: 10.1111/imr.12192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bain C.C., Mowat A.M. Intestinal macrophages: specialised adaptation to a unique environment. Eur J Immunol. 2011;41:2494–2498. doi: 10.1002/eji.201141714. [DOI] [PubMed] [Google Scholar]
- 6.Coombes J.L., Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–446. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mowat A.M., Bain C.C. Mucosal macrophages in intestinal homeostasis and inflammation. J Innate Immun. 2011;3:550–564. doi: 10.1159/000329099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pabst O., Bernhardt G. The puzzle of intestinal lamina propria dendritic cells and macrophages. Eur J Immunol. 2010;40:2107–2111. doi: 10.1002/eji.201040557. [DOI] [PubMed] [Google Scholar]
- 9.Smith P.D., Smythies L.E., Shen R., Greenwell-Wild T., Gliozzi M., Wahl S.M. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011;4:31–42. doi: 10.1038/mi.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith P.D., Ochsenbauer-Jambor C., Smythies L.E. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206:149–159. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 11.Abraham C., Medzhitov R. Interactions between the host innate immune system and microbes in inflammatory bowel disease. Gastroenterology. 2011;140:1729–1737. doi: 10.1053/j.gastro.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bain C.C., Bravo-Blas A., Scott C.L., Gomez Perdiguero E., Geissmann F., Henri S., Malissen B., Osborne L.C., Artis D., Mowat A.M. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bain C.C., Scott C.L., Uronen-Hansson H., Gudjonsson S., Jansson O., Grip O., Guilliams M., Malissen B., Agace W.W., Mowat A.M. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamoutounour S., Henri S., Lelouard H., de Bovis B., de Haar C., van der Woude C.J., Woltman A.M., Reyal Y., Bonnet D., Sichien D., Bain C.C., Mowat A.M., Reis e Sousa C., Poulin L.F., Malissen B., Guilliams M. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol. 2012;42:3150–3166. doi: 10.1002/eji.201242847. [DOI] [PubMed] [Google Scholar]
- 15.Zigmond E., Varol C., Farache J., Elmaliah E., Satpathy A.T., Friedlander G., Mack M., Shpigel N., Boneca I.G., Murphy K.M., Shakhar G., Halpern Z., Jung S. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 16.Fogg D.K., Sibon C., Miled C., Jung S., Aucouturier P., Littman D.R., Cumano A., Geissmann F. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311:83–87. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 17.Bogunovic M., Ginhoux F., Helft J., Shang L., Hashimoto D., Greter M., Liu K., Jakubzick C., Ingersoll M.A., Leboeuf M., Stanley E.R., Nussenzweig M., Lira S.A., Randolph G.J., Merad M. Origin of the lamina propria dendritic cell network. Immunity. 2009;31:513–525. doi: 10.1016/j.immuni.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacDonald K.P., Palmer J.S., Cronau S., Seppanen E., Olver S., Raffelt N.C., Kuns R., Pettit A.R., Clouston A., Wainwright B., Branstetter D., Smith J., Paxton R.J., Cerretti D.P., Bonham L., Hill G.R., Hume D.A. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood. 2010;116:3955–3963. doi: 10.1182/blood-2010-02-266296. [DOI] [PubMed] [Google Scholar]
- 19.Ryan G.R., Dai X.M., Dominguez M.G., Tong W., Chuan F., Chisholm O., Russell R.G., Pollard J.W., Stanley E.R. Rescue of the colony-stimulating factor 1 (CSF-1)-nullizygous mouse (Csf1(op)/Csf1(op)) phenotype with a CSF-1 transgene and identification of sites of local CSF-1 synthesis. Blood. 2001;98:74–84. doi: 10.1182/blood.v98.1.74. [DOI] [PubMed] [Google Scholar]
- 20.Varol C., Vallon-Eberhard A., Elinav E., Aychek T., Shapira Y., Luche H., Fehling H.J., Hardt W.D., Shakhar G., Jung S. Intestinal lamina propria dendritic cell subsets have different origin and functions. Immunity. 2009;31:502–512. doi: 10.1016/j.immuni.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 21.Hume D.A., Robinson A.P., MacPherson G.G., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: relationship between macrophages, Langerhans cells, reticular cells, and dendritic cells in lymphoid and hematopoietic organs. J Exp Med. 1983;158:1522–1536. doi: 10.1084/jem.158.5.1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denning T.L., Norris B.A., Medina-Contreras O., Manicassamy S., Geem D., Madan R., Karp C.L., Pulendran B. Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization. J Immunol. 2011;187:733–747. doi: 10.4049/jimmunol.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denning T.L., Wang Y.C., Patel S.R., Williams I.R., Pulendran B. Lamina propria macrophages and dendritic cells differentially induce regulatory and interleukin 17-producing T cell responses. Nat Immunol. 2007;8:1086–1094. doi: 10.1038/ni1511. [DOI] [PubMed] [Google Scholar]
- 24.Niess J.H., Brand S., Gu X., Landsman L., Jung S., McCormick B.A., Vyas J.M., Boes M., Ploegh H.L., Fox J.G., Littman D.R., Reinecker H.C. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 25.Medina-Contreras O., Geem D., Laur O., Williams I.R., Lira S.A., Nusrat A., Parkos C.A., Denning T.L. CX3CR1 regulates intestinal macrophage homeostasis, bacterial translocation, and colitogenic Th17 responses in mice. J Clin Invest. 2011;121:4787–4795. doi: 10.1172/JCI59150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulz O., Jaensson E., Persson E.K., Liu X., Worbs T., Agace W.W., Pabst O. Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med. 2009;206:3101–3114. doi: 10.1084/jem.20091925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rivollier A., He J., Kole A., Valatas V., Kelsall B.L. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scott C.L., Bain C.C., Wright P.B., Sichien D., Kotarsky K., Persson E.K., Luda K., Guilliams M., Lambrecht B.N., Agace W.W., Milling S.W., Mowat A.M. CCR2(+)CD103(-) intestinal dendritic cells develop from DC-committed precursors and induce interleukin-17 production by T cells. Mucosal Immunol. 2015;8:327–339. doi: 10.1038/mi.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smythies L.E., Sellers M., Clements R.H., Mosteller-Barnum M., Meng G., Benjamin W.H., Orenstein J.M., Smith P.D. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kamada N., Hisamatsu T., Okamoto S., Sato T., Matsuoka K., Arai K., Nakai T., Hasegawa A., Inoue N., Watanabe N., Akagawa K.S., Hibi T. Abnormally differentiated subsets of intestinal macrophage play a key role in Th1-dominant chronic colitis through excess production of IL-12 and IL-23 in response to bacteria. J Immunol. 2005;175:6900–6908. doi: 10.4049/jimmunol.175.10.6900. [DOI] [PubMed] [Google Scholar]
- 31.Takada Y., Hisamatsu T., Kamada N., Kitazume M.T., Honda H., Oshima Y., Saito R., Takayama T., Kobayashi T., Chinen H., Mikami Y., Kanai T., Okamoto S., Hibi T. Monocyte chemoattractant protein-1 contributes to gut homeostasis and intestinal inflammation by composition of IL-10-producing regulatory macrophage subset. J Immunol. 2010;184:2671–2676. doi: 10.4049/jimmunol.0804012. [DOI] [PubMed] [Google Scholar]
- 32.Zigmond E., Bernshtein B., Friedlander G., Walker C.R., Yona S., Kim K.W., Brenner O., Krauthgamer R., Varol C., Muller W., Jung S. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40:720–733. doi: 10.1016/j.immuni.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Shouval D.S., Biswas A., Goettel J.A., McCann K., Conaway E., Redhu N.S., Mascanfroni I.D., Al Adham Z., Lavoie S., Ibourk M., Nguyen D.D., Samsom J.N., Escher J.C., Somech R., Weiss B., Beier R., Conklin L.S., Ebens C.L., Santos F.G., Ferreira A.R., Sherlock M., Bhan A.K., Muller W., Mora J.R., Quintana F.J., Klein C., Muise A.M., Horwitz B.H., Snapper S.B. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–719. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubtsov Y.P., Rasmussen J.P., Chi E.Y., Fontenot J., Castelli L., Ye X., Treuting P., Siewe L., Roers A., Henderson W.R., Jr., Muller W., Rudensky A.Y. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 35.Maynard C.L., Harrington L.E., Janowski K.M., Oliver J.R., Zindl C.L., Rudensky A.Y., Weaver C.T. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3- precursor cells in the absence of interleukin 10. Nat Immunol. 2007;8:931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 36.Murai M., Turovskaya O., Kim G., Madan R., Karp C.L., Cheroutre H., Kronenberg M. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nat Immunol. 2009;10:1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hadis U., Wahl B., Schulz O., Hardtke-Wolenski M., Schippers A., Wagner N., Muller W., Sparwasser T., Forster R., Pabst O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity. 2011;34:237–246. doi: 10.1016/j.immuni.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Kitamura K., Farber J.M., Kelsall B.L. CCR6 marks regulatory T cells as a colon-tropic, IL-10-producing phenotype. J Immunol. 2010;185:3295–3304. doi: 10.4049/jimmunol.1001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ehirchiou D., Xiong Y., Xu G., Chen W., Shi Y., Zhang L. CD11b facilitates the development of peripheral tolerance by suppressing Th17 differentiation. J Exp Med. 2007;204:1519–1524. doi: 10.1084/jem.20062292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diehl G.E., Longman R.S., Zhang J.X., Breart B., Galan C., Cuesta A., Schwab S.R., Littman D.R. Microbiota restricts trafficking of bacteria to mesenteric lymph nodes by CX(3)CR1(hi) cells. Nature. 2013;494:116–120. doi: 10.1038/nature11809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu L.M., MacPherson G.G. Antigen acquisition by dendritic cells: intestinal dendritic cells acquire antigen administered orally and can prime naive T cells in vivo. J Exp Med. 1993;177:1299–1307. doi: 10.1084/jem.177.5.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Wilsem E.J., Van Hoogstraten I.M., Breve J., Scheper R.J., Kraal G. Dendritic cells of the oral mucosa and the induction of oral tolerance: a local affair. Immunology. 1994;83:128–132. [PMC free article] [PubMed] [Google Scholar]
- 43.Viney J.L., Mowat A.M., O'Malley J.M., Williamson E., Fanger N.A. Expanding dendritic cells in vivo enhances the induction of oral tolerance. J Immunol. 1998;160:5815–5825. [PubMed] [Google Scholar]
- 44.Worbs T., Bode U., Yan S., Hoffmann M.W., Hintzen G., Bernhardt G., Forster R., Pabst O. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med. 2006;203:519–527. doi: 10.1084/jem.20052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coombes J.L., Siddiqui K.R., Arancibia-Carcamo C.V., Hall J., Sun C.M., Belkaid Y., Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. J Exp Med. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mucida D., Park Y., Kim G., Turovskaya O., Scott I., Kronenberg M., Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 47.Sun C.M., Hall J.A., Blank R.B., Bouladoux N., Oukka M., Mora J.R., Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. J Exp Med. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwata M., Hirakiyama A., Eshima Y., Kagechika H., Kato C., Song S.Y. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 49.Mora J.R., Bono M.R., Manjunath N., Weninger W., Cavanagh L.L., Rosemblatt M., Von Andrian U.H. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 50.Persson E.K., Uronen-Hansson H., Semmrich M., Rivollier A., Hagerbrand K., Marsal J., Gudjonsson S., Hakansson U., Reizis B., Kotarsky K., Agace W.W. IRF4 transcription-factor-dependent CD103(+)CD11b(+) dendritic cells drive mucosal T helper 17 cell differentiation. Immunity. 2013;38:958–969. doi: 10.1016/j.immuni.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 51.Schlitzer A., McGovern N., Teo P., Zelante T., Atarashi K., Low D., Ho A.W., See P., Shin A., Wasan P.S., Hoeffel G., Malleret B., Heiseke A., Chew S., Jardine L., Purvis H.A., Hilkens C.M., Tam J., Poidinger M., Stanley E.R., Krug A.B., Renia L., Sivasankar B., Ng L.G., Collin M., Ricciardi-Castagnoli P., Honda K., Haniffa M., Ginhoux F. IRF4 transcription factor-dependent CD11b+ dendritic cells in human and mouse control mucosal IL-17 cytokine responses. Immunity. 2013;38:970–983. doi: 10.1016/j.immuni.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Satpathy A.T., Briseno C.G., Lee J.S., Ng D., Manieri N.A., Kc W., Wu X., Thomas S.R., Lee W.L., Turkoz M., McDonald K.G., Meredith M.M., Song C., Guidos C.J., Newberry R.D., Ouyang W., Murphy T.L., Stappenbeck T.S., Gommerman J.L., Nussenzweig M.C., Colonna M., Kopan R., Murphy K.M. Notch2-dependent classical dendritic cells orchestrate intestinal immunity to attaching-and-effacing bacterial pathogens. Nat Immunol. 2013;14:937–948. doi: 10.1038/ni.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Laffont S., Siddiqui K.R., Powrie F. Intestinal inflammation abrogates the tolerogenic properties of MLN CD103+ dendritic cells. Eur J Immunol. 2010;40:1877–1883. doi: 10.1002/eji.200939957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ivanov I.I., Atarashi K., Manel N., Brodie E.L., Shima T., Karaoz U., Wei D., Goldfarb K.C., Santee C.A., Lynch S.V., Tanoue T., Imaoka A., Itoh K., Takeda K., Umesaki Y., Honda K., Littman D.R. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Farache J., Koren I., Milo I., Gurevich I., Kim K.W., Zigmond E., Furtado G.C., Lira S.A., Shakhar G. Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity. 2013;38:581–595. doi: 10.1016/j.immuni.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mazzini E., Massimiliano L., Penna G., Rescigno M. Oral tolerance can be established via gap junction transfer of fed antigens from CX3CR1(+) macrophages to CD103(+) dendritic cells. Immunity. 2014;40:248–261. doi: 10.1016/j.immuni.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 57.McDole J.R., Wheeler L.W., McDonald K.G., Wang B., Konjufca V., Knoop K.A., Newberry R.D., Miller M.J. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012;483:345–349. doi: 10.1038/nature10863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Platt A.M., Bain C.C., Bordon Y., Sester D.P., Mowat A.M. An independent subset of TLR expressing CCR2-dependent macrophages promotes colonic inflammation. J Immunol. 2010;184:6843–6854. doi: 10.4049/jimmunol.0903987. [DOI] [PubMed] [Google Scholar]
- 59.Platt A.M., Mowat A.M. Mucosal macrophages and the regulation of immune responses in the intestine. Immunol Lett. 2008;119:22–31. doi: 10.1016/j.imlet.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 60.Su C.G., Wen X., Bailey S.T., Jiang W., Rangwala S.M., Keilbaugh S.A., Flanigan A., Murthy S., Lazar M.A., Wu G.D. A novel therapy for colitis utilizing PPAR-gamma ligands to inhibit the epithelial inflammatory response. J Clin Invest. 1999;104:383–389. doi: 10.1172/JCI7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seno H., Miyoshi H., Brown S.L., Geske M.J., Colonna M., Stappenbeck T.S. Efficient colonic mucosal wound repair requires Trem2 signaling. Proc Natl Acad Sci U S A. 2009;106:256–261. doi: 10.1073/pnas.0803343106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qualls J.E., Kaplan A.M., van Rooijen N., Cohen D.A. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006;80:802–815. doi: 10.1189/jlb.1205734. [DOI] [PubMed] [Google Scholar]
- 64.Hunter M.M., Wang A., Parhar K.S., Johnston M.J., Van Rooijen N., Beck P.L., McKay D.M. In vitro-derived alternatively activated macrophages reduce colonic inflammation in mice. Gastroenterology. 2010;138:1395–1405. doi: 10.1053/j.gastro.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 65.Rani R., Smulian A.G., Greaves D.R., Hogan S.P., Herbert D.R. TGF-beta limits IL-33 production and promotes the resolution of colitis through regulation of macrophage function. Eur J Immunol. 2011;41:2000–2009. doi: 10.1002/eji.201041135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pull S.L., Doherty J.M., Mills J.C., Gordon J.I., Stappenbeck T.S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc Natl Acad Sci U S A. 2005;102:99–104. doi: 10.1073/pnas.0405979102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hart A.L., Al-Hassi H.O., Rigby R.J., Bell S.J., Emmanuel A.V., Knight S.C., Kamm M.A., Stagg A.J. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 68.Spadoni I., Iliev I.D., Rossi G., Rescigno M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol. 2012;5:184–193. doi: 10.1038/mi.2011.64. [DOI] [PubMed] [Google Scholar]
- 69.Uematsu S., Fujimoto K., Jang M.H., Yang B.G., Jung Y.J., Nishiyama M., Sato S., Tsujimura T., Yamamoto M., Yokota Y., Kiyono H., Miyasaka M., Ishii K.J., Akira S. Regulation of humoral and cellular gut immunity by lamina propria dendritic cells expressing Toll-like receptor 5. Nat Immunol. 2008;9:769–776. doi: 10.1038/ni.1622. [DOI] [PubMed] [Google Scholar]
- 70.Kinnebrew M.A., Buffie C.G., Diehl G.E., Zenewicz L.A., Leiner I., Hohl T.M., Flavell R.A., Littman D.R., Pamer E.G. Interleukin 23 production by intestinal CD103(+)CD11b(+) dendritic cells in response to bacterial flagellin enhances mucosal innate immune defense. Immunity. 2012;36:276–287. doi: 10.1016/j.immuni.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chung H., Pamp S.J., Hill J.A., Surana N.K., Edelman S.M., Troy E.B., Reading N.C., Villablanca E.J., Wang S., Mora J.R., Umesaki Y., Mathis D., Benoist C., Relman D.A., Kasper D.L. Gut immune maturation depends on colonization with a host-specific microbiota. Cell. 2012;149:1578–1593. doi: 10.1016/j.cell.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.McDonald K.G., McDonough J.S., Dieckgraefe B.K., Newberry R.D. Dendritic cells produce CXCL13 and participate in the development of murine small intestine lymphoid tissues. Am J Pathol. 2010;176:2367–2377. doi: 10.2353/ajpath.2010.090723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoshi N., Schenten D., Nish S.A., Walther Z., Gagliani N., Flavell R.A., Reizis B., Shen Z., Fox J.G., Iwasaki A., Medzhitov R. MyD88 signalling in colonic mononuclear phagocytes drives colitis in IL-10-deficient mice. Nat Commun. 2012;3:1120. doi: 10.1038/ncomms2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ueda Y., Kayama H., Jeon S.G., Kusu T., Isaka Y., Rakugi H., Yamamoto M., Takeda K. Commensal microbiota induce LPS hyporesponsiveness in colonic macrophages via the production of IL-10. Int Immunol. 2010;22:953–962. doi: 10.1093/intimm/dxq449. [DOI] [PubMed] [Google Scholar]
- 75.Longman R.S., Diehl G.E., Victorio D.A., Huh J.R., Galan C., Miraldi E.R., Swaminath A., Bonneau R., Scherl E.J., Littman D.R. CX(3)CR1(+) mononuclear phagocytes support colitis-associated innate lymphoid cell production of IL-22. J Exp Med. 2014;211:1571–1583. doi: 10.1084/jem.20140678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mortha A., Chudnovskiy A., Hashimoto D., Bogunovic M., Spencer S.P., Belkaid Y., Merad M. Microbiota-dependent crosstalk between macrophages and ILC3 promotes intestinal homeostasis. Science. 2014;343:1249288. doi: 10.1126/science.1249288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Round J.L., Mazmanian S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci U S A. 2010;107:12204–12209. doi: 10.1073/pnas.0909122107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Atarashi K., Tanoue T., Shima T., Imaoka A., Kuwahara T., Momose Y., Cheng G., Yamasaki S., Saito T., Ohba Y., Taniguchi T., Takeda K., Hori S., Ivanov I.I., Umesaki Y., Itoh K., Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dasgupta S., Erturk-Hasdemir D., Ochoa-Reparaz J., Reinecker H.C., Kasper D.L. Plasmacytoid dendritic cells mediate anti-inflammatory responses to a gut commensal molecule via both innate and adaptive mechanisms. Cell Host Microbe. 2014;15:413–423. doi: 10.1016/j.chom.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mazmanian S.K., Round J.L., Kasper D.L. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 81.Singh N., Gurav A., Sivaprakasam S., Brady E., Padia R., Shi H., Thangaraju M., Prasad P.D., Manicassamy S., Munn D.H., Lee J.R., Offermanns S., Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geem D., Medina-Contreras O., McBride M., Newberry R.D., Koni P.A., Denning T.L. Specific microbiota-induced intestinal Th17 differentiation requires MHC class II but not GALT and mesenteric lymph nodes. J Immunol. 2014;193:431–438. doi: 10.4049/jimmunol.1303167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Goto Y., Panea C., Nakato G., Cebula A., Lee C., Diez M.G., Laufer T.M., Ignatowicz L., Ivanov I.I. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang Y., Torchinsky M.B., Gobert M., Xiong H., Xu M., Linehan J.L., Alonzo F., Ng C., Chen A., Lin X., Sczesnak A., Liao J.J., Torres V.J., Jenkins M.K., Lafaille J.J., Littman D.R. Focused specificity of intestinal TH17 cells towards commensal bacterial antigens. Nature. 2014;510:152–156. doi: 10.1038/nature13279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Atarashi K., Nishimura J., Shima T., Umesaki Y., Yamamoto M., Onoue M., Yagita H., Ishii N., Evans R., Honda K., Takeda K. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008;455:808–812. doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]
- 86.Hepworth M.R., Monticelli L.A., Fung T.C., Ziegler C.G., Grunberg S., Sinha R., Mantegazza A.R., Ma H.L., Crawford A., Angelosanto J.M., Wherry E.J., Koni P.A., Bushman F.D., Elson C.O., Eberl G., Artis D., Sonnenberg G.F. Innate lymphoid cells regulate CD4+ T-cell responses to intestinal commensal bacteria. Nature. 2013;498:113–117. doi: 10.1038/nature12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oliphant C.J., Hwang Y.Y., Walker J.A., Salimi M., Wong S.H., Brewer J.M., Englezakis A., Barlow J.L., Hams E., Scanlon S.T., Ogg G.S., Fallon P.G., McKenzie A.N. MHCII-mediated dialog between group 2 innate lymphoid cells and CD4(+) T cells potentiates type 2 immunity and promotes parasitic helminth expulsion. Immunity. 2014;41:283–295. doi: 10.1016/j.immuni.2014.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kamada N., Hisamatsu T., Okamoto S., Chinen H., Kobayashi T., Sato T., Sakuraba A., Kitazume M.T., Sugita A., Koganei K., Akagawa K.S., Hibi T. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kamada N., Hisamatsu T., Honda H., Kobayashi T., Chinen H., Kitazume M.T., Takayama T., Okamoto S., Koganei K., Sugita A., Kanai T., Hibi T. Human CD14+ macrophages in intestinal lamina propria exhibit potent antigen-presenting ability. J Immunol. 2009;183:1724–1731. doi: 10.4049/jimmunol.0804369. [DOI] [PubMed] [Google Scholar]
- 90.Kamada N., Hisamatsu T., Honda H., Kobayashi T., Chinen H., Takayama T., Kitazume M.T., Okamoto S., Koganei K., Sugita A., Kanai T., Hibi T. TL1A produced by lamina propria macrophages induces Th1 and Th17 immune responses in cooperation with IL-23 in patients with Crohn's disease. Inflamm Bowel Dis. 2010;16:568–575. doi: 10.1002/ibd.21124. [DOI] [PubMed] [Google Scholar]
- 91.Takedatsu H., Michelsen K.S., Wei B., Landers C.J., Thomas L.S., Dhall D., Braun J., Targan S.R. TL1A (TNFSF15) regulates the development of chronic colitis by modulating both T-helper 1 and T-helper 17 activation. Gastroenterology. 2008;135:552–567. doi: 10.1053/j.gastro.2008.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cho J.H., Weaver C.T. The genetics of inflammatory bowel disease. Gastroenterology. 2007;133:1327–1339. doi: 10.1053/j.gastro.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 93.Hedl M., Abraham C. NLRP1 and NLRP3 inflammasomes are essential for distinct outcomes of decreased cytokines but enhanced bacterial killing upon chronic Nod2 stimulation. Am J Physiol Gastrointest Liver Physiol. 2013;304:G583–G596. doi: 10.1152/ajpgi.00297.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hedl M., Li J., Cho J.H., Abraham C. Chronic stimulation of Nod2 mediates tolerance to bacterial products. Proc Natl Acad Sci U S A. 2007;104:19440–19445. doi: 10.1073/pnas.0706097104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brain O., Owens B.M., Pichulik T., Allan P., Khatamzas E., Leslie A., Steevels T., Sharma S., Mayer A., Catuneanu A.M., Morton V., Sun M.Y., Jewell D., Coccia M., Harrison O., Maloy K., Schonefeldt S., Bornschein S., Liston A., Simmons A. The intracellular sensor NOD2 induces microRNA-29 expression in human dendritic cells to limit IL-23 release. Immunity. 2013;39:521–536. doi: 10.1016/j.immuni.2013.08.035. [DOI] [PubMed] [Google Scholar]
- 96.Stappenbeck T.S., Rioux J.D., Mizoguchi A., Saitoh T., Huett A., Darfeuille-Michaud A., Wileman T., Mizushima N., Carding S., Akira S., Parkes M., Xavier R.J. Crohn disease: a current perspective on genetics, autophagy and immunity. Autophagy. 2011;7:355–374. doi: 10.4161/auto.7.4.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cooney R., Baker J., Brain O., Danis B., Pichulik T., Allan P., Ferguson D.J., Campbell B.J., Jewell D., Simmons A. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16:90–97. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- 98.Lassen K.G., Kuballa P., Conway K.L., Patel K.K., Becker C.E., Peloquin J.M., Villablanca E.J., Norman J.M., Liu T.C., Heath R.J., Becker M.L., Fagbami L., Horn H., Mercer J., Yilmaz O.H., Jaffe J.D., Shamji A.F., Bhan A.K., Carr S.A., Daly M.J., Virgin H.W., Schreiber S.L., Stappenbeck T.S., Xavier R.J. Atg16L1 T300A variant decreases selective autophagy resulting in altered cytokine signaling and decreased antibacterial defense. Proc Natl Acad Sci U S A. 2014;111:7741–7746. doi: 10.1073/pnas.1407001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Murthy A., Li Y., Peng I., Reichelt M., Katakam A.K., Noubade R., Roose-Girma M., DeVoss J., Diehl L., Graham R.R., van Lookeren Campagne M. A Crohn's disease variant in Atg16l1 enhances its degradation by caspase 3. Nature. 2014;506:456–462. doi: 10.1038/nature13044. [DOI] [PubMed] [Google Scholar]
- 100.Saitoh T., Fujita N., Jang M.H., Uematsu S., Yang B.G., Satoh T., Omori H., Noda T., Yamamoto N., Komatsu M., Tanaka K., Kawai T., Tsujimura T., Takeuchi O., Yoshimori T., Akira S. Loss of the autophagy protein Atg16L1 enhances endotoxin-induced IL-1beta production. Nature. 2008;456:264–268. doi: 10.1038/nature07383. [DOI] [PubMed] [Google Scholar]