Abstract
Hepatic expression levels of CXCL12, a chemokine important in inflammatory and stem cell recruitment, and its receptor, C-X-C chemokine receptor 4, are increased during all forms of liver injury. CXCL12 is expressed by both parenchymal and nonparenchymal hepatic cells, and on the basis of immunohistochemistry, biliary epithelial cells (BECs) are thought to be a predominant source of hepatic CXCL12, thereby promoting periportal recruitment of C-X-C chemokine receptor 4–expressing lymphocytes. Our study aims to show that BECs may, in fact, not be the predominant source of hepatic CXCL12. We measured CXCL12 secretion and expression from human and murine BECs using enzyme-linked immunosorbent assay and Western blot analysis from cell culture supernatants and whole cell lysates, respectively, whereas CXCL12 expression in murine livers was analyzed in a Cxcl12-Gfp reporter mouse. Cell culture supernatants and whole cell lysates from BECs failed to demonstrate their expression of CXCL12. Furthermore, we confirmed these results with a Cxcl12-Gfp reporter mouse in which green fluorescent protein expression is notably absent from BECs. Interestingly, on the basis of green fluorescent protein expression, we demonstrate a population of CXCL12-expressing cells within the portal tract that are distinct, yet intimately associated with BECs. These findings indicate that BECs are not a predominant source of CXCL12.
CXCL12, a chemokine important in hematopoietic stem cell homeostasis, and its receptor, C-X-C chemokine receptor 4 (CXCR4), are up-regulated in many disease pathologies and promote inflammation and tumor metastasis.1 Specifically, in patients with liver disease, CXCL12 expression is increased in both serum and hepatic tissue proportional to the extent of injury.2 CXCL12 expression has been documented in stellate cells, sinusoidal endothelial cells, and biliary epithelial cells (BECs) and is thought to drive inflammation and fibrogenesis, although its role, particularly in normal liver, remains largely unknown.3 During fetal development, B-cell lymphopoiesis is dependent on hepatic CXCL12,4 and in the adult, hepatic CXCL12 may support a hepatic hematopoietic stem cell niche.5 Finally, with injury, immunohistochemical data demonstrate robust CXCL12 expression by proliferating bile ductules in all forms of liver injury, and biliary CXCL12 expression is further supported by the accumulation of CXCR4-positive lymphocytes in the periportal region.2,6
A careful review of the literature, however, reveals that most data supporting BEC expression of CXCL12 are based on immunohistochemistry using a single monoclonal CXCL12 antibody (murine anti-human/mouse CXCL12, clone 79018). We believe that differentiated BECs may not express CXCL12 and that the observed pattern of BEC expression is a result of antibody cross-reactivity to an epitope found in BECs. Lack of CXCL12 expression by BECs has previously been alluded to by Mavier et al7 using in situ hybridization, where CXCL12 RNA expression in biliary cells lining interlobular bile ducts seemed to be absent. Herein, we show that despite previous studies demonstrating robust CXCL12 expression by BECs, their role in hepatic CXCL12 production may be more limited.2,6,8
Materials and Methods
Ethics Statement
All animal studies were conducted in accordance with and approved by the Institutional Animal Care and Use Committees/Ethics Committee of Kyoto University (Kyoto, Japan). For immunohistochemistry on human liver, deidentified waste or archived tissue was provided to the investigators. The Icahn School of Medicine at Mount Sinai (New York, NY) Institutional Review Board exempted this study from review (exempt category 4) because samples were considered waste or archived material and waived the need for consent because of the fact that the samples received were deidentified and the investigators had no way of tracking the samples back to the individual patients.
Cell Lines
Experiments were performed with human and murine BEC lines. H69 is a human SV40 immortalized BEC line derived from normal liver and grown in a hormone-supplemented medium (kindly provided by Dr. Douglas Jefferson, Tufts University, Medford, MA)9; MMNK-1 is a fetal human liver BEC line that was first transfected with SV40, followed by transduction with human telomerase reverse transcriptase10; 603B is a nontumorigenic murine cholangiocyte cell line immortalized with the SV40 T antigen (kindly provided by Dr. Yoshiyuki Ueno, Yamagata University, Yamagata, Japan)11; LX2 cells are a spontaneously immortalized human stellate cell line12; and JS1 cells are a murine SV40 immortalized hepatic stellate cell line with a highly activated phenotype.13
Isolation of Primary Murine Cholangiocytes
Primary murine cholangiocytes were isolated from wild-type C57/Bl6 mice (n = 3 independent isolations), as previously described.14,15 Briefly, intrahepatic bile ducts were microdissected, disassociated, and grown in Dulbecco’s modified Eagle’s medium with 10% fetal bovine serum.
Cxcl12-GFP Mice
Green fluorescent protein (GFP) expression was detected in livers from mice in which Gfp was knocked into exon 2 of the murine Cxcl12 locus, as previously described and well validated.16–21 GFP expression by a cell indicates transcription of the Cxcl12 gene. Mice are hemizygous for both Cxcl12 and GFP, with no abnormalities.
CXCL12 ELISA
To evaluate whether cholangiocytes secrete CXCL12, an enzyme-linked immunosorbent assay (ELISA) was performed on cell culture–conditioned media. Cells (2 × 104) were plated in 24-well plates and grown for 24 hours before adding 300 μL of fresh serum-free media. Cell culture supernatant was collected between 0 and 96 hours, centrifuged to remove particulate debris, and frozen at −80°C until time of assay. Samples were prepared, and assay was performed according to the manufacturer's protocol (catalog number DSA00; R&D Systems, Minneapolis, MN). Media incubated without cells were used as a negative control. Standard curves were generated using purified recombinant species-appropriate CXCL12. For cholangiocyte stimulation, cells were treated with either tumor necrosis factor-α (500 U/mL) or lipopolysaccharide (100 ng/mL) in 300 μL serum-free media for 24 hours.
Absolute Quantification of CXCL12 mRNA
To evaluate absolute copy numbers of CXCL12, unstimulated cholangiocyte and stellate cell lines and primary murine cholangiocytes were harvested and cell pellets frozen at −80°C. Total RNA was isolated using RNeasy Mini isolation kits (Qiagen, Valencia, CA). RNA concentration and purity were estimated using a NanoDrop 2000c (Thermo Scientific, Wilmington, DE) and 1 μg of RNA reverse transcribed into cDNA using EcoDry Premix (Clontech, Mountain View, CA). For production of standard curves, equal amounts of cDNA were amplified by PCRs (Thermo Scientific, Waltham, MA), with primers for the following genes: human CXCL12 (forward, 5′-AACACTCCAAACTGTGCCCT-3′; reverse, 5′-AGTGGGTCTAGCGGAAAGTC-3′), murine Cxcl12 (forward, 5′-GCTCTGCATCAGTGACGGTA-3′; reverse, 5′-AGATGCTTGACGTTGGCTCT-3′), and human/murine GAPDH (forward, 5′-CAATGACCCCTTCATTGACC-3′; reverse, 5′-GATCTCGCTCCTGGAAGATG-3′). PCR product concentrations were determined and converted to copy number on the basis of amplicon length. Serial dilutions of gene-specific PCR product were used in real-time quantitative PCR (qPCR) reactions to generate a standard curve of copy number versus cycle number. qPCR from cholangiocytes and stellate cells was performed with cDNA representing 2.5 ng of total RNA, using SYBR Green Mastermix (BioRad, Hercules, CA). Absolute copy number of CXCL12/μg of total RNA was calculated using the generated standard curves and normalized to GAPDH.
Immunohistochemistry
For tissue staining, fresh human or murine liver tissue was fixed in 4% paraformaldehyde for 2 hours, followed by immersion in graded sucrose solutions to a final concentration of 30%. Liver tissue was then flash frozen in OCT, and sections (8 μm thick) were cut and stored at −80°C until use. Sections were thawed, blocked in 1% bovine serum albumin–phosphate-buffered saline with Tween 20 (0.5%), and incubated with the following primary antibodies for 2 hours at room temperature: mouse anti-mouse/human monoclonal CXCL12, 1:200 dilution (clone number 79018; R&D Systems), mouse anti-human monoclonal cytokeratin 19, 1:100 dilution (clone number RCK108; Dako, Carpinteria, CA), rat anti-mouse monoclonal MIC1-1C3, 1:100 dilution (clone number MIC1-1C3; Thermo Scientific), rabbit anti-human/mouse polyclonal desmin, 1:100 dilution (catalog number 10570; Progen Biotechnik, Heidelberg, Germany), IgY-fraction chicken anti-GFP, 1:200 dilution (catalog number A10262; Life Technologies, Carlsbad, CA), rabbit anti-human/mouse polyclonal CXCL12, 1:100 dilution (catalog number sc-28876; Santa Cruz Biotechnology Inc., Santa Cruz, CA), rabbit anti-mouse/rat polyclonal CXCL12, 1:100 dilution (catalog number 14-7992; eBiosciences, San Diego, CA), and rabbit anti-human/mouse polyclonal CXCL12, 1:50 dilution (catalog number ab25117; Abcam, Cambridge, MA). Slides were then washed 3× in phosphate-buffered saline, followed by a 30-minute incubation with species-appropriate secondary antibodies: goat anti-chicken IgY fraction AlexaFluor 488, goat anti-mouse AlexaFluor 488, goat anti-rabbit AlexaFluor 594, donkey anti-rabbit AlexaFluor 594, goat anti-mouse AlexaFluor 647 (Life Technologies), and goat anti-rat AlexaFluor 647 (Life Technologies) at dilutions of 1:200 to 1:500. Slides were rinsed in phosphate-buffered saline 2× and mounted with ProLong Gold mounting media containing DAPI (Life Technologies). Images were acquired with either a Zeiss Axiophot 2 fluorescence microscope (Carl Zeiss Microscopy, Thornwood, NY) or a Leica upright scanning confocal microscope (Leica Microsystems, Buffalo Grove, IL). Pseudocolor was applied to fluorescent images for consistency throughout.
Quantification of Cxcl12-Expressing Cells within the Hepatic Lobule
The number of GFP-expressing cells was assessed by counting all GFP-positive cells between a portal tract and an adjacent central vein. The region of interest was defined as a 500-μm wide area encompassing the hepatic parenchyma between a portal tract and central vein. The number of GFP-positive cells is the average of 10 images at ×200 magnification per animal (n = 5).
Immunoblots
Expression of CXCL12 was examined in numerous cholangiocyte cell lines. Briefly, 40 μg of cellular protein in radioimmunoprecipitation assay buffer was separated by SDS-PAGE (4% to 12% gradient gel; NuPage, Carlsbad, CA), transferred to a polyvinylidene difluoride membrane, blocked overnight in 5% bovine serum albumin–phosphate-buffered saline with Tween 20, and probed for CXCL12 for 48 hours. GAPDH was used as a loading control. The following antibodies with corresponding dilutions were used: goat anti-mouse/human polyclonal CXCL12 (1:1000 dilution; R&D Systems; catalog number AF-310-NA), derived from the same immunogen as clone 79018, and rabbit anti-mouse polyclonal GAPDH (1:3000 dilution; Santa Cruz Biotechnology Inc.). Species appropriate HRP-conjugated secondary antibodies were used at 1:1000 to 1:3000 dilutions and blots developed using Immobilon Western Chemiluminescent HRP Substrate (Millipore, Billercia, MA).
Statistical Analysis
All results are expressed as the means ± SD. Statistical significance was tested using an unpaired Student's t and P < 0.05 indicated a significant difference.
Results
Detection of CXCL12 Staining in BECs with CXCL12 Antibody Clone 79081
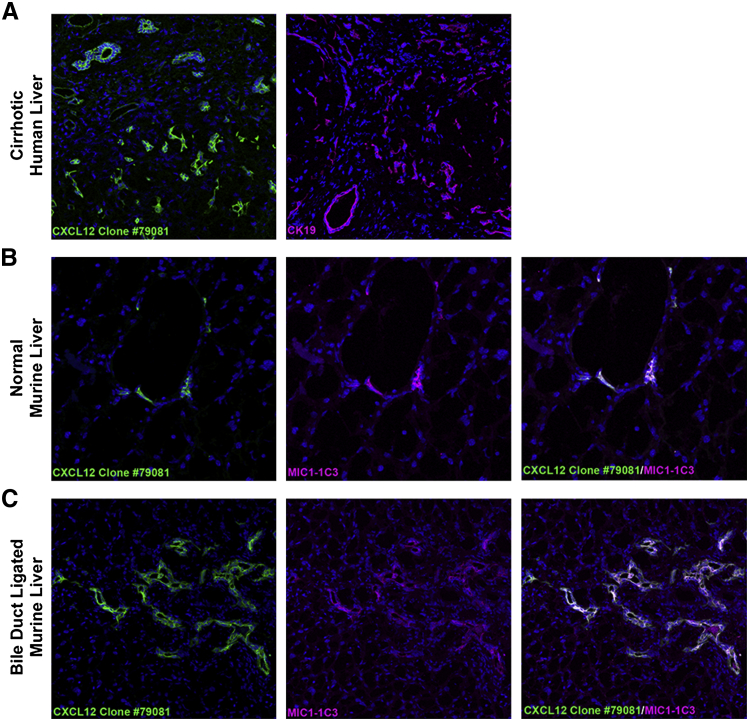
We confirmed previous reports suggesting CXCL12 expression by cholangiocytes using the same antibody (clone 79081) in both human and murine livers.2,25 In cirrhotic human livers, cholangiocytes, identified by cytokeratin 19, show similar distribution of staining as CXCL12 in hepatic sections from the same patient (Figure 1A). Similarly, in normal and bile duct ligated murine livers, CXCL12-expressing cholangiocytes were identified by dual immunofluorescence for CXCL12 and MIC1-1C3, a biliary progenitor cell marker (Figure 1, B and C, respectively). Similar to previous reports, we visualized strong CXCL12 staining in all BECs.2,8
Figure 1.
Colocalization of CXCL12 and biliary epithelial cells (BECs) in human and murine livers. A: Frozen sections from cirrhotic human livers show similar expression patterns of CXCL12 (green, clone 79081) and cholangiocytes, identified by expression of cytokeratin 19 (CK19; magenta). B and C: Uninjured (B) and bile duct–ligated (C) murine livers costained with CXCL12 (green) and BEC marker, MIC1-1C3 (magenta), show BEC expression of CXCL12 (white). n = 5 (A); n = 6 (B and C). Original magnification, ×400.
BEC Cultures neither Secrete nor Express CXCL12
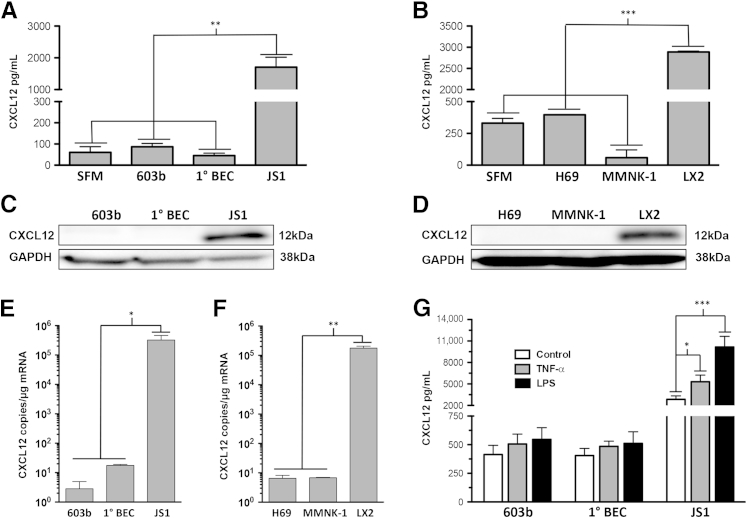
Despite immunofluorescence evidence of CXCL12 expression by BECs, using ELISA, we were unable to demonstrate CXCL12 secretion by BECs. By using CXCL12 antibody clone 79081 as the capture antibody, we failed to show any secreted CXCL12 from murine (Figure 2A) and human (Figure 2B) cholangiocyte cell culture supernatants. This was in contrast to cell culture supernatants from murine and human hepatic stellate cells that showed robust CXCL12 expression (Figure 2, A and B) and continued to increase the expression levels of CXCL12 up to 96 hours (Supplemental Figure S1). Consistent with our ELISA data, Western blot analysis failed to detect CXCL12 expression from whole cell protein lysates of murine (Figure 2C) and human (Figure 2D) biliary cell lines and primary murine cholangiocytes. Strong expression was nonetheless seen in the murine and human hepatic stellate cell lines (Figure 2, C and D). Similarly, absolute quantification of mRNA copy number from these cells demonstrated negligible CXCL12 mRNA transcripts (<20 copies per μg mRNA) in all cholangiocyte cell types, whereas in murine and human stellate cells, there are were between 1.8 × 105 and 3.2 × 105 copies per μg of mRNA (Figure 2, E and F, respectively). Finally, we showed by ELISA on cell culture supernatants that neither primary murine cholangiocytes nor a murine cholangiocyte cell line secreted CXCL12 after stimulation with known activators tumor necrosis factor-α and lipopolysaccharide, whereas in stellate cells, there were 1.8- and 3.5-fold increases in CXCL12 secretion, respectively (Figure 2G).
Figure 2.
Murine and human cholangiocytes do not express CXCL12. Enzyme-linked immunosorbent assay (ELISA) from cell culture supernatants collected at 24 hours from murine (A) and human (B) cholangiocytes shows no significant CXCL12 secretion compared with serum-free media (SFM). Murine (JS1) and human (LX2) hepatic stellate cell lines, used as positive controls, show robust CXCL12 secretion. Immunoblots from murine (C) and human (D) cholangiocytes demonstrate no band at the expected location for CXCL12, whereas strong bands are seen in stellate cell protein lysates. Absolute quantification of CXCL12 copy number per microgram of total mRNA in murine (E) and human (F) cholangiocytes shows minimal CXCL12 transcript compared with stellate cells. G: Treatment of a murine cholangiocyte cell line (603b) or primary murine cholangiocytes with 500 U/mL tumor necrosis factor-α (TNF-α) or 100 ng/mL lipopolysaccharide (LPS), factors known to stimulate cholangiocytes, does not lead to any CXCL12 production, whereas JS1 murine hepatic stellate cell secretion is increased by 1.8- and 3.5-fold, respectively. n = 3 independent experiments for ELISA, immunoblot assays, and real-time quantitative PCR. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. BEC, biliary epithelial cell; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
In Vivo CXCL12 Reporter Mouse Does Not Demonstrate CXCL12 BEC Expression
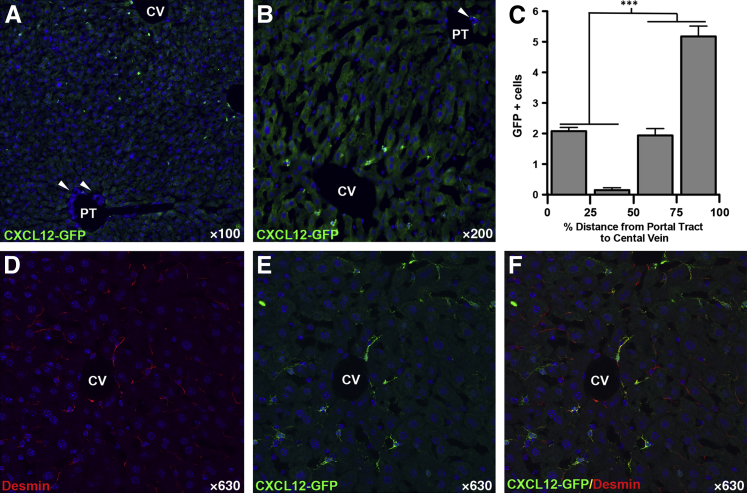
To confirm our results, we used an in vivo reporter mouse model in which the second exon of Cxcl12 was replaced with Gfp, as previously described.22 Any cells that express Cxcl12 are effectively tattooed with GFP, allowing for detection of endogenous Cxcl12 expression without concern of antibody cross-reactivity. We confirmed the presence of Cxcl12-epitope in GFP-positive cells with an alternate CXCL12 antibody that does not stain cholangiocytes (Supplemental Figure S2 and Supplemental Figure S3, C, D, I, and J). Livers from normal, uninjured mice, were stained with antibodies against GFP (Figure 3A) to enhance endogenous GFP fluorescence, and with antibodies recognizing MIC1-1C3, a cholangiocyte marker (Figure 3B), and desmin, a hepatic stellate cell marker (Figure 3D). Numerous GFP-positive, Cxcl12-expressing cells were seen within a portal tract (Figure 3A). Coexpression of Cxcl12 (green) with BEC marker MIC-1C3 (magenta) should appear white (Figure 3C); however, while there was a close association between Cxcl12-expressing cells and BECs, there was no evidence of Cxcl12 expression by BECs (Figure 3C). Staining for desmin, however, identified many of these Cxcl12-expressing cells as stellate cells (Figure 3E). Although none of the cholangiocytes expressed Cxcl12, all of the Cxcl12-expressing cells in the portal tract were in direct contact with bile ducts.
Figure 3.
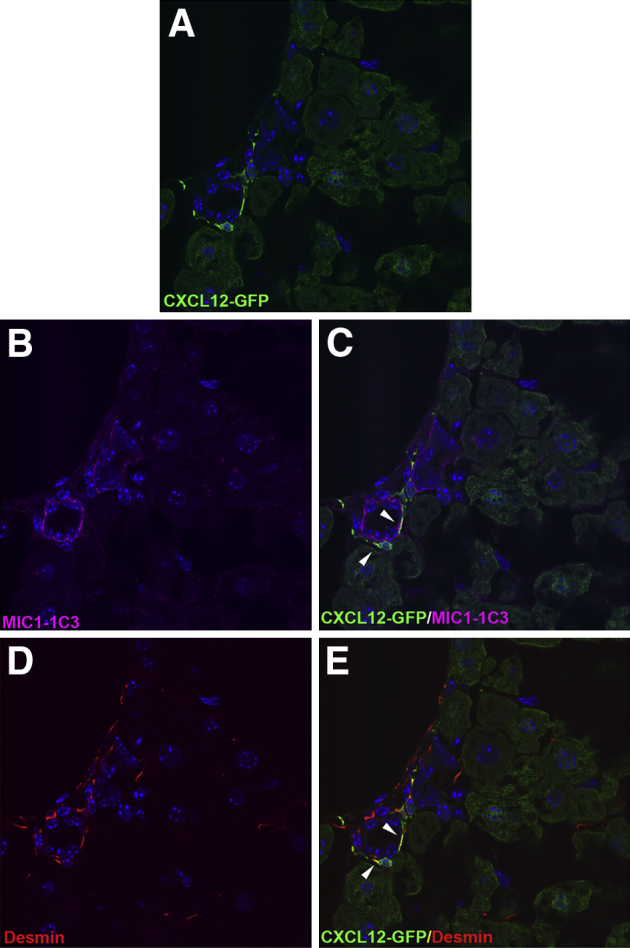
Cxcl12-GFP reporter mice show no green fluorescent protein (GFP) expression in biliary epithelial cells (BECs). Confocal images of liver sections from mice expressing Gfp in the Cxcl12 locus show no GFP expression in BECs. A and B: Cxcl12-expressing cells appear green (A), with MIC1-1C3–expressing cholangiocytes in magenta (B). C: Expression of Cxcl12 by BECs would appear white, which is notably absent. There is, however, an intimate association between Cxcl12-expressing cells and BECs (arrowheads). D and E: These Cxcl12-expressing cells surrounding the bile duct can be identified as hepatic stellate cells given their coexpression of desmin (arrowheads). n = 5. Original magnification, ×630.
Cxcl12-Expressing Cells Are Preferentially Located Near the Central Vein
Careful examination of Cxcl12-expressing cells within the hepatic parenchyma revealed a differential expression pattern throughout the liver. In the periportal region, these cells were found only in close association with bile ducts, whereas none were observed in the adjacent parenchyma. Conversely, around the central vein, Cxcl12-expressing cells were more abundant, with the most found near the central vein (Figure 4, A and B). Quantification of GFP-expressing cells showed that in the 50% of hepatic parenchyma closer to a central vein than an adjacent portal tract, there were threefold more Cxcl12-expressing cells (7.1 versus 2.2 cells; P < 0.001) (Figure 4C). Many, but not all, of these Cxcl12-positive cells were identified as stellate cells on the basis of desmin expression (Figure 4, C–F).
Figure 4.
Increased number of Cxcl12-expressing cells in central vein (CV) region compared with the portal tract (PT) are hepatic stellate cells. A and B: Cxcl12-expressing cells (green) can be seen directly adjacent to bile ducts in the PT (arrowheads pointing to bile duct) and around a CV, with more Cxcl12-expressing cells seen in the vicinity of the CV compared with the PT. C: Quantification of green fluorescent protein (GFP)–positive cells shows threefold more cells located in the proximity of the CV, defined as 50% to 100% of PT to CV distance. D–F: Coimmunostaining for stellate cell marker desmin (D, red) shows that many of these CXCL12-expressing cells (E, green) surrounding the CV are hepatic stellate cells (F, yellow). n = 5. ∗∗∗P < 0.001.
Discussion
BECs play a central role in hepatic injury by secreting chemokines, cytokines, and growth factors involved in driving fibrogenesis. Together with the canals of Herring, cholangiocytes are considered to constitute a special hepatic niche harboring hepatic and hematopoietic stem cells.23–25 CXCL12, and specifically cholangiocyte-derived CXCL12, plays a role in sustaining these unique hepatic niches and promoting accumulation of periportal CXCR4-expressing lymphocytes.2 Similar to its function in the bone marrow, hepatic CXCL12 can recruit CD34+ bone marrow stem cells to the periportal region and may be important in sustaining a population of hematopoietic stem cells within the liver.8 Furthermore, differential expression of CXCL12 and its receptors promotes profibrotic changes within the hepatic vascular niche during injury.26 Finally, in prostate and colon cancers, CXCR4 expression by tumor cells signifies their metastatic potential, and hepatic metastases of these tumors demonstrate a predilection for CXCL12-expressing niches within the liver.27–29
Despite numerous reports demonstrating immunohistochemical evidence of CXCL12 expression by cholangiocytes, we show that human and murine cholangiocytes do not appear to secrete or express CXCL12. Furthermore, we show that even stimulation with factors known to activate BECs fails to promote CXCL12 secretion from both cell line and primary cholangiocytes.
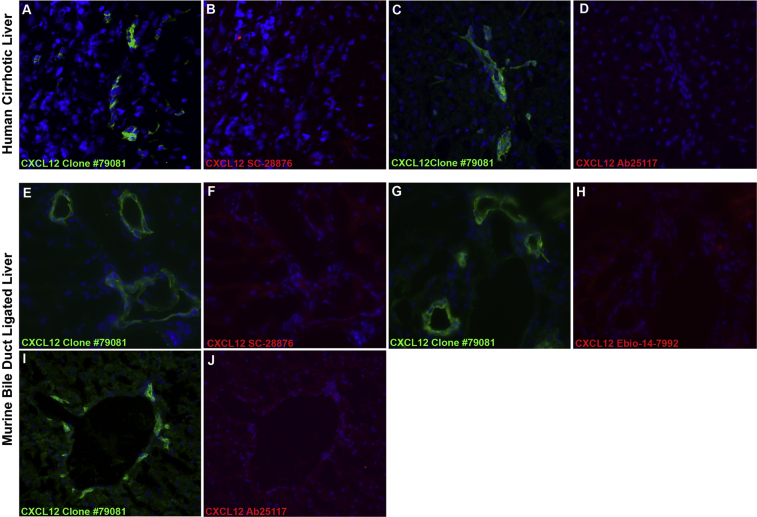
In our hands, immunoblots of cholangiocyte whole cell protein lysates fail to demonstrate a band correlating to the molecular weight of CXCL12. Because CXCL12 binds to cell surface heparan sulfates, it remains possible that the observed BEC expression of CXCL12 may be due to adherence of CXCL12 secreted by neighboring cells.30 This is unlikely, however, given the robust staining pattern seen with CXCL12 antibody clone 79081, which is notably absent from BECs when stained with three additional commercially available antibodies (Supplemental Figure S3, A–J), further suggesting a cross-reactive epitope specifically to clone 79081. We confirmed our results with a transgenic knock-in murine model in which the second exon of Cxcl12 is replaced with Gfp. Expression of GFP by a cell indicated active Cxcl12 transcription and, conversely, lack of GFP expression indicated no Cxcl12 expression by that cell. We show that BECs are not GFP positive, indicating that they do no express Cxcl12. Interestingly, we show a population of Cxcl12-expressing cells within the portal tract that are intimately associated with BECs and that universally express desmin. On the basis of their desmin expression, these cells are likely hepatic stellate cells which are known to play a major role in hepatic fibrogenesis.12,31
Finally, we make the novel observation that Cxcl12-expressing cells are differentially located throughout the liver, with a greater density found near the central vein. Cxcl12-expressing cells near the portal tract are exclusively found in direct contact to BECs and not in the adjacent parenchyma. Alternatively, a round the central vein, Cxcl12-expressing cells are more numerous and diffusely scattered.
Hypoxia and similarly, low oxygen tension are well-known regulators of CXCL12 expression through a hypoxia-inducible factor-1α–dependent mechanism.32 The relationship between CXCL12 and low oxygen tension is also seen within the bone marrow stem cell niche, where regions of hypoxia demonstrate increased CXCL12 expression.33,34 Similar to the bone marrow compartment, the liver is considered a low oxygen tension organ. The hepatic lobule can be divided into three functional zones, each with different oxygen tensions that decrease from zone 1 (60 to 65 mmHg), directly adjacent to the portal tract, to zone 3 (30 to 35 mmHg), at the level of the central vein.34 The increased number of Cxcl12-expressing cells observed specifically surrounding the central vein may, therefore, be due to the relative lower oxygen tension in zone 3 of the hepatic lobule, although this observation will require further investigation.
Our findings do not diminish the role of CXCL12 nor do they contradict previous findings regarding hepatic CXCL12 microenvironments and the accumulation of CXCR4-expressing cells in the periportal region.8 BECs were initially thought to play a role in supporting a stem cell niche, given their expression of CXCL12; however, their role may be more limited. We conclude that BECs are not a predominant source of hepatic CXCL12 and that there exists a unique population of desmin-positive CXCL12-expressing cells within the portal tract found exclusively in direct contact with BECs. Whether these cells play a role in portal inflammation or in supporting a stem cell niche remains to be determined, but the proposed role of cholangiocyte-derived CXCL12 in these processes needs to be reconsidered.
Acknowledgments
We thank Drs. Douglas Jefferson (Tufts University, Medford, MA) and Yoshiyuki Ueno (Yamagata University, Yamagata, Japan) for providing the H69 and 603B cell lines, respectively.
Footnotes
Supported by NIH grants DK-6047402, DK-071745, and R56DK-092128 (M.B.B.) and DK-090986 (Y.S.).
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2015.03.006.
Supplemental Data
Supplemental Figure S1.
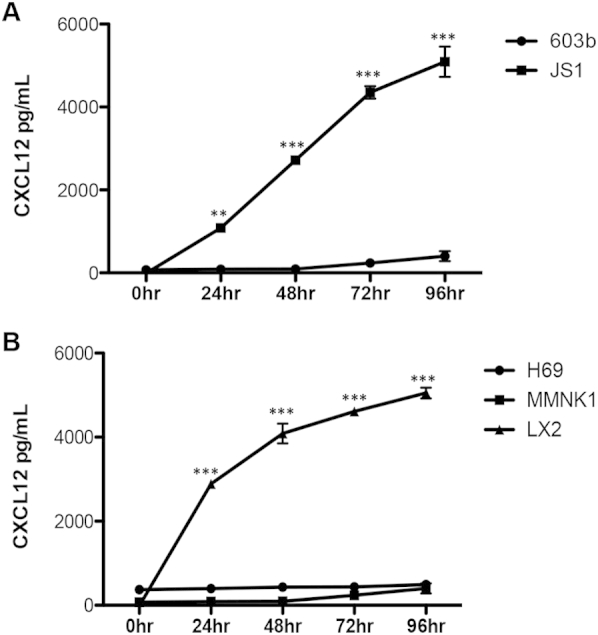
Time course of CXCL12 expression from murine and human cholangiocyte cell lines. Cell culture supernatants from both murine (A, 603b) and human (B, H69 and MMNK-1) cholangiocyte cell lines show no CXCL12 expression over a 96-hour time period. Conversely, murine (JS1) and human (LX2) stellate cell lines demonstrate increasing CXCL12 secretion between 0 and 96 hours. n = 3 independent experiments. ∗∗P < 0.01, ∗∗∗P < 0.001.
Supplemental Figure S2.
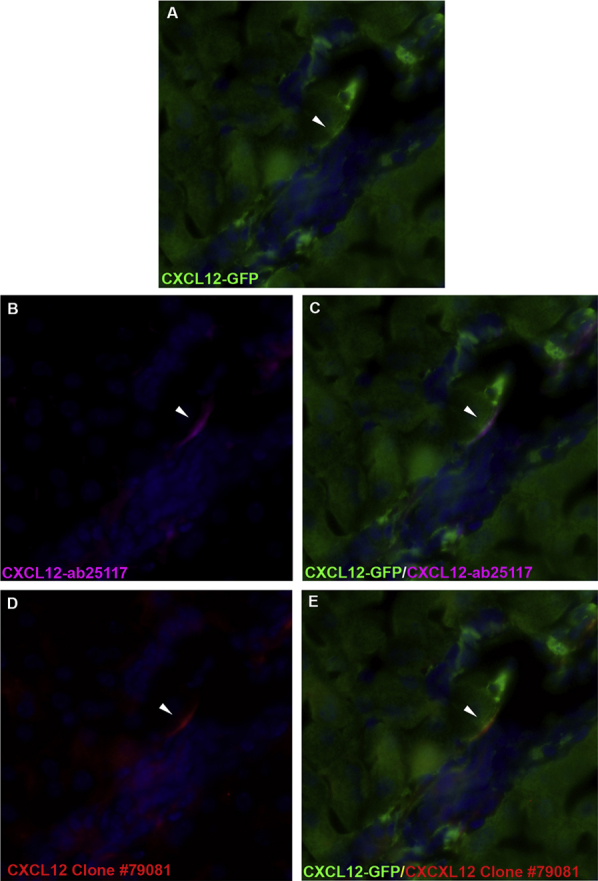
Antibody confirmation of CXCL12 epitope expression in CXCL12–green fluorescent protein (GFP) reporter-positive cells. Dual-immunofluorescence staining on liver sections from mice expressing Gfp in the Cxcl12 locus with either CXCL12-ab25117 or clone 79081. A and B: CXCL12-GFP reporter cells appear green (A, white), with CXCL12-ab25117–positive cells appearing magenta (B). C: Overlap of GFP and CXCL12-ab25117 confirms CXCL12 epitope expression in GFP-positive cells, which appears white. D and E: Similarly, CXCL12 clone 79081 (D, red) also identifies CXCL12 epitope in GFP-positive cells (E, yellow). Arrowheads identify CXCL12-positive cells. Original magnification, ×400.
Supplemental Figure S3.
Lack of CXCL12 expression in biliary epithelial cells (BECs) with alternate CXCL12 antibodies. CXCL12 staining on liver sections from cirrhotic human livers and mice with ligated bile ducts shows no CXCL12 expression using three alternate commercially available CXCL12 antibodies. Frozen sections were costained with anti-CXCL12 clone 79018 (A, C, E, G, and I, green) and either Santa Cruz Biotechnology Inc. rabbit anti-human/mouse CXCL12 (B and F, red), Abcam rabbit anti-human/mouse CXCL12 (D and J, red), or eBiosciences rabbit anti-mouse CXCL12 (H, red). Liver sections show bright fluorescence in BECs stained with CXCL12 antibody clone 79018 but not with the three alternate antibodies. Original magnification, ×400.
References
- 1.Sugiyama T., Nagasawa T. Bone marrow niches for hematopoietic stem cells and immune cells. Inflamm Allergy Drug Targets. 2012;11:201–206. doi: 10.2174/187152812800392689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wald O., Pappo O., Safadi R., Dagan-Berger M., Beider K., Wald H., Franitza S., Weiss I., Avniel S., Boaz P., Hanna J., Zamir G., Eid A., Mandelboim O., Spengler U., Galun E., Peled A. Involvement of the CXCL12/CXCR4 pathway in the advanced liver disease that is associated with hepatitis C virus or hepatitis B virus. Eur J Immunol. 2004;34:1164–1174. doi: 10.1002/eji.200324441. [DOI] [PubMed] [Google Scholar]
- 3.Saiman Y., Friedman S.L. The role of chemokines in acute liver injury. Front Physiol. 2012;3:213. doi: 10.3389/fphys.2012.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egawa T., Kawabata K., Kawamoto H., Amada K., Okamoto R., Fujii N., Kishimoto T., Katsura Y., Nagasawa T. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15:323–334. doi: 10.1016/s1074-7613(01)00185-6. [DOI] [PubMed] [Google Scholar]
- 5.Coulomb-L'Hermin A., Amara A., Schiff C., Durand-Gasselin I., Foussat A., Delaunay T., Chaouat G., Capron F., Ledee N., Galanaud P., Arenzana-Seisdedos F., Emilie D. Stromal cell-derived factor 1 (SDF-1) and antenatal human B cell lymphopoiesis: expression of SDF-1 by mesothelial cells and biliary ductal plate epithelial cells. Proc Natl Acad Sci U S A. 1999;96:8585–8590. doi: 10.1073/pnas.96.15.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Terada R., Yamamoto K., Hakoda T., Shimada N., Okano N., Baba N., Ninomiya Y., Gershwin M.E., Shiratori Y. Stromal cell-derived factor-1 from biliary epithelial cells recruits CXCR4-positive cells: implications for inflammatory liver diseases. Lab Invest. 2003;83:665–672. doi: 10.1097/01.lab.0000067498.89585.06. [DOI] [PubMed] [Google Scholar]
- 7.Mavier P., Martin N., Couchie D., Preaux A.M., Laperche Y., Zafrani E.S. Expression of stromal cell-derived factor-1 and of its receptor CXCR4 in liver regeneration from oval cells in rat. Am J Pathol. 2004;165:1969–1977. doi: 10.1016/S0002-9440(10)63248-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kollet O., Shivtiel S., Chen Y.Q., Suriawinata J., Thung S.N., Dabeva M.D., Kahn J., Spiegel A., Dar A., Samira S., Goichberg P., Kalinkovich A., Arenzana-Seisdedos F., Nagler A., Hardan I., Revel M., Shafritz D.A., Lapidot T. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grubman S.A., Perrone R.D., Lee D.W., Murray S.L., Rogers L.C., Wolkoff L.I., Mulberg A.E., Cherington V., Jefferson D.M. Regulation of intracellular pH by immortalized human intrahepatic biliary epithelial cell lines. Am J Physiol. 1994;266:G1060–G1070. doi: 10.1152/ajpgi.1994.266.6.G1060. [DOI] [PubMed] [Google Scholar]
- 10.Maruyama M., Kobayashi N., Westerman K.A., Sakaguchi M., Allain J.E., Totsugawa T., Okitsu T., Fukazawa T., Weber A., Stolz D.B., Leboulch P., Tanaka N. Establishment of a highly differentiated immortalized human cholangiocyte cell line with SV40T and hTERT. Transplantation. 2004;77:446–451. doi: 10.1097/01.TP.0000110292.73873.25. [DOI] [PubMed] [Google Scholar]
- 11.Hanada S., Harada M., Koga H., Kawaguchi T., Taniguchi E., Kumashiro R., Ueno T., Ueno Y., Ishii M., Sakisaka S., Sata M. Tumor necrosis factor-alpha and interferon-gamma directly impair epithelial barrier function in cultured mouse cholangiocytes. Liver Int. 2003;23:3–11. doi: 10.1034/j.1600-0676.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 12.Friedman S.L. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev. 2008;88:125–172. doi: 10.1152/physrev.00013.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo J., Loke J., Zheng F., Hong F., Yea S., Fukata M., Tarocchi M., Abar O.T., Huang H., Sninsky J.J., Friedman S.L. Functional linkage of cirrhosis-predictive single nucleotide polymorphisms of Toll-like receptor 4 to hepatic stellate cell responses. Hepatology. 2009;49:960–968. doi: 10.1002/hep.22697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spirli C., Locatelli L., Morell C.M., Fiorotto R., Morton S.D., Cadamuro M., Fabris L., Strazzabosco M. Protein kinase a-dependent pSer(675)-beta-catenin, a novel signaling defect in a mouse model of congenital hepatic fibrosis. Hepatology. 2013;58:1713–1723. doi: 10.1002/hep.26554. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 15.Fiorotto R., Spirli C., Fabris L., Cadamuro M., Okolicsanyi L., Strazzabosco M. Ursodeoxycholic acid stimulates cholangiocyte fluid secretion in mice via CFTR-dependent ATP secretion. Gastroenterology. 2007;133:1603–1613. doi: 10.1053/j.gastro.2007.08.071. [DOI] [PubMed] [Google Scholar]
- 16.Takabatake Y., Sugiyama T., Kohara H., Matsusaka T., Kurihara H., Koni P.A., Nagasawa Y., Hamano T., Matsui I., Kawada N., Imai E., Nagasawa T., Rakugi H., Isaka Y. The CXCL12 (SDF-1)/CXCR4 axis is essential for the development of renal vasculature. J Am Soc Nephrol. 2009;20:1714–1723. doi: 10.1681/ASN.2008060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugiyama T., Kohara H., Noda M., Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 18.Omatsu Y., Seike M., Sugiyama T., Kume T., Nagasawa T. Foxc1 is a critical regulator of haematopoietic stem/progenitor cell niche formation. Nature. 2014;508:536–540. doi: 10.1038/nature13071. [DOI] [PubMed] [Google Scholar]
- 19.Li W., Kohara H., Uchida Y., James J.M., Soneji K., Cronshaw D.G., Zou Y.R., Nagasawa T., Mukouyama Y.S. Peripheral nerve-derived CXCL12 and VEGF-A regulate the patterning of arterial vessel branching in developing limb skin. Dev Cell. 2013;24:359–371. doi: 10.1016/j.devcel.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greenbaum A., Hsu Y.M., Day R.B., Schuettpelz L.G., Christopher M.J., Borgerding J.N., Nagasawa T., Link D.C. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495:227–230. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omatsu Y., Sugiyama T., Kohara H., Kondoh G., Fujii N., Kohno K., Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Ara T., Itoi M., Kawabata K., Egawa T., Tokoyoda K., Sugiyama T., Fujii N., Amagai T., Nagasawa T. A role of CXC chemokine ligand 12/stromal cell-derived factor-1/pre-B cell growth stimulating factor and its receptor CXCR4 in fetal and adult T cell development in vivo. J Immunol. 2003;170:4649–4655. doi: 10.4049/jimmunol.170.9.4649. [DOI] [PubMed] [Google Scholar]
- 23.Barbera-Guillem E., Ayala R., Vidal-Vanaclocha F. Differential location of hemopoietic colonies within liver acini of postnatal and phenylhydrazine-treated adult mice. Hepatology. 1989;9:29–36. doi: 10.1002/hep.1840090106. [DOI] [PubMed] [Google Scholar]
- 24.Taniguchi H., Toyoshima T., Fukao K., Nakauchi H. Presence of hematopoietic stem cells in the adult liver. Nat Med. 1996;2:198–203. doi: 10.1038/nm0296-198. [DOI] [PubMed] [Google Scholar]
- 25.Kordes C., Haussinger D. Hepatic stem cell niches. J Clin Invest. 2013;123:1874–1880. doi: 10.1172/JCI66027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ding B.S., Cao Z., Lis R., Nolan D.J., Guo P., Simons M., Penfold M.E., Shido K., Rabbany S.Y., Rafii S. Divergent angiocrine signals from vascular niche balance liver regeneration and fibrosis. Nature. 2014;505:97–102. doi: 10.1038/nature12681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shanmugam M.K., Manu K.A., Ong T.H., Ramachandran L., Surana R., Bist P., Lim L.H., Kumar A.P., Hui K.M., Sethi G. Inhibition of CXCR4/CXCL12 signaling axis by ursolic acid leads to suppression of metastasis in transgenic adenocarcinoma of mouse prostate model. Int J Cancer. 2011;129:1552–1563. doi: 10.1002/ijc.26120. [DOI] [PubMed] [Google Scholar]
- 28.Ma L., Qiao H., He C., Yang Q., Cheung C.H., Kanwar J.R., Sun X. Modulating the interaction of CXCR4 and CXCL12 by low-molecular-weight heparin inhibits hepatic metastasis of colon cancer. Invest New Drugs. 2012;30:508–517. doi: 10.1007/s10637-010-9578-0. [DOI] [PubMed] [Google Scholar]
- 29.Matsusue R., Kubo H., Hisamori S., Okoshi K., Takagi H., Hida K., Nakano K., Itami A., Kawada K., Nagayama S., Sakai Y. Hepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis. Ann Surg Oncol. 2009;16:2645–2653. doi: 10.1245/s10434-009-0599-x. [DOI] [PubMed] [Google Scholar]
- 30.Amara A., Lorthioir O., Valenzuela A., Magerus A., Thelen M., Montes M., Virelizier J.L., Delepierre M., Baleux F., Lortat-Jacob H., Arenzana-Seisdedos F. Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J Biol Chem. 1999;274:23916–23925. doi: 10.1074/jbc.274.34.23916. [DOI] [PubMed] [Google Scholar]
- 31.Dranoff J.A., Wells R.G. Portal fibroblasts: underappreciated mediators of biliary fibrosis. Hepatology. 2010;51:1438–1444. doi: 10.1002/hep.23405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santiago B., Calonge E., Del Rey M.J., Gutierrez-Canas I., Izquierdo E., Usategui A., Galindo M., Alcami J., Pablos J.L. CXCL12 gene expression is upregulated by hypoxia and growth arrest but not by inflammatory cytokines in rheumatoid synovial fibroblasts. Cytokine. 2011;53:184–190. doi: 10.1016/j.cyto.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Ceradini D.J., Kulkarni A.R., Callaghan M.J., Tepper O.M., Bastidas N., Kleinman M.E., Capla J.M., Galiano R.D., Levine J.P., Gurtner G.C. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 34.Berniakovich I., Giorgio M. Low oxygen tension maintains multipotency, whereas normoxia increases differentiation of mouse bone marrow stromal cells. Int J Mol Sci. 2013;14:2119–2134. doi: 10.3390/ijms14012119. [DOI] [PMC free article] [PubMed] [Google Scholar]