Abstract
Fucoidan is a sulfated polysaccharide derived from brown seaweed, including Fucus vesiculosus. This compound is known to have immunostimulatory effects on various types of immune cells including macrophages and dendritic cells. A recent study described the application of fucoidan as a vaccine adjuvant. Vaccination is regarded as the most efficient prophylactic method for preventing harmful or epidemic diseases. To increase vaccine efficacy, effective adjuvants are needed. In the present study, we determined whether fucoidan can function as an adjuvant using vaccine antigens. Flow cytometric analysis revealed that fucoidan increases the expression of the activation markers major histocompatibility complex class II, cluster of differentiation (CD)25, and CD69 in spleen cells. In combination with Bordetella bronchiseptica antigen, fucoidan increased the viability and tumor necrosis factor-α production of spleen cells. Furthermore, fucoidan increased the in vivo production of antigen-specific antibodies in mice inoculated with Mycoplasma hyopneumoniae antigen. Overall, this study has provided valuable information about the use of fucoidan as a vaccine adjuvant.
Keywords: antigen-specific antibody, fucoidan, spleen cells, vaccine adjuvant, vaccine antigen
Introduction
Fucoidan is a sulfated polysaccharide primarily produced by various species of brown algae and seaweed. It is well known that fucoidan has many biological activities, such as immunostimulatory and anticancer effects [13]. Indeed, this compound regulates cellular and humoral immunity as well as hematopoietic mobilization [6]. Additionally, fucoidan potentiates the function of immune cells, including macrophages, natural killer cells, and dendritic cells (DCs) [1,19,20].
Vaccination is an effective method for preventing fatal infectious diseases. B lymphocytes play important roles in the mechanism underlying vaccination [2]. The efficacy of vaccines is attributed to the activation of B lymphocytes and plasma cells that produce pathogen-specific antibodies [16]. To enhance vaccine efficacy, appropriate adjuvants should be used to increase the immunogenicity of vaccine antigens [4]. Various adjuvants, such as alum, have been developed and used successfully in clinics [17].
We previously reported that fucoidan has immunostimulatory effects on DCs [11]. In the present study, we determined whether fucoidan enhances the expression of immune-related markers and cytokine production in spleen cells. In doing so, fucoidan could enhance the immunogenicity of vaccine antigens.
Materials and Methods
Animals and reagents
Female BALB/c mice 7 to 12 weeks old were purchased from Orient Bio (Korea), maintained in our animal facility, and used for all experiments. Animal experiments in this study were performed in accordance with the institutional guidelines of Jeju National University (Korea) for animal care and use. Two vaccine antigens used in this study, Mycoplasma (M.) hyopneumoniae and Bordetella (B.) bronchiseptica, were obtained from ChoongAng Vaccine Laboratories (Korea) and KBNP (Korea), respectively. The amount of protein in the formalin-inactivated intact bacteria administered as antigen was measured with the Bradford protein assay (Bio-Rad Laboratories, USA).
Preparation of fucoidan
Fucoidan derived from Fucus vesiculosus was purchased from Sigma (USA) and dissolved in phosphate buffered saline (PBS). Endotoxin levels in the fucoidan solutions were measured with QCL-1000 Chromogenic LAL endpoint assay (Lonza, USA). The endotoxin level in the solution with the highest concentration (50 µg/mL) was below than the detection limit of the kit (0.1 EU/mL) [11].
Preparation of spleen cells
The spleen cells were harvested as previously described [10]. The cells were acquired by mincing the spleens recovered from the BALB/c mice. Red blood cells were removed from the spleen cells with an ammonium chloride-potassium buffer. After incubating the cells in a T75 flask for 1 h, the floating cells were collected and cultured in RPMI1640 medium supplemented with 10% fetal bovine serum, L-glutamine, penicillin/streptomycin, sodium pyruvate, non-essential amino acids, and 2-mercaptoethanol. The cells were maintained at 37℃ in 5% CO2.
Measurement of spleen cell viability
The spleen cells were seeded at a concentration of 2 × 106 cells/mL in 96-well culture plates for a viability assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma). The cells were treated with fucoidan and B. bronchiseptica antigen for 2 days. Next, 10 µL/well of MTT solution (final concentration of 0.5 mg/mL) was added and the cells were incubated for 4 h. After this, 10% SDS solution (100 µL/well) was added to the wells and the plate was incubated for 2 h to dissolve the crystals generated by viable cells. Optical density of the wells was measured at 570 nm using a microplate reader (Molecular Devices, USA).
Enzyme-linked immunosorbent assay (ELISA)
A cytokine-specific ELISA kit was used to measure the amount of cytokines produced by the spleen cells. Culture supernatants were collected and used for the ELISA. The amount of tumor necrosis factor (TNF)-α in the supernatants was measured with CytoSet kit (Invitrogen, USA) according to the manufacturer's instruction.
Flow cytometry
To determine whether fucoidan affects subsets of spleen cells, flow cytometry analysis was performed with surface marker-specific antibodies. The spleen cells were seeded at a concentration of 2 × 106 cells/mL in 6-well culture plates. The staining procedure was performed as previously described in detail [8]. The cells were stained with biotin-labeled anti-B220, anti-CD19, anti-I-Ad, and anti-CD80 antibody followed by streptavidin-fluorescein isothiocyanate (FITC; all from BD Biosciences, USA). To examine activation markers, the spleen cells were stained with anti-CD25 antibody followed by phycoerythrin (PE)-labeled anti-rat IgM or PE-labeled anti-CD69 antibody. In addition, the cells were stained with biotin-labeled anti-B220, streptavidin-FITC, and PE-labeled anti-CD138 antibody to detect plasma cells. To assess the total effects of fucoidan in vivo, we measured the expression levels of fucoidan-treated cells without gating. The stained cells were analyzed with FACSCalibur (BD Biosciences) and WinMDI 2.9 software.
Measurement of antigen-specific antibody titer in the serum of mice inoculated with M. hyopneumoniae antigen
To measure the effect of fucoidan as an adjuvant in vivo, M. hyopneumoniae antigen (10 µg/mouse) and/or fucoidan (100 mg/kg) was injected into mice twice at a 2-week interval. The dose of fucoidan in vivo was determined based on efficacy and toxicity [12]. PBS was injected as a control. Two weeks after the final injection, serum was collected and antigen-specific antibody levels were measured. Maxisorp Nunc-Immuno module (Thermo Scientific, USA) was coated with M. hyopneumoniae antigen, and the plates were sequentially treated with blocking solution, the samples, horseradish peroxidase-conjugated anti-mouse IgG antibody, substrate, and stop solution. Optical density was measured at 405 nm using a microplate reader.
Statistical analysis
Data in Figs. 4,5,6 are presented as the mean ± standard deviation (SD). Differences were analyzed using ANOVA and Student t-test. A p value < 0.05 was considered significant.
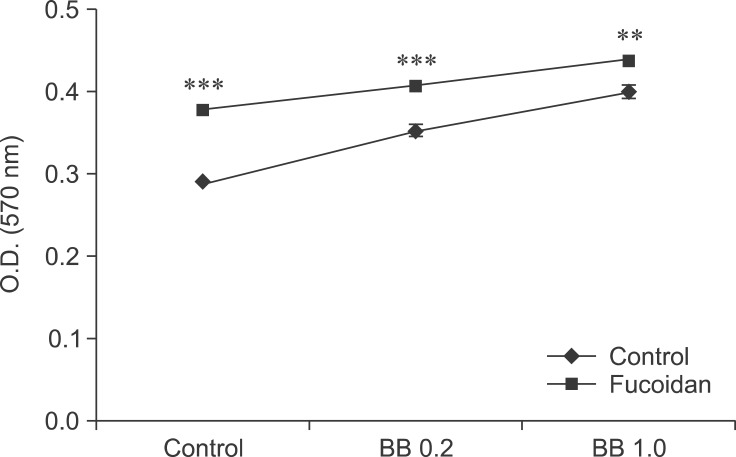
Fig. 4. The effect of fucoidan on the viability of Bordetella bronchiseptica (BB) antigen-treated spleen cells. The cells were cultured in 96-well plates, and treated with 50 µg/mL fucoidan and BB antigen at the indicated concentrations (µg/mL). An MTT assay was then performed.
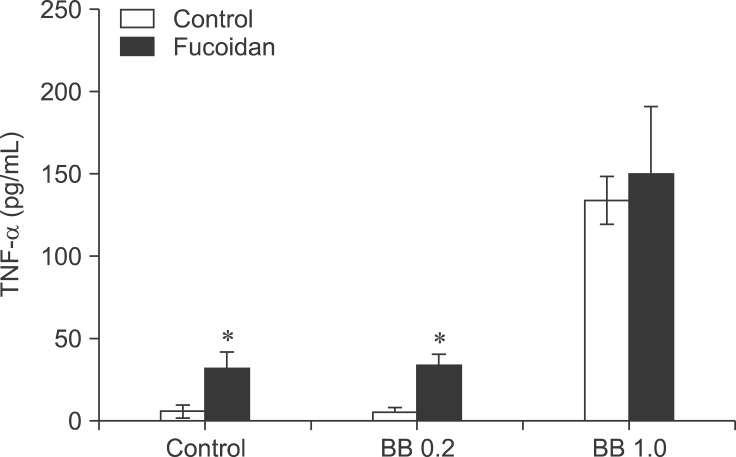
Fig. 5. Fucoidan up-regulates TNF-α production by BB antigen-treated spleen cells. The cells were cultured and treated as described in Fig. 4. The amount of TNF-α in the culture supernatants was then measured with ELISA.
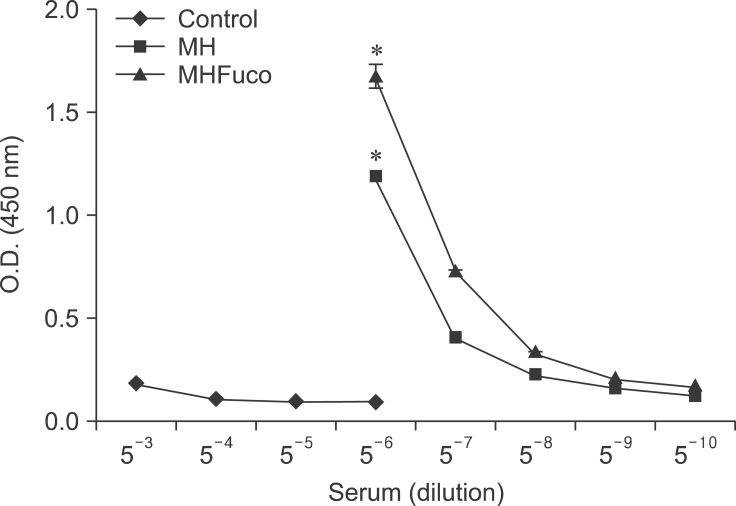
Fig. 6. Fucoidan enhances antigen-specific antibody production in mice. The mice were injected with fucoidan and M. hyopneumoniae (MH) antigen as described in "Materials and Methods". The serum was harvested and diluted to measure the amount of antigen-specific antibodies.
Results
Effect of fucoidan on the expression of B lymphocyte surface markers
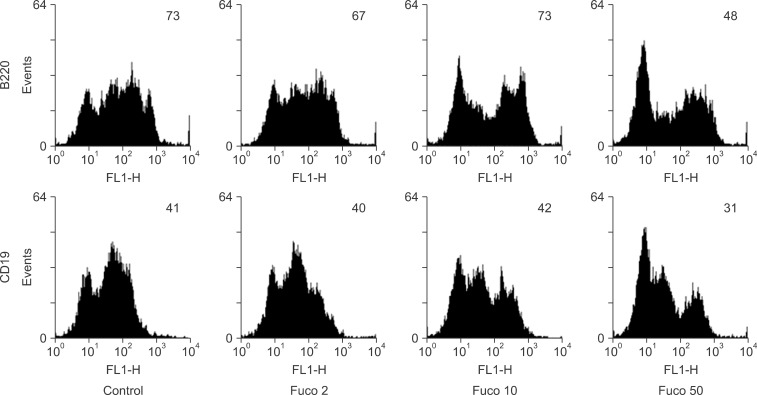
To investigate the effect of fucoidan on the expression of surface markers specific for B lymphocytes, we stained fucoidan-treated spleen cells with anti-B220 or -CD19 antibody. B220 is a pan B lymphocyte marker while CD19 is a mature B lymphocyte marker. The expression levels of both markers on spleen cells treated with 2 or 10 µg/mL fucoidan were similar to those on the control cells (Fig. 1). However, the expression of both markers was decreased on spleen cells treated with 50 µg/mL fucoidan.
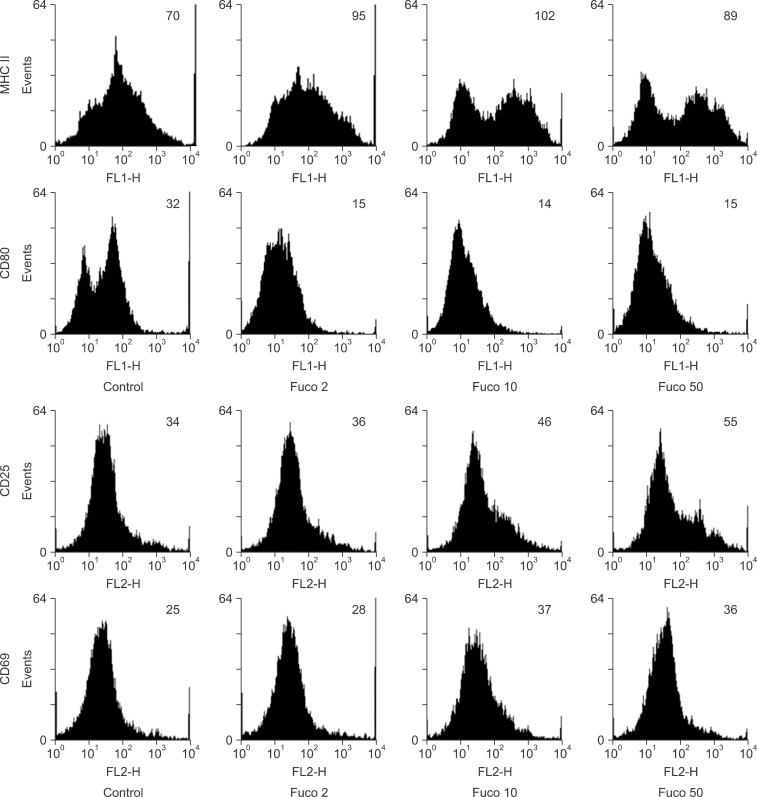
Fig. 1. The expression of B lymphocyte surface markers on fucoidan-treated spleen cells. Spleen cells were cultured in 6-well plates and treated with fucoidan (Fuco) at the indicated concentrations (µg/mL). After treatment, spleen cells were stained as described in "Materials and Methods". Numbers in the histograms represent the geometric mean fluorescence intensity.
Expression of immune response-related surface markers is up-regulated on fucoidan-treated spleen cells
We next measured the expression levels of various immune response-related markers, I-Ad, CD80, and CD25 with CD69, that are associated with antigen presentation, co-stimulatory signal transduction, and lymphocyte activation, respectively. Fucoidan consistently increased the expression level of I-Ad while CD80 expression was decreased (Fig. 2). Additionally, fucoidan increased the expression of both lymphocyte activation markers (CD25 and CD69) on spleen cells treated with 10 or 50 µg/mL fucoidan (Fig. 2). Flow cytometry analysis revealed that fucoidan activated the spleen cells as indicated by increased expression of the immune response-related markers.
Fig. 2. Up-regulated expression of lymphocyte activation markers on fucoidan-treated spleen cells. The cells were cultured, treated with fucoidan, and stained as described in "Materials and Methods". Numbers in the histograms represent the geometric mean fluorescence intensity.
Fucoidan enhances specific populations of plasma cells
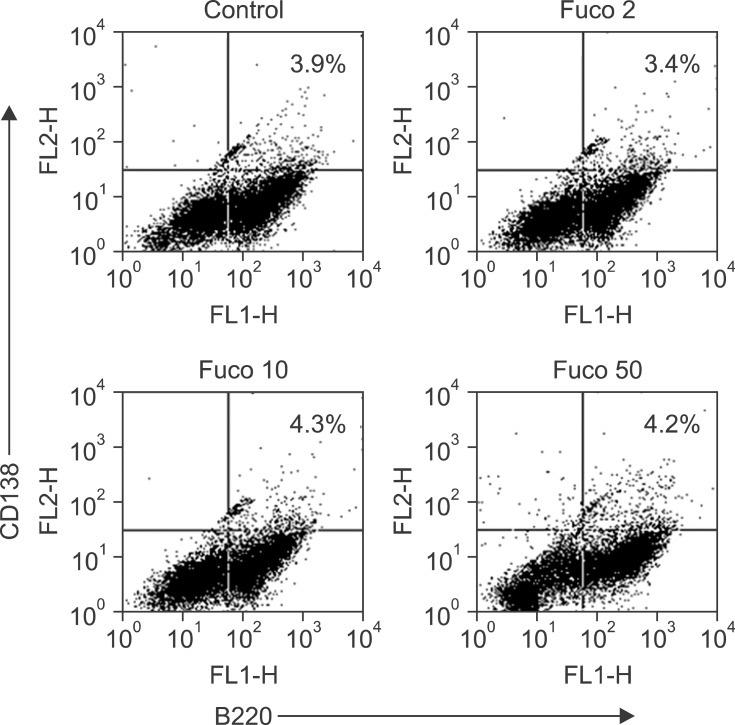
To determine whether fucoidan may induce the generation of antibody-producing plasma cells, we treated spleen cells with fucoidan and stained for two plasma cell-specific markers: B220 and CD138. The percentage of plasma cells (B220+ CD138+) was marginally enhanced among spleen cells treated with 10 or 50 µg/mL fucoidan (Fig. 3). However, the population of plasma cells was relatively small in our study.
Fig. 3. Fucoidan marginally increases the percentage of plasma cells. Spleen cells were cultured, treated with fucoidan, and stained as described in "Materials and Methods". Numbers in the dot plots represent the percentage of double-positive cells.
Effect of fucoidan on the viability of B. bronchiseptica antigen-treated spleen cells
To determine whether fucoidan can be used as an immunostimulatory adjuvant for vaccines, we administered fucoidan with B. bronchiseptica vaccine antigen. We then performed an MTT assay to assess the toxicity of fucoidan in combination with the antigen. Compared to the control, B. bronchiseptica antigen significantly increased the viability of spleen cells in a concentration-dependent manner. Addition of fucoidan further increased the viability (Fig. 4). Results of the MTT assay revealed that fucoidan does not cause significant toxicity when used in combination with B. bronchiseptica antigen.
Fucoidan up-regulates TNF-α production by B. bronchiseptica-treated spleen cells
Immunostimulatory cytokines are required to enhance the efficacy of vaccines. We measured the production of TNF-α by spleen cells treated with B. bronchiseptica and fucoidan. TNF-α is an inflammatory cytokine that increases immune responses essential for vaccine efficacy. Results of an ELISA demonstrated that B. bronchiseptica antigen significantly induced TNF-α production by spleen cells at 1.0 µg/mL. Addition of 50 µg/mL fucoidan increased TNF-α production at control and B. bronchiseptica antigen 0.2 µg/mL (Fig. 5). These findings seem to indicate that fucoidan may not have synergistic effects when used in combination with B. bronchiseptica antigen.
Fucoidan increases the production of antigen-specific antibodies in mice
We evaluated the adjuvant effect of fucoidan on the production of antigen-specific antibodies in mice using an immunoassay. For these in vivo experiments, M. hyopneumoniae was used as the antigen. No antigen-specific antibody production was detected in the control mice injected with PBS alone (5-3~5-6 dilution). Fucoidan enhanced the generation of M. hyopneumoniae-specific antibody compared to treatment with antigen alone (5-6~5-7 dilution; Fig. 6).
Discussion
Fucoidan is an edible sulfated polysaccharide derived from brown algae. This compound can modulate the function of immune cells, including lymphocytes [3,15]. In the present study, we determined whether fucoidan stimulated spleen cells and could be used as a vaccine adjuvant. We used killed B. bronchiseptica, a main causative pathogen of swine respiratory diseases including atrophic rhinitis [5,21], and M. hyopneumoniae bacteria, a main pathogen of swine enzootic pneumonia [14,18], as vaccine antigens. Flow cytometric analysis revealed that fucoidan stimulated the expression of an antigen-presenting molecule, major histocompatibility complex (MHC) class II, on spleen cells and further enhanced the expression of the lymphocyte activation markers CD25 and CD69. When administered in combination with B. bronchiseptica antigen, fucoidan significantly increased the viability and TNF-α production of spleen cells. These data indicate that fucoidan is a candidate vaccine adjuvant.
Interestingly, fucoidan enhanced the expression of MHC class II but not CD80, a major co-stimulatory molecule for immune responses. Thus, fucoidan may differentially regulate the molecules required for immune responses. During preparation of the spleen cells, we removed adherent cells, including macrophages, and used floating spleen cells that were mainly lymphocytes. Thus, B lymphocytes may express MHC class II, which can be up-regulated by fucoidan. To monitor changes in the proportion of B cells, a potential cause of enhanced antibody production following treatment with fucoidan, we measured the expression of B cell markers. However, the highest concentration of fucoidan (50 µg/mL) decreased the expression of B cell markers in vitro. It is possible that this effect was caused by cytotoxicity associated with higher concentrations of fucoidan and lower marker expression on lymphoblast/activated cells. Further studies are required to explain this phenomenon.
To evaluate plasma cell generation, we treated spleen cells with fucoidan for 3 days. A small difference in the percentage of plasma cells was noted between the control and treated spleen cells. The rather modest increase in plasma cells may be attributable to limitations associated with in vitro experiments such as duration of the treatment and culture conditions used to generate plasma cells.
We also investigated effects of the combination of fucoidan with the vaccine antigen B. bronchiseptica. Fucoidan in combination with B. bronchiseptica did not cause significant toxicity in terms of spleen cell activity. Additionally, fucoidan had an additive effect on the production of TNF-α, a cytokine critical for vaccine efficacy. Co-treatment with fucoidan resulted in increased TNF-α production with a lower vaccine antigen concentration. This is a promising finding as use of vaccine antigens at higher concentrations can induce side effects, including fever [7].
A recent study demonstrated that fucoidan can be used as an adjuvant [9]. Results from the present investigation also support this hypothesis. We provided additional data using two vaccine antigens commonly administered in veterinary clinics. It is likely that fucoidan may stimulate multiple types of cells, including DCs. In the present study, we confirmed that fucoidan can stimulate spleen cells in vitro, but it is not clear which cell type is stimulated by fucoidan. Future experiments should be performed using an in vivo system to evaluate the mobilization of cells following fucoidan treatment.
Taken together, our data demonstrated that fucoidan activates spleen cells and enhances antigen-specific antibody production in mice. The present study demonstrates that fucoidan can be used as a vaccine adjuvant. Additionally, this investigation has provided new insight into vaccine production and may broaden the use of fucoidan.
Acknowledgments
This research was supported by Bio-industry Technology Development Program, Ministry of Agriculture, Food and Rural Affairs, Korea.
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Ale MT, Maruyama H, Tamauchi H, Mikkelsen JD, Meyer AS. Fucoidan from Sargassum sp. and Fucus vesiculosus reduces cell viability of lung carcinoma and melanoma cells in vitro and activates natural killer cells in mice in vivo. Int J Biol Macromol. 2011;49:331–336. doi: 10.1016/j.ijbiomac.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Amanna IJ, Slifka MK. Contributions of humoral and cellular immunity to vaccine-induced protection in humans. Virology. 2011;411:206–215. doi: 10.1016/j.virol.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi EM, Kim AJ, Kim YO, Hwang JK. Immunomodulating activity of arabinogalactan and fucoidan in vitro. J Med Food. 2005;8:446–453. doi: 10.1089/jmf.2005.8.446. [DOI] [PubMed] [Google Scholar]
- 4.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horiguchi Y. Swine atrophic rhinitis caused by Pasteurella multocida toxin and Bordetella dermonecrotic toxin. Curr Top Microbiol Immunol. 2012;361:113–129. doi: 10.1007/82_2012_206. [DOI] [PubMed] [Google Scholar]
- 6.Irhimeh MR, Fitton JH, Lowenthal RM. Fucoidan ingestion increases the expression of CXCR4 on human CD34+ cells. Exp Hematol. 2007;35:989–994. doi: 10.1016/j.exphem.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 7.Jefferson T, Rudin M, DiPietrantonj C. Systematic review of the effects of pertussis vaccines in children. Vaccine. 2003;21:2003–2014. doi: 10.1016/s0264-410x(02)00770-3. [DOI] [PubMed] [Google Scholar]
- 8.Jeong BE, Ko EJ, Joo HG. Cytoprotective effects of fucoidan, an algae-derived polysaccharide on 5-fluorouracil-treated dendritic cells. Food Chem Toxicol. 2012;50:1480–1484. doi: 10.1016/j.fct.2012.01.034. [DOI] [PubMed] [Google Scholar]
- 9.Jin JO, Zhang W, Du JY, Wong KW, Oda T, Yu Q. Fucoidan can function as an adjuvant in vivo to enhance dendritic cell maturation and function and promote antigen-specific T cell immune responses. PLoS One. 2014;9:e99396. doi: 10.1371/journal.pone.0099396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JM, Joo HG. Immunostimulatory effects of β-glucan purified from Paenibacillus polymyxa JB115 on mouse splenocytes. Korean J Physiol Pharmacol. 2012;16:225–230. doi: 10.4196/kjpp.2012.16.4.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MH, Joo HG. Immunostimulatory effects of fucoidan on bone marrow-derived dendritic cells. Immunol Lett. 2008;115:138–143. doi: 10.1016/j.imlet.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 12.Ko EJ, Joo HG. Fucoidan enhances the survival and sustains the number of splenic dendritic cells in mouse endotoxemia. Korean J Physiol Pharmacol. 2011;15:89–94. doi: 10.4196/kjpp.2011.15.2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwak JY. Fucoidan as a marine anticancer agent in preclinical development. Mar Drugs. 2014;12:851–870. doi: 10.3390/md12020851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maes D, Segales J, Meyns T, Sibila M, Pieters M, Haesebrouck F. Control of Mycoplasma hyopneumoniae infections in pigs. Vet Microbiol. 2008;126:297–309. doi: 10.1016/j.vetmic.2007.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oomizu S, Yanase Y, Suzuki H, Kameyoshi Y, Hide M. Fucoidan prevents Cε transcription and NFκB p52 translocation for IgE production in B cells. Biochem Biophys Res Commun. 2006;350:501–507. doi: 10.1016/j.bbrc.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Pulendran B, Ahmed R. Translating innate immunity into immunological memory: implications for vaccine development. Cell. 2006;124:849–863. doi: 10.1016/j.cell.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Reed SG, Orr MT, Fox CB. Key roles of adjuvants in modern vaccines. Nat Med. 2013;19:1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- 18.Simionatto S, Marchioro SB, Maes D, Dellagostin OA. Mycoplasma hyopneumoniae: from disease to vaccine development. Vet Microbiol. 2013;165:234–242. doi: 10.1016/j.vetmic.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Yang JW, Yoon SY, Oh SJ, Kim SK, Kang KW. Bifunctional effects of fucoidan on the expression of inducible nitric oxide synthase. Biochem Biophys Res Commun. 2006;346:345–350. doi: 10.1016/j.bbrc.2006.05.135. [DOI] [PubMed] [Google Scholar]
- 20.Yang M, Ma C, Sun J, Shao Q, Gao W, Zhang Y, Li Z, Xie Q, Dong Z, Qu X. Fucoidan stimulation induces a functional maturation of human monocyte-derived dendritic cells. Int Immunopharmacol. 2008;8:1754–1760. doi: 10.1016/j.intimp.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Zhao Z, Wang C, Xue Y, Tang X, Wu B, Cheng X, He Q, Chen H. The occurrence of Bordetella bronchiseptica in pigs with clinical respiratory disease. Vet J. 2011;188:337–340. doi: 10.1016/j.tvjl.2010.05.022. [DOI] [PubMed] [Google Scholar]