Abstract
Scrapie is diagnosed antemortem in sheep by detecting misfolded isoforms of prion protein (PrPSc) in lymphoid follicles of the rectal mucosa and nictitating membranes. Assay sensitivity is limited if (a) the biopsy is collected early during disease development, (b) an insufficient number of follicles is collected, or (c) peripheral accumulation of PrPSc is reduced or delayed. A blood test would be convenient for mass live animal scrapie testing. Currently approved techniques, however, have their own detection limits. Novel detection methods may soon offer a non-animal-based, rapid platform with detection sensitivities that rival the prion bioassay. In anticipation, we sought to determine if diseased animals could be routinely identified with a bioassay using B lymphocytes isolated from blood sample volumes commonly collected for diagnostic purposes in small ruminants. Scrapie transmission was detected in five of six recipient lambs intravenously transfused with B lymphocytes isolated from 5~10 mL of blood from a naturally scrapie-infected sheep. Additionally, scrapie transmission was observed in 18 ovinized transgenic Tg338 mice intracerebrally inoculated with B lymphocytes isolated from 5~10 mL of blood from two naturally scrapie-infected sheep. Based on our findings, we anticipate that these blood sample volumes should be of diagnostic value.
Keywords: B lymphocytes, blood, prions, scrapie, Tg338 mice
Introduction
Scrapie, a slowly progressing fatal neurodegenerative disorder that affects domestic sheep and goats, belongs to a broad family of transmissible spongiform encephalopathies (TSEs). The infectious agent, a prion, primarily consists of an abnormal conformational isoform (PrPSc) of the host-encoded normal cellular prion protein (PrPC) [5,25]. A characteristic feature of all TSEs is accumulation of PrPSc in the central nervous system [25]. In most cases of classical ovine scrapie, PrPSc accumulation in the lymphoreticular system precedes accumulation in the central nervous system [1,31,32]. The presence of prions in lymphoid tissues of sheep with classical scrapie has been confirmed by bioassays in mice [26]. Infectious prions in peripheral blood and leukocyte subsets of sheep with classical scrapie have been identified with bioassays in lambs [2,6,15] and ovinized transgenic mice [2,18]. Relative susceptibility or resistance of sheep to classical scrapie infection is mainly associated with PrPC amino acids at codon 136 (valine [V] or alanine [A]), 154 (arginine [R] or histidine [H]), and 171 (glutamine [Q], R, H, or lysine [K]). Sheep with homozygous PRNP VRQ alleles are the most susceptible to classical scrapie infections whereas sheep with homozygous PRNP ARR alleles are the most resistant.
Antemortem diagnosis of scrapie in sheep is performed by immunohistochemical (IHC) analysis of rectal tissues [12,13], tonsils, [28] and nictitating membranes [22] in which PrPSc accumulates in the lymphoid follicles. Although rectal biopsy shows higher sensitivity [8] compared to a blood-based enzyme-linked immunosorbent assay (ELISA) [29] and protein misfolding cyclic amplification assay (PMCA) [30], test sensitivity is limited if the biopsy is collected early during disease development, if insufficient follicles are recovered, or if peripheral accumulation of PrPSc is reduced or delayed [8]. Several studies have been recently performed to identify infectious prions or PrPSc in scrapie-infected sheep blood; most were restricted to blood fractions collected from sheep experimentally inoculated with the highly infectious classical scrapie PG127 isolate [2,14,18]. This isolate was originally obtained from a naturally scrapie-infected VRQ/VRQ Cheviot-Welsh sheep [3,33]. Therefore, developing a blood-based diagnostic assay to detect natural scrapie infection in sheep (including the PG127 isolate) would be more useful for live animal testing under standard conditions.
Prion infectivity has been identified in platelet-rich plasma, monocytes, and B as well as T lymphocytes from ovine blood using bioassays [6,18], but only B lymphocytes were found to contain detectable PrPSc using TSE-ELISA (Idexx Laboratories, USA) [10]. A recent study in mice revealed that B lymphocytes acquire PrPSc within mesenteric lymph nodes, and mediate dissemination of the prion to more peripheral lymphoid tissues via the lymph and blood [19]. Thus, B lymphocytes may be a primary target for scrapie diagnostic studies in vitro. Previous attempts to detect PrPSc using the TSE-ELISA platform demonstrated that 10 million B lymphocytes are required for consistent detection [10]. Our previous bioassay in lambs revealed that B lymphocytes isolated from 50 mL of blood from a preclinical naturally scrapie-infected sheep efficiently transmit the disease [6]. Based on this information, the objective of the present study was to determine whether pan B lymphocytes isolated from routinely collected volumes of blood (e.g., 5 to 10 mL in one anticoagulant tube) harbor sufficient prion infectivity to be reliably detected by a bioassay in lambs and ovinized transgenic mice. This would help identify an optimal target volume for future development of blood-based scrapie diagnostic assays.
Materials and Methods
All experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Washington State University (USA) and the University of Washington (USA).
Blood donor sheep
Blood donor sheep (4124 and 4125, both homozygous for the PRNP VRQ allele) were obtained at 4 months of age from a scrapie-negative flock at the United States Sheep Experiment Station (USA). These sheep acquired classical scrapie while being housed with our research breeding flock (Agricultural Research Service [ARS] research facility, USA) in which naturally occurring classical scrapie is maintained with 100% transmission efficiency to VRQ/VRQ progeny. This type of natural scrapie transmission has been previously reported [27]. Infection of these blood donors was confirmed by observing strong immunolabeling in biopsy rectal tissue samples at approximately 29 months of age. At the time of blood collection for the inoculation studies, donor sheep 4125 was 32 months of age and in a preclinical stage of the disease. This animal developed clinical signs consistent with scrapie by 35 months of age and was humanely euthanized for postmortem examination. Donor sheep 4124 developed early clinical signs suggestive of scrapie at 40 months of age. Blood was collected for use in lamb and mouse bioassays when this sheep was 42 and 44 months of age, respectively. Postmortem examination of donor sheep 4124 was performed at 45 months of age. Immunolabeling revealed typical accumulation of PrPSc in lymphoid and nervous system tissues collected postmortem from both donors. Results from donor sheep 4125 have been previously reported [6].
Bioassay in lambs
Twelve mixed-breed 5-month-old lambs obtained from the same scrapie negative-flock at Dubois, ID were divided into three treatment groups, each comprised of four lambs housed together in isolation rooms (see Table 1 for experimental design). Ten of the recipient lambs were homozygous for the PRNP VRQ allele without any other mutation in the PRNP open reading frame. One of the VRQ/VRQ lambs and two additional ARQ/VRQ lambs were kept as uninoculated controls to assess the possibility of lateral scrapie transmission. Laboratory procedures used have been previously described [6]. In brief, the lambs were inoculated intravenously with either total peripheral blood mononuclear cells (PBMCs) isolated from 50 mL of blood (group 1; positive control group for the experimental procedure), CD72+ B lymphocytes isolated from 10 mL of blood (treatment group 2), or CD72+ B lymphocytes isolated from 5 mL of blood (treatment group 3). CD72+ B lymphocytes were separated from PBMCs with a CD72-specific monoclonal antibody (mAb) and magnetic-activated cell sorting system (MACS; Miltenyi Biotec, USA). Purity of the isolated cell population exceeded 95% as assessed by flow cytometry (data not shown). CD72+ B lymphocytes or PBMCs were suspended in 10 mL normal saline and directly transfused into the jugular veins of the recipient animals (Table 1).
Table 1. Intravenous transfusion of lambs with PBMCs and CD72+ B lymphocytes prepared from a clinically affected sheep naturally infected with classical scrapie.
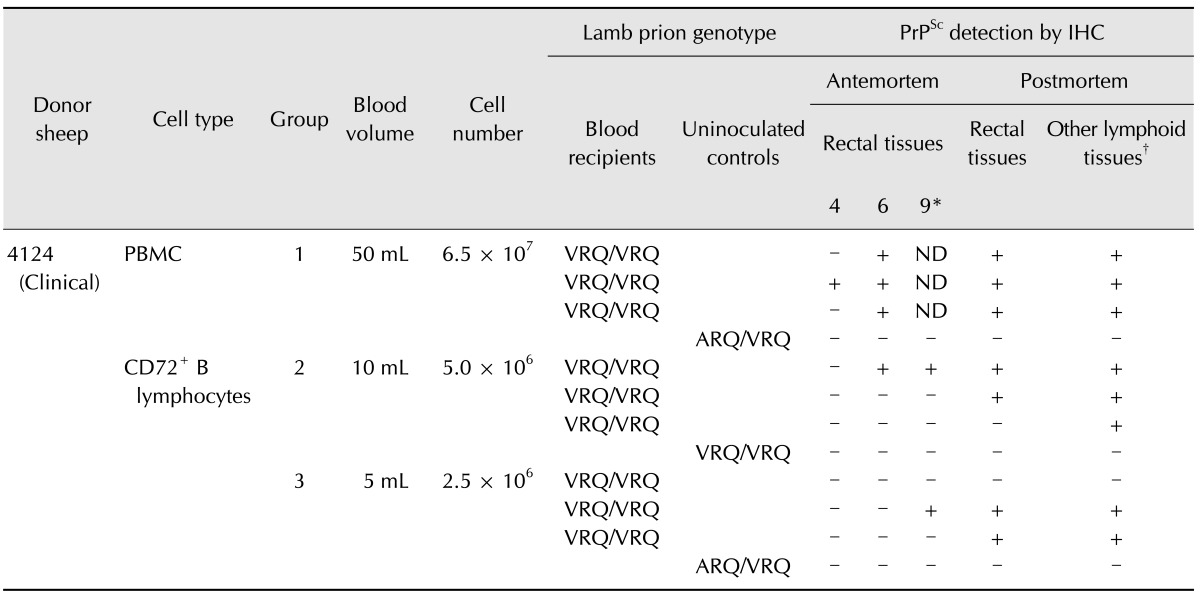
IHC: immunohistochemistry, ND: not done. PrPSc, misfolded prion protein isoform; CD72, pan B lymphocyte-specific marker; (+), positive for PrPSc; (-), negative for PrPSc. *Months post-inoculation. †Other lymphoid tissues included retropharyngeal lymph nodes, tonsils, spleens, mesenteric lymph nodes including ileocecal lymph nodes, distal ileum, ileocecal junction, prescapular lymph nodes, prefemoral lymph nodes, and popliteal lymph nodes.
Standard IHC specific for PrPSc in formalin-fixed paraffin embedded tissues was performed with an automated immunolabeler (Benchmark; Ventana Medical Systems, USA) to detect disease transmission to recipient lambs as previously described [7]. PrPSc was identified using a mixture of mAbs F99/97.6.1 [20] and F89/160.1.5 [21,22]. Tissues for evaluation were collected from rectal mucosa biopsies taken at 4, 6, and 9 months post-inoculation (mpi), and during the postmortem examination conducted at 10 mpi. The number of lymphoid follicles in the rectal biopsies ranged from 34 to 183 follicles per sample.
Bioassay using ovinized transgenic mice
Given the limited number of lambs available for our bioassay, scrapie-infectivity of B lymphocytes isolated from smaller volumes of blood not only from a clinical but also a preclinical scrapie-infected sheep was assessed in ovinized transgenic mice. Breeding pairs of transgenic mice overexpressing the VRQ allele of the ovine prion protein (Tg338 mice) were kindly provided by Dr. Hubert Laude [33] and maintained at the University of Washington. The mice were divided into five treatment groups (see Table 2 for the experimental design). We selected two different mAbs to isolate pan B lymphocytes (anti-CD72 and anti-sIgM), one anti-CD21 mAb to isolate the CD21+ subset of B lymphocytes, and anti-CD18 mAb to obtain PBMCs (Table 2). CD72 is known to be expressed in all lymphocytes except plasma cells while sIgM is expressed by most circulating B lymphocytes. In contrast, only a subset of mature B lymphocytes expresses CD21 [34].
Table 2. Intracerebral inoculation of Tg338 mice with PBMCs and B lymphocytes prepared from sheep naturally infected with classical scrapie.
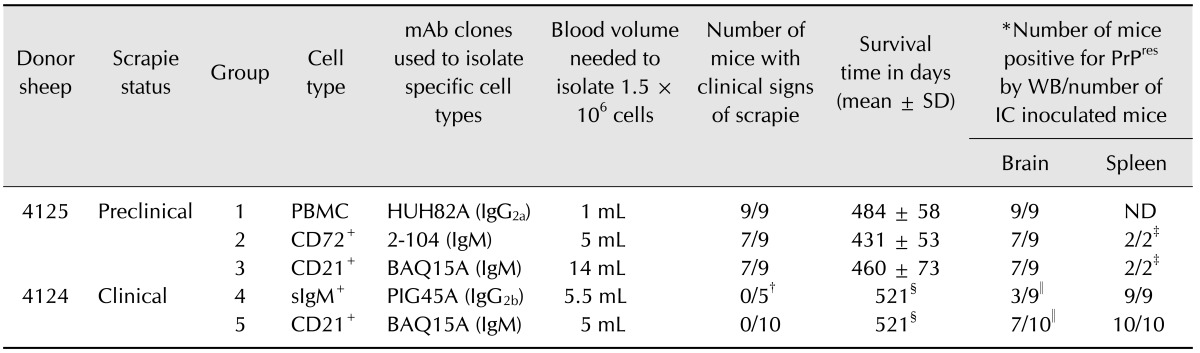
mAb: monoclonal antibody, SD: standard deviation, WB: Western blot, IC: intracerebral inoculation. PrPres, proteinase K-resistant prion protein; CD21, a subset of mature B lymphocytes express this marker; sIgM, surface IgM expressed on the majority of B lymphocytes. *Brains and/or spleens were positive for PrPres according to Western blot assays with mAb F99/97.6.1. †Four mice were culled early (338 days post-inoculation) due to reason unrelated to the experiment. ‡Only the spleens from mice negative for PrPres in the brain from groups 2 and 3 were tested. §Experiment was terminated at 521 days post-inoculation due to the development of intercurrent health issues. ∥PrPres was detected after concentrating the prions using PTA precipitation.
At 2 months of age, the mice were inoculated by intracerebral injection with 1.5 million of the following cells suspended in 20 µL of normal saline: PBMCs (CD18+; group 1, positive control for the experimental procedures), CD72+ B lymphocytes (group 2), sIgM+ B lymphocytes (group 4), or CD21+ B lymphocytes (groups 3 and 5). Uninoculated mice were kept in the same mouse room but in different cages. The animals were monitored daily for clinical signs indicative of scrapie. Mice displaying clear clinical symptoms or any signs of distress were euthanized immediately while animals that did not show any clinical signs were euthanized at a predetermined endpoint (Table 2). Mice in groups 4 and 5 had to be culled prematurely at 521 days post-inoculation (dpi) due to the development of intercurrent health issues. The brains and spleens were collected postmortem and assessed for proteinase K-resistant prion protein (PrPres) by Western blot using mAb F99/97.6.1 as previously described [23]. Precipitation using sodium phosphotungstic acid (PTA) was used to concentrate PrPSc if initial direct immunoblot results were negative [16,24].
Results
Lambs receiving PBMCs develop scrapie
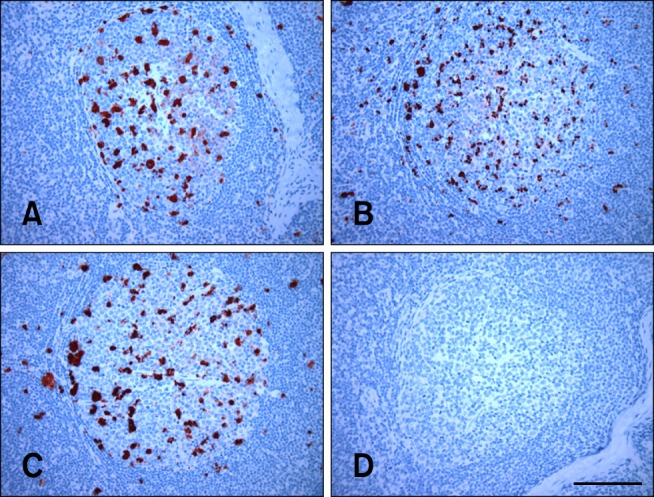
The results of lamb inoculation performed in the present study are reported in Table 1. As a positive control, treatment group 1 was inoculated with PBMCs. Disease transmission was evident in all recipient lambs when biopsied 6 mpi as the accumulation of PrPSc was clearly visible in the rectoanal mucosa-associated lymphoid tissue (RAMALT) follicles (Table 1). Widespread PrPSc accumulation was also observed in the retropharyngeal lymph nodes, tonsils, spleens, mesenteric lymph nodes, distal ileum (ileocecal junction), and peripheral lymph nodes such as prescapular, prefemoral, and popliteal collected at necropsy (panel A in Fig. 1).
Fig. 1. Detection of PrPSc-specific immunolabeling in the lymphoid tissues of inoculated sheep. PrPSc (dark red) was visible in the follicles of retropharyngeal lymph nodes collected during necropsy from sheep inoculated with PBMCs (A) or B lymphocytes recovered from 10 (B) and 5 mL (C) of blood. PrPSc immunolabeling was not detected in uninoculated control sheep (D). Immunohistochemistry (IHC) was performed using a mixture of mAbs F99/97.6.1. and F89/160.1.5. (2.5 µg/mL each) with 3-amino-9-ethylcarbazole (AEC) chromagen. Scale bar = 100 µm.
Lambs receiving B lymphocytes (isolated from 5 to 10 mL of blood) develop scrapie
In the present investigation, transmission of scrapie via CD72+ (pan) B lymphocytes (isolated using anti-CD72 mAb) derived from a 10-mL blood sample (group 2) was evident by 6 mpi in one lamb but delayed in the other two lambs as assessed by PrPSc accumulation in the RAMALT follicles of antemortem rectal tissues (Table 1). The time at which PrPSc was first detected in RAMALT appeared to be further delayed in lambs receiving CD72+ B lymphocytes recovered from 5 mL blood (group 3) with PrPSc accumulation first noted 9 mpi in one of the three lambs as determined by antemortem RAMALT biopsy (Table 1). When these animals were necropsied at 10 mpi, PrPSc immunolabeling was clearly viewed in the rectal tissues from two of the three recipients as well as retropharyngeal lymph nodes, tonsils, spleens, mesenteric lymph nodes, and peripheral lymph nodes collected from all three recipient lambs in group 2 (Table 1; panel B in Fig. 1). In two of the three recipient lambs in group 3 (Table 1; panel C in Fig. 1), PrPSc immunolabeling was found in rectal, mesenteric, and peripheral lymphoid tissues including the distal ileum. PrPSc immunolabeling was not detected in any of the lymphoid tissues examined (including the ileocecal junction and ileocecal lymph nodes) from uninoculated control sheep housed with other animals that received PBMCs or CD72+ B lymphocytes (Table 1; panel D in Fig. 1).
Ovinized transgenic mice treated with PBMCs and B lymphocytes develop scrapie
Most of the Tg338 mice receiving PBMCs (group 1), pan CD72+ lymphocytes (group 2), or the CD21+ subset of B lymphocytes (group 3) from the preclinical scrapie donor (4125) developed clinical signs of scrapie such as weight loss, lethargy, and kyphosis at 426 to 533 dpi (Table 2). Western immunoblot assays revealed PrPres accumulation in the brain and/or spleen of all inoculated mice (panel A in Fig. 2) with 100% disease transmission (Table 2). PrPres was not detected in the brain homogenates prepared from two of the nine mice inoculated with B lymphocytes (CD72+ or CD21+) in both groups 2 and 3 even after concentrating the prions using PTA precipitation. However, immunoblot assays of spleen homogenates from the same animals showed abundant accumulation of PrPres without need for PTA precipitation (Table 2).
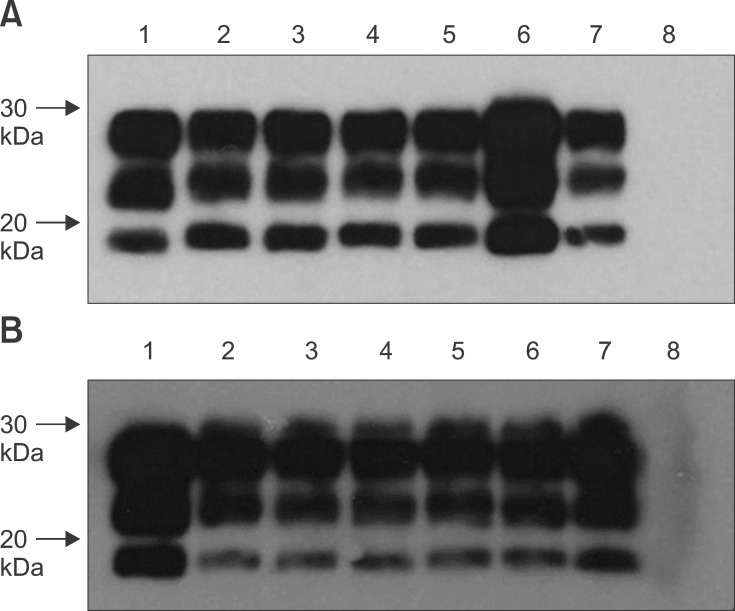
Fig. 2. Representative Western immunoblots for detecting PrPres in Tg338 mice inoculated with PBMCs and B lymphocytes. (A) PrPres-specific bands were detected for the brain homogenates of preclinical scrapie donor sheep 4125 (lane 1) along with brain homogenates of Tg338 mice inoculated with PBMCs (lanes 2 and 3), CD72+ B lymphocytes (lanes 4 and 5), and CD21+ B lymphocytes (lanes 6 and 7). These bands were not observed for the uninoculated control mouse (lane 8). (B) PrPres bands were also detected for the brain homogenates of clinical scrapie donor sheep 4124 (lane 1) as well as spleen homogenates of Tg338 mice inoculated with sIgM+ B lymphocytes (lanes 2~4) and CD21+ B lymphocytes (lanes 5~7). These bands were not observed for the uninoculated control mouse (lane 8). mAb F99/97.6.1 (2.5 µg/mL) was used to detect PrPres.
We also measured the prion infectivity in B lymphocytes prepared from 5 to 5.5 mL of blood collected from the same clinical scrapie-infected donor (4124) that was used for our lamb bioassay. Due to reason unrelated to the experiment, four mice from group 4 had to be euthanized at 338 dpi; PrPSc accumulation was clearly detected in the spleens of these mice. None of the remaining Tg338 mice receiving pan (sIgM+) B lymphocytes (isolated using anti-sIgM-specific mAb; group 4) or the CD21+ subset of B lymphocytes (group 5) developed clinical signs of scrapie. These mice were humanely euthanized at 521 dpi due to the development of intercurrent health issues (Table 2). Although PrPres was not detected in brain tissues by direct immunobloting, three of the nine mice in group 4 and seven of the nine mice in group 5 were positive for PrPres after concentrating prions from the brains by PTA precipitation (Table 2). Western blot assays performed with spleen homogenates prepared from all the mice in groups 4 and 5 were found positive for PrPres without the need for PTA precipitation (panel in B Fig. 2). These findings confirmed 100% transmission of scrapie to the recipient mice (Table 2).
Discussion
Most of the transfusion studies performed to detect infectious prions in blood from scrapie-affected sheep by other groups were limited to VRQ/VRQ donor and recipient sheep [2,14,15,18]. In our previous investigation, efficient transmission of classical scrapie was clearly observed when transfusion was performed between animals with the same or different prion genotypes such as ARQ/ARQ donors to ARQ/ARQ recipients, ARQ/VRQ donors to ARQ/VRQ recipients, and VRQ/VRQ donors to ARQ/VRQ or VRQ/VRQ recipients [6]. Those results demonstrated that the transmission of infection via intravenous inoculation of VRQ/VRQ lambs with PBMCs, CD72+ B lymphocytes, or CD21+ B lymphocytes was equivalently efficient and reliable when starting with 50 mL of blood from a VRQ/VRQ naturally scrapie-infected donor sheep [6]. Recovery of a 50 mL-blood sample in the field, however, requires prolonged sampling and collection into multiple tubes. Therefore, the objective of the present study was to determine whether pan B lymphocytes isolated from routinely collected volumes of blood harbor sufficient prion infectivity to be detected reliably by bioassays in lambs and ovinized transgenic mice. This would provide evidence for an optimal target volume for future development of blood-based scrapie diagnostic assays.
We used the same short-incubation scrapie-susceptible lamb (VRQ/VRQ genotype) bioassay model to determine the efficiency of scrapie transmission using B lymphocytes. Similar to the previous study, inoculation with PBMCs prepared from 50 mL of blood from a clinical scrapie-infected donor (4124) resulted in scrapie in all three recipient lambs by 6 mpi. This confirms that infectious prions were present in each of the 50-mL blood volumes from this particular donor at the time of blood collection. However, the time at which PrPSc was first detected in RAMALT follicles was delayed in lambs inoculated with CD72+ B lymphocytes isolated from 5 to 10 mL of blood. Furthermore, failure to demonstrate transmission of infection in one recipient lamb from group 3 by 10 mpi may indicate that a longer observation time is necessary to observe tissue accumulation of PrPSc. Alternatively, this inoculum derived from a 5-mL blood sample may not have contained a sufficient infectious prion dose. Konold et al. [17] reported the possibility of an early stage of infection via lateral transmission of scrapie from scrapie milk recipient lambs to control (scrapie milk unfed) lambs after mixing both groups of animals for 8 months [17]. The lack of PrPSc immunolabeling in any tissues from uninoculated lambs strongly suggests that the recipient lambs acquired scrapie only from the transfused cells and lateral transmission of scrapie did not occur during the 10-month observation period.
It would have been ideal to perform all the blood transfusion experiments using lambs. However, Tg338 mice were used in the present study to assess the prion infectivity in B lymphocytes isolated from both scrapie-infected donor sheep. These animals were selected due to the lack of an adequate number of lambs as well as the higher sensitivity of Tg338 mice [32] for detecting classical ovine- [3] and caprine- [23] derived scrapie. The absence of clinical scrapie in Tg338 mice receiving B lymphocytes from a sheep naturally infected with classical scrapie (4124) was unexpected since inoculation with B lymphocytes from a preclinical classical scrapie donor sheep (4125) produced clinical disease in most mice. The lack of clinical signs in mice from groups 4 and 5 was unlikely related to blood volumes or methods used in this study, but rather appeared to be associated with differences in scrapie blood inocula derived from the donor sheep. It is also possible that scrapie titers in the inocula might have been close to one infectious unit. We inoculated mice with an equal number of cells and used the same magnetic-assisted isolation technique to enrich the cell populations from both donor sheep. Although similar blood volumes (5~5.5 mL) were used to isolate pan B lymphocytes from both donors, only mice in group 2 developed clinical disease. Furthermore, PBMCs recovered from 1 mL of blood were able to produce clinical scrapie in group 1 mice, further suggesting that blood volume was unlikely to play a role in the lack of clinical signs in groups 4 and 5.
Although we did not have a reliable method for quantifying PrPres levels in the blood samples at the time of collection, brain tissues recovered from both donors at necropsy were analyzed with an immunoblot assay. Equivalent wet weight of brain homogenates (400 µg) prepared from both donors was loaded into the gels. Due to the band width, the immunoblot results appeared to indicate that donor sheep 4124 had relatively higher levels of PrPres compared to the other donor sheep (4125) at the time of necropsy. However, PrPres banding patterns in both donor sheep were identical. PrPSc immunolabeling patterns for the brains and lymphoid tissues from both donors were also similar.
PG127 scrapie isolate recovered from a clinically affected VRQ/VRQ Cheviot-Welsh sheep (Veterinary Laboratories Agency, UK) naturally infected with classical scrapie and LA404 scrapie isolate recovered from a clinically affected VRQ/VRQ Romanov sheep (Institut National de la Recherche Agronomique, France) naturally infected with classical scrapie have been extensively used for both sheep and mouse bioassays by other research groups [2,3,14,18,33]. When the incubation period and lesion profiles of the PG127 and LA404 scrapie isolates were compared in Tg338 mice, the PG127 isolate was found to have the shortest incubation period [3]. However, both isolates had similar infectious titers as well as similar PrPSc banding and glycosylation patterns. Recent studies using Tg338 mice [14,18] revealed that scrapie infectivity can be detected in sheep blood samples collected as early as 50 to 60 days post-oral challenge with PG127 isolate that possesses a relatively short incubation period (85 to 138 dpi). In contrast, a fairly long incubation period was reported (up to 783 dpi) when the same Tg338 mice were inoculated with PBMCs or WBCs prepared from another sheep naturally infected with classical scrapie [18]. Although the exact reason is not very clear, findings from these studies clearly suggest that PG127 is highly infectious to both VRQ/VRQ Cheviot-Welsh sheep and Tg338 mice. Based on the findings from these studies and our present study, the observed variation in incubation period of inoculated Tg338 mice might be due to the differences among natural scrapie isolates.
Currently approved scrapie diagnostic techniques have their own detection limits. For example, although rectal biopsy has greater sensitivity in sheep compared to goats, test sensitivity is limited if the biopsy is collected early during disease development, insufficient numbers of follicles are present in the biopsy, or PrPSc accumulation in peripheral tissues is reduced or delayed [8]. The lack of highly trained personnel and difficulty with collecting an adequate number of biopsies under field conditions can further affect the sensitivities and limit evaluation of large numbers of sheep. In vitro diagnostic assays such as the TSE-ELISA have limited sensitivity when detecting scrapie in the blood samples. In one study, only 55% of PBMCs samples prepared from sheep clinically affected with scrapie produced positive results [29]. Although PrPSc from the plasma samples was not detected by TSE-ELISA in a previous investigation, probably due the limited volume that can be loaded into the wells, surface-fluorescence intensity distribution analysis of plasma samples prepared from the same clinical donor sheep revealed the presence of PrP aggregates in six out of ten sheep [4]. Typical Western blot assays of scrapie-affected sheep brain homogenates including ones containing the PG127 isolate can detect PrPres up to a dilution of 10-3, but the Tg338 mouse bioassay can detect the prion up to a 10-5 dilution [3,18]. These observations clearly suggest the need for better platforms or improvement of existing assays such as the TSE-ELISA to detect scrapie using highly diluted tissue samples such as blood.
The PMCA has produced promising results of identifying prions in the brain and blood from sheep affected with classical scrapie [14,18,30]. For example, this assay can detect PrPSc in PG127 brain homogenates diluted up to 10-8 [18]. Although prions have been detected in smaller volumes of blood collected from sheep orally inoculated with the highly infectious PG127 scrapie isolate [2,14], using very small volumes of blood in which the infectious titers are possibly low is likely to produce false negative results. Although the PMCA can detect prions, this technique is limited by (a) a longer assay duration, (b) requirement of highly skilled personnel, (c) maintenance of Tg338 mice colonies to prepare the substrate, and (d) scrapie-free laboratories that are often difficult to maintain. The real-time quaking-induced conversion (RT-QuIC) assay has been successfully performed to detect prions in the blood of deer with chronic wasting disease [11]. Advantages of the RT-QuIC assay include (a) requirement of bacterial-expressed (recombinant) cellular prion protein and (b) a shorter assay time. We are currently performing experiments to determine whether the RT-QuIC assay can detect prions in blood samples collected from both naturally and experimentally scrapie-infected sheep.
One major limitation of the current study was the use of B lymphocytes isolated from only two VRQ/VRQ donor sheep for the bioassays. The decision to use two donor sheep in this investigation was based on our previous lamb bioassay findings showing that scrapie infectivity can be achieved with blood fractions isolated not only from clinically but also preclinically affected sheep naturally infected with classical scrapie [6]. Additionally, we used blood from one preclinical (rectal biopsy positive for PrPSc) and one clinical sheep as blood donors to identify scrapie infection and the presence of PrPSc in the blood. However, it has been reported in certain instances that the level of PrPSc does not necessarily correlate with the level of prion infectivity [9]. Detection of PrPSc in antemortem and postmortem tissues from lambs, development of clinical scrapie, and detection of PrPres in postmortem tissues from mice correlated with the presence of PrPSc in rectal and other tissues along with development of clinical scrapie in infected donor sheep.
Although none of the in vitro blood-based assays described above were able to match the sensitivities associated with rectal biopsies of sheep, novel detection methods may soon offer a rapid platform with detection sensitivities that rival biopsy as well as prion bioassays. The collection of smaller blood volumes (one anti-coagulated blood tube per animal) from sheep under field conditions is easier and faster than obtaining biopsy samples. Additionally, the current study demonstrated that infectious prions are present in smaller blood volumes. Thus, blood samples appear to be appropriate for identifying cases of classical scrapie infection in sheep. With the presence of infectious prions in CD72+ or sIgM+ pan B lymphocyte preparations, anti-CD72 or anti-sIgM mAbs can also be used to isolate pan B lymphocytes as seed for new assays. However, more studies should be performed using multiple donor sheep with other prion genotypes as well as multiple sheep breeds infected with different natural scrapie isolates (including PG127 isolates) to further assess the levels of prions in blood.
Acknowledgments
This work was supported by funds from the United States Department of Agriculture (USDA), Agricultural Research Service (ARS) (The Current Research Information System 5348-32000-030-00 D). The authors would like to thank Ms. Linda Hamburg and Ms. Lori Fuller for their assistance with the blood transfusion experiments; Ms. Desiree Lesiak for PRNP genotyping (USDA, ARS, Animal Disease Research Unit); and Mr. Christopher Schachtschneider and Ms. Jan Luft (Washington State University) for care of the animals. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the USDA.
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Andréoletti O, Berthon P, Marc D, Sarradin P, Grosclaude J, van Keulen L, Schelcher F, Elsen JM, Lantier F. Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J Gen Virol. 2000;81:3115–3126. doi: 10.1099/0022-1317-81-12-3115. [DOI] [PubMed] [Google Scholar]
- 2.Andréoletti O, Litaise C, Simmons H, Corbiére F, Lugan S, Costes P, Schelcher F, Vilette D, Grassi J, Lacroux C. Highly efficient prion transmission by blood transfusion. PLoS Pathog. 2012;8:e1002782. doi: 10.1371/journal.ppat.1002782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andréoletti O, Orge L, Benestad SL, Beringue V, Litaise C, Simon S, Le Dur A, Laude H, Simmons H, Lugan S, Corbiére F, Costes P, Morel N, Schelcher F, Lacroux C. Atypical/Nor98 scrapie infectivity in sheep peripheral tissues. PLoS Pathog. 2011;7:e1001285. doi: 10.1371/journal.ppat.1001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bannach O, Birkmann E, Reinartz E, Jaeger KE, Langeveld JP, Rohwer RG, Gregori L, Terry LA, Willbold D, Riesner D. Detection of prion protein particles in blood plasma of scrapie infected sheep. PLoS One. 2012;7:e36620. doi: 10.1371/journal.pone.0036620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bolton DC, McKinley MP, Prusiner SB. Identification of a protein that purifies with the scrapie prion. Science. 1982;218:1309–1311. doi: 10.1126/science.6815801. [DOI] [PubMed] [Google Scholar]
- 6.Dassanayake RP, Schneider DA, Truscott TC, Young AJ, Zhuang D, O'Rourke KI. Classical scrapie prions in ovine blood are associated with B lymphocytes and platelet-rich plasma. BMC Vet Res. 2011;7:75. doi: 10.1186/1746-6148-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dassanayake RP, Truscott TC, Ozyigit MO, Zhuang D, Schneider DA, O'Rourke KI. Accumulation profiles of PrPSc in hemal nodes of naturally and experimentally scrapie- infected sheep. BMC Vet Res. 2013;9:82. doi: 10.1186/1746-6148-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dennis MM, Thomsen BV, Marshall KL, Hall SM, Wagner BA, Salman MD, Norden DK, Gaiser C, Sutton DL. Evaluation of immunohistochemical detection of prion protein in rectoanal mucosa-associated lymphoid tissue for diagnosis of scrapie in sheep. Am J Vet Res. 2009;70:63–72. doi: 10.2460/ajvr.70.1.63. [DOI] [PubMed] [Google Scholar]
- 9.Dobie K, Barron R. Dissociation between transmissible spongiform encephalopathy (TSE) infectivity and proteinase K-resistant PrPSc levels in peripheral tissue from a murine transgenic model of TSE disease. J Virol. 2013;87:5895–5903. doi: 10.1128/JVI.03469-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edwards JC, Moore SJ, Hawthorn JA, Neale MH, Terry LA. PrPSc is associated with B cells in the blood of scrapie-infected sheep. Virology. 2010;405:110–119. doi: 10.1016/j.virol.2010.05.023. [DOI] [PubMed] [Google Scholar]
- 11.Elder AM, Henderson DM, Nalls AV, Wilham JM, Caughey BW, Hoover EA, Kincaid AE, Bartz JC, Mathiason CK. In vitro detection of prionemia in TSE-infected cervids and hamsters. PloS One. 2013;8:e80203. doi: 10.1371/journal.pone.0080203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Espenes A, Press CM, Landsverk T, Tranulis MA, Aleksandersen M, Gunnes G, Benestad SL, Fuglestveit R, Ulvund MJ. Detection of PrPSc in rectal biopsy and necropsy samples from sheep with experimental scrapie. J Comp Pathol. 2006;134:115–125. doi: 10.1016/j.jcpa.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 13.González L, Dagleish MP, Martin S, Dexter G, Steele P, Finlayson J, Jeffrey M. Diagnosis of preclinical scrapie in live sheep by the immunohistochemical examination of rectal biopsies. Vet Rec. 2008;162:397–403. doi: 10.1136/vr.162.13.397. [DOI] [PubMed] [Google Scholar]
- 14.Halliez S, Jaumain E, Huor A, Douet JY, Lugan S, Cassard H, Lacroux C, Béringue V, Andréoletti O, Vilette D. White blood cell-based detection of asymptomatic scrapie infection by ex vivo assays. PLoS One. 2014;9:e104287. doi: 10.1371/journal.pone.0104287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houston F, McCutcheon S, Goldmann W, Chong A, Foster J, Sisó S, González L, Jeffrey M, Hunter N. Prion diseases are efficiently transmitted by blood transfusion in sheep. Blood. 2008;112:4739–4745. doi: 10.1182/blood-2008-04-152520. [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Rendulich J, Stevenson D, O'Rourke K, Balachandran A. Evaluation of Western blotting methods using samples with or without sodium phosphotungstic acid precipitation for diagnosis of scrapie and chronic wasting disease. Can J Vet Res. 2005;69:193–199. [PMC free article] [PubMed] [Google Scholar]
- 17.Konold T, Moore SJ, Bellworthy SJ, Simmons HA. Evidence of scrapie transmission via milk. BMC Vet Res. 2008;4:14. doi: 10.1186/1746-6148-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lacroux C, Vilette D, Fernández-Borges N, Litaise C, Lugan S, Morel N, Corbiére F, Simon S, Simmons H, Costes P, Weisbecker JL, Lantier I, Lantier F, Schelcher F, Grassi J, Castilla J, Andréoletti O. Prionemia and leukocyte-platelet-associated infectivity in sheep transmissible spongiform encephalopathy models. J Virol. 2012;86:2056–2066. doi: 10.1128/JVI.06532-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mok SW, Proia RL, Brinkmann V, Mabbott NA. B cell-specific S1PR1 deficiency blocks prion dissemination between secondary lymphoid organs. J Immunol. 2012;188:5032–5040. doi: 10.4049/jimmunol.1200349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Rourke KI, Baszler TV, Besser TE, Miller JM, Cutlip RC, Wells GAH, Ryder SJ, Parish SM, Hamir AN, Cockett NE, Jenny A, Knowles DP. Preclinical diagnosis of scrapie by immunohistochemistry of third eyelid lymphoid tissue. J Clin Microbiol. 2000;38:3254–3259. doi: 10.1128/jcm.38.9.3254-3259.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O'Rourke KI, Baszler TV, Miller JM, Spraker TR, Sadler-Riggleman I, Knowles DP. Monoclonal antibody F89/160.1.5 defines a conserved epitope on the ruminant prion protein. J Clin Microbiol. 1998;36:1750–1755. doi: 10.1128/jcm.36.6.1750-1755.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'Rourke KI, Baszler TV, Parish SM, Knowles DP. Preclinical detection of PrPSc in nictitating membrane lymphoid tissue of sheep. Vet Rec. 1998;142:489–491. doi: 10.1136/vr.142.18.489. [DOI] [PubMed] [Google Scholar]
- 23.O'Rourke KI, Schneider DA, Spraker TR, Dassanayake RP, Highland MA, Zhuang D, Truscott TC. Transmissibility of caprine scrapie in ovine transgenic mice. BMC Vet Res. 2012;8:42. doi: 10.1186/1746-6148-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Rourke KI, Zhuang D, Truscott TC, Yan H, Schneider DA. Sparse PrPSc accumulation in the placentas of goats with naturally acquired scrapie. BMC Vet Res. 2011;7:7. doi: 10.1186/1746-6148-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prusiner SB. Novel proteinaceous infectious particles cause scrapie. Science. 1982;216:136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- 26.Race R, Jenny A, Sutton D. Scrapie infectivity and proteinase K-resistant prion protein in sheep placenta, brain, spleen, and lymph node: implications for transmission and antemortem diagnosis. J Infect Dis. 1998;178:949–953. doi: 10.1086/515669. [DOI] [PubMed] [Google Scholar]
- 27.Ryder S, Dexter G, Bellworthy S, Tongue S. Demonstration of lateral transmission of scrapie between sheep kept under natural conditions using lymphoid tissue biopsy. Res Vet Sci. 2004;76:211–217. doi: 10.1016/j.rvsc.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 28.Schreuder BE, van Keulen LJ, Vromans ME, Langeveld JP, Smits MA. Tonsillar biopsy and PrPSc detection in the preclinical diagnosis of scrapie. Vet Rec. 1998;142:564–568. doi: 10.1136/vr.142.21.564. [DOI] [PubMed] [Google Scholar]
- 29.Terry LA, Howells L, Hawthorn J, Edwards JC, Moore SJ, Bellworthy SJ, Simmons H, Lizano S, Estey L, Leathers V, Everest SJ. Detection of PrPSc in blood from sheep infected with the scrapie and bovine spongiform encephalopathy agents. J Virol. 2009;83:12552–12558. doi: 10.1128/JVI.00311-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorne L, Terry LA. In vitro amplification of PrPSc derived from the brain and blood of sheep infected with scrapie. J Gen Virol. 2008;89:3177–3184. doi: 10.1099/vir.0.2008/004226-0. [DOI] [PubMed] [Google Scholar]
- 31.van Keulen LJ, Schreuder BE, Meloen RH, Mooij-Harkes G, Vromans ME, Langeveld JP. Immunohistochemical detection of prion protein in lymphoid tissues of sheep with natural scrapie. J Clin Microbiol. 1996;34:1228–1231. doi: 10.1128/jcm.34.5.1228-1231.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Keulen LJ, Schreuder BE, Vromans ME, Langeveld JP, Smits MA. Pathogenesis of natural scrapie in sheep. Arch Virol Suppl. 2000;16:57–71. doi: 10.1007/978-3-7091-6308-5_5. [DOI] [PubMed] [Google Scholar]
- 33.Vilotte JL, Soulier S, Essalmani R, Stinnakre MG, Vaiman D, Lepourry L, Da Silva JC, Besnard N, Dawson M, Buschmann A, Groschup M, Petit S, Madelaine MF, Rakatobe S, Le Dur A, Vilette D, Laude H. Markedly increased susceptibility to natural sheep scrapie of transgenic mice expressing ovine PrP. J Virol. 2001;75:5977–5984. doi: 10.1128/JVI.75.13.5977-5984.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Young AJ, Marston WL, Dudler L. Subset-specific regulation of the lymphatic exit of recirculating lymphocytes in vivo. J Immunol. 2000;165:3168–3174. doi: 10.4049/jimmunol.165.6.3168. [DOI] [PubMed] [Google Scholar]