Abstract
Objectives
Dentin is composed of many minerals and growth factors. Based on this composition, we studied its effect as a possible regenerative material for alveolar healing.
Materials and Methods
This study was conducted using four 2.5-year-old mongrel dogs (male; weight, 25 to 30 kg). The third mandibular premolars were carefully mobilized with a dental elevator and then removed using forceps. The crown portions of the extracted teeth were removed with cutters, and the root portions of the remaining teeth were collectively trimmed as closely as possible to 350 to 500 µm. Dentin and cementum (DC) chips harvested from the extracted teeth were soaked in blood and packed into the fresh sockets (autograft). Biopsies were performed at the ends of day 14 and day 56 following implantation. Data were expressed as mean±standard deviation and compared with t-test results.
Results
The ratio of SA(bone) to total area of each probe was determined and was 170±16 µm2 for the control group and 71±14 µm2 for the DC group, a significant difference (P<0.05).
Conclusion
DC particulate grafts offered no improvement in bone regeneration in alveolar extraction sockets.
Keywords: Dentin, Cementum, Tooth extraction, Healing
I. Introduction
The preservation of the alveolus for jawbone health has been widely discussed in the scientific literature. In the majority of cases, when a tooth is extracted, the surrounding alveolar bone undergoes a series of resorptive processes1. Studies have shown that natural healing post-extraction is associated with a significant loss in vertical and horizontal bone2,3. Moreover, a variety of factors, including trauma and endodontic pathologies, contribute to the loss of alveolar bone and subsequently complicate restorative treatments such as dental implants and dentures4. Many studies have demonstrated the potential merits of socket preservation following tooth extraction. To date, various materials and techniques have been used to preserve bone after tooth extraction5. For instance, the use of dense hydroxyapatite (HA) and bovine bone mineral integrated in collagen matrix have been reported after tooth extraction6,7.
Extracted teeth can be used as grafting materials8, and dentin is of particular interest for this purpose due to its unique chemical composition9. Dentin is an acellular, avascular, collagen-rich tissue matrix, compared to bone that is a cellular tissue with vessels. However, dentin and bone have similar components; they both consist of 10% body fluid, 20% organic materials, and 70% minerals (mainly HA) and contain bone morphogenetic proteins (BMPs), insulin-like growth factor (IGF)-II, and transforming growth factor (TGF)-β. Cementum, a bone-like material surrounding tooth roots, also contains TGF-β, IGF-I, and type I and type III collagen10,11,12,13,14. The osteoinductive potential of dentin was discovered in 196715. Since then, several lines of studies have shown that animal demineralized dentine matrix induces ectopic bone formation in subcutaneous and intramuscular pockets in rodents9,11. Regarding the biochemical properties of dentin, we sought to assess the regenerative properties of fresh dentin as a novel graft material for alveolar bone regeneration.
II. Materials and Methods
This study was conducted on four 2.5-year-old mongrel dogs (male; weight, 25 to 30 kg). These animals were housed in temperature-controlled rooms and lived under a standard 12-hour light/dark cycle. The protocol of this prospective, randomized controlled trial was approved by the ethics committee of the Dental Research Center at Shahid Beheshti University of Medical Sciences (Tehran, Iran). The animals were sedated with an intramuscular injection of 2% acepromazin (0.1 mg/kg; Ingelheim Vetmedica Inc., St. Joseph, MO, USA) and subsequently anesthetized with 10% ketamine hydrochloride (25 mg/kg; Parke-Davis, Morris Plains, NJ, USA) administered intravenously and maintained during anesthesia; 2% lidocaine with 1:80,000 epinephrine (Darouphakhsh, Tehran, Iran) was injected to control any hemorrhaging. The third mandibular premolars on both sides were carefully mobilized with a dental elevator and then removed using forceps without the elevation of a muco-periosteal flap or compromising the marginal gingiva. The sockets were checked after extraction and thoroughly debrided with a curette to remove the periodontal ligament. After irrigation with saline solution, the depth of each socket was measured with a probe. The pulp tissues of the roots were extirpated with a hand instrument, and the root surfaces were cleaned with sterile gauze and a soft periodontal curette. The crown portions of the extracted teeth were removed with cutters, and the root portions of the remaining teeth were collectively trimmed as closely as possible to 350 to 500 µm using a calibrated mesh filter. In one quadrant, dentin and cementum (DC) chips harvested from the extracted teeth were soaked in blood and packed into the fresh sockets (autograft) so that they were completely filled with graft material. In the other quadrant, no bone substitute was placed. The sockets were sutured (Seralene 4-0s; Serag-Wiessner, Naila, Germany), and the animals were observed once a day for any clinical abnormality. Antimicrobial prophylaxis (cephalosporin 15 mg/kg, twice a day) was continued for 48 hours after surgery. Postoperative pain control was achieved via carprofen (50 mg/os/day, Rimadyls; Pfizer Santé Animale, Orsay, France) for 13 days. The dogs were placed on a soft diet throughout the entire observation period. The sutures were removed under a brief period of general anesthesia two weeks after the surgery.
Experiments performed and conducted in accordance with Regional Committee of Ethic complied with the regulations of the European Convention on Vertebrate Animals protection (2005).
1. Collection and storage of the specimens
The biopsy procedure was performed at the ends of 14 and 56 days after implantation16. At that time, short-lasting general anesthesia was induced, and local infiltration anesthesia was administered. A crestal incision was made, and the mucoperiosteal flap was elevated on both the buccal and lingual/palatal sides using a microsurgical periosteal elevator (P-TROM; Hu-Friedy, Chicago, IL, USA) whenever the concomitant surgical procedure required a full-thickness flap. When this was not the case (e.g., implant positioning with a flapless approach), the soft tissues overlying the extraction socket were included. The tissue specimens were collected with a trephine bur (2 mm internal diameter and 8 mm length; Hu-Friedy) from the centers of the sockets. The depth of trephine bur insertion was related to the measurements previously made by the stent. Tissue was obtained from the center of each socket, with the insertion axis of the trephine kept parallel to the long axis of the adjacent tooth. The apical portion of the specimen was marked with a fine indelible pen. The specimens were immediately fixed in 10% formalin.
2. Histological analysis
The specimens were rinsed thoroughly with water. After decalcification6, the tissue blocks were embedded in paraffin. Several transverse sections with a diameter of 5 µm were cut through the center of each defect using a microtome (Jung, Frankfort, Germany). We used systematic random sampling to select sections with 20-µm intervals17. The samples were then stained with H&E and examined with an optical microscope at 40× magnification (Nikon Eclipse E400; Nikon, Tokyo, Japan) that was linked via a digital camera (Nikon Fuji HC-300 ZI; Nikon) to a personal computer. For quantification of the studied fields, a simple morphometric analysis was used; tissues of the specimens were divided into two compartments: newly formed bone and connective tissue.
A stereological frame was superimposed on each randomized field.(Fig. 1) Based on the tissue contained within each randomized field, the boundary of each phase was defined on the image. And then the frame was superimposed on it; the hit points or intersections for each phase were counted and recorded. Using this method, the proportions of the histological phases (new bone and connective tissue) were obtained for each studied field. The surface area (SA) of each counting frame (150×120 µm) was 18,000 µm2. Finally, the number of hit of points was inserted into the following formula:
| SA=I phase A/total number |
where SA indicates the proportion of tissue, and I indicates the number of intersections for each phase. As mentioned earlier, the surface ratios for each section were calculated17.
Fig. 1. A stereological counting frame was superimposed on each randomized field. This frame is composed of 30 squares (each square, 25 µm). ×40.
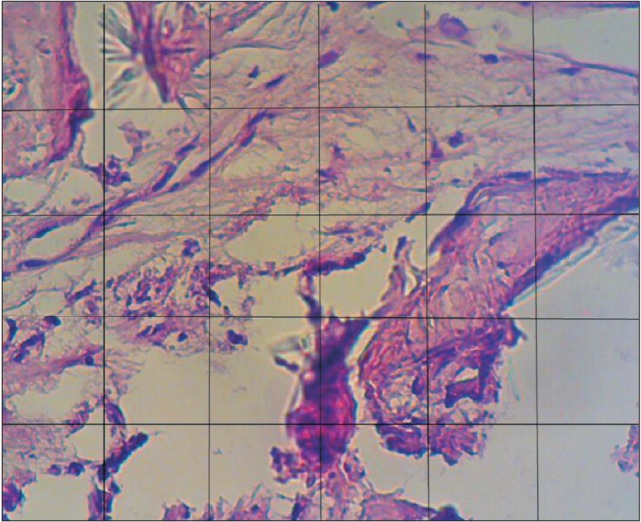
3. Statistical analysis
All data are expressed as mean±standard deviation. The comparison was made by paired t-test, and P-values<0.05 were considered statistically significant. Calculations were performed using the SPSS Statistics version 17.0 software (SPSS Inc., Chicago, IL, USA).
III. Results
Histological examination of group DC at the end of the second week showed particles of unreacted ground dentin and some dilated vessels.(Fig. 2) On the control side, no specific reaction was noted. At the end of eight weeks in group DC, granular tissue with fibroblast cells and collagen fibers was the dominant histological feature, and sparse bone formation was noted in the vicinity of the particles.(Fig. 3) On the control side after eight weeks, the histological examination showed collagen fiber deposition, a few scattered osteoblasts, bone formation in a centripetal pattern, and venules packed with red blood cells.(Fig. 4, 5)
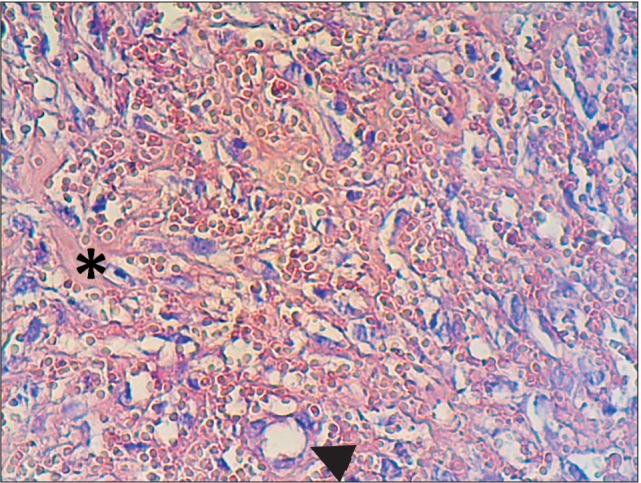
Fig. 2. Dentin/cementum particle (asterisk) and a blood vessel (arrow) in an alveolar socket at the end of the second week. H&E staining, ×40.
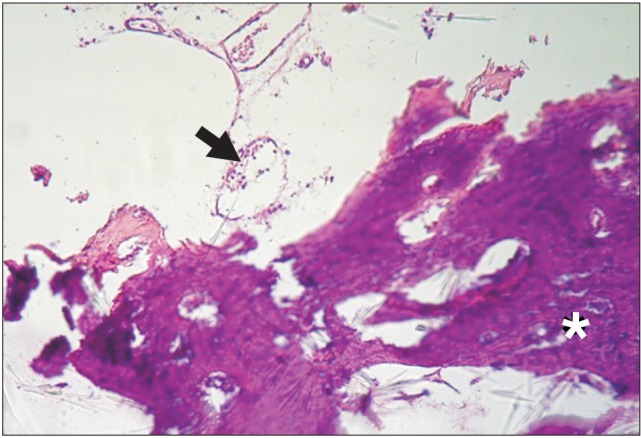
Fig. 3. New bone formation (arrowhead), particles of dentin/cementum (arrow) in the periphery, and newly formed bone adjacent to the particles at the end of the eighth week. H&E staining, ×20.
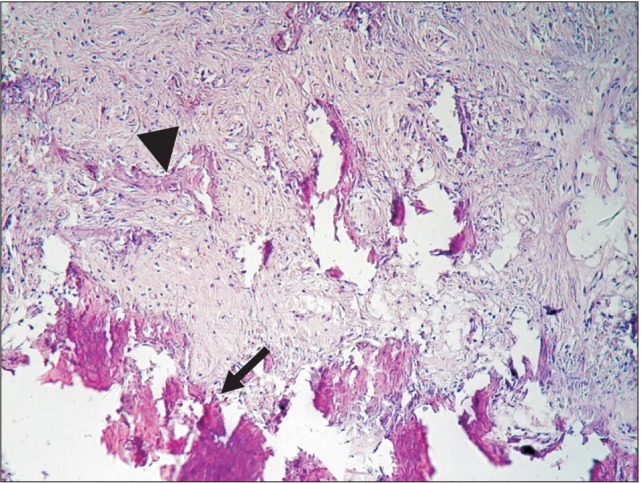
Fig. 4. Bone formed in a centripetal pattern. Bone spicules (arrowhead), connective tissue (asterisk) and a blood vessel (arrow) in the control group at the eighth week. H&E staining, ×20.
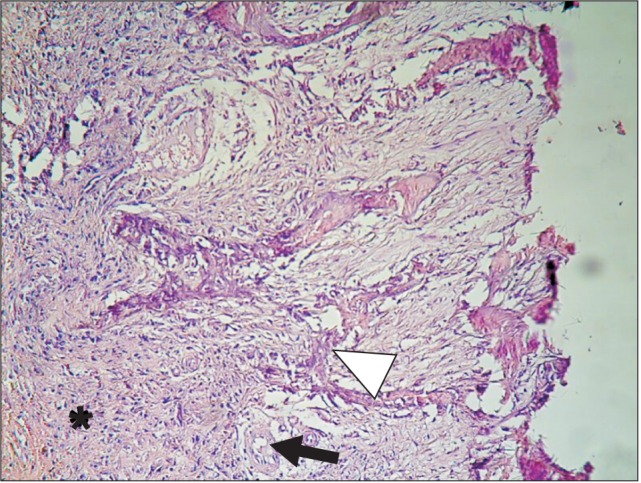
Fig. 5. Red blood cells, venules (arrowhead), and connective tissue (asterisk) in the control group at the eighth week. H&E staining, ×20.
1. Morphometry
The ratio of SA(bone) to total area of each probe was assessed and was 170±16 µm2 for the control group at the end of eight weeks and 71±14 µm2 for the DC group, a significant difference (P<0.05).
IV. Discussion
This animal study was conducted to assess the healing of tooth extraction sockets using fresh DC. The results showed that the application of DC induced no effects on the healing process before two weeks. However, after eight weeks, granular connective tissue was prominent in DC sockets. We used descriptive examination in conjunction with morphometric analysis to estimate the SA of newly formed bone. This method provides tangible and quantitative results from histological sections and therefore enables the precise comparison of data18. The results of morphometric analysis revealed that bone formation proceeded at a lower rate in sockets treated with fresh DC. The present results are likely the first report on the use of fresh DC in reconstructive surgery. The application of biomaterials such as dentin has been studied extensively in the field of reconstructive surgery. Our data suggest that the route of preparation is a crucial step in the osteoinductivity of biomaterials19. Studies have shown that demineralizing treatments of bone and dentin increase their osteoinductivity and decrease their antigenicity20,21. Nampo et al.22 studied the effect of iliac bone and teeth on new bone formation in the jawbones of rats. The results of their study showed that both types of materials could be used as suitable grafting materials. Kim et al.21 reported that a mixture of heat-pulverized dentin and plaster repaired large jaw defects 57 months after surgery. They concluded that a dentin/plaster mixture was a useful biomaterial in the reconstructive surgery of jaw defects13. Register found that dentin allografts activated bone formation14, and Jeong et al.23 reported that autogenous teeth and bone could be used as good alternatives to autogenous bone. Collectively, the aforementioned studies have reported the potential of dentin as a grafting material. However, our results are not compatible with these studies. The discrepancies between our results and those of other studies may be explained by the wounds or defects, species, age, and method of material preparation. For instance, Nampo et al.22 used the extracted tooth with its pulp as a source of stem cells and neural crest cells. Devecioğlu et al.24 studied the effects of demineralized, freeze-dried DC on periodontal ligaments and fibroblasts. Their results implied that the applied materials had greater potential to form mineral-like nodules than did other applied materials. Gomes et al.25 studied the effects of demineralized dentin matrix on bone repair in diabetic rabbits and showed that dematerialized dentin was biocompatible in diabetic rabbits.
Given the results of these studies and our findings, fresh DC has little capability to induce significant bone formation in the alveolar socket. This result may be explained by the fact that such mineral acts as a barrier to chemotactic and morphogenic molecules of dentin, while acid initiates a demineralizing process that exposes osteoinductive molecules like BMPs. It seems that demineralization of biomaterials such as DC would be a key determinant factor in subsequent inductive/conductive potential of in vivo grafted materials19,20,21. Studies have documented that demineralization of dentin exposes the sequestrated BMPs in the insoluble collagen of the dentin matrix. Decalcified dentin has revealed better bone induction activity due to the activation of BMPs, which bind to collagen matrices through the demineralization process17,18,19,20,21,26. Recent data provide evidence that addition of BMP2 to demineralized dentin accelerates its osteoinductivity16,26.
V. Conclusion
In conclusion, the strength of our pilot study is the evidence that fresh mineralized DC autografts have little to no effect on the induction of new bone in the alveolar socket milieu. We suggest using ultrastructural and molecular approaches in similar studies in order to determine the underlying mechanisms.
Footnotes
Conflict of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Irinakis T. Rationale for socket preservation after extraction of a single-rooted tooth when planning for future implant placement. J Can Dent Assoc. 2006;72:917–922. [PubMed] [Google Scholar]
- 2.Botticelli D, Berglundh T, Lindhe J. Hard-tissue alterations following immediate implant placement in extraction sites. J Clin Periodontol. 2004;31:820–828. doi: 10.1111/j.1600-051X.2004.00565.x. [DOI] [PubMed] [Google Scholar]
- 3.Tan WL, Wong TL, Wong MC, Lang NP. A systematic review of post-extractional alveolar hard and soft tissue dimensional changes in humans. Clin Oral Implants Res. 2012;23(Suppl 5):1–21. doi: 10.1111/j.1600-0501.2011.02375.x. [DOI] [PubMed] [Google Scholar]
- 4.Dimova C. Socket preservation procedure after tooth extraction. Key Engineering Materials. 2014;587:325–330. [Google Scholar]
- 5.Vignoletti F, Matesanz P, Rodrigo D, Figuero E, Martin C, Sanz M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin Oral Implants Res. 2012;23(Suppl 5):22–38. doi: 10.1111/j.1600-0501.2011.02331.x. [DOI] [PubMed] [Google Scholar]
- 6.Sattayasanskul W, Brook IM, Lamb DJ. Dense hydroxyapatite root replica implantation: measurement of mandibular ridge preservation. Int J Oral Maxillofac Implants. 1988;3:203–207. [PubMed] [Google Scholar]
- 7.Jung RE, Siegenthaler DW, Hämmerle CH. Postextraction tissue management: a soft tissue punch technique. Int J Periodontics Restorative Dent. 2004;24:545–553. [PubMed] [Google Scholar]
- 8.Kim YK, Kim SG, Oh JS, Jin SC, Son JS, Kim SY, et al. Analysis of the inorganic component of autogenous tooth bone graft material. J Nanosci Nanotechnol. 2011;11:7442–7445. doi: 10.1166/jnn.2011.4857. [DOI] [PubMed] [Google Scholar]
- 9.Huggins C, Wiseman S, Reddi AH. Transformation of fibroblasts by allogeneic and xenogeneic transplants of demineralized tooth and bone. J Exp Med. 1970;132:1250–1258. doi: 10.1084/jem.132.6.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finkelman RD, Mohan S, Jennings JC, Taylor AK, Jepsen S, Baylink DJ. Quantitation of growth factors IGF-I, SGF/IGF-II, and TGF-beta in human dentin. J Bone Miner Res. 1990;5:717–723. doi: 10.1002/jbmr.5650050708. [DOI] [PubMed] [Google Scholar]
- 11.Bang G, Urist MR. Bone induction in excavation chambers in matrix of decalcified dentin. Arch Surg. 1967;94:781–789. doi: 10.1001/archsurg.1967.01330120035008. [DOI] [PubMed] [Google Scholar]
- 12.Bessho K, Tagawa T, Murata M. Purification of rabbit bone morphogenetic protein derived from bone, dentin, and wound tissue after tooth extraction. J Oral Maxillofac Surg. 1990;48:162–169. doi: 10.1016/s0278-2391(10)80204-6. [DOI] [PubMed] [Google Scholar]
- 13.Butler WT, Mikulski A, Urist MR, Bridges G, Uyeno S. Noncollagenous proteins of a rat dentin matrix possessing bone morphogenetic activity. J Dent Res. 1977;56:228–232. doi: 10.1177/00220345770560030601. [DOI] [PubMed] [Google Scholar]
- 14.Murata M, Kawai T, Kawakami T, Akazawa T, Tazaki J, Ito K, et al. Human acid-insoluble dentin with BMP2 accelerates bone induction in subcutaneous and intramuscular tissues. J Ceram Soc Jpn. 2010;118:438–441. [Google Scholar]
- 15.Yeomans JD, Urist MR. Bone induction by decalcified dentine implanted into oral, osseous and muscle tissues. Arch Oral Biol. 1967;12:999–1008. doi: 10.1016/0003-9969(67)90095-7. [DOI] [PubMed] [Google Scholar]
- 16.Murata M. Bone engineering using human demineralized dentin matrix and recombinant human BMP2. J Hard Tissue Biol. 2005;14:80–81. [Google Scholar]
- 17.Reed MG, Howard CV, de Yanés GS. One-stop stereology: the estimation of 3D parameters using isotropic rulers. J Microsc. 2010;239:54–65. doi: 10.1111/j.1365-2818.2009.03356.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Zauel RR, Rao DS, Fyhrie DP. Cancellous bone lamellae strongly affect microcrack propagation and apparent mechanical properties: separation of patients with osteoporotic fracture from normal controls using a 2D nonlinear finite element method (biomechanical stereology) Bone. 2008;42:1184–1192. doi: 10.1016/j.bone.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewandrowski KU, Tomford WW, Schomacker KT, Deutsch TF, Mankin HJ. Improved osteoinduction of cortical bone allografts: a study of the effects of laser perforation and partial demineralization. J Orthop Res. 1997;15:748–756. doi: 10.1002/jor.1100150518. [DOI] [PubMed] [Google Scholar]
- 20.Reddi AH. Bone matrix in the solid state: geometric influence on differentiation of fibroblasts. Adv Biol Med Phys. 1974;15:1–18. doi: 10.1016/b978-0-12-005215-8.50007-3. [DOI] [PubMed] [Google Scholar]
- 21.Kim YK, Kim SG, Byeon JH, Lee HJ, Um IU, Lim SC, et al. Development of a novel bone grafting material using autogenous teeth. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:496–503. doi: 10.1016/j.tripleo.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 22.Nampo T, Watahiki J, Enomoto A, Taguchi T, Ono M, Nakano H, et al. A new method for alveolar bone repair using extracted teeth for the graft material. J Periodontol. 2010;81:1264–1272. doi: 10.1902/jop.2010.100016. [DOI] [PubMed] [Google Scholar]
- 23.Jeong HR, Hwang JH, Lee JK. Effectiveness of autogenous tooth bone used as a graft material for regeneration of bone in miniature pig. J Korean Assoc Oral Maxillofac Surg. 2011;37:375–379. [Google Scholar]
- 24.Devecioğlu D, Tözüm TF, Sengün D, Nohutcu RM. Biomaterials in periodontal regenerative surgery: effects of cryopreserved bone, commercially available coral, demineralized freeze-dried dentin, and cementum on periodontal ligament fibroblasts and osteoblasts. J Biomater Appl. 2004;19:107–120. doi: 10.1177/0885328204043818. [DOI] [PubMed] [Google Scholar]
- 25.Gomes MF, Destro MF, Banzi EC, Vieira EM, Morosolli AR, Goulart Md. Optical density of bone repair after implantation of homogenous demineralized dentin matrix in diabetic rabbits. Braz Oral Res. 2008;22:275–280. doi: 10.1590/s1806-83242008000300015. [DOI] [PubMed] [Google Scholar]
- 26.Urist MR, Granstein R, Nogami H, Svenson L, Murphy R. Transmembrane bone morphogenesis across multiple-walled diffusion chambers. New evidence for a diffusible bone morphogenetic property. Arch Surg. 1977;112:612–619. doi: 10.1001/archsurg.1977.01370050072012. [DOI] [PubMed] [Google Scholar]


