Abstract
Hepatocellular carcinoma (HCC) is ranked as the 5th common type of cancer worldwide and is considered as the 3rd common reason for cancer-related deaths. HCC often occurs on top of a cirrhotic liver. The prognosis is determined by several factors; tumour extension, alpha-fetoprotein (AFP) concentration, histologic subtype of the tumour, degree of liver dysfunction, and the patient’s performance status. HCC prognosis is strongly correlated with diagnostic delay. To date, no ideal screening modality has been developed. Analysis of recent studies showed that AFP assessment lacks adequate sensitivity and specificity for effective surveillance and diagnosis. Many tumour markers have been tested in clinical trials without progressing to routine use in clinical practice. Thus, surveillance is still based on ultrasound (US) examination every 6 mo. Imaging studies for diagnosis of HCC can fall into one of two main categories: routine non-invasive studies such as US, computed tomography (CT), and magnetic resonance imaging, and more specialized invasive techniques including CT during hepatic arteriography and CT arterial portography in addition to the conventional hepatic angiography. This article provides an overview and spotlight on the different diagnostic modalities and treatment options of HCC.
Keywords: Diagnosis of hepatocellular carcinoma, Surgical resection, Hepatocellular carcinoma, Liver transplantation, Radiofrequency ablation, Microwave ablation, Percutaneous ethanol or acetic acid ablation, Radio-embolisation, Systemic chemotherapy, Trans-arterial chemoembolisation
Core tip: This review aims to spotlight on the different diagnostic modalities, and treatment options of hepatocellular carcinoma (HCC). Despite lack of adequate sensitivity of ultrasound (US) examination and alpha-fetoprotein, both are still considered the cornerstone for surveillance for HCC. So, a plethora of clinical studies searching for a more ideal tool are running. One of these tools is the microRNAs which can be considered as a promising diagnostic as well as prognostic tool for HCC. This review discusses the diagnostic utility of computed tomography and magnetic resonance imaging, as well as the enhanced US efficacy in diagnosis of HCC. Management of HCC depends on the tumour stage, liver function reserve, and patient performance status, and requires a multidisciplinary approach for optimal treatment. Liver transplantation and hepatic resection are the only curative options in early stage of disease. In addition, radiofrequency ablation is equivalent to surgical resection in well-selected patients. Radioembolization with use of resin or glass sphere appear promising. Novel molecular therapies are also discussed. For patients with advanced disease, sorafenib is the only approved therapy, but novel targeted agents and their combinations are emerging.
INTRODUCTION
Hepatocellular carcinoma (HCC) is the 5th common type of cancer all over the world; after lung, prostate, colorectal, and stomach cancers. It evolves at a fairly constant rate of 3% per year, and is often preceded by liver cirrhosis[1]. In Egypt, incidence of HCC has increased over the last decade from 4% to 7.2%[2,3]. This remarkable increase may be explained by an increase in risk factors, such as the hepatitis C virus (HCV) infection that emerges over the same period of time[4]. Additionally, Egypt has a high prevalence of HCV affecting approximately 12% of the general population[5,6]. Cirrhosis represents an entity of diffuse hepatic disease that results from exposure to chronic injury with subsequent regeneration of liver cells and formation of abnormal structural nodules surrounded by fibrosis. It occurs secondary to chronic viral hepatitis, metabolic liver diseases (e.g., hemochromatosis, α1-antitrypsin deficiency, Wilson’s disease, non-alcoholic steatohepatitis), alcoholic liver disease, and autoimmune diseases (e.g., primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis)[7]. The prognosis of HCC is strongly correlated with diagnostic delay. To date, no ideal screening modality has been developed. Recent studies revealed poor sensitivity and specificity of alpha-fetoprotein (AFP) for proper surveillance and diagnosis. Thus, the basis for surveillance is still an abdominal ultrasound (US) every 6 mo despite inadequate sensitivity[8-10]. The barcelona-clinic liver cancer (BCLC) system has been certified for use by both European (EASL) and American (AASLD) associations for the Study of Liver Diseases[9,10]. The best treatment modality for liver cirrhosis as well as HCC is orthotopic liver transplantation (OLT)[11]. Different treatment options are available, including both surgical modalities (resection and liver transplantation) and radiological techniques [radiofrequency ablation (RFA), microwave ablation, percutaneous ethanol (or acetic acid) ablation and transarterial chemoembolisation (TACE)] in addition to systemic chemotherapy and molecularly targeted therapies.
DIAGNOSIS OF HCC
A multidisciplinary approach includes clinical, radiological, and laboratory modalities with or without liver biopsy (in certain cases) to establish the diagnosis of HCC.
CLINICAL PRESENTATION
In early stages, HCC usually runs a silent course making clinical diagnosis difficult. This may be due to the deep position of the liver underneath the lower ribs, making the liver difficult to feel; moreover, the tumour must reach a substantial size before it invades adjacent structures or organs. The liver has considerable functional reserves, so clinical manifestation as jaundice and other evidence of hepatic dysfunction do not appear until a large part of the organ has been replaced by tumour. Additionally, no pathognomonic symptoms or signs are attributable to HCC; tumours generally spread to distant sites late in the disease. As a result, the clinical picture is extremely variable and the patient may be completely asymptomatic with no physical signs other than of cirrhosis. Alternatively, the presentation may be florid, indicative that liver failure has occurred[12]. These complications are frequently associated with the tumour spread into the portal or hepatic veins or through arteriovenous shunt induced by the tumour[13].
Few patients may have weight loss, sense of poor appetite or early fullness of the stomach, or a visible mass in the upper abdominal part. These symptoms often signify the presence of an advanced hepatic lesion[13].
Pain
Pain is a common feature, which is dull aching in nature, of mild to moderate severity, continuous, felt as a non-specific, located in the epigastrium, and right upper quadrant or back. Rarely, pain of severe degree occurs and is due to perihepatitis or infiltration of the diaphragm. Acute abdomen may occur as a result of sudden intra-peritoneal bleeding when the tumour ruptures. The most important determinant of this complication is the superficial location of the tumour in the liver. Rupture of the tumour is either spontaneous, or follow mild blunt abdominal trauma[14]. This complication is associated with a severe drop in haematocrit and hypotension and diagnosis is made by peritoneal lavage and laparotomy. A liver mass and free intraperitoneal blood are typically seen on computed tomography (CT) scan[15]. This complication is a life-threatening, and control of bleeding is a goal. It may require emergent angiography and embolization of the bleeding vessel or even surgery[16]. Delayed resection may be considered, although there is a high risk of peritoneal dissemination.
Other unusual clinical presentations include: (1) Obstructive jaundice owing to intrahepatic duct compression, infiltration of biliary tract or rarely, as a result of hemobilia. It occurs in less than 10% of patients from a high incidence population[17]; (2) Diarrhoea; (3) Bone aches or dyspnoea due to metastases; (4) Fever might develop in association with central tumour necrosis[18]; (5) Pyogenic liver abscess (very rare)[19]; (6) Paraneoplastic syndromes: Occasionally, patients with HCC presented by a paraneoplastic syndrome that can manifest as hypoglycaemia, thrombocytosis, erythrocytosis, hypercalcemia, or diarrhoea of watery nature. The presence of any of these manifestations, other than erythrocytosis, is generally associated with a poor prognosis[20]; (7) Hypoglycaemia: In advanced HCC, hypoglycaemia usually occurs as a reflection of increased metabolic demands of the tumour. Typically, the hypoglycaemia is mild degree and present without symptoms; however, severe reductions in the levels of plasma glucose can happen, resulting in state of lethargy and confusion. The types of hypoglycaemia have been described in HCC are two: Type A: hypoglycaemia that occurs in the late stages of the disease, the severity is a mild to moderate degree and associated with a poorly differentiated tumour; and Type B (less common): hypoglycaemia of severe degree that occurs in the early stages of the HCC and is associated with a well-differentiated slow growing tumour. The possible explanations of hypoglycaemia that occurs in HCC include high secretion of insulin-like growth factor II by the tumour tissue (< 5% of patients) and impaired gluconeogenesis due to liver decompensation (glucose underproduction)[21-27]; (8) Erythrocytosis: In HCC, tumour secretion of erythropoietin raises in up to 23% of patients, this is probably the cause of erythrocytosis. However, elevations in packed cell volume or haemoglobin concentration are uncommon. Indeed, at the time of diagnosis most patients are anaemic, because of other effects of the tumour[28,29]; (9) Thrombocytosis: Underestimation of thrombocytosis might represent a difficult issue in HCC cases owing to the fact that most patients already have low platelet count caused by the underlying liver cirrhosis. Mean serum thrombopoietin level is significantly increased in HCC patients with thrombocytosis rather than those with normal or low platelet count. In addition, interventions like hepatic resection or TACE in HCC patients with thrombocytosis result in drop of both serum thrombopoietin level and platelet count which rise again in case of tumour recurrence[30,31]; (10) Hypercalcaemia: HCC cases might present with hypercalcaemia due to associated osteolytic metastases or secretion of parathyroid hormone-related protein[32,33]; (11) Watery diarrhoea: In one study, diarrhoea was statistically significantly more frequent among cirrhotic patients with HCC when compared to those without HCC (48% vs 9%, P < 0.05)[18]. It might be intractable and severe, leading to achlorhydria and hypokalaemia[34]. The actual mechanism of watery diarrhoea in HCC is not clearly understood. An increase in intestinal production of different peptides including vasoactive intestinal polypeptide, prostaglandin-like immunoreactivity peptides and gastrin might be a plausible explanation[34]; (12) Hyperthyroidism: may be due to increased thyroid-stimulating hormone production[35]; (13) Gynaecomastia: is painful and associated with increased level of oestrogen[36]; and (14) Systemic arterial hypertension in few patients with HCC[37].
Budd-Chiari syndrome
Budd-Chiari syndrome (BCS) is found in less than 1% of all HCC patients. Clinical features that occur in HCC patients complicated by BCS are diverse. These range from complete absence of symptoms and signs to a variable degree of abdominal or chest pain, dyspnoea, and even variceal haemorrhage. BCS diagnosis can be exclusively done by using imaging techniques only without the need to do percutaneous liver biopsy.
The diagnostic accuracy (sensitivity and specificity) of Doppler ultrasonography for the diagnosis of BCS is considered to be high ranging from 85% to 90%[38]. Bargalló et al[39] classified the ultrasonographic features of BCS into three categories: (1) Specific: Obstructed hepatic vein; (2) Suggestive: Collaterals between intrahepatic, portal or caval veins and caudate vein diameter more than 3 mm; and (3) Nonspecific: Hypertrophied caudate lobe, portal vein thrombosis, extrahepatic collaterals, regenerating nodules, non-homogeneous liver parenchyma, and ascites.
The most frequent US signs of BCS are altered hepatic (71.1%) and/or caval (28.9%) veins. Combination of these two signs together with caudate lobe hypertrophy has the highest positive predictive value (PPV) of 97.8% for the diagnosis of BCS[38].
CT scan allows for more comprehensive assessment of the patency of both hepatic and caval veins. It also allows for more accurate measurement of the degree of hypertrophy of caudate lobe. Magnetic resonance imaging (MRI) helps in the differentiation of chronic BCS from acute form as well as further delineation of vascular anatomy[39,40].
Cutaneous features
Numerous cutaneous manifestations have been reported in association with HCC; however, none of them are clue for the diagnosis of HCC[41]. These comprise; dermatomyositis, pemphigus foliaceus, pityriasis rotunda, Leser-Trélat sign, and porphyria cutanea tarda[42,43].
The physical findings in most patients with HCC are manifestations of decompensated cirrhosis[44]. Rarely, bruits over the liver are heard.
Laboratory examination is often nonspecific. The majority of HCCs patients have underlying hepatic disease, and subsequent cirrhosis, so hyperbilirubinaemia, hypoalbuminemia, and hypoprothrombinaemia are frequently encountered. Patients are often mildly anaemic and may have thrombocytopenia. They may have electrolyte disturbances (e.g., hyponatraemia, hypokalaemia, metabolic alkalosis) that are associated with defective water handling. Liver enzymes as serum aminotransferases, alkaline phosphatase, and gamma glutamyl transpeptidase are often abnormal with nonspecific patterns[45].
RADIOLOGICAL DIAGNOSIS
Imaging studies for diagnosis of HCC can fall into one of two main categories: routine non-invasive studies such as US, CT, and MRI, and more specialized invasive techniques including CT during hepatic arteriography, iodised oil-CT, and CT arterial portography in addition to the conventional hepatic angiography[46]. Diagnostic accuracy assessed by the sensitivity and specificity of these different radiological modalities in HCC detection was shown in Table 1.
Table 1.
Sensitivity and specificity of different radiological modalities in hepatocellular carcinoma
| Sensitivity (%) | Specificity (%) | |
| US | 60 | 97 |
| Colour Doppler US | 92 | 100 |
| MPCT | 68 | 93 |
| MRI | 81 | 85 |
| Angiography | 82-93 | 73 |
MPCT: Multiphasic helical computed tomography; MRI: Magnetic resonance imaging; US: Ultrasound.
US
To date, the surveillance for hepatic focal lesions in high risk patients is based on US. Different appearances in US may be present; either hypoechoic, hyperechoic or target lesions. Unfortunately, all of these signs are non-specific. Suspicion for HCC is raised when any lesion is recognized in US, particularly if it is more than 1 cm in size in the background of liver cirrhosis[47] (Figure 1). Several studies assessed the diagnostic accuracy of US as a screening tool for early detection of HCC[48-51]. One systematic review concluded that the specificity was 97% (95%CI: 95%-98%) and sensitivity was 60% (95%CI: 44%-76%) compared with pathologic assessment of resected or explanted liver as a standard reference[52,53]. In a large prospective study enrolling non-cirrhotic hepatitis B carriers, the specificity, sensitivity, and PPV of US were 93%, 71%, and 15%, respectively[49]. The sensitivity increased up to 79%, when combined with AFP assessment. In other studies, sensitivity values have ranged from 78%[50] to much lower values of 40% to 50%, mostly when the diagnosis is established by examination of the resected liver after transplantation or autopsy specimens[54].
Figure 1.
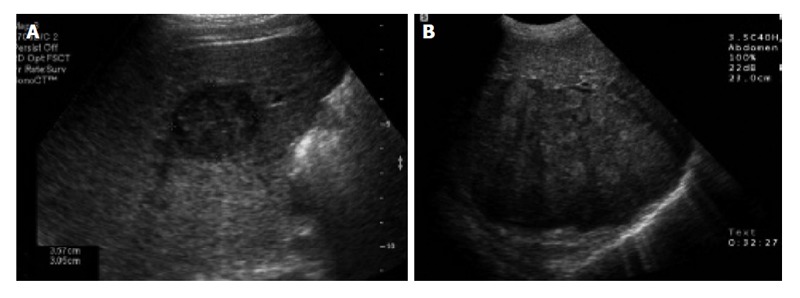
Abdominal ultrasound of the liver. A: Transverse sonogram shows a small, 3 cm, hypoechoic mass in the right lobe of the liver; B: Transverse sonogram shows a heterogeneous large mass in the right lobe of the liver[47].
Abdominal US allows the recognition of tumours as small as 1 cm in size. Furthermore, the identification of metastases by US in a normal liver is much easier than the recognition of small HCC in a cirrhotic liver[46]. In advanced tumour stage, the patency of vascular structures and the existence of adenopathies at the hilum can be assessed by US[55]. In addition, small tumour nodules can be detected by intraoperative US during hepatic resection.
US offers many signs that raise the suspicion of malignant transformation, including the presence of intrahepatic venous thrombosis[38] (Figure 2), a mass protruding from the hepatic surface or dilated intrahepatic bile duct, even in the absence of a definite liver mass[56].
Figure 2.
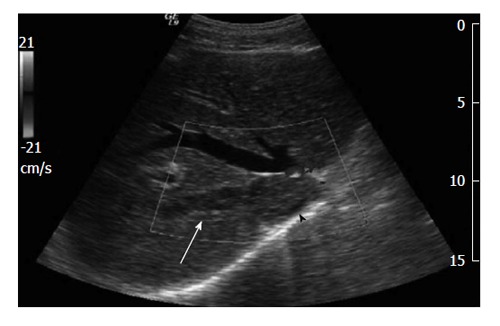
Doppler abdominal ultrasound shows the disappearance of Doppler flow in the intrahepatic segment of the inferior vena cava (arrowhead) and the right hepatic vein[38].
Colour Doppler US
When combining Doppler with US in patients with HCC, the detection rate of malignant portal vein thrombosis can be increased. Thus, the diagnostic accuracy can be improved, to achieve a sensitivity of 92% and a specificity of nearly 100%[47] (Figure 3). Portal thrombosis is ruled out, when US-Doppler shows no obstruction and evidence of normal permeability of the portal venous system[55].
Figure 3.
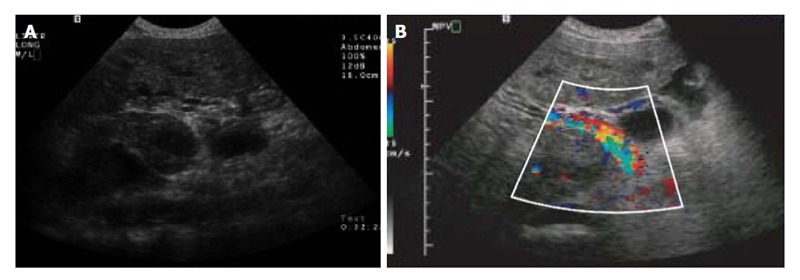
Colour Doppler ultrasound of the liver. A: Transverse sonogram shows portal vein thrombus; B: Transverse colour Doppler sonogram of the right upper quadrant shows heterogeneous flow within the tumour thrombus[47].
Contrast-enhanced US
Ultrasonographic visualization and characterization of hepatic tumor vascularity can be improved by using contrast-enhanced US (CEUS) that utilizes on-linear imaging modes[57]. CEUS can provide useful data about the characteristics of hepatic lesions that are not recognized with conventional US. CEUS has comparable diagnostic performance of CT and MRI in the characterization of hepatic focal lesions that are identified during US screening in patients with chronic liver disease or with a past history of prior malignancy[58]. CEUS is well tolerated, safe and may be the best choice when the CT or MRI are contraindicated[59]. However, a meta-analysis of 18 studies was unable to determine whether CEUS was adequate to exclude HCC lesions of size less than 30 mm[60].
Endoscopic US
Endoscopic US can establish a diagnosis of HCC especially if combined with fine needle aspiration (FNA) and has the probability to enhance the diagnostic accuracy of staging of HCC compared with CT and MRI[61]. The role of endoscopic US (EUS) in the assessment of patients with suspected HCC is still being investigated. Early experience in assessment of EUS-FNA diagnostic accuracy in detection of focal liver lesions, suggests that EUS-FNA is comparable to the results of CT-FNA. However, EUS-FNA is limited anatomically to only a portion of the hepatic parenchyma including the left hepatic lobe, hilum and proximal biliary tree. Meanwhile, extrahepatic biliary tree, gallbladder, and perihilar lymph nodes are all readily accessible[62].
Abdominal CT multiphase perfusion CT
Currently, multiphase perfusion CT is the technique of choice for diagnosis of HCC with an excellent performance in the early detection of hepatic focal lesions and staging. It comprises four subsequent phases; namely pre-contrast, hepatic arterial, portal venous and finally the delayed phases. This technique utilizes a single detector spiral scanner of a high-speed and images are taken out after injection of the contrast at a delay of 25, 70 and 300 s that correspond to the three phases of arterial, portal venous, and equilibrium, respectively. From the anatomical view, hepatic artery supplies the principal blood flow to the tumour tissues, owing to their hypervascular appearance throughout the hepatic arterial phase. Conversely, throughout the delayed phase due to early washout of contrast, HCCs appear hypodense. It was observed that images of delayed phase could assist in confirming the diagnosis of HCC in 14% of patients[56]. The gold standard imaging modality for evaluating the response after loco-regional intervention of HCC is Spiral CT[63]. In some centers, CT scan is also used as a principal screening modality for HCC in cirrhotic patients. Numerous studies have assessed test characteristics of CT for diagnosis of HCC. A systematic review estimated that the specificity was 93% (95%CI: 89%-96%) and the sensitivity was 68% (95%CI: 55%-80%) compared with pathologic tissue examination of an explanted or resected liver as the reference standard[52].
Multi-detector helical CT
Multi-detector helical CT (MDCT) has permitted us to collect early arterial phase images (18-28 s post contrast injection) as well as late (early parenchymal) arterial phase images (35-45 s post contrast injection). Although the lesions are demonstrated better in the “late” rather than the “early” arterial phase, the latter clarifies more optimally the vessels in patients who are considered for surgical resection[64]. The capability and sensitivity of MDCT in the identification of HCC lesions within the cirrhotic parenchyma of the liver is very high. This can be attributed to its elasticity and rapid speed of the device which lead to gaining images of high quality by using both thin sections and 3-D capabilities[65]. In the equilibrium phase (3-5 min after injection), the appearance of vascular tumors is hypodense relative to the surrounding liver tissue. In addition, focal hepatic lesions that measure less than 2 cm can be accurately identified in this phase due to washout of contrast rapidly from the tumor tissue than from the normal parenchyma of the liver[66,67] (Figure 4). It was observed that MDCT scan is valuable in early diagnosis of HCC and in monitoring the successful treatment of HCC lesions particularly the small ones. This is the most important issue especially during follow-up of patients with chronic viral hepatitis and/or cirrhosis[46]. Non-contrast CT scan has very low sensitivity for detecting small HCCs. If contrast cannot be given safely, US or MRI is the preferred diagnostic modality[62].
Figure 4.
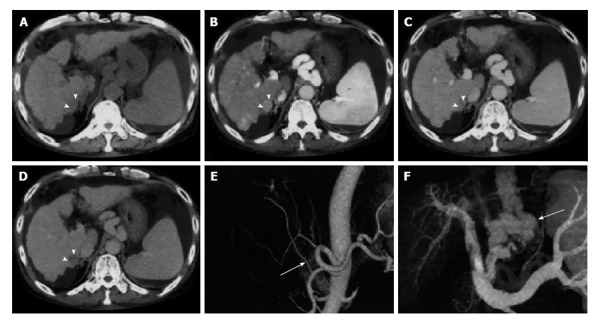
Multi-detector computed tomography of hepatocellular carcinoma. A: Pre-contrast; B: Late arterial phase; C: Portal venous phase; D: Equilibrium phase dynamic multi-detector computed tomography images. The hepatocellular carcinoma (arrowheads) is visualised as an enhanced nodule in the late arterial phase and as a hypo-attenuated nodule in the equilibrium phase; E: Three-dimensional computed tomography angiography of hepatic arteries shows that the right hepatic artery branches (arrow in E) from the superior mesenteric artery; F: The portal venous system with creation of varices (arrow)[66].
MRI
The advantage of MRI over CT is obtaining images of the liver of high-resolution without using ionizing radiation or nephrotoxic contrast agents. MRI has an equivalent diagnostic accuracy as helical CT in the early detection and diagnosis of HCC[68,69]. On T2-weighted MRI images, HCC gives a high intensity pattern, while on T1-weighted MRI images it gives a low intensity pattern[70] (Figure 5). MRI is superior to both US and CT in differentiating the nature of regenerative nodules from HCC nodules in the patient with cirrhosis[71]. A systematic review described that the sensitivity was 81% (95%CI: 70%-91%) and specificity was 85% (95%CI: 77%-93%) compared with pathologic tissue assessment of an explanted or resected liver tissue as the reference standard[52]. The sensitivity may be higher when used in conjunction with US. In one series of patients with known HCC, the combination of MRI and US detected 85 out of 87 HCCs[72]. The sensitivity also appears to be augmented when gadoxetic acid-enhanced and diffusion-weighted imaging are combined[73,74]. In a study of 130 patients with small (≤ 2.0 cm) HCCs and 130 cirrhotic patients without HCC[73], the sensitivity of the combined approach was 91% to 93%, compared with 81% to 82% for gadoxetic acid-enhanced imaging and 78% to 80% for diffusion-weighted imaging alone. The specificity did not differ among the groups. In patients with advanced renal failure, gadolinium used for MRI can cause nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy, and thus alternatives should be used. As a novel technique, MRI angiography during a single breath-hold, allows for the achievement of a three-dimensional data set. This will include the 3 subsequent phases; arterial phase, portal venous phase and late venous phase[75]. In one series, comparing this procedure to triphasic CT found that it had higher sensitivity (76% vs 61%) for detection of HCC nodules ≥ 10 mm. Indeed, helical CT scanning is still the preferred technique used by most radiologists, because of both the high cost of MRI and the extended duration necessitated to achieve standard MRI images with good quality. MRI may be important in patients presented with renal impairment or those who had a hypersensitivity to CT contrast agents. MRI may also be valuable in cases in which CT results are unclear; this is predominantly true when the liver is severely nodular, because MRI can distinguish HCC focal lesions from dysplastic nodules[71-76]. MRI has higher performance compared to CT scanning in the characterization of focal fat lesion from HCC and vascular lesions (such as a haemangioma).
Figure 5.
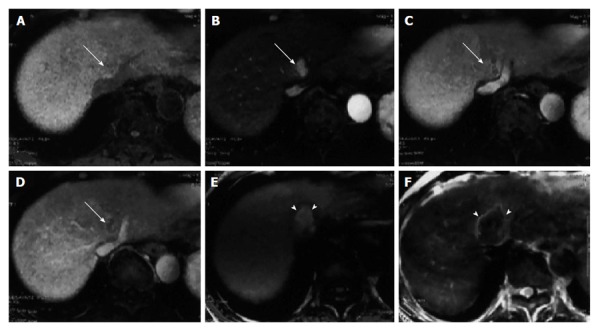
Dynamic magnetic resonance imaging of hepatocellular carcinoma. A: Precontrast; B: Arterial phase; C: Portal venous phase; D: Equilibrium phase; dynamic magnetic resonance imaging obtained with the LAVA sequence prior to therapeutic treatment. The hepatocellular carcinoma (HCC) (arrow) is seen adjacent to the hepatic vein as an enhanced nodule in the late arterial phase and as hypo-attenuated nodule in the portal venous and equilibrium phases; E: T1-weighted gradient echo image; F: Spin echo T2-weighted image two days after radiofrequency ablation therapy for HCC. The completely ablated area shows as hyperintense on T1-weighted images and as hypointense on T2–weighted images (arrowheads)[66].
Angiography
Owing to the hypervascular characteristic of HCC, the tumor frequently has dilated, distorted, tortuous, and displaced arterial supply[77] (Figure 6). Strong tumour staining, presence of vascular lakes and venous pools are the most common features described in angiography[56]. It can enhance the detection and characterization of HCC if combined with CT or MRI scanning[78-82]. The technique includes injection of intra-arterial contrast dye (usually in the superior mesenteric, splenic, or hepatic artery) instantly before performing CT or MRI, followed by achieving images through both arterial and portal phases. The diagnostic performance of hepatic arteriography largely depends on the size of hepatic tumour and the pattern of vascularization[83]. The overall diagnostic accuracy of angiography in the diagnosis of smaller HCCs (< 5 cm) is up to 89%, with sensitivity of 82%-93%, and specificity of 73%. These estimated values were reduced, when the size of the tumor was smaller than 2 cm[55]. Recently, the less invasive and novel techniques discussed above have substituted conventional angiography for the diagnosis and early detection of HCC. So, angiography became reserved for therapeutic use during chemoembolization of tumors and for the control of bleeding from ruptured focal HCC[65]. In a randomized trial with 280 HCC patients eligible for RFA, performing CT hepatic arteriography and portography led to the identification of 75 nodules that were not detected by conventional CT scan, but this fact did not improve the recurrence-free survival rates[84].
Figure 6.
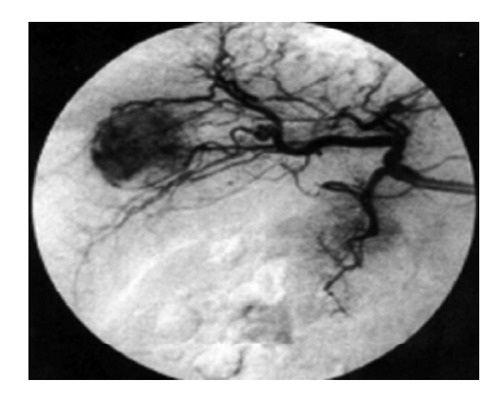
Hepatic angiography. Typical hyper-vascular pattern, corresponding to the findings of enhanced Doppler study, is clearly demonstrated by angiography[77].
LABORATORY DIAGNOSIS
Serum markers
The ideal tumour marker should possess the following properties: (1) high specificity and not detected in benign diseases and healthy subjects; (2) high sensitivity and detectable very early when few cancer cells are present; (3) organ specificity; (4) correlation with tumour stage or tumour mass; (5) correlation with prognosis; and (6) reliable predictive value. Such a tumour marker could be used for diagnosis (in screening programs), prognosis, monitoring the effects of the therapy and as a target for localisation and therapy.
The most frequently used marker for diagnosis of HCC is the serum concentration level of AFP. Numerous serologic markers (such as des-gamma-carboxyprothrombin) may be used; the diagnostic accuracy of which may be improved when they are combined with serum AFP. While in clinical practice, these markers are not routinely used, they represent a fruitful area of investigation (Table 2).
Table 2.
Currently used and investigational serum tumour markers[101]
| Markers | Character | Cut-off level | Sensitivity | Specificity | Comments |
| AFP | Oncofoetal glycoprotein | 10-16 ng/dL | 60%-80% | 70%-90% | Poor marker alone |
| 20 ng/dL | 39%-66% | 76%-97% | |||
| AFP-L3 | AFP variant (subtype) | 10% | 39.9% | 93.4% | Useful in combination |
| 15% | 36.1%-96% | 92%-99.5% | with other markers | ||
| GP73 | Golgi-specific membrane protein | 10 relative units | 69% | 86% | Promising marker |
| GPC3 | Oncofoetal glycoprotein | 2 ng/dL | 51% | 90% | Limited utility as a marker |
| DCP | Abnormal prothrombin | 40 mAU/mL | 48%-62% | 81%-98% | Useful in combination |
| HS-GGT | Abnormal prothrombin | 5.5 IU/mL | 43.8%-74% | Not available | Non specific |
| AFU | Lysosomal enzyme | 870 nmol/mL per hour | 82% | 71% | Lower specificity and poor marker |
AFP: Alpha-fetoprotein; AFP-L3: Lens culinaris-reactive AFP; GP73: Golgi protein 73; GPC3: Glypican-3; DCP: Des-gamma-carboxyprothrombin; GGT: Gamma-glutamyl transferase; AFU: Alpha-L-fucosidase.
AFP is a glycoprotein of an oncofoetal origin. Its level is increased in patients with cirrhosis complicated by HCC. Primarily, it is a foetal-specific antigen produced in the liver of the foetus. After birth, its serum concentration falls rapidly and decreases through adult life. Elevation of serum AFP occurs normally during pregnancy, and an abnormal rise has been reported with gonadal tumours (both germ cell and non-germ cell)[85] and in a different variety of other malignancies, especially cancer stomach[86]. Several studies reported a rise of serum AFP in patients with chronic hepatic disease without HCC, and in patients with acute or chronic viral hepatitis[87,88]. In addition, patients with HCV-related cirrhosis may have a slightly higher concentration level of AFP. In one series, patients who had been treated with a combination of pegylated interferon plus ribavirin for HCV-related cirrhosis demonstrated significant decrease in AFP level after treatment[89]. Additionally, in small HCCs the serum concentrations of AFP are normal in up to 40%, as not all tumours secrete AFP[90].
A fibrolamellar carcinoma is a variant of HCC characterized by normal AFP levels in the majority of cases[91]. In one report, patients with cirrhosis and persistently elevated AFP values compared with those who have fluctuating or normal levels have an increased risk of developing HCC (29% vs 13% and 2.4%, respectively)[92].
Assessment of diagnostic performance of serum AFP as a screening tool in the early detection of HCC using certain parameters as sensitivity, specificity, and predictive values depends upon the value of cut-off chosen for confirming the diagnosis, the natural characteristics of the population under study, and the gold standard test used to establish the diagnosis of HCC. Test characteristics in different situations have described in several studies[49,93]. A large systematic review that enrolled five studies revealed the following prognostic values; sensitivity of 41% to 65%, specificity of 80% to 94%, negative likelihood ratio of 0.4 to 0.6 and a positive likelihood ratio of 3.1 to 6.8 when a cut-off value based upon > 20 mcg/L[89-94].
Although values of AFP above 400 ng/dL are considered diagnostic for HCC, such a high level of AFP present only in a small percentage of patients[95]. Patients with high AFP levels (above 400 ng/dL) tend to have larger tumour mass, diffuse or multilobar involvement, thrombosis of the portal vein and a lower survival rate[96].
The utility of AFP in differentiating HCC from benign liver diseases has also been noted to be limited with false negative rates and high false positive rates[97]. Although AFP is considered to be the gold standard as a serum bio-marker of HCC, its utility as a screening tool for HCC detection is of a questionable issue due to its poor performance. The role of variant forms of AFP has been investigated, to improve the diagnostic performance of AFP.
Three different AFP variants (AFP-L1, AFP-L2, and AFP-L3) have been investigated. Each variant has a different sugar chain with a differential affinity for lectins, such as Lens culinaris agglutinin. AFP-L3 (Lens culinaris-reactive AFP) is more specific and superior for HCC than is global AFP[98]. The United States Food and Drug Administration approved AFP-L3 as a screening marker for HCC. The AFP-L3 percentage is calculated as a ratio of AFP-L3 to whole AFP. Initial investigations demonstrated a specificity and sensitivity of 93.9% and 55.3%, respectively, when a cut-off value of 15% AFP-L3 was used[95].
More recent studies using the same cut-off value showed a sensitivity of 96% and specificity of 92%[89]. Another series, using cut-off values of AFP-L3 about 10% to 15% exhibited a lower sensitivity (30.9%-36.1%) but comparable specificity (93.4%-99.5%)[99]. However, the diagnostic accuracy of AFP-L3 vs whole AFP in distinguishing patients with HCC from patients who have only cirrhosis and regenerating nodules, is still a matter of debate and has not been well investigated. Interestingly, several studies have reported that AFP-L3 may be a useful prognostic bio-marker for HCC, and higher percentage values are associated with increased tumour size, poorly differentiated HCC, vascular invasion, and metastasis[100]. Therefore, it appears that because of inconsistencies in its sensitivity and specificity data for predicting HCC occurrence, AFP-L3 is still unreliable, even though it is more precise and specific than whole AFP. Also, AFP-L3 may not be very useful for surveillance even though it may be a valuable prognostic bio-marker in patients with known HCC[101].
Des-gamma-carboxyprothrombin (also recognized as “prothrombin produced by vitamin K absence or antagonism II”) has also shown advances in the detection of HCC. In one series, 69 out of total 76 patients diagnosed as HCC, had a mean serum level concentration of this marker in the range of 900 mcg/L; much lower mean values were reported in chronic hepatitis patients, metastatic liver disease, and healthy individuals (10 and 42 mcg/L and undetectable, respectively). Tumours of less than 3 cm in size show less frequent elevations in des-gamma-carboxyprothrombin. In addition, there is no significant correlation between serum levels of abnormal prothrombin and AFP[102-107].
MicroRNAs
The plasma microRNA expression has also been investigated as a possible marker of HCC[108-110]. One study examined 934 participants in 4 groups; healthy, chronic HBV, cirrhosis, and HBV-related HCC[108]. Regardless of the stage of HCC, a microRNA panel that included miR-122, miR-192, miR-21, miR-223, miR-26a, and miR-801 accurately identified patients with HCC (AUC 0.89 with a specificity of 84% and a sensitivity of 82% for the validation set). This microRNA panel also accurately differentiated patients with HCC from healthy individuals and those with cirrhosis or chronic HBV. Additionally, microRNAs have a potential prognostic impact in patients with HCC (Table 3).
Table 3.
MicroRNAs with potential prognostic impact in patients with hepatocellular carcinoma
| MiRNAs | Molecular alteration | Clinical significance | Ref. |
| 20 miRNAs | Signature | Venous metastasis, overall survival | [154] |
| 19 mi NAs | Signature | Poor survival | [155] |
| MiR-19a, miR-886-5p, miR-126, miR-233, miR-24, and miR-147 | Signature | Predictor of overall survival and recurrence-free survival after LT | [156] |
| MiR-26a | Down-regulation | Poor survival | [157] |
| MiR-122 | Down-regulation | Gain of metastasis properties | [158,159] |
| MiR-122 | Down-regulation | Early recurrence | [160] |
| Let-7 members | Down-regulation | Early recurrence | [161] |
| MiR-199a-3p | Down-regulation | Reduced time to recurrence | [162] |
| MiR-199b-5p | Down-regulation | Poor overall survival and progression-free survival rates | [163] |
| MiR-101 | Down-regulation | Advanced tumour progression, poor prognosis | [164] |
| MiR-125a | Up-regulation | Better survival | [165] |
| MiR-92, miR-20, miR-18 | Up-regulation | Poor differentiation | [166] |
| MiR-372 | Up-regulation | Advanced TNM stage | [167] |
| MiR-221 | Up-regulation | Multi-nodularity, reduced time to recurrence | [168] |
| MiR-221 | Up-regulation | Gain of metastatic properties | [169] |
| MiR-221 | Up-regulation | High tumour capsular infiltration | [170] |
| MiR-17-5p | Up-regulation | Multiple tumour nodules, vein invasion, shortened overall survival | [171] |
| MiR-155 | Up-regulation | High recurrence and poor prognosis following OLT | [172] |
| MiR-203 | Up-regulation | Good prognosis | [173] |
| MiR-18 | Up-regulation | Poor prognosis | [174] |
Data adapted from Negrini et al[175]. MiRNAs: MicroRNAs; TNM: Tumour-node-metastasis; LT: Liver transplantation; OLT: Orthotopic liver transplantation.
Other serum bio-markers of HCC that have been studied include: (1) Tumour-associated isoenzymes of gamma-glutamyl transpeptidase[111]; (2) Transforming growth factor-β-1 in urine[112]; (3) Serum circulating intercellular adhesion molecule-1 level[113]; (4) Serum alpha-L-fucosidase activity[114]; (5) Glypican-3 (a cell-surface heparin sulfate proteoglycan)[115]; (6) Dickkopf-1[116]; (7) Human carboxylesterase 1[117]; and (8) Plasma proteasome[118,119].
PERCUTANEOUS LIVER BIOPSY
It should only be considered when diagnostic imaging results are doubtful, for example, in patients with cirrhosis and nodules of hypovascular nature, and the result would directly have an impact on management[120,121]. Both the European (EASL) and the American (AASLD) Associations for the Study of Liver Diseases have proposed a role for liver biopsy in confirming the diagnosis and identification of HCC[9,10]. In addition, clinical criteria have been developed that can be designed for prioritising patients with any suspicion of HCC prior to liver transplantation without confirming the diagnosis with a biopsy[63]. Possible risks encountered of a biopsy include bleeding, and tumour spread through the track of the needle. The magnitude of the risk ranges from 1.6% to 5%[122-126]. A large meta-analysis of eight clinical studies estimated that the overall risk was 2.7%[127]. However, some reports have not observed any adverse impact of preoperative FNA, or an increased risk of needle tract seeding, on long-term outcome and survival in patients who were considered for elective surgical resection of HCC[120,128,129]. Although the potential risk of spreading tumour through the biopsy tract should always be considered, liver biopsy might be performed in certain circumstances as surgical resection and liver transplantation.
TREATMENT OF HCC
Surveillance programs have been conducted in the high-risk group patients; and led to an increasing number of early detection of HCC[130]. Nevertheless, HCC is usually diagnosed late along its course, and the median survival is approximately 6 to 20 mo after the establishment of the diagnosis[9,10]. Based upon pre-established prognostic criteria, the BCLC classification categorizes HCC patients into five stages (0, A, B, C and D)[10] (Figure 7). Different treatment modalities are available including: (1) Surgical resection; (2) Liver transplantation; (3) RFA; (4) Microwave ablation; (5) Percutaneous ethanol or acetic acid ablation; (6) TACE; (7) Radioembolization; (8) Cryoablation; (9) Radiation therapy (RT) and stereotactic radiotherapy; and (10) Systemic chemotherapy and molecularly targeted therapies.
Figure 7.
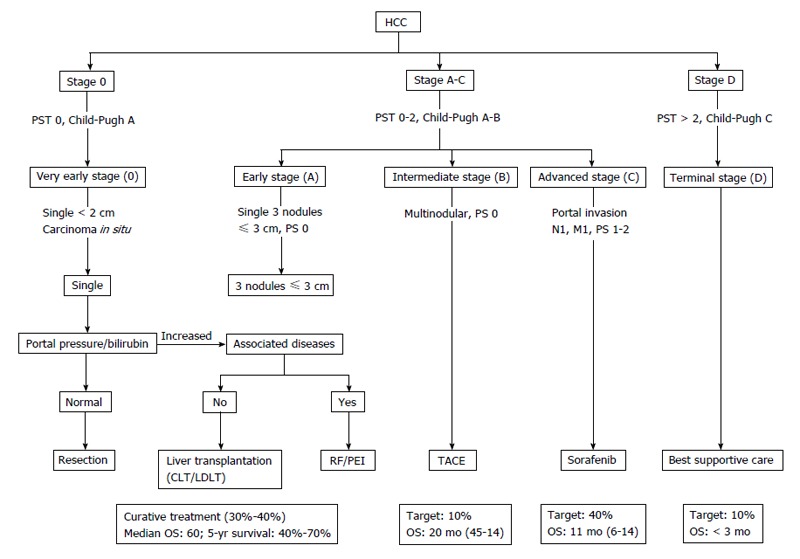
Updated Barcelona Clinic Liver Cancer staging system and treatment strategy, 2011[10]. The BCLC algorithm classifies HCC into five stages-based on the extent of disease, Child-Pugh score, and ECOG performance status-that enables prognostication and informs allocation of first-line treatment. BCLC: Barcelona Clinic Liver Cancer (group); HCC: Hepatocellular carcinoma; PST: Performance status test; TACE: Transarterial chemoembolization; RF: Radiofrequency; PEI: Percutaneous ethanol infusion; CLT: Cadaveric liver transplant; LDLT: Living donor liver transplant.
SURGICAL RESECTION
In patients with adequate liver functional reserve, potentially curative partial hepatectomy is the optimal treatment for HCC. Solitary HCC confined to the liver is ideal for surgical resection, which shows no radiographic evidence of portal hypertension, no evidence of invasion of the hepatic vasculature, and preserved hepatic function. In carefully selected patients, Relapse-free Long-term survival rates, average 40% or better and five-year survival rates as high as 90% are reported.
The preoperative evaluation should focus on the probability of disease being confined to the liver, and whether the anatomic bonds of the intrahepatic tumour and the underlying liver function will permit resection. Although surgeons restrict suitability for resection of patients with tumours that are ≤ 5 cm in diameter, there is no general basis regarding tumour size for determination of patients for resection. A comparable survival rate reported in patients with a solitary HCC without vascular invasion regardless of tumour size, although patients with smaller tumours tend to have a better outcome.
Hepatic reserve assessment is of paramount importance in the selection of patients for resection. Perioperative mortality is twice as high in cirrhotic as in non-cirrhotic patients unless proper selection of patient is applied. Patients with Child-Pugh class (A) cirrhosis, who have a normal bilirubin and well-preserved hepatic function, can undergo operative surgical resection safely. Also, liver volumetry, and portal vein patency should be assessed before major surgical liver resection in those patients.
The utilization of intraoperative US allows accurate localisation and staging of tumours. Anatomical consideration is favoured by some gastrointestinal surgeons during surgical resection, where segment-wise hepatectomies is carried out whenever feasible. This depends on the fact that microscopic intrahepatic metastasis is expected to rise in the same original segment from which the tumour rise. Interestingly, even after apparently curative surgical resection, recurrent HCC develops in 80% of patients within five years because of latent metachronous multicentric carcinogenesis or intrahepatic metastasis[131].
LIVER TRANSPLANTATION
Practically all patients who are candidate for liver transplantation are unresectable because of the degree of underlying liver dysfunction rather than tumour extent. Liver transplantation can be considered as an appropriate treatment option for patients with end stage liver disease and earlier stage HCC. OLT is an appropriate option for patients with chronic liver disease (usually cirrhosis) who would not tolerate liver resection and according to Milan criteria have a single HCC focal mass with size ≤ 5 cm in diameter or up to three separate focal lesions, with any one of them is not larger than 3 cm, with no distant metastases or regional nodal and no evidence of gross invasion of vascular structure.
When these strict selection criteria used, excellent overall three- to four-year actuarial (75% to 85%) and recurrence-free survival rates (83% to 92%) could be achieved. Long-term survival is similar to or only a little worse in precisely designated patients experiencing OLT for HCC, than survival for patients performing OLT for non-malignant aetiologies. Patel et al[132] found that patients transplanted within Milan criteria and those transplanted outside of Milan criteria but within the University of California, San Francisco criteria (one tumor ≤ 6.5 cm, or ≤ 3 tumors with largest tumor diameter ≤ 4.5 cm and total tumor diameter ≤ 8 cm) had equivalent outcomes.
A major disadvantage with OLT (in addition to the need for lifelong immunosuppression with its attendant risks) is the long waiting time for donor organs. The requirement for livers continues to go up as the waiting list of possible recipients remains to be extended. The shortage of donor organs is a universal problem. With 12 mo duration waiting lists, many patients (up to 25%) are expected to be excluded from liver transplantation due to tumour progression. A scarcity of donor livers has led to the development of schema, whereby preference for donor organs is given to the most critically ill patients. In the United States, the selection depends on the “model for end-stage liver disease” score which is used in the prediction of median survival in cirrhotic patients putting into consideration that diagnosis of HCC will increase the priority score for donor organs[131,133].
RFA
As regards loco-regional intervention ablation therapy of HCC, RFA has been the most commonly evaluated option. Distinctive types of electrode are available including internally cooled electrodes and multi-tined expandable electrodes with or without perfusion for clinical RF ablation[134]. The raising of temperature with subsequent damage is dependent on both the degree of the temperature achieved within the tissue and the whole length of exposure to the heating process. Irreversible cellular damage produced by raising the temperature of tumour tissue to 50 °C-55 °C for 4-6 min. In addition, tissue vaporises and carbonises at more than 100 °C-110 °C. A key factor that affects the achievement of successfully RF ablation is the capability to destroy all viable tumour tissues and probably a satisfactory tumour-free margin. Ideally, ablative margin at 360°, and 0.5-1 cm-thick should be created around the tumour. This cutoff would verify that microscopic invasions around the margin of a tumour have been eliminated[135].
While there is no precise tumour size beyond which RFA should not be applied, single tumour < 4 cm in diameter have the best outcome. As noted above, RFA has also been used as a “bridging” therapy[136].
Five randomised controlled trials (RCTs) have compared the efficacy of RFA vs percutaneous ethanol infusion (PEI) in the management of early-stage HCC. RF ablation showed superiority in the local control of the HCC than PEI due to its higher anti-cancer effects[137]. In addition, two large meta-analyses endorsed RF ablation as the standard percutaneous modality for HCC treatment[138].
A question arises here, whether RFA can replace the surgical resection as the first-line option for patients with small, single HCCs. A RCT comparing RFA ablation vs surgical resection in Child A patients with a solitary HCC lesion of diameter 5 cm or less, has failed to demonstrate any statistically significant differences in disease-free survival and overall survival rate between the two treatment groups[139].
Another limitation of RFA applicability in the treatment of HCC is the anatomical location of the HCC lesions; those who are close to the gastrointestinal system or near portahepatis or gall bladder are risky, with the potential for major complications[140]. Up to 30% of the small size tumours may not be appropriate for RFA due to their unfavourable position. Consequently, there are no clear data to support RFA as an alternative technique for surgical resection as a first-line in the management plan for patients with early-stage HCC[141].
MICROWAVE ABLATION
Microwave ablation is a medical term used for all electromagnetic methods that result in tumour damage by using different devices with various frequencies (equal to or more than 900 kHz). These microwaves move through the cells or other materials containing water leading to rotation of the different molecules. The rapid rotation of these molecules produces a consistent and evenly distributed hotness, which is continued until the radiation is completely stopped. A necrosis area in the form of round or column shape, created by microwave irradiation around the needle is formed, depending on the type of needle used and the amount of power generated[142].
One RCT compared the efficacy of both microwave ablation and RF ablation in the management of HCC. There is a propensity favouring RFA over microwave ablation despite taking into account the rate of both complications and local recurrences and that there was no statistically significant differences in the efficacy of the two procedures. It has to be noted that technology of microwave ablation has improved dramatically. Newer devices seem to have overcome the limitation encountered by the small size of the coagulation area produced by a single probe insertion. Microwave ablation offers an important advantage over RF ablation in that treatment outcome is not influenced by the site of the tumour[135].
PEI OR ACETIC ACID ABLATION
PEI is often indicated in patients who are not suitable for resection and with small HCCs due to their poor functional hepatic reserve. The ethanol produces coagulation necrosis of the tumour mass, through different mechanisms as dehydration of cells, denaturation of protein, and occlusion of small vessels. PEI is a well-established technique for the management of HCC nodular-type. Nodules of HCC have soft consistency within a firm cirrhotic liver. Consequently, the injected ethanol diffuses easily and selectively within these lesions. Multiple (4-6) sessions of PEI have been shown to be effective with complete response (CR) in approximately 70% of all lesions of small size[143].
An inherent advantages of PEI are low financial cost and low morbidity, but the higher local recurrence rate is a major limitation, that may occur in up to 33% in small lesions (less than 3 cm) and in up to 43% in larger lesions[144].
The CR is not usually achieved by ethanol injection, due to the uneven allocation of the ethanol within the lesion (particularly in the existence of intra-tumoural septa) and a restricted influence on extracapsular cancer cells spread. The current advance of dedicated multi-pronged PEI needles has been shown to overcome some of these limitations, leading to the achievement of 90% sustained rate of CR, when the size of the tumour is smaller than 3 cm and furthermore treated only with a single session ablation. Acetic acid injection as another method of chemical ablation has been utilized for the treatment of HCC. However, this procedure has been investigated by very few researchers worldwide[145].
TACE
TACE has been commonly used for treatment of unresectable type of HCC. The principle of TACE depends on deprivation of HCC lesions from their blood supply which is predominantly derived from the hepatic artery, whereas portal system supplies blood to surrounding liver tissue. TACE is preferable for multiple or large size focal HCC lesions and even in cases of impaired hepatic reserve. The tip of the catheter is introduced at the nearest and the achievable site of the feeding artery of HCC lesion. Under fluoroscopic monitoring, an emulsion of anti-cancer agents combined with lipidol followed by careful injection of gelatin sponge particles. The dose of chemotherapeutic agent and lipidol emulsion used in TACE is calculated and determined according to tumour size and extension of the lesions[131]. A more recent method of chemoembolisation involves the use of drug-eluting polyvinyl alcohol microspheres (“beads”), which seems to cause less toxicity with similar efficacy. Use of drug-eluting beads causes simultaneous or sequential occlusion of the feeding branch of hepatic artery until blood flow to the tumour tissue ceases, which may lead to greater efficacy of anti-cancer drug than chemotherapy alone.
TACE is the first-line strategy in the plan of downstaging of HCC tumours that exceed the criteria for transplantation[146]. During follow up, dynamic CT or MRI has been done every 3 to 4 mo and TACE was considered when local recurrence, second primary HCCs and/or intrahepatic metastasis were found. Limitations of TACE include invasion of liver capsule, extracapsular growth of the tumour, or invasion of the vessels with thrombosis. Rarely, complete ablation and necrosis of whole lesions is obtained, so the TACE should be considered for advanced HCC lesions that cannot be treated by resection or ablation.
Takayaso et al[146] noticed that TACE resulted in a five-year survival rate of 26%. A mortality rate of 0.5% was reported in 8510 patients with unresectable tumour of HCC. Patient survival can be predicted by assessment of hepatic reserve, characteristics of HCC (size, number of lesions, portal vein patency, and presence of tumour invasion) and values of alpha fetoprotein[147,148].
Adverse events associated with TACE were described in approximately 10% of treated patients; these events include abdominal pain, nausea, vomiting, bone marrow suppression, and ischemic cholecystitis. A post-embolisation syndrome has been observed in > 50% of patients treated with TACE and the patients usually presented with fever, abdominal pain, and intestinal obstruction of moderate degree. Treatment-related mortality is less than 5%[147].
Absolute contraindications to TACE include portal vein thrombosis (absence of hepatopetal blood flow), encephalopathy, and biliary obstruction. Relative contraindications include increased level of bilirubin > 2 mg/dL, aspartate aminotransferase > 100 unit/L, lactate dehydrogenase > 425 unit/L, tumour burden occupying > 50% of the liver, cardiac or renal co-morbidities, ascites, significant thrombocytopaenia, or recent variceal hemorrhage[149].
TACE PLUS RFA
Combined TACE and RFA can be applied to overcome the limitations of RFA when used alone. Three meta-analyses have concluded that combined TACE together with RFA was associated with an improvement of overall survival rate than RFA alone. While the combination of TACE plus RFA may be better than RFA alone, there is no obvious indication that it is better than TACE alone[10].
CRYOABLATION
Cryoablation is the application of alternating freeze-thaw cycles through the use of a cryoprobe inserted directly into the tumour; this procedure is used intraoperatively more frequently in HCC patients with unresectable lesions. RFA has supplanted the use of percutaneous cryoablation[10].
RT AND STEREOTACTIC BODY RADIOTHERAPY
External-beam RT is an emerging utility in the management of liver cancer, although its place among other treatment modalities for patients with unresectable HCC, its role has yet to be clarified. HCC is considered one of the radiosensitive tumours, and the liver is an extremely radiosensitive organ. As a whole, the liver can only tolerate radiation doses of approximately 20 Gy, although newer techniques using three-dimensional conformal treatment planning or stereotactic focusing may permit the guided delivery of up to 100 Gy.
Stereotactic body radiotherapy (SBRT) is a technique in which a high-dose radiation fractions used as a single or in limited numbers are delivered and condensed to a small, accurately distinct target lesion by utilizing multiple, non-parallel beams of radiation. These beams focus and converge exactly on the precise target lesion, minimising adjacent healthy tissue from exposure to radiation. This precise targeting facilitate treatment of small- or medium-sized tumours in extracranial sites in either both types of single or limited number of dose fractions, but experience with SBRT is still limited[150-152].
RADIOEMBOLISATION
A variety of hepatic artery-directed treatment is radionuclide yttrium-90 therapy. Microspheres containing yttrium-90 of approximately 25 mm in diameter are introduced through a catheter precisely inserted into the hepatic artery at the lobar or segmental level, and discharge of local radiation with minimal radiation exposure to neighbouring healthy tissue[125].
High intensity focused US (HIFU) therapy has been evolved for the treatment of solid organs tumours. The principle of HIFU is based on an extracorporal US source that focuses to a predetermined target lesion inside the body. The US energy passes safely through overlying tissues to a predetermined specific target area. The energy deposition rapidly produces a rapid elevation of the temperature of this area, resulting in an irreversible death of cells with an obvious area of tissue necrosis. The reported disadvantages of HIFU therapy include the long duration of the procedure (3-4 h) and the rarely required rib resection when the tumour is situated behind the rib bone[131]. In addition to the high cost, certain anatomical constraints (e.g., pass-through of the radioactive material to the lung in some patients with shunting) and a question of less effective tumour necrosis than is seen with TACE limit the utility of this treatment. It is under active study for patients with thrombosis of the portal vein and those with advanced disease, but still there is an appropriate liver reserve. No RCTs are currently available.
SYSTEMIC THERAPY
Molecularly targeted therapy (sorafenib)
One of the oral multi-tyrosine kinase inhibitor is sorafenib which is currently considered as the first drug that might improve survival in patients with advanced HCC. The multicentre European randomised SHARP trial in 2007, demonstrated monotherapy with sorafenib agent as a standard systemic treatment for advanced tumour of HCC.
In a large series, double-blinded placebo-controlled phase III, the estimated median overall survival estimated in months was found to increase from 7.9 in the placebo arm to 10.7 in the sorafenib arm (HR = 0.69; 95%CI: 0.55-0.87; P = 0.00058), which results in a 31% decrease in the relative risk of death.
A preliminary data from a randomised phase II trial comparing the results of sorafenib plus doxorubicin vs doxorubicin alone suggested an advantage for combined therapy; however, whether this combination is superior to sorafenib alone will necessitate a randomised trial in which the sorafenib is the control arm.
Cytotoxic chemotherapy
Chemotherapy has not been used consistently in the treatment of advanced HCC for a several reasons: (1) the relatively chemotherapy-refractory nature of HCC tumour. This is partially due to the high rate of drug resistance gene expression, including mutations in p53, glutathione-S-transferase, P-glycoprotein, and the heat shock proteins; (2) systemic chemotherapy is typically not properly acknowledged and endured by patients owing to the presence of significant underlying poor hepatic reserve. The overall survival of the patients is most often estimated neither by tumour aggressiveness nor the impact of a systemic treatment, but by the degree of impairment of hepatic reserve; and (3) clinical trials of systemic chemotherapy in patients with advanced stage of HCC have been conducted in different variety of populations.
Despite these issues, emerging data suggest a modest degree of antitumour efficacy for several cytotoxic agents and/or combined drug regimens; a chemotherapy trial may be warranted in many individuals, particularly if they have normal underlying liver. Reactivation of viral hepatitis may happen in patients with HCC who are experiencing intense systemic chemotherapy; it is therefore important to continue their antiviral medications.
Using a combination therapy of intra-arterial infusion of 5-Fluorouracil (5-FU) and subcutaneous interferon in 116 HCC patients with portal vein invasion has shown promising results[153].
The treatment cycle comprises of 4 wk; 5000000 u (5 MU) IFU was administered three times weekly, given intramuscularly at days 1, 3, and 5 from each week, with a total dose of 60 MU per cycle. 5-FU (500 mg/d) was injected into the hepatic artery through a portable infusion pump on days 1-5 of the first and second weeks through the intra-arterial catheter (5 gm per cycle) over a period of five hours each time. A CR achieved in 19 (16%) patients and a partial response obtained in the other 42 (36%). Only, nausea and appetite loss were noticed as adverse events. The overall survival rates reported at 12 and 14 mo among those patients were 34% and 18%, respectively, and those among patients who achieved CR were 81% and 59%, respectively[131].
Other molecular targets under clinical development are shown in Table 4.
Table 4.
Ongoing randomised phase II-III trials aimed at changing the standard of care in hepatocellular carcinoma management during the period 2012-2013[10]
| Indication | Randomised studies |
| Adjuvant | Sorafenib vs placebo |
| Intermediate HCC | Chemoembolisation ± sorafenib |
| Chemoembolisation ± brivanib | |
| Chemoembolisation ± everolimus | |
| Advanced HCC | |
| First line | Sorafenib ± erlotinib |
| Sorafenib vs brivanib | |
| Sorafenib vs sunitinib | |
| Sorafenib vs linifanib | |
| Sorafenib ± yttrium-90 | |
| Sorafenib ± doxorubicin | |
| Second line | Brivanib vs placebo |
| Everolimus vs placebo | |
| Ramucirmab vs placebo |
HCC: Hepatocellular carcinoma.
CONCLUSION
At present, the surveillance of HCC is based on US examination every 6 mo because AFP lacks the satisfactory sensitivity and specificity necessary for successful surveillance and diagnosis. Numerous clinical trials searching for a more ideal tool are running. One of these tools is the microRNAs which can be considered as a promising diagnostic as well as prognostic tool for HCC. Treatment of HCC depends on assessment of the tumour stage using BCLC or other scoring systems, preserved hepatic function, and performance status of the patients. Thus, a multidisciplinary approach is required for an optimal treatment of HCC. Indeed, hepatic surgical resection and liver transplantation are the only curative treatment options in the early stages of disease. RFA is equivalent to surgical resection in highly-selected patients with an early stage of HCC. Radioembolisation using resin or glass spheres appears to be a promising tool. Molecular studies of HCC have recognized peculiar activation of different signalling pathways, which signify key targets for emerging molecular therapies. The only approved therapy in patients with advanced disease is sorafenib, but novel and emerging targeted agents and their combinations are promising and being used in several clinical trials.
Footnotes
P- Reviewer: Sazci A, Takaki A, Wong GLH S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Conflict-of-interest: The authors declare that they have no competing interests.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 30, 2014
First decision: September 28, 2014
Article in press: May 27, 2015
References
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273.e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman El-Zayadi A, Abaza H, Shawky S, Mohamed MK, Selim OE, Badran HM. Prevalence and epidemiological features of hepatocellular carcinoma in Egypt-a single center experience. Hepatol Res. 2001;19:170–179. doi: 10.1016/s1386-6346(00)00105-4. [DOI] [PubMed] [Google Scholar]
- 3.el-Zayadi AR, Badran HM, Barakat EM, Attia Mel-D, Shawky S, Mohamed MK, Selim O, Saeid A. Hepatocellular carcinoma in Egypt: a single center study over a decade. World J Gastroenterol. 2005;11:5193–5198. doi: 10.3748/wjg.v11.i33.5193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.el-Serag HB. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2001;5:87–107, vi. doi: 10.1016/s1089-3261(05)70155-0. [DOI] [PubMed] [Google Scholar]
- 5.Arafa N, El Hoseiny M, Rekacewicz C, Bakr I, El-Kafrawy S, El Daly M, Aoun S, Marzouk D, Mohamed MK, Fontanet A. Changing pattern of hepatitis C virus spread in rural areas of Egypt. J Hepatol. 2005;43:418–424. doi: 10.1016/j.jhep.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Strickland GT, Elhefni H, Salman T, Waked I, Abdel-Hamid M, Mikhail NN, Esmat G, Fix A. Role of hepatitis C infection in chronic liver disease in Egypt. Am J Trop Med Hyg. 2002;67:436–442. doi: 10.4269/ajtmh.2002.67.436. [DOI] [PubMed] [Google Scholar]
- 7.Moradpour D, Blum HE. Pathogenesis of hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2005;17:477–483. doi: 10.1097/00042737-200505000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Singal A, Volk ML, Waljee A, Salgia R, Higgins P, Rogers MA, Marrero JA. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. doi: 10.1016/j.jhep.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 12.Stevens WR, Johnson CD, Stephens DH, Batts KP. CT findings in hepatocellular carcinoma: correlation of tumor characteristics with causative factors, tumor size, and histologic tumor grade. Radiology. 1994;191:531–537. doi: 10.1148/radiology.191.2.8153335. [DOI] [PubMed] [Google Scholar]
- 13.Sugano S, Miyoshi K, Suzuki T, Kawafune T, Kubota M. Intrahepatic arteriovenous shunting due to hepatocellular carcinoma and cirrhosis, and its change by transcatheter arterial embolization. Am J Gastroenterol. 1994;89:184–188. [PubMed] [Google Scholar]
- 14.Kew MC, Hodkinson J. Rupture of hepatocellular carcinoma as a result of blunt abdominal trauma. Am J Gastroenterol. 1991;86:1083–1085. [PubMed] [Google Scholar]
- 15.Choi BG, Park SH, Byun JY, Jung SE, Choi KH, Han JY. The findings of ruptured hepatocellular carcinoma on helical CT. Br J Radiol. 2001;74:142–146. doi: 10.1259/bjr.74.878.740142. [DOI] [PubMed] [Google Scholar]
- 16.Chearanai O, Plengvanit U, Asavanich C, Damrongsak D, Sindhvananda K, Boonyapisit S. Spontaneous rupture of primary hepatoma: report of 63 cases with particular reference to the pathogenesis and rationale treatment by hepatic artery ligation. Cancer. 1983;51:1532–1536. doi: 10.1002/1097-0142(19830415)51:8<1532::aid-cncr2820510829>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 17.Kew MC, Dos Santos HA, Sherlock S. Diagnosis of primary cancer of the liver. Br Med J. 1971;4:408–411. doi: 10.1136/bmj.4.5784.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruix J, Castells A, Calvet X, Feu F, Bru C, Solé M, Bruguera M, Rodés J. Diarrhea as a presenting symptom of hepatocellular carcinoma. Dig Dis Sci. 1990;35:681–685. doi: 10.1007/BF01540166. [DOI] [PubMed] [Google Scholar]
- 19.Lin YT, Liu CJ, Chen TJ, Chen TL, Yeh YC, Wu HS, Tseng CP, Wang FD, Tzeng CH, Fung CP. Pyogenic liver abscess as the initial manifestation of underlying hepatocellular carcinoma. Am J Med. 2011;124:1158–1164. doi: 10.1016/j.amjmed.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Luo JC, Hwang SJ, Wu JC, Lai CR, Li CP, Chang FY, Chiang JH, Lui WY, Chu CW, Lee SD. Clinical characteristics and prognosis of hepatocellular carcinoma patients with paraneoplastic syndromes. Hepatogastroenterology. 2002;49:1315–1319. [PubMed] [Google Scholar]
- 21.Eastman RC, Carson RE, Orloff DG, Cochran CS, Perdue JF, Rechler MM, Lanau F, Roberts CT, Shapiro J, Roth J. Glucose utilization in a patient with hepatoma and hypoglycemia. Assessment by a positron emission tomography. J Clin Invest. 1992;89:1958–1963. doi: 10.1172/JCI115803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeung RT. Hypoglycaemia in hepatocellular carcinoma: a review. Hong Kong Med J. 1997;3:297–301. [PubMed] [Google Scholar]
- 23.Tietge UJ, Schöfl C, Ocran KW, Wagner S, Böker KH, Brabant G, Zapf J, Manns MP. Hepatoma with severe non-islet cell tumor hypoglycemia. Am J Gastroenterol. 1998;93:997–1000. doi: 10.1111/j.1572-0241.1998.00297.x. [DOI] [PubMed] [Google Scholar]
- 24.Thipaporn T, Bubpha P, Varaphon V. Hepatocellular carcinoma with persistent hypoglycemia: successful treatment with corticosteroid and frequent high carbohydrate intake. J Med Assoc Thai. 2005;88:1941–1946. [PubMed] [Google Scholar]
- 25.Nikeghbalian S, Bananzadeh A, Yarmohammadi H. Hypoglycemia, the first presenting sign of hepatocellular carcinoma. Saudi Med J. 2006;27:387–388. [PubMed] [Google Scholar]
- 26.Jayaprasad N, Anees T, Bijin T, Madhusoodanan S. Severe hypoglycemia due to poorly differentiated hepatocellular carcinoma. J Assoc Physicians India. 2006;54:413–415. [PubMed] [Google Scholar]
- 27.Sorlini M, Benini F, Cravarezza P, Romanelli G. Hypoglycemia, an atypical early sign of hepatocellular carcinoma. J Gastrointest Cancer. 2010;41:209–211. doi: 10.1007/s12029-010-9137-0. [DOI] [PubMed] [Google Scholar]
- 28.Kew MC, Fisher JW. Serum erythropoietin concentrations in patients with hepatocellular carcinoma. Cancer. 1986;58:2485–2488. doi: 10.1002/1097-0142(19861201)58:11<2485::aid-cncr2820581122>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 29.Sakisaka S, Watanabe M, Tateishi H, Harada M, Shakado S, Mimura Y, Gondo K, Yoshitake M, Noguchi K, Hino T. Erythropoietin production in hepatocellular carcinoma cells associated with polycythemia: immunohistochemical evidence. Hepatology. 1993;18:1357–1362. [PubMed] [Google Scholar]
- 30.Hwang SJ, Luo JC, Li CP, Chu CW, Wu JC, Lai CR, Chiang JH, Chau GY, Lui WY, Lee CC, et al. Thrombocytosis: a paraneoplastic syndrome in patients with hepatocellular carcinoma. World J Gastroenterol. 2004;10:2472–2477. doi: 10.3748/wjg.v10.i17.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr BI, Guerra V. Thrombocytosis and hepatocellular carcinoma. Dig Dis Sci. 2013;58:1790–1796. doi: 10.1007/s10620-012-2527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knill-Jones RP, Buckle RM, Parsons V, Calne RY, Williams R. Hypercalcemia and increased parathyroid-hormone activity in a primary hepatoma. Studies before and after hepatic transplantation. N Engl J Med. 1970;282:704–708. doi: 10.1056/NEJM197003262821302. [DOI] [PubMed] [Google Scholar]
- 33.Yen TC, Hwang SJ, Wang CC, Lee SD, Yeh SH. Hypercalcemia and parathyroid hormone-related protein in hepatocellular carcinoma. Liver. 1993;13:311–315. doi: 10.1111/j.1600-0676.1993.tb00651.x. [DOI] [PubMed] [Google Scholar]
- 34.Steiner E, Velt P, Gutierrez O, Schwartz S, Chey W. Hepatocellular carcinoma presenting with intractable diarrhea. A radiologic-pathologic correlation. Arch Surg. 1986;121:849–851. doi: 10.1001/archsurg.1986.01400070119025. [DOI] [PubMed] [Google Scholar]
- 35.Helzberg JH, McPhee MS, Zarling EJ, Lukert BP. Hepatocellular carcinoma: an unusual course with hyperthyroidism and inappropriate thyroid-stimulating hormone production. Gastroenterology. 1985;88:181–184. doi: 10.1016/s0016-5085(85)80152-9. [DOI] [PubMed] [Google Scholar]
- 36.Summerskill WH, Adson MA. Gynecomastia as a sign of hepatoma. Report of a case. Am J Dig Dis. 1962;7:250–254. doi: 10.1007/BF02231817. [DOI] [PubMed] [Google Scholar]
- 37.Kew MC, Leckie BJ, Greeff MC. Arterial hypertension as a paraneoplastic phenomenon in hepatocellular carcinoma. Arch Intern Med. 1989;149:2111–2113. [PubMed] [Google Scholar]
- 38.Kao WY, Hung HH, Lu HC, Lin HC, Wu JC, Lee SD, Su CW. Hepatocellular carcinoma with presentation of budd-Chiari syndrome. J Chin Med Assoc. 2010;73:93–96. doi: 10.1016/S1726-4901(10)70008-3. [DOI] [PubMed] [Google Scholar]
- 39.Bargalló X, Gilabert R, Nicolau C, García-Pagán JC, Ayuso JR, Brú C. Sonography of Budd-Chiari syndrome. AJR Am J Roentgenol. 2006;187:W33–W41. doi: 10.2214/AJR.04.0918. [DOI] [PubMed] [Google Scholar]
- 40.Bălăceanu LA, Diaconu CC, Aron G. Budd-Chiari syndrome as an initial presentation of hepatocellular carcinoma: a case report. Med Ultrason. 2014;16:172–174. doi: 10.11152/mu.201.3.2066.162.lab1. [DOI] [PubMed] [Google Scholar]
- 41.Gregory B, Ho VC. Cutaneous manifestations of gastrointestinal disorders. Part II. J Am Acad Dermatol. 1992;26:371–383. doi: 10.1016/0190-9622(92)70059-o. [DOI] [PubMed] [Google Scholar]
- 42.Berkowitz I, Hodkinson HJ, Kew MC, DiBisceglie AM. Pityriasis rotunda as a cutaneous marker of hepatocellular carcinoma: a comparison with its prevalence in other diseases. Br J Dermatol. 1989;120:545–549. doi: 10.1111/j.1365-2133.1989.tb01329.x. [DOI] [PubMed] [Google Scholar]
- 43.Lim HW, Mascaro JM. The porphyrias and hepatocellular carcinoma. Dermatol Clin. 1995;13:135–142. [PubMed] [Google Scholar]
- 44.Kew MC. Tumors of the liver. Hepatology: A textbook of liver disease. Philadelphia: WB Saunders Company; 1996. p. 1513. [Google Scholar]
- 45.Lai CL, Ng RP, Lok AS. The diagnostic value of the ratio of serum gamma-glutamyl transpeptidase to alkaline phosphatase in alcoholic liver disease. Scand J Gastroenterol. 1982;17:41–47. doi: 10.3109/00365528209181042. [DOI] [PubMed] [Google Scholar]
- 46.Gomaa AI, Khan SA, Leen EL, Waked I, Taylor-Robinson SD. Diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2009;15:1301–1314. doi: 10.3748/wjg.15.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bhosale P, Szklaruk J, Silverman PM. Current staging of hepatocellular carcinoma: imaging implications. Cancer Imaging. 2006;6:83–94. doi: 10.1102/1470-7330.2006.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fasani P, Sangiovanni A, De Fazio C, Borzio M, Bruno S, Ronchi G, Del Ninno E, Colombo M. High prevalence of multinodular hepatocellular carcinoma in patients with cirrhosis attributable to multiple risk factors. Hepatology. 1999;29:1704–1707. doi: 10.1002/hep.510290604. [DOI] [PubMed] [Google Scholar]
- 49.Sherman M, Peltekian KM, Lee C. Screening for hepatocellular carcinoma in chronic carriers of hepatitis B virus: incidence and prevalence of hepatocellular carcinoma in a North American urban population. Hepatology. 1995;22:432–438. [PubMed] [Google Scholar]
- 50.Pateron D, Ganne N, Trinchet JC, Aurousseau MH, Mal F, Meicler C, Coderc E, Reboullet P, Beaugrand M. Prospective study of screening for hepatocellular carcinoma in Caucasian patients with cirrhosis. J Hepatol. 1994;20:65–71. doi: 10.1016/s0168-8278(05)80468-4. [DOI] [PubMed] [Google Scholar]
- 51.Larcos G, Sorokopud H, Berry G, Farrell GC. Sonographic screening for hepatocellular carcinoma in patients with chronic hepatitis or cirrhosis: an evaluation. AJR Am J Roentgenol. 1998;171:433–435. doi: 10.2214/ajr.171.2.9694470. [DOI] [PubMed] [Google Scholar]
- 52.Colli A, Fraquelli M, Casazza G, Massironi S, Colucci A, Conte D, Duca P. Accuracy of ultrasonography, spiral CT, magnetic resonance, and alpha-fetoprotein in diagnosing hepatocellular carcinoma: a systematic review. Am J Gastroenterol. 2006;101:513–523. doi: 10.1111/j.1572-0241.2006.00467.x. [DOI] [PubMed] [Google Scholar]
- 53.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 54.Dodd GD, Miller WJ, Baron RL, Skolnick ML, Campbell WL. Detection of malignant tumors in end-stage cirrhotic livers: efficacy of sonography as a screening technique. AJR Am J Roentgenol. 1992;159:727–733. doi: 10.2214/ajr.159.4.1326883. [DOI] [PubMed] [Google Scholar]
- 55.França AV, Elias Junior J, Lima BL, Martinelli AL, Carrilho FJ. Diagnosis, staging and treatment of hepatocellular carcinoma. Braz J Med Biol Res. 2004;37:1689–1705. doi: 10.1590/s0100-879x2004001100015. [DOI] [PubMed] [Google Scholar]
- 56.Yu SC, Yeung DT, So NM. Imaging features of hepatocellular carcinoma. Clin Radiol. 2004;59:145–156. doi: 10.1016/s0009-9260(03)00316-7. [DOI] [PubMed] [Google Scholar]
- 57.Albrecht T, Blomley M, Bolondi L, Claudon M, Correas JM, Cosgrove D, Greiner L, Jäger K, Jong ND, Leen E, et al. Guidelines for the use of contrast agents in ultrasound. January 2004. Ultraschall Med. 2004;25:249–256. doi: 10.1055/s-2004-813245. [DOI] [PubMed] [Google Scholar]
- 58.Quaia E. Microbubble ultrasound contrast agents: an update. Eur Radiol. 2007;17:1995–2008. doi: 10.1007/s00330-007-0623-0. [DOI] [PubMed] [Google Scholar]
- 59.Pompili M, Riccardi L, Semeraro S, Orefice R, Elia F, Barbaro B, Covino M, Grieco A, Gasbarrini G, Rapaccini GL. Contrast-enhanced ultrasound assessment of arterial vascularization of small nodules arising in the cirrhotic liver. Dig Liver Dis. 2008;40:206–215. doi: 10.1016/j.dld.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 60.Westwood M, Joore M, Grutters J, Redekop K, Armstrong N, Lee K, Gloy V, Raatz H, Misso K, Severens J, et al. Contrast-enhanced ultrasound using SonoVue® (sulphur hexafluoride microbubbles) compared with contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging for the characterisation of focal liver lesions and detection of liver metastases: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2013;17:1–243. doi: 10.3310/hta17160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh P, Erickson RA, Mukhopadhyay P, Gopal S, Kiss A, Khan A, Ulf Westblom T. EUS for detection of the hepatocellular carcinoma: results of a prospective study. Gastrointest Endosc. 2007;66:265–273. doi: 10.1016/j.gie.2006.10.053. [DOI] [PubMed] [Google Scholar]
- 62.Crowe DR, Eloubeidi MA, Chhieng DC, Jhala NC, Jhala D, Eltoum IA. Fine-needle aspiration biopsy of hepatic lesions: computerized tomographic-guided versus endoscopic ultrasound-guided FNA. Cancer. 2006;108:180–185. doi: 10.1002/cncr.21912. [DOI] [PubMed] [Google Scholar]
- 63.Bruix J, Sherman M, Llovet JM, Beaugrand M, Lencioni R, Burroughs AK, Christensen E, Pagliaro L, Colombo M, Rodés J. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol. 2001;35:421–430. doi: 10.1016/s0168-8278(01)00130-1. [DOI] [PubMed] [Google Scholar]
- 64.Baron RL, Brancatelli G. Computed tomographic imaging of hepatocellular carcinoma. Gastroenterology. 2004;127:S133–S143. doi: 10.1053/j.gastro.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 65.Bialecki ES, Di Bisceglie AM. Diagnosis of hepatocellular carcinoma. HPB (Oxford) 2005;7:26–34. doi: 10.1080/13651820410024049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Onishi H, Murakami T, Nakamura H. Diagnosis of hepatocellular carcinoma: multidetector-row computed tomography and magnetic resonance imaging. In: Hayat MA, editor. Methods of cancer diagnosis, therapy and prognosis, Volume 5. Netherlands: Springer; 2009. pp. 221–235. [Google Scholar]
- 67.Saar B, Kellner-Weldon F. Radiological diagnosis of hepatocellular carcinoma. Liver Int. 2008;28:189–199. doi: 10.1111/j.1478-3231.2007.01655.x. [DOI] [PubMed] [Google Scholar]
- 68.Szklaruk J, Silverman PM, Charnsangavej C. Imaging in the diagnosis, staging, treatment, and surveillance of hepatocellular carcinoma. AJR Am J Roentgenol. 2003;180:441–454. doi: 10.2214/ajr.180.2.1800441. [DOI] [PubMed] [Google Scholar]
- 69.Yu NC, Chaudhari V, Raman SS, Lassman C, Tong MJ, Busuttil RW, Lu DS. CT and MRI improve detection of hepatocellular carcinoma, compared with ultrasound alone, in patients with cirrhosis. Clin Gastroenterol Hepatol. 2011;9:161–167. doi: 10.1016/j.cgh.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 70.Ishiguchi T, Shimamoto K, Fukatsu H, Yamakawa K, Ishigaki T. Radiologic diagnosis of hepatocellular carcinoma. Semin Surg Oncol. 1996;12:164–169. doi: 10.1002/(SICI)1098-2388(199605/06)12:3<164::AID-SSU4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 71.Libbrecht L, Bielen D, Verslype C, Vanbeckevoort D, Pirenne J, Nevens F, Desmet V, Roskams T. Focal lesions in cirrhotic explant livers: pathological evaluation and accuracy of pretransplantation imaging examinations. Liver Transpl. 2002;8:749–761. doi: 10.1053/jlts.2002.34922. [DOI] [PubMed] [Google Scholar]
- 72.Kunishi Y, Numata K, Morimoto M, Okada M, Kaneko T, Maeda S, Tanaka K. Efficacy of fusion imaging combining sonography and hepatobiliary phase MRI with Gd-EOB-DTPA to detect small hepatocellular carcinoma. AJR Am J Roentgenol. 2012;198:106–114. doi: 10.2214/AJR.10.6039. [DOI] [PubMed] [Google Scholar]
- 73.Park MJ, Kim YK, Lee MW, Lee WJ, Kim YS, Kim SH, Choi D, Rhim H. Small hepatocellular carcinomas: improved sensitivity by combining gadoxetic acid-enhanced and diffusion-weighted MR imaging patterns. Radiology. 2012;264:761–770. doi: 10.1148/radiol.12112517. [DOI] [PubMed] [Google Scholar]
- 74.Granito A, Galassi M, Piscaglia F, Romanini L, Lucidi V, Renzulli M, Borghi A, Grazioli L, Golfieri R, Bolondi L. Impact of gadoxetic acid (Gd-EOB-DTPA)-enhanced magnetic resonance on the non-invasive diagnosis of small hepatocellular carcinoma: a prospective study. Aliment Pharmacol Ther. 2013;37:355–363. doi: 10.1111/apt.12166. [DOI] [PubMed] [Google Scholar]
- 75.Burrel M, Llovet JM, Ayuso C, Iglesias C, Sala M, Miquel R, Caralt T, Ayuso JR, Solé M, Sanchez M, et al. MRI angiography is superior to helical CT for detection of HCC prior to liver transplantation: an explant correlation. Hepatology. 2003;38:1034–1042. doi: 10.1053/jhep.2003.50409. [DOI] [PubMed] [Google Scholar]
- 76.Lencioni R, Mascalchi M, Caramella D, Bartolozzi C. Small hepatocellular carcinoma: differentiation from adenomatous hyperplasia with color Doppler US and dynamic Gd-DTPA-enhanced MR imaging. Abdom Imaging. 1996;21:41–48. doi: 10.1007/s002619900007. [DOI] [PubMed] [Google Scholar]
- 77.Nanda NC, Schlief R. Advances in echo imaging using contrast enhancement. Dordrecht: Kluwer Academic Publishers; 1993. [Google Scholar]
- 78.Heiken JP, Weyman PJ, Lee JK, Balfe DM, Picus D, Brunt EM, Flye MW. Detection of focal hepatic masses: prospective evaluation with CT, delayed CT, CT during arterial portography, and MR imaging. Radiology. 1989;171:47–51. doi: 10.1148/radiology.171.1.2538862. [DOI] [PubMed] [Google Scholar]
- 79.Yu JS, Kim KW, Lee JT, Yoo HS. MR imaging during arterial portography for assessment of hepatocellular carcinoma: comparison with CT during arterial portography. AJR Am J Roentgenol. 1998;170:1501–1506. doi: 10.2214/ajr.170.6.9609162. [DOI] [PubMed] [Google Scholar]
- 80.Kanematsu M, Hoshi H, Murakami T, Inaba Y, Kim T, Yamada T, Kato M, Yokoyama R, Nakamura H. Detection of hepatocellular carcinoma in patients with cirrhosis: MR imaging versus angiographically assisted helical CT. AJR Am J Roentgenol. 1997;169:1507–1515. doi: 10.2214/ajr.169.6.9393154. [DOI] [PubMed] [Google Scholar]
- 81.Murakami T, Oi H, Hori M, Kim T, Takahashi S, Tomoda K, Narumi Y, Nakamura H. Helical CT during arterial portography and hepatic arteriography for detecting hypervascular hepatocellular carcinoma. AJR Am J Roentgenol. 1997;169:131–135. doi: 10.2214/ajr.169.1.9207512. [DOI] [PubMed] [Google Scholar]
- 82.Kanematsu M, Hoshi H, Imaeda T, Murakami T, Inaba Y, Yokoyama R, Nakamura H. Detection and characterization of hepatic tumors: value of combined helical CT hepatic arteriography and CT during arterial portography. AJR Am J Roentgenol. 1997;168:1193–1198. doi: 10.2214/ajr.168.5.9129410. [DOI] [PubMed] [Google Scholar]
- 83.Ikeda K, Saitoh S, Koida I, Tsubota A, Arase Y, Chayama K, Kumada H. Imaging diagnosis of small hepatocellular carcinoma. Hepatology. 1994;20:82–87. doi: 10.1016/0270-9139(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 84.Ohki T, Tateishi R, Akahane M, Mikami S, Sato M, Uchino K, Arano T, Enooku K, Kondo Y, Yamashiki N, et al. CT with hepatic arterioportography as a pretreatment examination for hepatocellular carcinoma patients: a randomized controlled trial. Am J Gastroenterol. 2013;108:1305–1313. doi: 10.1038/ajg.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.El-Bahrawy M. Alpha-fetoprotein-producing non-germ cell tumours of the female genital tract. Eur J Cancer. 2010;46:1317–1322. doi: 10.1016/j.ejca.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 86.Liu X, Cheng Y, Sheng W, Lu H, Xu Y, Long Z, Zhu H, Wang Y. Clinicopathologic features and prognostic factors in alpha-fetoprotein-producing gastric cancers: analysis of 104 cases. J Surg Oncol. 2010;102:249–255. doi: 10.1002/jso.21624. [DOI] [PubMed] [Google Scholar]
- 87.Collier J, Sherman M. Screening for hepatocellular carcinoma. Hepatology. 1998;27:273–278. doi: 10.1002/hep.510270140. [DOI] [PubMed] [Google Scholar]
- 88.Sterling RK, Wright EC, Morgan TR, Seeff LB, Hoefs JC, Di Bisceglie AM, Dienstag JL, Lok AS. Frequency of elevated hepatocellular carcinoma (HCC) biomarkers in patients with advanced hepatitis C. Am J Gastroenterol. 2012;107:64–74. doi: 10.1038/ajg.2011.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Di Bisceglie AM, Sterling RK, Chung RT, Everhart JE, Dienstag JL, Bonkovsky HL, Wright EC, Everson GT, Lindsay KL, Lok AS, et al. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43:434–441. doi: 10.1016/j.jhep.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 90.Chen DS, Sung JL, Sheu JC, Lai MY, How SW, Hsu HC, Lee CS, Wei TC. Serum alpha-fetoprotein in the early stage of human hepatocellular carcinoma. Gastroenterology. 1984;86:1404–1409. [PubMed] [Google Scholar]
- 91.Soreide O, Czerniak A, Bradpiece H, Bloom S, Blumgart L. Characteristics of fibrolamellar hepatocellular carcinoma. A study of nine cases and a review of the literature. Am J Surg. 1986;151:518–523. doi: 10.1016/0002-9610(86)90117-0. [DOI] [PubMed] [Google Scholar]
- 92.Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, Donato MF, Piva A, Di Carlo V, Dioguardi N. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675–680. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 93.Trevisani F, D’Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S, Roda E, Bernardi M. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34:570–575. doi: 10.1016/s0168-8278(00)00053-2. [DOI] [PubMed] [Google Scholar]
- 94.Gupta S, Bent S, Kohlwes J. Test characteristics of alpha-fetoprotein for detecting hepatocellular carcinoma in patients with hepatitis C. A systematic review and critical analysis. Ann Intern Med. 2003;139:46–50. doi: 10.7326/0003-4819-139-1-200307010-00012. [DOI] [PubMed] [Google Scholar]
- 95.Nguyen MH, Garcia RT, Simpson PW, Wright TL, Keeffe EB. Racial differences in effectiveness of alpha-fetoprotein for diagnosis of hepatocellular carcinoma in hepatitis C virus cirrhosis. Hepatology. 2002;36:410–417. doi: 10.1053/jhep.2002.34744. [DOI] [PubMed] [Google Scholar]
- 96.Fujioka M, Nakashima Y, Nakashima O, Kojiro M. Immunohistologic study on the expressions of alpha-fetoprotein and protein induced by vitamin K absence or antagonist II in surgically resected small hepatocellular carcinoma. Hepatology. 2001;34:1128–1134. doi: 10.1053/jhep.2001.29202. [DOI] [PubMed] [Google Scholar]
- 97.Bae JS, Park SJ, Park KB, Paik SY, Ryu JK, Choi CK, Hwang TJ. Acute exacerbation of hepatitis in liver cirrhosis with very high levels of alpha-fetoprotein but no occurrence of hepatocellular carcinoma. Korean J Intern Med. 2005;20:80–85. doi: 10.3904/kjim.2005.20.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Johnson PJ, Poon TC, Hjelm NM, Ho CS, Blake C, Ho SK. Structures of disease-specific serum alpha-fetoprotein isoforms. Br J Cancer. 2000;83:1330–1337. doi: 10.1054/bjoc.2000.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Oka H, Saito A, Ito K, Kumada T, Satomura S, Kasugai H, Osaki Y, Seki T, Kudo M, Tanaka M. Multicenter prospective analysis of newly diagnosed hepatocellular carcinoma with respect to the percentage of Lens culinaris agglutinin-reactive alpha-fetoprotein. J Gastroenterol Hepatol. 2001;16:1378–1383. doi: 10.1046/j.1440-1746.2001.02643.x. [DOI] [PubMed] [Google Scholar]
- 100.Khien VV, Mao HV, Chinh TT, Ha PT, Bang MH, Lac BV, Hop TV, Tuan NA, Don LV, Taketa K, et al. Clinical evaluation of lentil lectin-reactive alpha-fetoprotein-L3 in histology-proven hepatocellular carcinoma. Int J Biol Markers. 2001;16:105–111. doi: 10.1177/172460080101600204. [DOI] [PubMed] [Google Scholar]
- 101.Mohanty S, Jensen D. Tumor markers and molecular biology. In: Al-Knawy B, Reddy KR, Bolondi L, editors. Hepatocellular carcinoma: a practical approach. New York: Informa Healthcare; 2009. pp. 63–74. [Google Scholar]
- 102.Liebman HA, Furie BC, Tong MJ, Blanchard RA, Lo KJ, Lee SD, Coleman MS, Furie B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N Engl J Med. 1984;310:1427–1431. doi: 10.1056/NEJM198405313102204. [DOI] [PubMed] [Google Scholar]
- 103.Nomura F, Ishijima M, Horikoshi A, Nakai T, Ohnishi K. Determination of serum des-gamma-carboxy prothrombin levels in patients with small-sized hepatocellular carcinoma: comparison of the conventional enzyme immunoassay and two modified methods. Am J Gastroenterol. 1996;91:1380–1383. [PubMed] [Google Scholar]
- 104.Aoyagi Y, Oguro M, Yanagi M, Mita Y, Suda T, Suzuki Y, Hata K, Ichii K, Asakura H. Clinical significance of simultaneous determinations of alpha-fetoprotein and des-gamma-carboxy prothrombin in monitoring recurrence in patients with hepatocellular carcinoma. Cancer. 1996;77:1781–1786. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1781::AID-CNCR4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 105.Weitz IC, Liebman HA. Des-gamma-carboxy (abnormal) prothrombin and hepatocellular carcinoma: a critical review. Hepatology. 1993;18:990–997. doi: 10.1002/hep.1840180434. [DOI] [PubMed] [Google Scholar]
- 106.Marrero JA, Su GL, Wei W, Emick D, Conjeevaram HS, Fontana RJ, Lok AS. Des-gamma carboxyprothrombin can differentiate hepatocellular carcinoma from nonmalignant chronic liver disease in american patients. Hepatology. 2003;37:1114–1121. doi: 10.1053/jhep.2003.50195. [DOI] [PubMed] [Google Scholar]
- 107.Nakamura S, Nouso K, Sakaguchi K, Ito YM, Ohashi Y, Kobayashi Y, Toshikuni N, Tanaka H, Miyake Y, Matsumoto E, et al. Sensitivity and specificity of des-gamma-carboxy prothrombin for diagnosis of patients with hepatocellular carcinomas varies according to tumor size. Am J Gastroenterol. 2006;101:2038–2043. doi: 10.1111/j.1572-0241.2006.00681.x. [DOI] [PubMed] [Google Scholar]
- 108.Borel F, Konstantinova P, Jansen PL. Diagnostic and therapeutic potential of miRNA signatures in patients with hepatocellular carcinoma. J Hepatol. 2012;56:1371–1383. doi: 10.1016/j.jhep.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 109.Zhou J, Yu L, Gao X, Hu J, Wang J, Dai Z, Wang JF, Zhang Z, Lu S, Huang X, et al. Plasma microRNA panel to diagnose hepatitis B virus-related hepatocellular carcinoma. J Clin Oncol. 2011;29:4781–4788. doi: 10.1200/JCO.2011.38.2697. [DOI] [PubMed] [Google Scholar]
- 110.Li L, Guo Z, Wang J, Mao Y, Gao Q. Serum miR-18a: a potential marker for hepatitis B virus-related hepatocellular carcinoma screening. Dig Dis Sci. 2012;57:2910–2916. doi: 10.1007/s10620-012-2317-y. [DOI] [PubMed] [Google Scholar]
- 111.Kew MC, Wolf P, Whittaker D, Rowe P. Tumour-associated isoenzymes of gamma-glutamyl transferase in the serum of patients with hepatocellular carcinoma. Br J Cancer. 1984;50:451–455. doi: 10.1038/bjc.1984.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tsai JF, Jeng JE, Chuang LY, Yang ML, Ho MS, Chang WY, Hsieh MY, Lin ZY, Tsai JH. Clinical evaluation of urinary transforming growth factor-beta1 and serum alpha-fetoprotein as tumour markers of hepatocellular carcinoma. Br J Cancer. 1997;75:1460–1466. doi: 10.1038/bjc.1997.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hamazaki K, Gochi A, Shimamura H, Kaihara A, Maruo Y, Doi Y, Orita K, Lygidakis NJ. Serum levels of circulating intercellular adhesion molecule 1 in hepatocellular carcinoma. Hepatogastroenterology. 1996;43:229–234. [PubMed] [Google Scholar]
- 114.Takahashi H, Saibara T, Iwamura S, Tomita A, Maeda T, Onishi S, Yamamoto Y, Enzan H. Serum alpha-L-fucosidase activity and tumor size in hepatocellular carcinoma. Hepatology. 1994;19:1414–1417. [PubMed] [Google Scholar]
- 115.International Consensus Group for Hepatocellular Neoplasia; The International Consensus Group for Hepatocellular Neoplasia. Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658–664. doi: 10.1002/hep.22709. [DOI] [PubMed] [Google Scholar]
- 116.Shen Q, Fan J, Yang XR, Tan Y, Zhao W, Xu Y, Wang N, Niu Y, Wu Z, Zhou J, et al. Serum DKK1 as a protein biomarker for the diagnosis of hepatocellular carcinoma: a large-scale, multicentre study. Lancet Oncol. 2012;13:817–826. doi: 10.1016/S1470-2045(12)70233-4. [DOI] [PubMed] [Google Scholar]
- 117.Na K, Jeong SK, Lee MJ, Cho SY, Kim SA, Lee MJ, Song SY, Kim H, Kim KS, Lee HW, et al. Human liver carboxylesterase 1 outperforms alpha-fetoprotein as biomarker to discriminate hepatocellular carcinoma from other liver diseases in Korean patients. Int J Cancer. 2013;133:408–415. doi: 10.1002/ijc.28020. [DOI] [PubMed] [Google Scholar]
- 118.Henry L, Lavabre-Bertrand T, Vercambre L, Ramos J, Carillo S, Guiraud I, Pouderoux P, Bismuth M, Valats JC, Demattei C, Duny Y, Chaze I, Funakoshi N, Bureau JP, Daurès JP, Blanc P. Plasma proteasome level is a reliable early marker of malignant transformation of liver cirrhosis. Gut. 2009;58:833–838. doi: 10.1136/gut.2008.157016. [DOI] [PubMed] [Google Scholar]
- 119.Hamed M, El-Etreby S, Bahgat M, Attia M, Abdaziz S, Salem D, Kamr W. Is plasma proteasome level a reliable marker of hepatocellular carcinoma in hepatitis c virus related cirrhosis? Hepatology. 2012;56 Suppl 2:S281. [Google Scholar]
- 120.Bialecki ES, Ezenekwe AM, Brunt EM, Collins BT, Ponder TB, Bieneman BK, Di Bisceglie AM. Comparison of liver biopsy and noninvasive methods for diagnosis of hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2006;4:361–368. doi: 10.1016/S1542-3565(05)00977-8. [DOI] [PubMed] [Google Scholar]
- 121.Crippin JS. Biopsy of suspicious liver nodules: does it change management? Clin Gastroenterol Hepatol. 2006;4:296–298. doi: 10.1016/j.cgh.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 122.John TG, Garden OJ. Needle track seeding of primary and secondary liver carcinoma after percutaneous liver biopsy. HPB Surg. 1993;6:199–203; discussion 203-204. doi: 10.1155/1993/39539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Durand F, Regimbeau JM, Belghiti J, Sauvanet A, Vilgrain V, Terris B, Moutardier V, Farges O, Valla D. Assessment of the benefits and risks of percutaneous biopsy before surgical resection of hepatocellular carcinoma. J Hepatol. 2001;35:254–258. doi: 10.1016/s0168-8278(01)00108-8. [DOI] [PubMed] [Google Scholar]
- 124.Huang GT, Sheu JC, Yang PM, Lee HS, Wang TH, Chen DS. Ultrasound-guided cutting biopsy for the diagnosis of hepatocellular carcinoma--a study based on 420 patients. J Hepatol. 1996;25:334–338. doi: 10.1016/s0168-8278(96)80120-6. [DOI] [PubMed] [Google Scholar]
- 125.Kim SH, Lim HK, Lee WJ, Cho JM, Jang HJ. Needle-tract implantation in hepatocellular carcinoma: frequency and CT findings after biopsy with a 19.5-gauge automated biopsy gun. Abdom Imaging. 2000;25:246–250. doi: 10.1007/s002610000025. [DOI] [PubMed] [Google Scholar]
- 126.Ohlsson B, Nilsson J, Stenram U, Akerman M, Tranberg KG. Percutaneous fine-needle aspiration cytology in the diagnosis and management of liver tumours. Br J Surg. 2002;89:757–762. doi: 10.1046/j.1365-2168.2002.02111.x. [DOI] [PubMed] [Google Scholar]
- 127.Silva MA, Hegab B, Hyde C, Guo B, Buckels JA, Mirza DF. Needle track seeding following biopsy of liver lesions in the diagnosis of hepatocellular cancer: a systematic review and meta-analysis. Gut. 2008;57:1592–1596. doi: 10.1136/gut.2008.149062. [DOI] [PubMed] [Google Scholar]
- 128.Ng KK, Poon RT, Lo CM, Liu CL, Lam CM, Ng IO, Fan ST. Impact of preoperative fine-needle aspiration cytologic examination on clinical outcome in patients with hepatocellular carcinoma in a tertiary referral center. Arch Surg. 2004;139:193–200. doi: 10.1001/archsurg.139.2.193. [DOI] [PubMed] [Google Scholar]
- 129.Liu YW, Chen CL, Chen YS, Wang CC, Wang SH, Lin CC. Needle tract implantation of hepatocellular carcinoma after fine needle biopsy. Dig Dis Sci. 2007;52:228–231. doi: 10.1007/s10620-006-9354-3. [DOI] [PubMed] [Google Scholar]
- 130.Bruix J, Llovet JM. Major achievements in hepatocellular carcinoma. Lancet. 2009;373:614–616. doi: 10.1016/S0140-6736(09)60381-0. [DOI] [PubMed] [Google Scholar]
- 131.Masuzaki R, Omata M. HCC screening and surveillance. In: Al-Knawy B, Reddy KR, Bolondi L, editors. Hepatocellular carcinoma: a practical approach. London: Informa Healthcare; 2009. pp. 26–35. [Google Scholar]
- 132.Patel SS, Arrington AK, McKenzie S, Mailey B, Ding M, Lee W, Artinyan A, Nissen N, Colquhoun SD, Kim J. Milan Criteria and UCSF Criteria: A Preliminary Comparative Study of Liver Transplantation Outcomes in the United States. Int J Hepatol. 2012;2012:253517. doi: 10.1155/2012/253517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A, Gennari L. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334:693–699. doi: 10.1056/NEJM199603143341104. [DOI] [PubMed] [Google Scholar]
- 134.Lencioni R, Crocetti L. Radiofrequency ablation of liver cancer. Tech Vasc Interv Radiol. 2007;10:38–46. doi: 10.1053/j.tvir.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 135.Lencioni R, Crocetti L, De Simone P, Filipponi F. Loco-regional interventional treatment of hepatocellular carcinoma: techniques, outcomes, and future prospects. Transpl Int. 2010;23:698–703. doi: 10.1111/j.1432-2277.2010.01109.x. [DOI] [PubMed] [Google Scholar]
- 136.Lu DS, Yu NC, Raman SS, Limanond P, Lassman C, Murray K, Tong MJ, Amado RG, Busuttil RW. Radiofrequency ablation of hepatocellular carcinoma: treatment success as defined by histologic examination of the explanted liver. Radiology. 2005;234:954–960. doi: 10.1148/radiol.2343040153. [DOI] [PubMed] [Google Scholar]
- 137.Brunello F, Veltri A, Carucci P, Pagano E, Ciccone G, Moretto P, Sacchetto P, Gandini G, Rizzetto M. Radiofrequency ablation versus ethanol injection for early hepatocellular carcinoma: A randomized controlled trial. Scand J Gastroenterol. 2008;43:727–735. doi: 10.1080/00365520701885481. [DOI] [PubMed] [Google Scholar]
- 138.Orlando A, Leandro G, Olivo M, Andriulli A, Cottone M. Radiofrequency thermal ablation vs. percutaneous ethanol injection for small hepatocellular carcinoma in cirrhosis: meta-analysis of randomized controlled trials. Am J Gastroenterol. 2009;104:514–524. doi: 10.1038/ajg.2008.80. [DOI] [PubMed] [Google Scholar]
- 139.Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, Lau WY. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243:321–328. doi: 10.1097/01.sla.0000201480.65519.b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lencioni R, Crocetti L. Image-guided thermal ablation of hepatocellular carcinoma. Crit Rev Oncol Hematol. 2008;66:200–207. doi: 10.1016/j.critrevonc.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 141.Ebara M, Okabe S, Kita K, Sugiura N, Fukuda H, Yoshikawa M, Kondo F, Saisho H. Percutaneous ethanol injection for small hepatocellular carcinoma: therapeutic efficacy based on 20-year observation. J Hepatol. 2005;43:458–464. doi: 10.1016/j.jhep.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 142.Shibata T, Iimuro Y, Yamamoto Y, Maetani Y, Ametani F, Itoh K, Konishi J. Small hepatocellular carcinoma: comparison of radio-frequency ablation and percutaneous microwave coagulation therapy. Radiology. 2002;223:331–337. doi: 10.1148/radiol.2232010775. [DOI] [PubMed] [Google Scholar]
- 143.Lencioni R, Pinto F, Armillotta N, Bassi AM, Moretti M, Di Giulio M, Marchi S, Uliana M, Della Capanna S, Lencioni M, et al. Long-term results of percutaneous ethanol injection therapy for hepatocellular carcinoma in cirrhosis: a European experience. Eur Radiol. 1997;7:514–519. doi: 10.1007/s003300050194. [DOI] [PubMed] [Google Scholar]
- 144.Khan KN, Yatsuhashi H, Yamasaki K, Yamasaki M, Inoue O, Koga M, Yano M. Prospective analysis of risk factors for early intrahepatic recurrence of hepatocellular carcinoma following ethanol injection. J Hepatol. 2000;32:269–278. doi: 10.1016/s0168-8278(00)80072-0. [DOI] [PubMed] [Google Scholar]
- 145.Kuang M, Lu MD, Xie XY, Xu HX, Xu ZF, Liu GJ, Yin XY, Huang JF, Lencioni R. Ethanol ablation of hepatocellular carcinoma Up to 5.0 cm by using a multipronged injection needle with high-dose strategy. Radiology. 2009;253:552–561. doi: 10.1148/radiol.2532082021. [DOI] [PubMed] [Google Scholar]
- 146.Takayasu K, Shima Y, Muramatsu Y, Moriyama N, Yamada T, Makuuchi M, Hasegawa H, Hirohashi S. Hepatocellular carcinoma: treatment with intraarterial iodized oil with and without chemotherapeutic agents. Radiology. 1987;163:345–351. doi: 10.1148/radiology.163.2.3031724. [DOI] [PubMed] [Google Scholar]
- 147.El-Serag HB, Marrero JA, Rudolph L, Reddy KR. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology. 2008;134:1752–1763. doi: 10.1053/j.gastro.2008.02.090. [DOI] [PubMed] [Google Scholar]
- 148.Guan YS, Ahmad Al-Shatouri M, He Q, Liu WM. Hepatocellular carcinoma: carcinogenesis, establishment, progression, and therapies. Biomed Res Int. 2014;2014:706142. doi: 10.1155/2014/706142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Rammohan A, Sathyanesan J, Ramaswami S, Lakshmanan A, Senthil-Kumar P, Srinivasan UP, Ramasamy R, Ravichandran P. Embolization of liver tumors: Past, present and future. World J Radiol. 2012;4:405–412. doi: 10.4329/wjr.v4.i9.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Park W, Lim DH, Paik SW, Koh KC, Choi MS, Park CK, Yoo BC, Lee JE, Kang MK, Park YJ, et al. Local radiotherapy for patients with unresectable hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2005;61:1143–1150. doi: 10.1016/j.ijrobp.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 151.Seong J, Park HC, Han KH, Chon CY. Clinical results and prognostic factors in radiotherapy for unresectable hepatocellular carcinoma: a retrospective study of 158 patients. Int J Radiat Oncol Biol Phys. 2003;55:329–336. doi: 10.1016/s0360-3016(02)03929-9. [DOI] [PubMed] [Google Scholar]
- 152.Seo YS, Kim JN, Keum B, Park S, Kwon YD, Kim YS, Jeen YT, Chun HJ, Kim CY, Kim CD, et al. Radiotherapy for 65 patients with advanced unresectable hepatocellular carcinoma. World J Gastroenterol. 2008;14:2394–2400. doi: 10.3748/wjg.14.2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Obi S, Yoshida H, Toune R, Unuma T, Kanda M, Sato S, Tateishi R, Teratani T, Shiina S, Omata M. Combination therapy of intraarterial 5-fluorouracil and systemic interferon-alpha for advanced hepatocellular carcinoma with portal venous invasion. Cancer. 2006;106:1990–1997. doi: 10.1002/cncr.21832. [DOI] [PubMed] [Google Scholar]
- 154.Budhu A, Jia HL, Forgues M, Liu CG, Goldstein D, Lam A, Zanetti KA, Ye QH, Qin LX, Croce CM, et al. Identification of metastasis-related microRNAs in hepatocellular carcinoma. Hepatology. 2008;47:897–907. doi: 10.1002/hep.22160. [DOI] [PubMed] [Google Scholar]
- 155.Jiang J, Gusev Y, Aderca I, Mettler TA, Nagorney DM, Brackett DJ, Roberts LR, Schmittgen TD. Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin Cancer Res. 2008;14:419–427. doi: 10.1158/1078-0432.CCR-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Han ZB, Zhong L, Teng MJ, Fan JW, Tang HM, Wu JY, Chen HY, Wang ZW, Qiu GQ, Peng ZH. Identification of recurrence-related microRNAs in hepatocellular carcinoma following liver transplantation. Mol Oncol. 2012;6:445–457. doi: 10.1016/j.molonc.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, Ambs S, Chen Y, Meltzer PS, Croce CM, et al. MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med. 2009;361:1437–1447. doi: 10.1056/NEJMoa0901282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW, Chen CM, Lin CD, Liao YL, Wang JL, Chau YP, et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49:1571–1582. doi: 10.1002/hep.22806. [DOI] [PubMed] [Google Scholar]
- 159.Coulouarn C, Factor VM, Andersen JB, Durkin ME, Thorgeirsson SS. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene. 2009;28:3526–3536. doi: 10.1038/onc.2009.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fornari F, Gramantieri L, Giovannini C, Veronese A, Ferracin M, Sabbioni S, Calin GA, Grazi GL, Croce CM, Tavolari S, et al. MiR-122/cyclin G1 interaction modulates p53 activity and affects doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2009;69:5761–5767. doi: 10.1158/0008-5472.CAN-08-4797. [DOI] [PubMed] [Google Scholar]
- 161.Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, O’Sullivan M, Lu J, Phillips LA, Lockhart VL, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nat Genet. 2009;41:843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fornari F, Milazzo M, Chieco P, Negrini M, Calin GA, Grazi GL, Pollutri D, Croce CM, Bolondi L, Gramantieri L. MiR-199a-3p regulates mTOR and c-Met to influence the doxorubicin sensitivity of human hepatocarcinoma cells. Cancer Res. 2010;70:5184–5193. doi: 10.1158/0008-5472.CAN-10-0145. [DOI] [PubMed] [Google Scholar]
- 163.Wang C, Song B, Song W, Liu J, Sun A, Wu D, Yu H, Lian J, Chen L, Han J. Underexpressed microRNA-199b-5p targets hypoxia-inducible factor-1α in hepatocellular carcinoma and predicts prognosis of hepatocellular carcinoma patients. J Gastroenterol Hepatol. 2011;26:1630–1637. doi: 10.1111/j.1440-1746.2011.06758.x. [DOI] [PubMed] [Google Scholar]
- 164.Zhang Y, Guo X, Xiong L, Kong X, Xu Y, Liu C, Zou L, Li Z, Zhao J, Lin N. MicroRNA-101 suppresses SOX9-dependent tumorigenicity and promotes favorable prognosis of human hepatocellular carcinoma. FEBS Lett. 2012;586:4362–4370. doi: 10.1016/j.febslet.2012.10.053. [DOI] [PubMed] [Google Scholar]
- 165.Li W, Xie L, He X, Li J, Tu K, Wei L, Wu J, Guo Y, Ma X, Zhang P, et al. Diagnostic and prognostic implications of microRNAs in human hepatocellular carcinoma. Int J Cancer. 2008;123:1616–1622. doi: 10.1002/ijc.23693. [DOI] [PubMed] [Google Scholar]
- 166.Murakami Y, Yasuda T, Saigo K, Urashima T, Toyoda H, Okanoue T, Shimotohno K. Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene. 2006;25:2537–2545. doi: 10.1038/sj.onc.1209283. [DOI] [PubMed] [Google Scholar]
- 167.Gu H, Guo X, Zou L, Zhu H, Zhang J. Upregulation of microRNA-372 associates with tumor progression and prognosis in hepatocellular carcinoma. Mol Cell Biochem. 2013;375:23–30. doi: 10.1007/s11010-012-1521-6. [DOI] [PubMed] [Google Scholar]
- 168.Gramantieri L, Fornari F, Ferracin M, Veronese A, Sabbioni S, Calin GA, Grazi GL, Croce CM, Bolondi L, Negrini M. MicroRNA-221 targets Bmf in hepatocellular carcinoma and correlates with tumor multifocality. Clin Cancer Res. 2009;15:5073–5081. doi: 10.1158/1078-0432.CCR-09-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fu X, Wang Q, Chen J, Huang X, Chen X, Cao L, Tan H, Li W, Zhang L, Bi J, et al. Clinical significance of miR-221 and its inverse correlation with p27Kip¹ in hepatocellular carcinoma. Mol Biol Rep. 2011;38:3029–3035. doi: 10.1007/s11033-010-9969-5. [DOI] [PubMed] [Google Scholar]
- 170.Rong M, Chen G, Dang Y. Increased miR-221 expression in hepatocellular carcinoma tissues and its role in enhancing cell growth and inhibiting apoptosis in vitro. BMC Cancer. 2013;13:21. doi: 10.1186/1471-2407-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen L, Jiang M, Yuan W, Tang H. miR-17-5p as a novel prognostic marker for hepatocellular carcinoma. J Invest Surg. 2012;25:156–161. doi: 10.3109/08941939.2011.618523. [DOI] [PubMed] [Google Scholar]
- 172.Han ZB, Chen HY, Fan JW, Wu JY, Tang HM, Peng ZH. Up-regulation of microRNA-155 promotes cancer cell invasion and predicts poor survival of hepatocellular carcinoma following liver transplantation. J Cancer Res Clin Oncol. 2012;138:153–161. doi: 10.1007/s00432-011-1076-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chen HY, Han ZB, Fan JW, Xia J, Wu JY, Qiu GQ, Tang HM, Peng ZH. miR-203 expression predicts outcome after liver transplantation for hepatocellular carcinoma in cirrhotic liver. Med Oncol. 2012;29:1859–1865. doi: 10.1007/s12032-011-0031-9. [DOI] [PubMed] [Google Scholar]
- 174.Murakami Y, Tamori A, Itami S, Tanahashi T, Toyoda H, Tanaka M, Wu W, Brojigin N, Kaneoka Y, Maeda A, et al. The expression level of miR-18b in hepatocellular carcinoma is associated with the grade of malignancy and prognosis. BMC Cancer. 2013;13:99. doi: 10.1186/1471-2407-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Negrini M, Gramantieri L, Sabbioni S, Croce CM. microRNA involvement in hepatocellular carcinoma. Anticancer Agents Med Chem. 2011;11:500–521. doi: 10.2174/187152011796011037. [DOI] [PubMed] [Google Scholar]


