Abstract
AIM: To elucidate the efficacies of tolvaptan (TLV) as a treatment for refractory ascites compared with conventional treatment.
METHODS: We retrospectively enrolled 120 refractory ascites patients between January 1, 2009 and September 31, 2014. Sixty patients were treated with oral TLV at a starting dose of 3.75 mg/d in addition to sodium restriction (> 7 g/d), albumin infusion (10-20 g/wk), and standard diuretic therapy (20-60 mg/d furosemide and 25-50 mg/d spironolactone) and 60 patients with large volume paracentesis in addition to sodium restriction (less than 7 g/d), albumin infusion (10-20 g/wk), and standard diuretic therapy (20-120 mg/d furosemide and 25-150 mg/d spironolactone). Patient demographics and laboratory data, including liver function, were not matched due to the small number of patients. Continuous variables were analyzed by unpaired t-test or paired t-test. Fisher’s exact test was applied in cases comparing two nominal variables. We analyzed factors affecting clinical outcomes using receiver operating characteristic curves and multivariate regression analysis. We also used multivariate Cox’s proportional hazard regression analysis to elucidate the risk factors that contributed to the increased incidence of ascites.
RESULTS: TLV was effective in 38 (63.3%) patients. The best cut-off values for urine output and reduced urine osmolality as measures of refractory ascites improvement were > 1800 mL within the first 24 h and > 30%, respectively. Multivariate regression analysis indicated that > 25% reduced urine osmolality [odds ratio (OR) = 20.7; P < 0.01] and positive hepatitis C viral antibodies (OR = 5.93; P = 0.05) were positively correlated with an improvement of refractory ascites, while the total bilirubin level per 1.0 mg/dL (OR = 0.57; P = 0.02) was negatively correlated with improvement. In comparing the TLV group and controls, only the serum sodium level was significantly lower in the TLV group (133 mEq/L vs 136 mEq/L; P = 0.02). However, there were no significant differences in the other parameters between the two groups. The cumulative incidence rate was significantly higher in the control group with a median incidence time of 30 d in the TLV group and 20 d in the control group (P = 0.01). Cox hazard proportional multivariate analysis indicated that the use of TLV (OR = 0.58; P < 0.01), uncontrolled liver neoplasms (OR = 1.92; P < 0.01), total bilirubin level per 1.0 mg/dL (OR = 1.10; P < 0.01), and higher sodium level per 1.0 mEq/L (OR = 0.94; P < 0.01) were independent factors that contributed to incidence.
CONCLUSION: Administration of TLV results in better control of refractory ascites and reduced the incidence of additional invasive procedures or hospitalization compared with conventional ascites treatments.
Keywords: Refractory ascites, Tolvaptan, Paracentesis, Decompensated cirrhosis
Core tip: Tolvaptan (TLV) was effective in 38 (63.3%) refractory ascites patients. The best cut-off values for urine output and reduced urine osmolality as measures of refractory ascites improvement were > 1800 mL within the first 24 h and > 30%, respectively. The cumulative incidence rate was significantly higher in the control group with a median incidence time of 30 d in the TLV group and 20 d in the control group. Administration of TLV results in better control of refractory ascites and reduced the incidence of additional invasive procedures or hospitalization compared with conventional ascites treatments.
INTRODUCTION
Hepatic edema and ascites are common complications in decompensated liver cirrhosis patients[1,2]. Refractory ascites is defined as non-responsiveness to sodium dietary restriction and high dose diuretic therapy that occurs in 15%-20% of all ascites patients[3]. Refractory ascites is also associated with a poor quality of life (QOL) and prognosis due to restricted treatment options[4,5].
Tolvaptan (TLV) is a new oral, selective vasopressin V2 receptor antagonist originally developed for the treatment of hypervolemic or euvolemic hyponatremia in patients with heart failure, cirrhosis or syndrome of inappropriate antidiuretic hormone[6,7]. Inhibition of the vasopressin V2 receptor by TLV prevents the insertion of aquaporin 2 water channels into the apical cell membrane of the collecting duct, which increases free water excretion without significantly affecting urinary sodium or potassium secretion[8]. This allows for reduced water retention with elevated serum sodium levels, which is an ideal outcome in decompensated liver cirrhosis patients with refractory ascites.
In Japan, the addition of TLV to conventional diuretic therapy has been useful for the treatment of refractory ascites. A phase 3 study showed a remarkable reduction in ascites with a median loss of 2 kg body weight compared with the placebo controls. However, the administration of TLV was limited to 7 d because of the study design[9], and since decompensated cirrhosis is a progressive disease, even transient improvement of refractory ascites could eventually result in uncontrolled ascites.
Our principal objective was to conduct an observational retrospective study to elucidate the clinical outcomes of TLV. These outcomes included assessing the safety and efficacy of long-term administration, determining the effectiveness cut-off level, and identifying factors that contribute to improved refractory ascites in a clinical setting. In addition, since decompensated cirrhosis is a progressive disease, we examined the time to progression by comparing TLV to conventional treatment.
MATERIALS AND METHODS
Study design
A single center, open label, observational retrospective study was conducted in Mitsui Memorial Hospital (Tokyo, Japan) between January 1st 2009 and September 30 2014. The last follow-up date was October 31 2014.
Inclusion criteria
This study enrolled liver cirrhosis patients 20-80 years of age with refractory ascites who had been receiving loop diuretic and/or anti-aldosterone agents. Refractory ascites was defined as follows: existence of ascites detected by ultrasound under the treatment of a loop diuretic at a daily dose equivalent to ≥ 40 mg/d furosemide and ≥ 25 mg/d spironolactone, a loop diuretic at a daily dose equivalent to ≥ 20 mg/d furosemide and ≥ 50 mg/d spironolactone, or a loop diuretic alone at a daily dose equivalent to ≥ 60 mg/d furosemide. Patients were required to be hospitalized or to be available for hospitalization during the treatment period. Exclusion criteria were existence of hepatic encephalopathy, inability to take oral medication, or end stage renal disease on hemodialysis.
Patients
We enrolled 60 refractory ascites patients treated with TLV until September 30, 2014. We included another 60 refractory ascites patients treated with conventional large volume paracentesis as a control group from our liver disease database between January 1, 2009 and September 30, 2012. Patient demographics and laboratory data, including liver function, were not matched due to the small number of patients. The final analysis included 120 patients.
Therapeutic protocol
Sixty patients received oral TLV at a starting dose of 3.75 mg/d in addition to sodium restriction (> 7 g/d), albumin infusion (10-20 g/wk), and standard diuretic therapy (20-60 mg/d furosemide and 25-50 mg/d spironolactone). Patients could drink water without restriction. The dose of TLV was increased to 7.5 mg/d if insufficient effects were seen. Because the effect of TLV is closely related to serum creatinine levels[9], in some patients with poor renal function, the TLV dose was increased to 15.0 mg/d as directed by the primary physician. TLV was discontinued if patients had encephalopathy, hematemesis, hemodialysis or side effects of > grade 3 assessed using the common terminology criteria for adverse events (CTCAE) version 4.0 or if the patients were unable to take the medication orally. If there were no improvements with TLV, the patients received large-volume paracentesis as a rescue treatment for refractory ascites.
The control patients received conventional large volume paracentesis as a treatment for refractory ascites in addition to sodium restriction (less than 7 g/d), albumin infusion (10-20 g/wk), and standard diuretic therapy (20-120 mg/d furosemide and 25-150 mg/d spironolactone). In all cases, total paracentesis was achieved by removal of all ascites by supplementing 10-20 g albumin per each liter exceeding 5 L. If ascites re-accumulated during the follow-up period, large-volume paracentesis was repeated.
Efficacy assessment
In the TLV-treated group, the primary endpoint was improvement of symptoms, such as bloating sensation or respiratory discomfort, or a > 2-kg reduction in body weight. We also assessed factors that contributed to the effectiveness of TLV by comparing the TLV-treated group with the controls. The primary endpoint was the cumulative incidence rate. In the controls, all ascites was removed transiently by large volume paracentesis. The cumulative incidence rate was defined as the necessity of an additional invasive procedure to treat refractory ascites, including large volume paracentesis, or admission for the treatment of refractory ascites. The secondary endpoint was overall survival rate.
Safety assessment
Patients were monitored throughout the study period, and any incidences of adverse events or deaths were recorded. Adverse events were evaluated using CTCAE version 4.0.
Statistical analysis
Data was expressed as medians (25-75th percentile range) or means ± SD deviations, unless otherwise indicated. Continuous variables were analyzed by unpaired t-test or paired t-test. Fisher’s exact test was applied in cases comparing two nominal variables. We applied receiver operating characteristic (ROC) curve analysis to determine the ideal cut-off levels that indicate the potency of TLV. Univariate and multivariate logistic regression analyses were used to assess the predictors for improvement of refractory ascites by TLV. The cumulative incidence rate and survival rate were estimated using the Kaplan-Meier method compared with the log-rank test. We used univariate and multivariate Cox’s proportional hazard regression analysis to elucidate the risk factors that contributed to the increased incidence of ascites. Differences with a P < 0.05 were considered statistically significant. Data processing and analysis were performed using StatView version 5 (SAS institute, Cary, NC, United States).
RESULTS
Characteristics of patients treated with TLV
Sixty patients were treated with TLV. The demographics and other baseline characteristics of these patients are shown in Table 1. The mean dosing period was 54 d, and the mean observational period was 168 d. There were 27 (45.0%) Child-Pugh class C patients and 26 (43.3%) patients who had uncontrollable liver neoplasms, defined as TNM stage 3, 4a or 4b. There were four patients with a small amount of ascites who had severe hepatic hydrothorax. The mean estimated glomerular filtration rate (eGFR) was 43.1 mL/min per 1.73 m2, which indicated the existence of moderate chronic kidney disease.
Table 1.
Baseline characteristics of the patients treated with tolvaptan and controls
| Characteristic | TLV group (n = 60) | Controls (n = 60) | P |
| Age (yr) | 67.1 ± 11.2 | 69.5 ± 9.0 | 0.21 |
| Male | 46 (76.7%) | 46 (76.7%) | 1.00 |
| Bodyweight (kg) | 61 (54-69) | 64 (55-73) | 0.58 |
| HCV antibody positive | 36 (60.0%) | 35 (58.3%) | 1.00 |
| Child-Pugh class C | 27 (45.0%) | 24 (40.0%) | 0.71 |
| Refractory ascites | 56 (93.3%) | 60 (100%) | 0.12 |
| Hepatic hydrothorax | 32 (53.3%) | 29 (48.3%) | 0.72 |
| Liver neoplasms stage 3, 4a, or 4b | 26 (43.3%) | 25 (41.7%) | 1.00 |
| Serum albumin (g/dL) | 2.8 (2.5-3.1) | 2.8 (2.5-3.1) | 0.98 |
| Total bilirubin (mg/dL) | 2.7 (0.7-3.1) | 2.8 (1.1-3.6) | 0.81 |
| ALT (IU/L) | 42 (20-44) | 36 (20-53) | 0.27 |
| Serum creatinine (mg/dL) | 1.40 (0.90-1.61) | 1.45 (0.78-1.59) | 0.30 |
| eGFR (mL/min per 1.73 m2) | 43.1 (31.0-62.7) | 49.8 (33.5-67.8) | 0.95 |
| Serum sodium (mEq/L) | 133 (130-136) | 136 (132-139) | 0.02 |
| Platelet count (× 103/μL) | 114 (58-147) | 95 (69-139) | 0.80 |
| Prothrombin activity (%) | 58.7 (45.0-70.0) | 57.3 (42.3-70.2) | 0.71 |
ALT: Alanine aminotransferase; eGFR: Estimated glomerular filtration rate; HCV: Hepatitis C virus; TLV: Tolvaptan.
Changes after administration of TLV
Body weight was significantly reduced during the treatment period. The median reduction in bodyweight was 3 kg (P < 0.01), and 38 (63.3%) patients had improved bloating sensation or respiratory discomfort or achieved a > 2-kg weight reduction (Figure 1). The serum sodium concentration increased, peaking 3 d after administration of TLV. The median elevated serum sodium concentration was 4.5 mEq/L (P < 0.01, Figure 2). The eGFR was decreased significantly after administration of TLV from 43.1 mL/min per 1.73 m2 to 38.1 mL/min per 1.73 m2 (P < 0.01, Figure 3). The minimum urine osmolality was markedly reduced 7 d after administration of TLV with a 34% reduction in the urine osmolality rate (Table 2).
Figure 1.
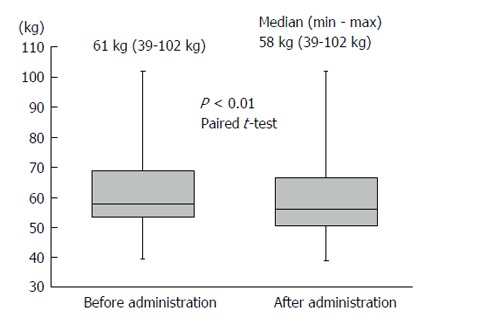
The median reduction in body weight was 3 kg (P < 0.01) during the tolvaptan treatment period.
Figure 2.
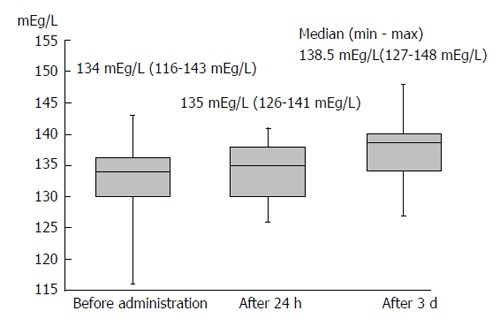
The serum sodium concentration peaked 3 d after administration of tolvaptan. The median elevated serum sodium concentration was 4.5 mEq/L.
Figure 3.
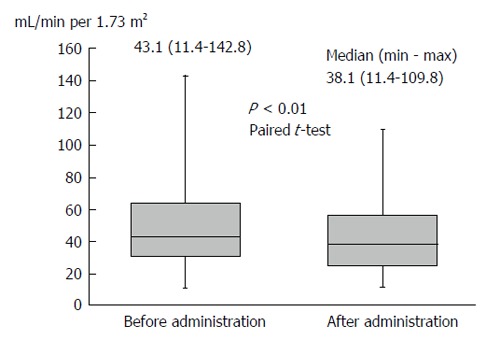
The estimated glomerular filtration rate significantly decreased after administration of tolvaptan from 43.1 to 38.1 mL/min per 1.73 m2.
Table 2.
Changes in the urine volume and osmolality after administration of tolvaptan
| TLV group (n = 60) | |
| 24 h urine volume (mL) | 1844 (1200-2400) |
| 24 h water intake (mL) | 1231 (894-1463) |
| Pre-urine osmolality (OSM) | 417 (366-487) |
| Time to achieve the minimum urine osmolality (d) | 7 (2-6) |
| The minimum urine osmolality (OSM) | 274 (230-311) |
| Pre-post-urine osmolality rate (%) | 66 (55-79) |
OSM: Osmole; TLV: Tolvaptan.
Follow-up of TLV treated patients
The median follow-up period was 168 d. During this follow-up period, 33 of 38 patients (86.8%) treated with TLV who improved then experienced exacerbated symptoms, such as bloating sensation or respiratory discomfort, or an increase in body weight, which indicates the progression of ascites. The median time to progression was 48 d. Although administration of TLV transiently improved refractory ascites, decompensated cirrhosis is a progressive disease that will eventually lead to the development of uncontrolled ascites.
TLV adverse events during the hospitalization period
Patients received TLV while hospitalized for 6-8 d. Adverse events were observed in 19 (31.7%) patients during the hospitalization period. The most common adverse event was thirst. Polydipsia was observed in 14 (23.3%) patients (CTCAE grade 1 to 2). There were no other severe side-effects higher than grade 3 as defined by CTCAE version 4 during the hospitalization period; CTCAE grade 2 tachycardia was seen in 1 patient, CTCAE grade 2 fatigue in 1 patient, CTCAE grade 2 cough in 1 patient, and CTCAE grade 2 acute kidney injury in 1 patient. One patient discontinued TLV due to the necessity for frequent blood tests. During the entire follow-up period, 8 (13.3%) patients developed hepatic encephalopathy. However, it is difficult to determine whether this was due to adverse events or the natural course of decompensated cirrhosis.
Comparison of TLV effectiveness
There were 38 patients who had improved symptoms, such as a bloating sensation or respiratory discomfort, or a loss of 2 kg body weight. There were significant differences in TLV effectiveness related to the proportion of male patients, comorbidity with hepatitis C virus (HCV), severe liver dysfunction, and uncontrolled liver neoplasms. Blood tests showed significantly higher levels of serum bilirubin and lower levels of sodium in patients in whom TLV was ineffective (Table 3).
Table 3.
Comparison of baseline characteristics (effective vs ineffective with tolvaptan)
| Characteristic | Effective (n = 38) | Ineffective (n = 22) | P |
| Dosing period (d) | 73 (12-109) | 22 (7-36) | 0.02 |
| TLV (mg/d) | 7.5 (7.5-7.5) | 7.5 (7.5-7.5) | 0.36 |
| Age (yr) | 66.7 ± 11.1 | 67.0 ± 11.4 | 0.95 |
| Male | 33 (86.8%) | 13 (59.1%) | 0.02 |
| Bodyweight (kg) | 62 (54-68) | 60 (48-71) | 0.58 |
| 1Bodyweight (kg) | 3.6 (2.1-4.7) | 0.2 (0.1-0.8) | < 0.01 |
| HCV antibody positive | 27 (71.1%) | 9 (40.9%) | 0.03 |
| Child-Pugh class C | 11 (28.9%) | 16 (72.7%) | < 0.01 |
| Refractory ascites | 35 (92.1%) | 32 (95.5%) | 1.00 |
| Hepatic hydrothorax | 21 (55.3%) | 11 (50.0%) | 0.79 |
| Liver neoplasms stage 3, 4a, or 4b | 11 (28.9%) | 15 (68.2%) | < 0.01 |
| Serum albumin (g/dL) | 2.9 (2.6-3.2) | 2.7 (2.3-3.0) | 0.20 |
| Total bilirubin (mg/dL) | 1.7 (0.7-1.9) | 4.5 (1.5-6.3) | < 0.01 |
| ALT (IU/L) | 37 (20-41) | 50 (25-77) | 0.18 |
| Serum creatinine (mg/dL) | 1.53 (0.89-2.09) | 1.17 (0.95-1.40) | 0.11 |
| eGFR (mL/min per 1.73 m2) | 49.8 (26.7-62.7) | 51.7 (34.6-62.5) | 0.80 |
| Serum sodium (mEq/L) | 134 (132-138) | 131 (128-136) | 0.03 |
| Platelet count (× 103/μL) | 107 (58-144) | 127 (65-190) | 0.27 |
| Prothrombin activity (%) | 61.5 (46.3-73.2) | 53.8 (41.2-64.2) | 0.10 |
Reduction. ALT: Alanine aminotransferase; eGFR: Estimated glomerular filtration rate; HCV: Hepatitis C virus; TLV: Tolvaptan.
Comparison of the changes between effective and ineffective patients after administration of TLV
We evaluated the changes in urine volume and osmolality between the two groups after administration of TLV (Table 4). Urine volume recorded at 24 h after administration of TLV was significantly higher in the effective group (2154 mL vs 1352 mL; P < 0.01). The minimum urine osmolality was also significantly lower in the effective group (251 osmole vs 313 osmole; P < 0.01). The time to reach minimum urine osmolality was significantly longer in the effective group (median: 7 d vs 4 d; P = 0.02).
Table 4.
Comparison of the changes after administration of tolvaptan (effective vs ineffective with tolvaptan)
| Effective (n = 38) | Ineffective (n = 22) | P | |
| 1Bodyweight | 3.6 (2.1-4.7) | 0.2 (0.1-0.8) | < 0.01 |
| 24 h urine volume (mL) | 2154 (1448-2516) | 1352 (871-1675) | < 0.01 |
| 24 h water intake (mL) | 1238 (800-1429) | 1235 (1000-1463) | 0.96 |
| Pre-urine osmolality (OSM) | 445 (411-485) | 417 (335-495) | 0.42 |
| Time to achieve the minimum urine osmolality (d) | 7 (3-9) | 4 (1-6) | 0.02 |
| The minimum urine osmolality (OSM) | 251 (213-288) | 313 (256-359) | < 0.01 |
| Pre-post-urine osmolality rate (%) | 58 (51-69) | 78 (66-90) | < 0.01 |
Reduction. OSM: Osmole.
eGFR analysis to determine the cut-off level
Figure 4A indicated that a reduction in urine osmolality over 25% was the single best cut-off level to clarify the improvement of refractory ascites with 89.5% sensitivity and 59.1% specificity. A combined measure of urine > 1800 mL within the first 24 h and a reduction in urine osmolality > 30% were the best cut-off levels to clarify refractory ascites improvements with 84.2% sensitivity and 81.8% specificity (Figure 4B).
Figure 4.
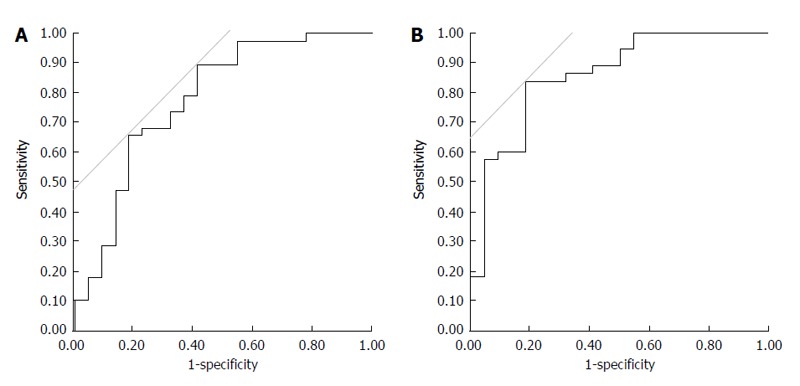
Receiver operating characteristic analysis. A: A reduction in urine osmolality > 25% was the single best cut-off level for improvement of refractory ascites with 89.5% sensitivity and 59.1% specificity; B: A combination of urine output > 1800 mL within the first 24 h and a 30% reduction in urine osmolality were the best cut-off levels for improvement of refractory ascites with 84.2% sensitivity and 81.8% specificity.
Multivariate regression analysis to elucidate the factor contributing to improved refractory ascites
Multivariate regression analysis was performed to evaluate the factors found to be significant in univariate analysis. As a result, a reduction in urine osmolality > 25% [odds ratio (OR) = 20.7; P < 0.01] and the presence of positive HCV antibodies (OR = 5.93; P = 0.05) were positively correlated with an improvement of refractory ascites, while the total bilirubin level per 1.0 mg/dL (OR = 0.57; P = 0.02) was negatively correlated with improvement (Table 5).
Table 5.
Multivariate regression analysis assessing the effectiveness of tolvaptan
| Variables | HR (95%CI) | P |
| Reduction of urine osmolality over 25% | 20.7 (3.26-132) | < 0.01 |
| Age (yr) | 1.00 (0.93-1.08) | 0.91 |
| HCV antibody positive | 5.93 (1.01-34.8) | 0.05 |
| Uncontrollable liver neoplasms | 0.68 (0.03-1.20) | 0.21 |
| Total bilirubin (per 1.0 mg/dL) | 0.57 (0.35-0.93) | 0.02 |
| Na (per 1.0 mEq/mL) | 0.99 (0.84-1.17) | 0.93 |
HCV: Hepatitis C virus.
Comparing the patients backgrounds between the TLV group and historical controls
Due to the small number of patients, their backgrounds and laboratory data, including liver function, were not matched. In comparing the TLV group and controls, only the serum sodium level was significantly lower in the TLV group (133 mEq/L vs 136 mEq/L; P = 0.02). However, there were no significant differences in the other parameters between the two groups (Table 1).
Cumulative incidence rate
The cumulative incidence rate was defined as the need for an additional invasive procedure to treat refractory ascites, including large volume paracentesis, or hospital admission for the treatment of refractory ascites. The cumulative incidence rate was significantly higher in the control group, with a median incidence time of 30 d in the TLV group and 20 d in the control group (P = 0.01, Figure 5).
Figure 5.
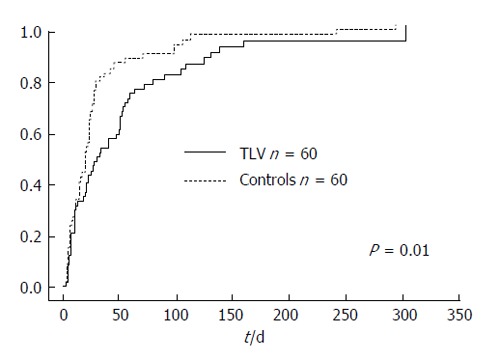
The cumulative incidence rate was significantly higher in the control group, with a median incidence time of 30 d in the tolvaptan group and 20 d in the control group.
Factors affecting the incidence of refractory ascites
We used univariate and multivariate Cox’s proportional hazard regression analysis to elucidate the risk factors predicting incidence. Cox hazard proportional multivariate analysis indicated that the use of TLV (OR = 0.58; P < 0.01), uncontrolled liver neoplasms (OR = 1.92; P < 0.01), a total bilirubin level per 1.0 mg/dL (OR = 1.10; P < 0.01), and a higher sodium level per 1.0 mEq/L (OR = 0.94; P < 0.01) were independent factors contributing to the incidence of refractory ascites (Table 6).
Table 6.
Cox’s proportional hazard multivariate regression analysis assessing the factors contributing to the incidence of refractory ascites
| Variables | HR (95%CI) | P |
| Use of TLV | 0.58 (0.39-0.87) | < 0.01 |
| Age (yr) | 1.01 (0.99-1.03) | 0.19 |
| Uncontrollable liver neoplasms | 1.92 (1.23-2.94) | < 0.01 |
| ALT (IU/L) | 1.00 (0.98-1.01) | 0.11 |
| Total bilirubin (mg/dL) | 1.10 (1.03-1.18) | < 0.01 |
| Na (mEq/mL) | 0.94 (0.91-0.98) | < 0.01 |
HCV: Hepatitis C virus; ALT: Alanine aminotransferase; TLV: Tolvaptan.
Cumulative survival rate
There was no significant difference in cumulative survival rate between the TLV group (median survival time = 121 d) and control group (median survival time = 123 d; P = 0.57, Figure 6).
Figure 6.
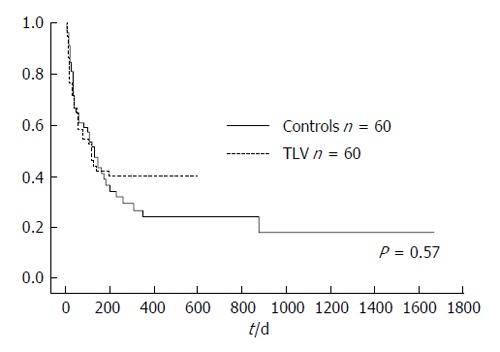
There was no significant difference in cumulative survival rate between the groups, with a median survival time of 121 d in the tolvaptan group and 123 d in the control group.
DISCUSSION
This study demonstrated that administration of TLV improved refractory ascites in 38 (63.3%) patients. Before the introduction of TLV, treatment of refractory ascites was initially based on invasive procedures, such as paracentesis, concentrated ascites reinfusion therapy, the Denver® shunt, or transjugular intrahepatic portosystemic shunt[10-12]. Although, increased doses of loop diuretic agents or spironolactone were also allowed, the effectiveness was limited and renal function deteriorated[13]. TLV is a less invasive novel oral aquaresis treatment that complements conventional refractory ascites therapies. The effectiveness of TLV is limited, however, because decompensated liver cirrhosis is a progressive disease, although compared with conventional large volume paracentesis treatment, TLV may prolong the time to disease progression. Thus, TLV may be an important therapy that transiently improves refractory ascites to avoid early invasive treatment or hospitalization.
Determining the best timing of TLV administration is difficult, since TLV was not effective in 22 cases of patients with either uncontrolled liver neoplasms or severe liver dysfunction Child-Pugh class C. In this study, we defined refractory ascites as follows: existence of ascites detected by ultrasound under the treatment of a loop diuretic at a daily dose equivalent to ≥ 40 mg/d furosemide and ≥ 25 mg/d spironolactone, a loop diuretic at a daily dose equivalent to ≥ 20 mg/d furosemide and ≥ 50 mg/d spironolactone, or a loop diuretic alone at a daily dose equivalent to ≥ 60 mg/d furosemide. If refractory ascites was not controlled with these doses of standard diuretic medicines, TLV administration should be considered. If TLV is initiated later, its effects may be restricted due to liver dysfunction or progression of liver neoplasms.
The change in urine osmolality is an important factor to consider when evaluating the effectiveness of TLV. It has been reported that a reduction in the rate of urine osmolality can predict TLV effectiveness in chronic heart failure patients[14]. In these patients, urine osmolality was measured before and 4-6 h after administration of TLV. However, in refractory ascites patients, our current study showed that the minimum urine osmolality was reached at a median of 7 d after TLV administration (Table 2), which indicated that the reduction rate in urine osmolality was a promising measure of TLV effectiveness. However, the sensitivity and specificity were more accurate using the combination of 24-h urine volume and urine osmolality reduction rate after the administration of TLV. The mechanisms underlying this difference remain unknown, although it has been reported that elevated intra-abdominal pressure due to refractory ascites might affect renal function[15]. Treating refractory ascites with TLV may lead to a gradual improvement of glomerular blood flow. Thus, the decrease in urine osmolality was slower in refractory ascites patients compared with the change in chronic heart failure patients. At any rate, the results showed that it was difficult to predict the effectiveness of TLV in a short-term study.
TLV is thought to be a relatively safe drug with little impact on renal function[9,16]. However, in our study, TLV administration deteriorated renal function. Although there is a possibility that TLV could lead to dehydration and decrease eGFR, progressive liver disease induces renal impairment, a phenomenon known as hepatorenal syndrome[17]. The control patients also had a significant decrease in eGFR during the follow-up period. These patients were treated with large-volume paracentesis under infusion of albumin, which was also reported to have less impact on renal function[18]. In a comprehensive manner, the decrease in eGFR did not depend on TLV but on progressive liver disease itself. Thus, we concluded that TLV could be used safely in patients with refractory ascites.
An elevation in the serum sodium level is a major side effect of TLV[19]. Advanced liver cirrhosis tends to result in hyponatremia, and TLV is used as a treatment option for hyponatremia in patients with euvolemic hyponatremia[20]. In this study, we did not experience any side effects of hypernatremia in patients treated with TLV. Although the median level of serum sodium was elevated to a maximum of 138.5 mEq/L, this was preferable to hyponatremia in advanced liver cirrhosis patients. Comorbidity with hyponatremia has a high risk for mortality[21]. In this current study, there were no significant differences in the cumulative survival rate between the TLV and control group. However, the patients’ backgrounds between the two groups were not matched, and it was difficult to elucidate the true effect of treating hyponatremia with TLV. A further prospective study is needed to clarify the outcome of improving hyponatremia on the cumulative survival rate.
There were no severe adverse events that exceeded CTCAE grade 2 during the hospitalization period. The most common side effect was thirst observed in 14 (23.3%) patients, which was similar to previous reports[22,23]. Hypernatremia was also reported as an adverse effect of TLV treatment[20]. However, there were no patients with a severe elevation in serum sodium. During the follow-up period after hospital discharge, hepatic encephalopathy occurred in 8 patients. It is difficult to clarify whether the cause of encephalopathy was administration of TLV or part of the natural course of severe liver dysfunction. On the whole, TLV is a safe treatment for refractory ascites patients with advanced liver cirrhosis.
This study has several limitations. It was not a randomized retrospective study, and the control group was not matched to the TLV group. However, this study was conducted in a realistic clinical setting. We propose that the clinical outcomes of TLV will have significant meaning for the treatment of refractory ascites, and the current results revealed that the best new indicators to predict efficacy of TLV were a 24-h urine volume > 1800 mL and > 30% urine osmolality reduction rate, as well as prolongation of progression-free survival. Thus, this retrospective study will serve as a reference for using TLV in refractory ascites patients.
In conclusion, administration of TLV achieved not only better control of refractory ascites but also improved QOL by avoiding additional invasive procedures, including paracentesis, or the need for hospitalization compared with conventional ascites treatments.
COMMENTS
Background
Hepatic edema and ascites are common complications in decompensated liver cirrhosis patients. In Japan, the addition of tolvaptan (TLV) to conventional diuretic therapy has been useful for the treatment of refractory ascites. The principal objective was to conduct an observational retrospective study to elucidate the safety and efficacy of long-term administration, determining the effectiveness cut-off level, and identifying factors that contribute to improved refractory ascites in a clinical setting. In addition, since decompensated cirrhosis is a progressive disease, the authors examined the time to progression by comparing TLV to conventional treatment.
Research frontiers
TLV was reported as a new oral, selective vasopressin V2 receptor antagonist originally developed for the treatment of hypervolemic or euvolemic hyponatremia in patients with heart failure, cirrhosis or syndrome of inappropriate antidiuretic hormone. A phase 3 study showed a remarkable reduction in ascites with a median loss of 2 kg body weight compared with the placebo controls. However, the administration of TLV was limited to 7 d because of the study design.
Innovations and breakthroughs
The authors concluded that administration of TLV resulted in better control of refractory ascites and reduced the incidence of additional invasive procedures or hospitalization compared with conventional ascites treatments. The authors also determined the best values for urine output and reduced urine osmolality as measures of refractory ascites.
Applications
This study may provide a new treatment option for refractory ascites. TLV could use safely in refractory ascites patients with over 60% of effectiveness. Administration of TLV may be considered before invasive procedure such as paracentesis.
Peer-review
This is an interesting topic.
Footnotes
P- Reviewer: De Ponti F, Enomoto H, He JY, Qin JM S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Ethics approval: This is a retrospective study. We have no statement about institutional review board.
Informed consent: For this type of retrospective study formal consent is not required.
Conflict-of-interest: We have no conflict of interests.
Data sharing: Technical appendix, statistical code, and dataset available from the corresponding author at following e-mail address (anb72547@nifty.com). Participants gave informed consent for data sharing.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: March 6, 2015
First decision: April 17, 2015
Article in press: June 11, 2015
References
- 1.Ginès P, Cárdenas A, Arroyo V, Rodés J. Management of cirrhosis and ascites. N Engl J Med. 2004;350:1646–1654. doi: 10.1056/NEJMra035021. [DOI] [PubMed] [Google Scholar]
- 2.Moore KP, Wong F, Gines P, Bernardi M, Ochs A, Salerno F, Angeli P, Porayko M, Moreau R, Garcia-Tsao G, et al. The management of ascites in cirrhosis: report on the consensus conference of the International Ascites Club. Hepatology. 2003;38:258–266. doi: 10.1053/jhep.2003.50315. [DOI] [PubMed] [Google Scholar]
- 3.Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651–1653. doi: 10.1002/hep.26359. [DOI] [PubMed] [Google Scholar]
- 4.Solà E, Watson H, Graupera I, Turón F, Barreto R, Rodríguez E, Pavesi M, Arroyo V, Guevara M, Ginès P. Factors related to quality of life in patients with cirrhosis and ascites: relevance of serum sodium concentration and leg edema. J Hepatol. 2012;57:1199–1206. doi: 10.1016/j.jhep.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Planas R, Montoliu S, Ballesté B, Rivera M, Miquel M, Masnou H, Galeras JA, Giménez MD, Santos J, Cirera I, et al. Natural history of patients hospitalized for management of cirrhotic ascites. Clin Gastroenterol Hepatol. 2006;4:1385–1394. doi: 10.1016/j.cgh.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Vaidya C, Ho W, Freda BJ. Management of hyponatremia: providing treatment and avoiding harm. Cleve Clin J Med. 2010;77:715–726. doi: 10.3949/ccjm.77a.08051. [DOI] [PubMed] [Google Scholar]
- 7.Orlandi C, Zimmer CA, Gheorghiade M. Tolvaptan for the treatment of hyponatremia and congestive heart failure. Future Cardiol. 2006;2:627–634. doi: 10.2217/14796678.2.6.627. [DOI] [PubMed] [Google Scholar]
- 8.Decaux G, Soupart A, Vassart G. Non-peptide arginine-vasopressin antagonists: the vaptans. Lancet. 2008;371:1624–1632. doi: 10.1016/S0140-6736(08)60695-9. [DOI] [PubMed] [Google Scholar]
- 9.Sakaida I, Kawazoe S, Kajimura K, Saito T, Okuse C, Takaguchi K, Okada M, Okita K. Tolvaptan for improvement of hepatic edema: A phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44:73–82. doi: 10.1111/hepr.12098. [DOI] [PubMed] [Google Scholar]
- 10.Sanyal AJ, Genning C, Reddy KR, Wong F, Kowdley KV, Benner K, McCashland T. The North American Study for the Treatment of Refractory Ascites. Gastroenterology. 2003;124:634–641. doi: 10.1053/gast.2003.50088. [DOI] [PubMed] [Google Scholar]
- 11.Ginès P, Arroyo V, Vargas V, Planas R, Casafont F, Panés J, Hoyos M, Viladomiu L, Rimola A, Morillas R. Paracentesis with intravenous infusion of albumin as compared with peritoneovenous shunting in cirrhosis with refractory ascites. N Engl J Med. 1991;325:829–835. doi: 10.1056/NEJM199109193251201. [DOI] [PubMed] [Google Scholar]
- 12.Zaak D, Paquet KJ, Kuhn R. Prospective study comparing human albumin vs. reinfusion of ultrafiltrate-ascitic fluid after total paracentesis in cirrhotic patients with tense ascites. Z Gastroenterol. 2001;39:5–10. doi: 10.1055/s-2001-10707. [DOI] [PubMed] [Google Scholar]
- 13.Peacock WF, Costanzo MR, De Marco T, Lopatin M, Wynne J, Mills RM, Emerman CL. Impact of intravenous loop diuretics on outcomes of patients hospitalized with acute decompensated heart failure: insights from the ADHERE registry. Cardiology. 2009;113:12–19. doi: 10.1159/000164149. [DOI] [PubMed] [Google Scholar]
- 14.Imamura T, Kinugawa K, Shiga T, Kato N, Muraoka H, Minatsuki S, Inaba T, Maki H, Hatano M, Yao A, et al. Novel criteria of urine osmolality effectively predict response to tolvaptan in decompensated heart failure patients--association between non-responders and chronic kidney disease. Circ J. 2013;77:397–404. doi: 10.1253/circj.cj-12-0971. [DOI] [PubMed] [Google Scholar]
- 15.Mullens W, Abrahams Z, Skouri HN, Francis GS, Taylor DO, Starling RC, Paganini E, Tang WH. Elevated intra-abdominal pressure in acute decompensated heart failure: a potential contributor to worsening renal function? J Am Coll Cardiol. 2008;51:300–306. doi: 10.1016/j.jacc.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Wang SZ, Zheng JF, Zhao WM, Li P, Fan CL, Li B, Dong PL, Li L, Ding HG. Clinical efficacy of tolvaptan for treatment of refractory ascites in liver cirrhosis patients. World J Gastroenterol. 2014;20:11400–11405. doi: 10.3748/wjg.v20.i32.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arroyo V, Ginès P, Gerbes AL, Dudley FJ, Gentilini P, Laffi G, Reynolds TB, Ring-Larsen H, Schölmerich J. Definition and diagnostic criteria of refractory ascites and hepatorenal syndrome in cirrhosis. International Ascites Club. Hepatology. 1996;23:164–176. doi: 10.1002/hep.510230122. [DOI] [PubMed] [Google Scholar]
- 18.Ginès P, Titó L, Arroyo V, Planas R, Panés J, Viver J, Torres M, Humbert P, Rimola A, Llach J. Randomized comparative study of therapeutic paracentesis with and without intravenous albumin in cirrhosis. Gastroenterology. 1988;94:1493–1502. doi: 10.1016/0016-5085(88)90691-9. [DOI] [PubMed] [Google Scholar]
- 19.Ambrosy A, Goldsmith SR, Gheorghiade M. Tolvaptan for the treatment of heart failure: a review of the literature. Expert Opin Pharmacother. 2011;12:961–976. doi: 10.1517/14656566.2011.567267. [DOI] [PubMed] [Google Scholar]
- 20.Schrier RW, Gross P, Gheorghiade M, Berl T, Verbalis JG, Czerwiec FS, Orlandi C. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355:2099–2112. doi: 10.1056/NEJMoa065181. [DOI] [PubMed] [Google Scholar]
- 21.Umemura T, Shibata S, Sekiguchi T, Kitabatake H, Nozawa Y, Okuhara S, Kimura T, Morita S, Komatsu M, Matsumoto A, et al. Serum sodium concentration is associated with increased risk of mortality in patients with compensated liver cirrhosis. Hepatol Res. 2014:Epub ahead of print. doi: 10.1111/hepr.12412. [DOI] [PubMed] [Google Scholar]
- 22.Sakaida I, Yamashita S, Kobayashi T, Komatsu M, Sakai T, Komorizono Y, Okada M, Okita K. Efficacy and safety of a 14-day administration of tolvaptan in the treatment of patients with ascites in hepatic oedema. J Int Med Res. 2013;41:835–847. doi: 10.1177/0300060513480089. [DOI] [PubMed] [Google Scholar]
- 23.Okita K, Kawazoe S, Hasebe C, Kajimura K, Kaneko A, Okada M, Sakaida I. Dose-finding trial of tolvaptan in liver cirrhosis patients with hepatic edema: A randomized, double-blind, placebo-controlled trial. Hepatol Res. 2014;44:83–91. doi: 10.1111/hepr.12099. [DOI] [PubMed] [Google Scholar]


