Abstract
Attention deficit hyperactivity disorder (ADHD) is associated with decreased ventral-striatal responsiveness during reward anticipation. However, previous research mostly focused on adults with heterogeneous ADHD subtype and divers drug treatment status while studies in children with ADHD are sparse. Moreover, it remains unclear to what degree ADHD is characterized by a delay of normal brain structure or function maturation. We therefore attempt to determine whether results from structural and functional magnetic resonance imaging (fMRI) are associated with childhood and adult ADHD combined subtype (ADHD-CT). This study used fMRI to compare VS structure and function of 30 participants with ADHD-CT (16 adults, 14 children) and 30 controls (20 adults, 10 children), using a monetary incentive delay task. Joint analyses of structural and functional imaging data were conducted with Biological Parametric Mapping. Reward anticipation elicited decreased ventral-striatal responsiveness in adults but not in children with ADHD-CT. Children and adults with ADHD showed reduced ventral-striatal volume. Taking these gray matter differences into account, the results remained the same. These results suggest that decreased ventral-striatal responsiveness during reward anticipation is present in adults but not in children with ADHD-CT, irrespective of structural characteristics. The question arises whether ventral-striatal hypoactivity is an ADHD correlate that develops during the course of illness.
Keywords: reward anticipation, ADHD, ventral striatum
INTRODUCTION
Attention deficit hyperactivity disorder (ADHD) is a heterogeneous childhood onset, neurodevelopmental disorder that affects up to 5.3% of children and 4.7% of adults worldwide (Biederman and Faraone, 2005; Polanczyk et al., 2007; Döpfner et al., 2008; Huss et al., 2008a,b). A hallmark characteristic of ADHD is the diminished ability to tolerate delayed reward. For example, compared with healthy controls, children, adolescents and adults with ADHD show altered responses to reinforcement by having very strong preferences for immediate small rewards rather than larger delayed rewards (Sonuga-Barke et al., 2008; Marx et al., 2010; Scheres et al., 2010). They require higher reinforcers to modify their behavior and learn faster when behavior is reinforced directly (Solanto et al., 2001; Bitsakou et al., 2009; Marco et al., 2009). This contributes to impulsive behavior and serious motivational and learning difficulties that negatively affect occupational performance. Understanding the factors that mediate altered reward processing may be essential for the prevention of symptom onset and treatment of its effects on learning, motivation, and overall functioning.
At the neural level, the mesolimbic dopaminergic system plays a central role in reward processing (e.g. Schultz et al., 1997; Haber and Knutson et al., 2010). Key components of this network are the ventral striatum (VS), the ventral pallidum, the anterior cingulate cortex, the orbitofrontal cortex and the dopaminergic midbrain. The dual-pathway model of ADHD by Sonuga-Barke (2003) assumes an underlying hypofunctioning of the mesolimbic reward circuitry that contributes to the pathophysiology of ADHD. Functional neuroimaging findings reinforce this assumption and describe the involvement of developmentally abnormal brain networks related to reward processing and delay aversion (Dickstein et al., 2006; Shaw and Rabin, 2009; Nakao et al., 2011). However, other studies did not find a relation between delay aversion and ADHD (Solanto et al., 2007; Sjöwall et al., 2013) and therefore multiple pathways are likely. Next to the reward pathway, the dual-pathway model includes an executive pathway assuming executive deficits, such as inhibition and working memory, in a subgroup of patients with ADHD (Sonuga-Barke, 2003). It has to be noted that delay aversion is an important characteristic of some, but not all, patients with ADHD. Because the executive pathway is very well explored, this study focuses on the reward pathway.
Neurobiological correlates of reward processing can be investigated with the MID task in combination with functional magnetic resonance imaging (fMRI) (Knutson et al., 2001; Ströhle et al., 2008). Alterations of brain activity during reward anticipation are supposed to be associated with abnormalities within the dopaminergic reward system. Furthermore, previous studies suggest ventral-striatal hyporesponsiveness during reward anticipation in adolescents (Scheres et al., 2007) and adults with ADHD (Ströhle et al., 2008; Plichta et al., 2009; Hoogman et al., 2011; Carmona et al., 2012) that increases with the severity of hyperactivity and impulsivity. However, other studies did not replicate ventral-striatal-hypoactivity in ADHD adults (Stoy et al., 2011) and adolescents (Paloyelis et al., 2012). The latter group used a variant of the MID task, which may have contributed to these inconsistent results. Moreover, the inclusion of mixed ADHD subtypes and medication status make direct comparisons difficult. Only one study investigated ventral-striatal activation in homogeneous samples of drug-naïve adults with ADHD using the MID task (Edel et al., 2013). Interestingly, the group with predominantly inattentive ADHD, and not the group with ADHD combined subtype (ADHD-CT), exhibited ventral-striatal hypoactivation. Moreover, a recent study in a population-based sample of adolescent boys revealed that the association between ADHD symptoms and ventral-striatal activity varied by monoamine oxidase A (MAOA) genotype. Only in participants with lower MAOA levels, ADHD symptoms were associated with ventral-striatal hypoactivation, whereas in participants with higher levels of MAOA, ADHD symptoms were associated with increased ventral-striatal activation during the MID task (Nymberg et al., 2013). To date, it remains unclear, how these contrasting findings can be integrated and therefore further research on homogeneous samples is necessary.
Although ADHD is defined as a childhood onset disorder, it remains unclear whether alterations in the processing of reward anticipation are present in children with ADHD. Given the importance of brain development prior to adulthood, the exploration of reward anticipation in children is crucial to identify and analyze critical periods during early development when mesolimbic dysfunction might create a predisposition to neurodevelopmental disorders such as ADHD. To date, no study has examined processing of reward anticipation in children with ADHD with the MID task. The examination of children with ADHD during reward anticipation may help to determine whether ventral-striatal hypoactivity during reward anticipation is present at disease onset or if it is a correlate that appears later in the course of ADHD.
Brain function in patients with ADHD may be partly affected by altered brain maturation processes. Lesion studies as well as MRI-studies suggest that brain structure is at least partly related to brain functioning. For example, there is strong evidence that hippocampal-formation size is positively associated with memory (Visser et al., 1999; Kaup et al., 2011). There is also evidence that the strength of blood oxygenation level-dependent (BOLD) activation is related to structural characteristics such as brain volume (e.g. Venkatraman et al., 2010). Moreover, developmental and age-related structural changes (such as pruning, synaptic formation and myelinization in children) are likely to be correlated to functional changes. Further, altered cortical development is likely to lead to changes in the configurations of brain networks (cerebral plasticity). It has to be noted, that developmental cortical malformations may provoke a functional reorganization through alternative anatomical pathways, which may result in restoration or compensation of functions (Wieshmann et al., 2001; Rykhlevskaia et al., 2008). To date, the exact interplay between brain structure and function is far from known and therefore it is important to analyze possible connections. Very little is known about how structural and functional abnormalities are related in ADHD. In structural MRI, children with ADHD have shown consistent abnormalities in late developing frontostriatal networks. These networks mediate reward processing and associated cognitive functions (delay discounting, motivation, inhibition) that are impaired in ADHD. Longitudinal MRI studies demonstrated that structural discrepancies in frontal, striatal, parietal and cerebellar regions of children with ADHD, may be due to a delay in structural maturation (Castellanos et al., 2002; Shaw et al., 2007). A meta-analytic approach revealed that these changes seem to diminish from childhood to adulthood (Frodl and Skokauskas, 2012). There is evidence that children with ADHD, whose developmental trajectory of cortical thickness is more similar to that of typically developing children, have better clinical outcomes than children with persistent thickness reductions (Shaw et al., 2006). Based on these findings, we also performed a new statistical approach using a local voxel-wise correction for grey matter (GM) alterations as additional information. To the best of our knowledge, there is no imaging study in ADHD patients using this additional information of potentially altered brain structure as a voxel-wise covariate, although this most likely interferes with brain function.
The aim of this study is to determine whether results from structural and fMRI are associated with ADHD-CT in drug-naïve children and unmedicated adults. Drawing on findings obtained by previous neuroimaging studies, we hypothesized (i) decreased ventral-striatal GM volume in children but not in adults with ADHD-CT, and (ii) decreased ventral-striatal brain response during reward anticipation in children and adults with ADHD-CT. Moreover, we will compare standard functional imaging analyses (to ensure methodological consistency with previous studies) with a new statistical approach using a local voxel-wise correction for GM alterations as additional information.
MATERIALS AND METHODS
Participants
Twenty unmedicated adults with ADHD-CT (and with the same confirmed childhood diagnosis), 16 drug-naïve children with ADHD-CT and 30 healthy controls (CON, 20 adults, 10 children) participated in the study. Adults with ADHD-CT were recruited via a longitudinal sample of former patients with childhood ADHD-CT (Huss et al., 2008a,b). Children with ADHD-CT were recruited via our inpatient and outpatient unit. Current ADHD-CT was diagnosed according to DSM-IV criteria (Diagnostic and Statistic Manual of Mental Disorders, American Psychiatric Association, 2000) by clinical experts using the ADHD-Diagnostic Checklist (Rösler et al., 2004). In adults, ‘childhood’ ADHD-CT was diagnosed as part of a longitudinal study according to DSM-III-R or DSM-IV diagnostic criteria by experienced psychiatrists at our clinic. To exclude other Axis I and Axis II psychiatric disorders in patients and to ensure mental health in controls, a standardized diagnostic assessment was conducted before to the MRI data acquisition. The assessment in adults included the German version of the structured clinical interview for DSM-IV diagnoses (SCID I & II, Wittchen and Zaudig, 1997). Due to its high comorbidity rates and its relevant association with reward processing (Beck et al., 2009), addiction was examined via the Composite International Diagnostic Interview, module addiction (CIDI/DIA-X, Wittchen and Pfister, 1997). None of the adults with ADHD-CT fulfilled lifetime or current criteria for drug addiction (excluding nicotine), two fulfilled the criteria for alcohol abuse during the past 12 months, and one fulfilled the criteria of multiple drug use abuse >12 months ago. Psychiatric examination in children included the diagnostic interview Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime Version (Kiddie-SADS-PL, Kaufmann et al., 1997; German translation: Delmo et al., 2001). The severity of ADHD was assessed using the German version of the Conners’ adult ADHD rating scale (CAARS, Connors et al., 1999) in adults, and the Attention-Deficit/Hyperactivity Disorder Rating Scale-IV-Parent Version in children (DuPaul et al., 1998). The socioeconomic status was measured with the Hollingshead Index of Social Position (Hollingshead, 1975). IQ was assessed using the short form of the Culture Fair Intelligence Test (CFT-20-R, Weiß, 2006). Handedness was examined with the Edinburgh Handedness Inventory (Oldfield, 1971).
Due to technical problems or excessive head motion (translation larger than 3 mm and/or rotation larger than 2° in any direction), respectively, four ADHD adults and two ADHD children had to be excluded from further analyses. Thus, data of 16 young adults with ADHD-CT (1 female) between 19 and 31 years of age (M = 23.5, s.d. = 4.1), and 14 drug-naïve children with ADHD-CT (4 female) between 8 and 12 years of age (M = 9.8, s.d. = 1.3) were finally analyzed. Because distribution of males and females was skewed, we reanalyzed the sample with only male participants. All subanalyses with only male participants revealed comparable results. Of the final sample, 13 ADHD adults had been treated with methylphenidate (MPH) in childhood, while 3 were drug-naïve. Adults with ADHD were free of medication for at least 2 weeks before imaging procedures.
Control participants were recruited from the local community through advertisements and (i) were right handed, (ii) had no psychiatric diagnosis according to ICD-10 or Axis I and II of the DSM-IV, (iii) had no history of dependence on illicit drugs and alcohol, (iv) had no first-degree relatives with a neurological or psychiatric disorder, (v) were currently not taking any psychotropic medication and (vi) had no sensory-motor deficits or other neurological disorders.
Behavioral and clinical data were analyzed with IBM SPSS 21 (Statistical Package for the Social Sciences, Stanford, USA) and reported at P < 0.05 using two-sample t-tests with exceptions for group differences in self-reported motivation (1 × 3 analysis of variance, ANOVA), Hollingshead Index of Social Position (Mann-Whitney-U-test) and gender (χ2-test). Demographic characteristics are presented in Table 1.
Table 1.
Sample characteristics
| CON adults (n = 20) |
ADHD adults (n = 16) |
P-value | CON children (n = 10) |
ADHD children (n = 14) |
P-value | Adults vs Childrend | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | s.d. | M | s.d. | M | s.d. | M | s.d. | ||||
| Agea | 23.7 | 3.4 | 23.5 | 4.1 | t(34) = 0.16, P = 0.87 | 11.0 | 1.3 | 9.8 | 1.3 | t(22) = 2.30, P = 0.03 | F = 269.3, P < 0.001 |
| Gender (male/female)b | 20/0 | 15/1 | χ2(1) = 1.29, P = 0.26 | 8/2 | 10/4 | χ2(1) = 0.23, P = 0.63 | χ2(1) = 6.9, P = 0.013 | ||||
| SESc | 5.55 | 1.38 | 4.41 | 0.92 | P = 0.02 | 5.85 | 0.74 | 4.50 | 1.69 | P = 0.04 | F = 0.34, P = 0.564 |
| Education in yearsa | 12.1 | 1.41 | 9.69 | 1.89 | t(34) = .44, P < 0.001 | 5.05 | 1.32 | 3.78 | 1.33 | t(22) = 2.30, P = 0.03 | F = 225.0, P < 0.001 |
| CFT-IQa | 108.45 | 11.3 | 97.8 | 12.9 | t(34) = 2.62, P = 0.01 | 111.9 | 16.2 | 104.6 | 15.5 | t(22) = 1.11, P = 0.28 | F = 2.0, P = 0.164 |
| Edinburgh Handedness Inventorya | 95.84 | 8.73 | 71.29 | 67.17 | t(15.4) = 1.45, P = 0.17 | 75.79 | 47.28 | 90.53 | 26.28 | t(22) = −0.98, P = 0.34 | F = 50.3, P < 0.001 |
| ADHD total scorea | 9.00 | 5.24 | 23.56 | 9.98 | t(21.5) = −5.28, P < 0.001 | 8.40 | 2.80 | 40.21 | 5.83 | t(19.8) = −17.75, P < 0.001 | n.a. |
| ADHD Inattentiona | 4.55 | 3.65 | 12.56 | 6.20 | t(21.2) = −5.18, P < 0.001 | 5.20 | 3.425 | 23.00 | 4.37 | t(22) = −10.73, P < 0.001 | n.a. |
| ADHD Hyperactivity/Impulsivitya | 4.45 | 2.35 | 11.00 | 4.60 | t(21.2) = −5.18, P < 0.001 | 3.00 | 2.98 | 21.36 | 8.87 | t(22) = −6.26, P < 0.001 | n.a. |
| ADHD severityd | 0 | 0 | 14.44 | 1.93 | t(15) = −29.91, P < 0.001 | 0 | 0 | 13.36 | 0.84 | t(13) = −59.36, P < 0.001 | F = 3.53, P = 0.065 |
| Cigarettes per dayb | 1.9 (n = 6) | 3.8 | 6.3 (n = 8) | 7.8 | χ2(11) = 11.3, P = 0.42 | 0 | 0 | 0 | 0 | n.a. | |
| Alcohol grams per montha | 304.3 | 284.2 | 310.2 | 400.9 | t(34) = −0.05, P = 0.96 | 0 | 0 | 0 | 0 | n.a. | |
ADHD, attention deficit hyperactivity disorder; ADHD scores in adults are determined via the Conner’s adult ADHD Rating Scale, ADHD scores in children are determined via the ADHD Rating Scale; ADHD severity is determined via symptom counts of DSM-IV based interviews (in children via the Kiddie-Sads-Present and Lifetime Version, in adults via the ADHD-Diagnostic Checklist), CFT, Culture Fair Intelligence Test, part 1, minimal time; CON, healthy controls; SES, Socioeconomic status using Hollingshead Index of Social Position.
aStudent’s t-test.
bχ2-test.
cMann-Whitney-U-test.
d2 × 2 ANOVA.
Approval for the study was obtained from the responsible Ethics Committee of the Charité-Universitätsmedizin Berlin and the German Psychological Society, and informed written consent was obtained from all participants and, in the case of children, from a legal guardian.
Functional magnetic resonance imaging
MID
Adults completed a monetary incentive delay (MID) task according to Knutson et al. (2001), while children completed a child-friendly version (CID, Kappel et al., 2013). The child-friendly version was chosen to provide a less abstract feedback and to assure a clear and prompt comprehension even for younger children. Therefore, the original MID was modified by inserting a feedback phase that used points (instead of numbers) as a rewarding stimulus. Apart from the modified feedback condition, MID and CID did not differ and were conducted in the exact same way. Both tasks are event-related fMRI designs that consist of two sessions of 72 trials, yielding a total of 144 trials per task with the same delay intervals. Each run lasted about 11 min. Before entering the scanner, all participants completed a short practice version to minimize later learning effects and to ensure that they had completely understood the task. After scanning, participants retrospectively rated their own exertion in response to each of the three cues on a visual analog scale (VAS effort). Adults and children received monetary rewards. Although adults were paid directly, children received a voucher for a toy store. Details and evidence for the validity of the CID task are presented elsewhere (Kappel et al., 2013).
MRI data acquisition and analysis
Scanning was conducted on a 3T GE Signa Scanner with an eight channel head coil.
Data processing and analysis were performed with the Statistical Parametric Mapping 8 (SPM8) software package (http://www.fil.ion.ucl.ac.uk/spm/; Wellcome Department of Imaging Neuroscience, London, Great Britain), the ArtRepair software (http://cibsr.stanford.edu/tools/human-brain-project/artrepair-software.html, Stanford, CA, Mazaika et al., 2005), the voxel-based morphometry toolbox for SPM 8 (VBM8) developed by Gaser and colleagues (http://dbm.neuro.uni-jena.de/vbm8/), and the Biological Parametric Mapping (BPM) toolbox (http://fmri.wfubmc.edu/cms/software, Casanova et al., 2007). Corresponding brain regions were identified with reference to the Anatomy Toolbox for SPM (version 1.8, http://www.fz-juelich.de/inm/inm-1/DE/Forschung/_docs/SPMAnatomyToolbox/SPMAnatomyToolbox_node.html, Jülich, Germany, Eickhoff et al., 2007).
Voxel-based morphometry was conducted using standard procedures as implemented in the VBM8 toolbox. For investigating the children’s data, the low-dimensional SPM8 normalization approach combined with customized Tissue Probability Maps from the Template-O-Matic toolbox (Wilke et al., 2008) (http://dbm.neuro.uni-jena.de/software/tom/, Jena, Germany) was used. Statistical analysis for the smoothed GM segments was carried out by means of whole brain comparison of GM volume between ADHD and CON groups. Age and sex were entered as covariates of no interest. To correct for multiple comparisons Monte Carlo simulation based cluster size correction was applied (as provided in REST toolbox, Song et al., 2011). Thousand Monte Carlo simulations revealed an alpha error probability of P < 0.05, when using a minimum clustersize of 75 voxels (adults) or 78 voxels (children) with a significance level of P < 0.001. For further quantification, each subject’s ventral-striatal mean GM volumes were extracted in terms of the first eigenvariate using a priori regions of interest (ROI). The ROI was created by combining anatomical hypotheses with functional findings as reported in the literature of comparable experimental designs for the left and right ventral striatum. The resulting mean GM values (extracted mean ROI GM) were used for further statistical analysis (two-sample t-test) in SPSS 21 to compare ventral-striatal mean GM volumes in children (ADHD, CON) and adults (ADHD, CON).
BOLD fMRI: On the first level, the three different cue conditions (anticipation of gain, anticipation of loss and anticipation of neutral outcome), and five feedback conditions (successful gain, non-successful gain, successful loss avoidance, non-successful loss avoidance and neutral outcome) were modeled as events of interest. Movement parameters and the target were modeled as events of no interest and convolved with the canonical hemodynamic response function. On the second level, one-sample t-tests were performed to determine activations within groups using the individual contrast images ‘anticipation of gain > anticipation of neutral outcome’. Two-sample t-tests were then used to compare these contrasts between CON and ADHD groups. Age and IQ were entered as covariates of no interest.
A Monte Carlo simulation based cluster size correction was used for correction of multiple comparisons (Song et al., 2011). Therefore, 1000 Monte Carlo simulations were computed and revealed a minimum cluster size of 25 voxels for children and adults with a statistical threshold of P < 0.005 to correct with an alpha error probability of P < 0.05. Only results were reported surviving this multiple comparison corrected threshold. Due to a priori hypothesis of ventral-striatal activation, we additionally performed a small volume correction (SVC) with the same literature based ROI as mentioned for VBM mean value extraction analysis. SPM’s SVC was performed with a significance level set at P < 0.05 (family-wise error corrected) for the contrast images ‘anticipation of gain > anticipation of neutral outcome’.
In order to explore ventral-striatal activation while controlling for the influence of local GM volume differences, we used the BPM toolbox (Casanova et al., 2007) in which the voxel-wise GM volume maps of each subject were used as a covariate. The resulting maps were thresholded to 10 voxels and P < 0.05 for ROI-based analyses and for whole brain analyses Monte Carlo-based cluster size correction was conducted as described in the fMRI-section earlier. For detailed information on data acquisition and analysis please view Supplement 1.
RESULTS
Behavioral data
(i) Adult sample: reaction times were analyzed using a three-factorial ANOVA for repeated measures with the between subject factor group (CON vs ADHD) and the within subject factor condition (gain, loss, neutral). ANOVA revealed a significant main effect of condition [F(1, 34) = 7.52; P = 0.009]. There was no significant main effect of group [F(1, 34) = 0.82; P = 0.37] and no significant interaction [F(1, 34) = 0.31; P = 0.59] between the factors group and condition. Post hoc within group paired t-tests revealed faster responses during gain and loss trials compared with neutral trials in the ADHD group, and a trend in CON (Supplement 2).
(ii) Children sample: The same 2 × 3 ANOVA as conducted for the adult sample revealed a significant main effect of condition [F(1.5, 22) = 14.45; P < 0.001]. There was no significant main effect of group [F(1, 22) = 0.93; P = 0.35] and no significant interaction [F(1.5, 22) = 1.06; P = 0.34] between the factors group and condition. Post hoc within group paired t-tests revealed faster responses during gain and loss trials compared with neutral trials in the ADHD and CON groups (Supplement 2).
Neuroimaging results
Voxel-based morphometry
(i) Adult sample: the two-sample t-test, with the extracted mean GM volume per participant within the ventral-striatal ROIs, revealed significantly decreased volumes in the ADHD group compared with the CON group in the bilateral VS (left T = 5.69, P < 0.001; right T = 4.07, P < 0.001). Exploratory whole brain analyses (AlphaSim correction P < 0.05) revealed significantly less GM volume in ADHD in the right supramarginal gyrus, right precuneus, right hippocampus, left orbital frontal gyrus and left rectal gyrus. No significant differences appeared for ADHD > CON.
(ii) Children sample: the two-sample t-test for the extracted ROI data revealed significantly decreased volumes in the ADHD group compared with the CON group in the left ventral striatum (T = 4.77, P < 0.001) but not in the right VS (T = 1.72, P = 0.10). Exploratory whole brain analyses (AlphaSim correction P < 0.05) revealed significantly less GM volume in ADHD in the right superior temporal gyrus, right heschls gyrus and right rolandic operculum and significantly increased GM volume in the ADHD group compared with CON in the left paracentral lobule, bilateral middle orbital gyrus, right fusiform gyrus and left rectal gyrus (Supplement 3).
Neural activity between groups during gain anticipation
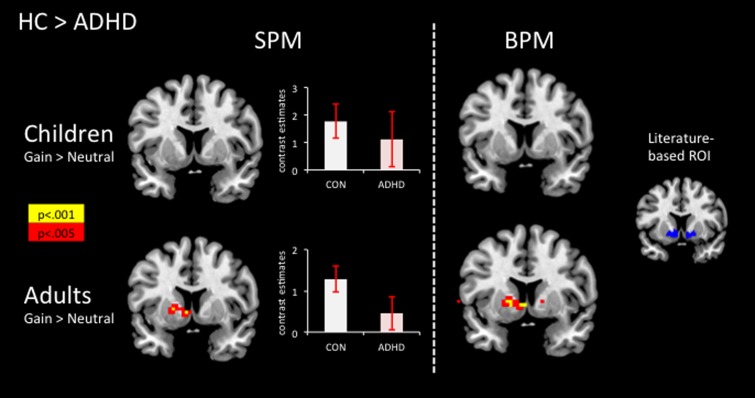
(i) Adult sample: the two-sample t-test revealed significant hypoactivity in the ADHD group compared with the CON group in the left VS (SVC analysis FWE-corrected T = 3.02, P = 0.047, x = −5, y = 7, z = −4) but not in the right ventral striatum (T = 2.40, P = 0.14; Figure 1). Exploratory whole brain analyses (AlphaSim correction P < 0.05) revealed significant hypoactivation in the ADHD group compared with the CON group in the left caudate head and putamen and significant hyperactivation in the right superior medial and frontal gyrus, right middle and superior temporal gyrus, right insula and right precuneus and left superior medial gyrus and left middle frontal gyrus (Supplement 4).
Fig. 1.
SPM (left panels): Increase in ventral-striatal activation during gain anticipation in healthy adults compared with adults with ADHD, but not in children comparing CON > ADHD; displayed at MNI coordinate y = 7. Contrast estimates (mean ± SEM) during gain anticipation for the left ventral striatum in children (MNI coordinate: x = −12, y = 6.8, z = −10.4) and adults (MNI coordinate: −16.3, 10.1, −7.1). BPM (middle panels): Increase in ventral-striatal activation during gain anticipation in healthy adults compared with adults with ADHD, but not in children comparing CON > ADHD as revealed by BPM analysis; displayed at MNI coordinate y = 7. Literature-based ROI (right panel): a priori-defined, literature-based probabilistic ROI, volumes: left ventral striatum 1130 mm3 (center of gravity: x = −13, y = 9, z = −8), right ventral striatum 1133 mm3 (center of gravity: x = 14, y = 11, z = −9). The overlays were mapped on the standard MNI template as provided by MRIcroN.
(ii) Children sample: during gain anticipation, the two-sample t-test revealed no significant differences in ventral-striatal activation between ADHD and CON (SVC analysis FWE-corrected P > 0.05; Figure 1). Exploratory whole brain analyses (AlphaSim correction P < 0.05) revealed significant hypoactivation in ADHD compared with CON in the left inferior parietal lobule, left angular gyrus and left occipital gyrus. No significant differences appeared for ADHD > CON (Supplement 4).
Biological parametric mapping
Ventral-striatal activity during reward anticipation with voxel-wise gray matter covariation
(i) Adult sample: between-group analysis revealed the following findings: during gain anticipation, the two-sample t-test revealed significant hypoactivity in the ADHD group compared with the CON group in the left VS (peak coordinate in Montreal Neurological Institute (MNI) space: −5, 7, −4; T = 3.27, P = 0.04 SVC FWE corrected) but not in the right VS (T = 2.41, P = 0.35; Figure 1). Exploratory whole brain analyses (AlphaSim correction P < 0.05) revealed significant hypoactivation in ADHD compared with CON in the left caudate head and pallidum, left precuneus, left middle temporal gyrus, and left superior occipital gyrus and significant hyperactivation in the left superior medial gyrus, left middle temporal gyrus, left cingulate gyrus, bilateral middle temporal gyrus, bilateral superior temporal gyrus, left supramarginal gyrus, right medial and superior frontal gyrus, right insula and right middle frontal gyrus (Supplement 5).
(ii) Children sample: during gain anticipation, the two-sample t-test revealed no significant differences in ventral-striatal activation between ADHD and CON (SVC analysis FWE P > 0.05; Figure 1). Exploratory whole brain analyses (AlphaSim correction P < 0.05) revealed no significant differences between CON and ADHD (Supplement 5).
DISCUSSION
The findings suggest that decreased ventral-striatal responsiveness during reward anticipation processing is present in unmedicated adults but not in drug-naïve children with ADHD-CT. Ventral-striatal responsiveness seems to be independent of morphometric ventral-striatal differences because results remained stable after controlling for ventral-striatal GM differences in children and adults with ADHD-CT compared with healthy controls.
The findings in adults are in line with previous studies showing decreased ventral-striatal responsiveness in adolescents and adults with ADHD (Scheres et al., 2007; Ströhle et al., 2008; Plichta et al., 2009; Hoogman et al., 2011; Carmona et al., 2012). Our data add several aspects to the existing literature. Previous studies explored heterogeneous samples with different ADHD subtypes and medication history so that direct comparisons and interpretation were difficult. Only one study explored reward anticipation in drug-naïve homogeneous ADHD samples (Edel et al., 2013) demonstrating ventral-striatal hypoactivity in patients with ADHD predominantly inattentive subtype, whereas the ADHD-CT group did not differ from healthy controls. Our results contradict this. This difference may be attributable to different sample characteristics, such as symptom and medication history. Although Edel et al. (2013) recruited adult outpatients, we explored former ADHD patients of our clinic with both confirmed childhood diagnosis and present diagnosis of ADHD-CT. Therefore, symptom load and duration of illness may differ between study samples and this may have impacted results. Further, Edel et al. (2013) examined drug-naïve adult patients, while most of our ADHD-CT adults had been treated with MPH in childhood. MPH blocks the reuptake of dopamine in the striatum (Volkow et al., 2001) and may enhance the saliency of rewarding stimuli (Volkow et al., 2004). Although adults of our sample were unmedicated for at least 2 weeks before the MRI examination, it may still be possible that medication during childhood influenced ventral-striatal reward responsiveness in adulthood. (Shiels et al., 2009; Volkow et al., 2012). To date, the long-term effects of MPH on reward responsiveness, even after the medication phase, remain unclear and prospective studies on long-term effects in subtype homogeneous ADHD groups are needed (Stoy et al., 2011).
To our knowledge, this is the first study examining ventral-striatal responsiveness during reward anticipation in drug-naïve children with ADHD-CT via the MID task. In contrast to our hypotheses, children with ADHD-CT did not show ventral-striatal hypoactivity during reward anticipation, which is consistent with behavioral results indicating no differences in reaction times between patients and healthy controls (while within groups, children with ADHD and healthy children reacted faster during gain and loss trials compared with neutral trails). Therefore, children and adults with ADHD seem to differ with respect to reward anticipation processing and the question arises whether ventral-striatal hypoactivity is an ADHD correlate that develops during the course of illness. Structural and functional brain imaging studies aim to establish how much ADHD interferes with normal trajectories of brain changes. During normal development, child and adult brains differ highly regarding volumetric and neurotransmitter development; therefore, different brain structures may mediate reward processing in children compared with adults. A recent review supports this assumption. Although reward-based learning impairments in children seem to be due to premature prefrontal structures involved in executive control, impairments in adults seem to be associated with deficits in the integration of reward information. These deficits may reflect reduced dopaminergic input from the midbrain via the ventral striatum to the ventromedial prefrontal cortex (Hämmerer and Eppinger, 2012). Furthermore, the hormonal changes that occur during puberty may play an important role in the development of the ventral striatum. Stress during puberty and adolescence may also affect brain development and vulnerability to psychopathologies. In ADHD, the manifold developmental changes during adolescence might interact with the disease not only in the domain of reward processing but also in various other disease and non-disease-related domains. Thus, more research, especially with a longitudinal sample, is needed.
Our morphometric analyses revealed ventral-striatal GM reductions in both children and adults with ADHD. This is only partly in line with previous studies. Whereas ventral-striatal GM reduction seems to be a typical correlate in children with ADHD, this has not been shown in adults with ADHD (Frodl and Skokauskas, 2012). Hence, in contrast to previous studies, we followed a hypothesis driven ROI based approach and focused only on the VS. The functional BPM analysis (which takes individual differences in brain volumes into account in a voxel-by-voxel manner) revealed a decreased ventral-striatal BOLD response during reward anticipation processing in adults but not in children with ADHD combined subtype compared with healthy controls. To the best of our knowledge, this is the first study examining the impact of structural ventral-striatal alterations on functional ventral-striatal brain response in children and adults with ADHD. We consider this as highly relevant because Shaw et al. (2007) reported delayed brain maturation in a large sample of children with ADHD compared with healthy controls. Therefore, differences in brain response might partly be mediated by structural discrepancies between ADHD patients and healthy controls. Our results strengthen the association of adult ADHD with ventral-striatal hypoactivity during reward anticipation. However, the influence of structural changes on functional activity is far from clear. Therefore, longitudinal studies with larger samples should clarify the association between ventral-striatal structure and functioning in children with ADHD.
Some limitations of the study need to be addressed. First, the relatively small sample sizes, especially of the children groups, may have biased results possibly due to large variance. To address this, we reported only whole brain results that survived clustersize based multiple comparison correction. Second, results may potentially be skewed when comparing ADHD-CT adults on medication washout vs ADHD-CT drug-naïve children. Although acute MPH doses normalized frontostriatal activation in children with ADHD (Rubia et al., 2009), it remains unclear whether MPH influences brain functioning, even after medication washout. Although a comparison between drug-naïve and MPH-treated adults with ADHD revealed no differences in their ventral-striatal activations (Stoy et al., 2011), more studies are needed to clarify this. Third, children in the control group were slightly older than the ADHD group; therefore, we included age as a covariate of no interest. Fourth, the MID tasks differed in children and adults and it cannot be completely ruled out that this influenced the results of this study. However, we previously conducted a study to examine the validity and comparability of both MID tasks in healthy adults. Both, the child friendly and the adult version induced comparable ventral-striatal responses (Kappel et al., 2013). Thus, we expect the influence of task differences to be minimal. Fifth, the structural whole brain analyses of the ventral striatum did not reveal volume differences between patients and controls. However, changes in smaller regions like the ventral striatum are more difficult to detect when large cluster threshold corrections for the whole brain are used. Based on a meta-analysis, VBM studies did not provide evidence for changes in the striatum, whereas manual tracing studies found reduced striatal volumes in patients with ADHD (Frodl and Skokauskas, 2012). We therefore used a ROI approach to detect ventral-striatal volume differences between patients and controls. Longitudinal research in larger samples is required to look more closely at the development of the ventral striatum with regard to ADHD symptomatology. Sixth, we abstained from direct comparisons of children and adult brain data because their brains differ in many structural aspects due to pervasive morphological changes that occur during normal development. Developmental imaging studies show cortical and subcortical gray matter decreases during childhood and adolescence. Importantly, our ROI, the VS, is not fully developed in childhood and shows distinct maturational changes into adulthood (e.g. Wierenga et al., 2014). Moreover, the VS is a very small structure and it is therefore very important to refer preprocessing to an age-appropriate template.
In summary, we observed decreased ventral-striatal brain response during reward anticipation in unmedicated adults but not in drug-naïve children with ADHD-CT in comparison to healthy controls. This association was still present after controlling for ventral-striatal GM differences. Decreased brain response during reward anticipation may thus be perceived as an ADHD correlate that develops during the course of illness. To date, there are very mixed findings regarding reward processing in patients with ADHD. Studies with larger sample sizes are essential to verify our results. Next to reward processing, future studies should explore executive functioning and consider other factors that might discriminate between subgroups of patients with ADHD. Our results may sensitize clinicians to consider motivational characteristics of patients with ADHD with respect to diagnostic and therapeutic approaches, such as performing contingency management and validating motivational fluctuations, next to treatment of executive functioning deficits. To avoid potential brain alterations, it may be important to prevent dysfunctional reward anticipation processing via psychological interventions.
Supplementary Material
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th edn, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Beck A, Schlagenhauf F, Wüstenberg T, et al. Ventral striatal activation during reward anticipation correlates with impulsivity in alcoholics. Biological Psychiatry. 2009;66:734–42. doi: 10.1016/j.biopsych.2009.04.035. [DOI] [PubMed] [Google Scholar]
- Biederman J, Faraone SV. Attention-deficit hyperactivity disorder. Lancet. 2005;366:237–48. doi: 10.1016/S0140-6736(05)66915-2. [DOI] [PubMed] [Google Scholar]
- Bitsakou P, Psychogiou L, Thompson M, Sonuga-Barke EJ. Delay Aversion in Attention Deficit/Hyperactivity Disorder: an empirical investigation of the broader phenotype. Neuropsychologia. 2009;47:446–56. doi: 10.1016/j.neuropsychologia.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Carmona S, Hoekzema E, Ramos-Quiroga JA, et al. Response inhibition and reward anticipation in medication-naive adults with attention-deficit/hyperactivity disorder: a within-subject case-control neuroimaging study. Human Brain Mapping. 2012;33:2350–61. doi: 10.1002/hbm.21368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova R, Srikanth R, Baer A, et al. Biological parametric mapping: a statistical toolbox for multimodality brain image analysis. Neuroimage. 2007;34:137–43. doi: 10.1016/j.neuroimage.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos FX, Lee PP, Sharp W, et al. Developmental trajectories of brain volume abnormalities in children and adolescents with attention-deficit/hyperactivity disorder. JAMA. 2002;288:1740–48. doi: 10.1001/jama.288.14.1740. [DOI] [PubMed] [Google Scholar]
- Connors C, Erhardt D, Sparrow E. Connors’ Adult Rating Scales (CAARS) North Tonawada, NY: Multi-Health Systems; 1999. [Google Scholar]
- Coupe P, Yger P, Prima S, Hellier P, Kervrann C, Barillot C. An optimized blockwise nonlocal means denoising filter for 3-D magnetic resonance images. IEEE Transactions on Medical Imaging. 2008;27:425–41. doi: 10.1109/TMI.2007.906087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmo C, Weiffenbach O, Gabriel M, Stadler C, Poustka F. Kiddie-Sads-Present and Lifetime Version, K-SADS-PL. Frankfurt am Main: Klinik für Psychiatrie und Psychotherapie des Kindes- und Jugendalters; 2001. [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. Journal of Child Psychology and Psychiatry. 2006;47:1051–62. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Döpfner M, Breuer D, Wille N, Erhart M, Ravens-Sieberer U BELLA study group. How often do children meet ICD-10/DSM-IV criteria of attention deficit-/hyperactivity disorder and hyperkinetic disorder? Parent-based prevalence rates in a national sample—results of the BELLA study. European Child and Adolescent Psychiatry. 2008;17(Suppl 1):59–70. doi: 10.1007/s00787-008-1007-y. [DOI] [PubMed] [Google Scholar]
- DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. AD/HD Rating Scale-IV: Checklist, Norms, and Clinical Interpretation. New York: Guilford; 1998. [Google Scholar]
- Edel MA, Enzi B, Witthaus H, et al. Differential reward processing in subtypes of adult attention deficit hyperactivity disorder. Journal of Psychiatric Research. 2013;47:350–6. doi: 10.1016/j.jpsychires.2012.09.026. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36:511–21. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Evans AC, Group BDC. The NIH MRI study of normal brain development. Neuroimage. 2006;30:184–202. doi: 10.1016/j.neuroimage.2005.09.068. [DOI] [PubMed] [Google Scholar]
- Frodl T, Skokauskas N. Meta-analysis of structural MRI studies in children and adults with attention deficit hyperactivity disorder indicates treatment effects. Acta Psychiatrica Scandinavica. 2012;125:114–26. doi: 10.1111/j.1600-0447.2011.01786.x. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollingshead AA. Four-Factor Index of Social Status. New Haven: Yale University; 1975. [Google Scholar]
- Hoogman M, Aarts E, Zwiers M, et al. Nitric oxide synthase genotype modulation of impulsivity and ventral striatal activity in adult ADHD patients and healthy comparison subjects. American Journal of Psychiatry. 2011;168:1099–106. doi: 10.1176/appi.ajp.2011.10101446. [DOI] [PubMed] [Google Scholar]
- Huss M, Holling H, Kurth BM, Schlack R. How often are German children and adolescents diagnosed with ADHD? Prevalence based on the judgment of health care professionals: results of the German health and examination survey, KiGGS. European Child and Adolescent Psychiatry. 2008a;17(Suppl 1):52–8. doi: 10.1007/s00787-008-1006-z. [DOI] [PubMed] [Google Scholar]
- Huss M, Poustka F, Lehmkuhl G, Lehmkuhl U. No increase in long-term risk for nicotine use disorders after treatment with methylphenidate in children with attention-deficit/hyperactivity disorder (ADHD): evidence from a non-randomized retrospective study. Journal of Neural Transmission. 2008b;115:335–9. doi: 10.1007/s00702-008-0872-3. [DOI] [PubMed] [Google Scholar]
- Hämmerer D, Eppinger B. Dopaminergic and prefrontal contributions to reward-based learning and outcome monitoring during child development and aging. Developmental Psychology. 2012;48:862–74. doi: 10.1037/a0027342. [DOI] [PubMed] [Google Scholar]
- Kappel V, Koch A, Lorenz RC, et al. CID: a valid incentive delay paradigm for children. Journal of Neural Transmission. 2013;120:1259–70. doi: 10.1007/s00702-012-0962-0. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- Kaup AR, Mirzakhanian H, Jeste DV, Eyler LT. A review of the brain structure correlates of successful cognitive aging. The Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23:6–15. doi: 10.1176/appi.neuropsych.23.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco R, Miranda A, Schlotz W, et al. Delay and reward choice in ADHD: an experimental test of the role of delay aversion. Neuropsychology. 2009;23:367–80. doi: 10.1037/a0014914. [DOI] [PubMed] [Google Scholar]
- Marx I, Hübner T, Herpertz SC, et al. Cross-sectional evaluation of cognitive functioning in children, adolescents and young adults with ADHD. Journal of Neural Transmission. 2010;117:403–19. doi: 10.1007/s00702-009-0345-3. [DOI] [PubMed] [Google Scholar]
- Mazaika PK, Whitfield S, Cooper JC. Detection and repair of transient artifacts in fMRI data. 2005 Paper presented at the HBM 2005. http://cibsr.stanford.edu/documents/ArtRepairHBM2005.pdf. [Google Scholar]
- Nakao T, Radua J, Rubia K, Mataix-Cols D. Gray matter volume abnormalities in ADHD: voxel-based meta-analysis exploring the effects of age and stimulant medication. American Journal of Psychiatry. 2011;168:1154–63. doi: 10.1176/appi.ajp.2011.11020281. [DOI] [PubMed] [Google Scholar]
- Nymberg C, Jia T, Lubbe S, et al. Neural mechanisms of attention-deficit/hyperactivity disorder symptoms are stratified by MAOA genotype. Biological Psychiatry. 2013;74:607–14. doi: 10.1016/j.biopsych.2013.03.027. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Mehta MA, Faraone SV, Asherson P, Kuntsi J. Striatal sensitivity during reward processing in attention-deficit/hyperactivity disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2012;51:722–32. doi: 10.1016/j.jaac.2012.05.006. e729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plichta MM, Vasic N, Wolf RC, et al. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biological Psychiatry. 2009;65:7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: a systematic review and metaregression analysis. American Journal of Psychiatry. 2007;164:942–8. doi: 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Rösler M, Retz W, Retz-Junginger P, et al. Tools for the diagnosis of attention-deficit/hyperactivity disorder in adults. Self-rating behaviour questionnaire and diagnostic checklist. Nervenarzt. 2004;75:888–95. doi: 10.1007/s00115-003-1622-2. [DOI] [PubMed] [Google Scholar]
- Rubia K, Halari R, Cubillo A, Mohammad AM, Brammer M, Taylor E. Methylphenidate normalises activation and functional connectivity deficits in attention and motivation networks in medication-naive children with ADHD during a rewarded continuous performance task. Neuropharmacology. 2009;57:640–52. doi: 10.1016/j.neuropharm.2009.08.013. [DOI] [PubMed] [Google Scholar]
- Rykhlevskaia E, Gratton G, Fabiani M. Combining structural and functional neuroimaging data for studying brain connectivity: a review. Psychophysiology. 2008;45:173–87. doi: 10.1111/j.1469-8986.2007.00621.x. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biological Psychiatry. 2007;61:720–4. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Scheres A, Sumiya M, Thoeny AL. Studying the relation between temporal reward discounting tasks used in populations with ADHD: a factor analysis. International Journal of Methods in Psychiatric Research. 2010;19:167–76. doi: 10.1002/mpr.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lerch JP, Greenstein D, et al. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63:540–9. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, et al. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19649–54. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Rabin C. New insights into attention-deficit/hyperactivity disorder using structural neuroimaging. Current Psychiatry Reports. 2009;11:393–8. doi: 10.1007/s11920-009-0059-0. [DOI] [PubMed] [Google Scholar]
- Shiels K, Hawk LW, Reynolds B, et al. Effects of methylphenidate on discounting of delayed rewards in attention deficit/hyperactivity disorder. Experimental and Clinical Psychopharmacology. 2009;17:291–301. doi: 10.1037/a0017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöwall D, Roth L, Lindqvist S, Thorell LB. Multiple deficits in ADHD: executive dysfunction, delay aversion, reaction time variability, and emotional deficits. Journal of Child Psychology and Psychiatry. 2013;54:619–27. doi: 10.1111/jcpp.12006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solanto MV, Abikoff H, Sonuga-Barke E, et al. The ecological validity of delay aversion and response inhibition as measures of impulsivity in AD/HD: a supplement to the NIMH multimodal treatment study of AD/HD. Journal of Abnormal Child Psychology. 2001;29:215–28. doi: 10.1023/a:1010329714819. [DOI] [PubMed] [Google Scholar]
- Song X-W, Dong Z-Y, Long X-Y, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. Public Library of Science One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ. The dual pathway model of AD/HD: an elaboration of neuro-developmental characteristics. Neuroscience and Biobehavioral Reviews. 2003;27:593–604. doi: 10.1016/j.neubiorev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Sergeant JA, Nigg J, Willcutt E. Executive dysfunction and delay aversion in attention deficit hyperactivity disorder: nosologic and diagnostic implications. Child and Adolescent Psychiatric Clinics of North America. 2008;17:367–84. doi: 10.1016/j.chc.2007.11.008. ix. [DOI] [PubMed] [Google Scholar]
- Stoy M, Schlagenhauf F, Schlochtermeier L, et al. Reward processing in male adults with childhood ADHD—a comparison between drug-naive and methylphenidate-treated subjects. Psychopharmacology. 2011;215:467–81. doi: 10.1007/s00213-011-2166-y. [DOI] [PubMed] [Google Scholar]
- Ströhle A, Stoy M, Wrase J, et al. Reward anticipation and outcomes in adult males with attention-deficit/hyperactivity disorder. Neuroimage. 2008;39:966–72. doi: 10.1016/j.neuroimage.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Venkatraman VK, Aizenstein H, Guralnik J, et al. Executive control function, brain activation and white matter hyperintensities in older adults. Neuroimage. 2010;49:3436–42. doi: 10.1016/j.neuroimage.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser PJ, Scheltens P, Verhey FR, et al. Medial temporal lobe atrophy and memory dysfunction as predictors for dementia in subjects with mild cognitive impairment. Journal of Neurology. 1999;246:477–85. doi: 10.1007/s004150050387. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, et al. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. The Journal of Neuroscience. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, et al. Evidence that methylphenidate enhances the saliency of a mathematical task by increasing dopamine in the human brain. American Journal of Psychiatry. 2004;161:1173–80. doi: 10.1176/appi.ajp.161.7.1173. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, et al. (2012).Methylphenidate-elicited dopamine increases in ventral striatum are associated with long-term symptom improvement in adults with attention deficit hyperactivity disorder. Journal of Neuroscience. 2012;32:841–9. doi: 10.1523/JNEUROSCI.4461-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiß RH. CFT 20-R Grundintelligenztest, Revision. Göttingen: Hogrefe; 2006. [Google Scholar]
- Wierenga L, Langen M, Ambrosino S, van Dijk S, Oranje B, Duston S. Typical development of basal ganglia, hippocampus, amygdala and cerebellum from age 7 to 24. Neuroimage. 2014;96:67–72. doi: 10.1016/j.neuroimage.2014.03.072. [DOI] [PubMed] [Google Scholar]
- Wieshmann UC, Krakow K, Symms MR, et al. Combined functional magnetic resonance imaging and diffusion tensor imaging demonstrate widespread modified organization in malformation of cortical development. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;70:521–3. doi: 10.1136/jnnp.70.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Altaye M, Gaser C. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage. 2008;41:903–13. doi: 10.1016/j.neuroimage.2008.02.056. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Pfister H. Instruktionsmanual zur Durchführung von DIA-X-Interviews. Frankfurt: Swets & Zeitlinger; 1997. [Google Scholar]
- Wittchen HU, Zaudig M. Strukturiertes Klinisches Interview für DSM-IV. Göttingen: Hogrefe; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.