Abstract
As a social species in a constantly changing environment, humans rely heavily on the informational richness and communicative capacity of the face. Thus, understanding how the brain processes information about faces in real-time is of paramount importance. The N170 is a high-temporal resolution electrophysiological index of the brain’s early response to visual stimuli that is reliably elicited in carefully controlled laboratory-based studies. Although the N170 has often been reported to be of greatest amplitude to faces, there has been debate regarding whether this effect might be an artefact of certain aspects of the controlled experimental stimulation schedules and materials. To investigate whether the N170 can be identified in more realistic conditions with highly variable and cluttered visual images and accompanying auditory stimuli we recorded EEG ‘in the wild’, while participants watched pop videos. Scene-cuts to faces generated a clear N170 response, and this was larger than the N170 to transitions where the videos cut to non-face stimuli. Within participants, wild-type face N170 amplitudes were moderately correlated to those observed in a typical laboratory experiment. Thus, we demonstrate that the face N170 is a robust and ecologically valid phenomenon and not an artefact arising as an unintended consequence of some property of the more typical laboratory paradigm.
Keywords: faces, EEG, N170
INTRODUCTION
Electrophysiological studies of visual-evoked potentials almost invariably try to compensate for the limited signal to noise ratio of Event Related Potentials (ERPs) by exercising as much control as possible over the stimuli and the testing environment. Typically, stimuli are presented one at a time in a series of separate trials in a quiet room and participants are asked to fixate centrally before and often during each trial. The stimuli themselves will be carefully matched for overall luminance and often a number of other properties as well.
A remarkably consistent finding from such studies has been the N170, an early negative potential following visual stimulus onset that is often reported as larger to faces than to other visual stimuli (Bentin et al., 1996; Rossion and Jacques, 2008; Eimer, 2011). Recorded maximally at occipito-temporal electrode sites, the N170 is a negative inflection of the ERP that occurs ∼150–210 ms following stimulus onset. Since first reported by Bentin et al. (1996), the N170 has proven to be a highly reliable and useful index of early visual cognition. Although the N170 response is elicited to a wide variety of visually presented stimuli (e.g. Mercure et al., 2011), and may be modulated by expertise (e.g. Tanaka and Curran, 2001) and signalling value (e.g. Levita et al., 2014) its amplitude is generally somewhat greater to faces than to other classes of stimuli, and it is as an index of face processing that it has been most widely studied (Eimer, 2011).
The face N170 appears to be sensitive to a range of characteristics relating to face stimuli, including for instance head orientation (Itier et al., 2007; Nummenmaa and Calder, 2009), facial motion (Puce et al., 2000) including gaze shifts (Tipples et al., 2013), facial inversion (e.g. Rossion et al., 2000), and facial expression of emotion (Blau et al., 2007). Other studies have focussed on the vertex positive potential (VPP; Botzel and Grusser, 1989; Jeffreys, 1989; Johnston et al., 2005), a positivity measured with similar latency at the vertex, which is believed to reflect the same underlying generators and processes (Joyce and Rossion, 2005). The dominant view with respect to the processes indexed by the N170 and VPP is that they reflect the early structural encoding of face features preceding the identification of unique identity.
Although the majority of studies report a larger N170/VPP to faces, the field has not been free from controversy. For example, a particularly lively debate was sparked by Thierry et al.'s (2007) claim that the larger N170 to faces was not a face-specific response but instead reflects poorly controlled ‘inter-stimulus perceptual variability’ (ISPV) because most studies used only full-face stimuli but more variable non-faces. Thierry et al. (2007) claimed to demonstrate that when ISPV was properly controlled the N170 no longer showed a greater sensitivity to faces than to objects. However, this claim has not been widely accepted. Instead, a number of aspects of Thierry et al.'s (2007) study cast doubt on their interpretation, including the choice of electrode site (Bentin et al. 2007) and the measure used to characterize the N170 amplitude (Rossion and Jacques, 2008). Moreover, contrary to Thierry et al.’s claims, a number of previous studies had carefully controlled low-level stimulus differences between faces and objects and had, nevertheless, shown larger N170 amplitudes to faces (Eimer, 2011). More recently, a study by Ganis and colleagues (2012), using a single exemplar stimulus and single exemplar house stimulus, well matched in terms of low-level characteristics, showed category sensitivity of the N170 which could not be attributed to poorly controlled ISPV.
Although most researchers have not accepted Thierry et al.'s (2007) claims (Bentin et al., 2007; Eimer, 2011) the debate serves to illustrate an important general point, which is that tight experimental control always carries a risk that important aspects of the phenomenon of interest are overlooked or distorted, and critiques of lack of ‘ecological validity’ have become commonplace in some areas of psychology. The limited ecological validity of the standard ERP paradigms is obvious. In everyday life faces are encountered in very varied lighting conditions and in cluttered visual environments, they do not usually appear suddenly ‘out of the black’ at the fixation point but have to be found in different parts of the visual field, they are often moving, they are seen at all kinds of sizes and visual angles, they are attached to bodies, and there is usually relevant or irrelevant concurrent auditory stimulation. Any or all of these might impact on the N170 in at present unknown ways (although see Joassin et al., 2006, and Focker et al., 2011, for evidence of cross-modal interactions of voice and face identity on N170).
We therefore sought to determine whether the N170 (and VPP) can be identified in a more realistic context (in the wild) by recording EEG while participants watched popular music videos. We chose pop videos because they are commonplace in the lives of young people, they contain plenty of faces but also lots of other stimuli, they have an abundance of arbitrary transitions between scenes, and there is a continual accompanying soundtrack with a varying relation to the visual action. Our reasoning was that if the N170 is evident to faces seen under such conditions, it is clearly a robust phenomenon and the case for its significance is strengthened.
Based on established characteristics of ERPs reported in laboratory studies, we set a series of criteria as being necessary and sufficient for establishing that the face N170 occurs ‘in the wild’, and that the ‘wild-type’ N170 reflects similar mechanisms to its laboratory counterpart. The first two of these criteria relate to establishing the presence of meaningful ERPs comparable to those that are typically observed in standard laboratory visual ERP experiments (i.e. the P1-N1 complex). The P1 is a positive inflection of the visual ERP that occurs at ∼80–130 ms post-stimulus onset and is thought to be primarily generated in early visual brain areas (Spehlmann, 1965), and to relate to the processing of low-level visual features (Eimer, 2011). Whilst the P1 can be modulated by attention (i.e. Luck et al., 2000), it is generated by both simple and complex visual stimuli and is not generally believed to show categorical sensitivity (Eimer, 2011). To establish that N170 occurs ‘in the wild', there should be a clearly observable positive peak in the ERP waveform occurring at around 90–130 ms following face onsets (i.e. a P1) observable at posterior electrode sites. This positive peak should be followed by a negative inflection of the ERP peaking between 140 and 210 ms post-onset (the N170). The third and fourth criteria relate to the face sensitivity of the N170 and VPP. The N170 to faces should be of greater magnitude than to non-faces over posterior lateralised electrode sites (i.e. P8 and P7). Furthermore, there should be an observable VPP (positivity measured at the vertex between 140 and 210 ms) that is greater to faces than to non-faces. The final criterion involves the relationship between the laboratory and ‘wild type’ N170/VPP. To the extent that it is a manifestation of the same phenomenon, the amplitude of the ‘wild-type' face N170/VPP should be correlated with that observed in a standard laboratory paradigm.
METHOD
Participants took part in two EEG experiments, the order of which was counterbalanced. In one experiment (WILD), the participants passively viewed a series of three contemporary popular music videos, in the other experiment (LAB), they performed a simple vigilance task whilst viewing a series of static images (of faces, objects and phase scrambled images).
Participants
EEG data were collected from 28 adults with normal or corrected-to-normal vision. Six participants were excluded from the analyses since they had too few artefact-free EEG epochs for one or more of the experimental conditions in the WILD task (see EEG Recording and Analysis section below). Of the 22 participants whose data contributed to the final analyses 15 were female; the average age was 21.64 years (SD: 2.04 years; range: 19–26 years); 17 were Caucasian, 5 were Asian. None had any known neurological abnormalities. The study was approved by the University of York Psychology Department’s Ethics Committee, and participants gave informed consent.
Materials and tasks
WILD task
In selecting materials for the WILD task, we were motivated by a number of desiderata and constraints, as follows. We wished to use materials that more closely resemble the kind of visual stimulation that occurs in real-life than that generally seen in a tightly controlled laboratory setting—we therefore resolved to adopt a ‘found object' approach by making use of pre-existing video materials that participants might choose to watch in their everyday lives. We also required that the videos should not be excessively lengthy but that in order to be able to produce averaged ERPs they should include a reasonably high number of frame transitions both to faces and to other foci of potential interest (objects, scenes, bodies etc.).
We therefore selected three contemporary pop videos (Cher Lloyd—‘Swagger Jagger’; The Fray—‘How To Save A Life’ and All American Rejects—‘Dirty Little Secret’). These videos fulfilled our criteria, including many points where the scene cut rapidly from one type of stimulus to shots of faces and other foci. We selected a subset of the available scene-cuts in the videos to act as time-points on which to base ERP averages to Faces and to Non-Faces (objects and body-parts) on the basis that the transition should cut to a scene where the initial focus was clearly definable as belonging to one of these categories and that another scene-cut should not occur within 500 ms. The videos were edited using Adobe Premiere Pro CS6 to usually contain a small black square in the left hand corner, but for transitions (i.e. scene-cuts) that were designated as triggers for ERPs of interest this black square was replaced by a white square. This black-to-white transition in the corner of the video frame was used to trigger a light-detecting diode placed in the corner of the screen whilst the participants watched the video. The diode recorded a trigger pulse alongside the EEG data which could be used during the analysis as a basis for time-locking stimulus events.
The duration of the sequence of three videos was 11 min and 4 s. In total, across the three videos, there were 137 coded transitions to Faces and 86 coded transitions to Non-Faces. This discrepancy in the number of trials across these conditions was a consequence of the general nature of the content of pop-videos, and is addressed in the analysis through a down-sampling of the number of Face trials when direct comparisons to Non-Faces are made (see EEG Recording and Analysis section below). Age, sex and ethnicity of stimuli were not coded nor used as a factor in the analyses. Example Face and Non-Face trigger-screens are shown in Figure 1.
Fig. 1.
Examples of frames following scene-cuts cuts coded as Face and Non-Face onsets for the WILD task (Top) and examples of Face and Object and Scrambled stimuli used in the LAB task (Bottom).
LAB task
Stimuli for the LAB task were static images of faces, everyday objects and phase scrambled images. The Face category consisted of 40 images—four images of each of 10 individuals (5 males and 5 female) taken from the ‘Aberdeen’ set of faces from the University of Stirling image database (http://pics.psych.stir.ac.uk/2D_face_sets.htm). All images were frontal views of faces with a neutral expression presented in greyscale on backgrounds which were scrambled composites of luminance of the original face photograph. The Object category also consisted of 40 greyscale images (four slightly different views of each of 10 distinct objects). The depicted objects included a hat, a vase, a cooking pot, a tea-pot, a bunch of bananas, a capsicum, a bicycle helmet, a wellington boot, sunglasses and a toy cash-register. There were also 80 Scrambled images produced by phase scrambling each of the Face and Object images. In addition, a number of images were edited to contain a small randomly placed red-dot for the incidental monitoring task. Examples of Face, Object and Scrambled stimuli are shown in Figure 1.
The LAB task involved the participants viewing a pseudo-random sequence in which stimuli were presented one at a time for 600 ms, each preceded either by a 600 ms duration central fixation cross on a blank screen, or immediately following the previous stimulus with no inter-stimulus-interval. Participants were instructed to watch the sequence, and to press a response key every time they saw an image containing a small red spot. The trials in which the red spot was present comprised approximately 10% of the total number of trials. The task of inspecting each image for a red spot thus served to ensure that participants looked at all stimuli. It should be noted that the attentional demands of the LAB task may have differed from those of the WILD task, where there was no explicit task. In total, there were 650 stimuli presented including approximately equal numbers of Faces, Objects and Scrambled images. For the purpose of this article, to maintain a degree of consistency between analyses of LAB and WILD ERPs only transitions where Faces, Objects and Scrambled images appeared directly following the presentation of another image (rather than a fixation screen) were included in the analysis of the LAB data. Thus, for both the LAB and WILD tasks the stimulus onsets used as time-locking events for the generation of ERPs involved transitions from a visual stimulus which had a comparable degree of complexity, and variation of brightness and contrast to the target stimulus, rather than ‘hard onsets’ from a fixation screen. After excluding red spot trials to eliminate the possibility of motor artefacts contaminating visually induced ERPs, there were 158 trials on which there was a transition to a Face and 151 trials on which there was a transition to an Object and 167 trials on which there was a transition to a Scrambled Image. Stimuli were presented using E-Prime 2.0 software. The LAB task ran for a period of approximately 7 min and 40 s.
Both experimental tasks were delivered using an Intel Pentium 4 HT computer, and the visual stimuli were presented on a 23″ TFT LCD widescreen monitor with a 1340 x 1084 pixel resolution. Participants were seated ∼60 cm away from the screen. For the WILD task, the video stimuli filled the entire screen. Participants were not instructed to fixate centrally, and their eye-movements were not measured during the experiment. For the LAB task, Faces and Objects were presented centrally with a visual angle subtending ∼3°, and participants were instructed to fixate centrally.
EEG recording and analyses
For both WILD and LAB task, continuous EEG was collected with a sampling rate of 1000 Hz on 64 channels using an ANT ASAlab high-speed amplifier, from scalp sites corresponding to the extended International 10–20 electrode montage using a WaveGuard cap. An averaged reference was used and impedance values were kept below 20 kΩ. Vertical and horizontal EOG measures were taken using bipolar electrode pairs placed above and below the left eye, and proximal to the outer canthus of each eye, as a basis for attenuating eye-movement artefacts in the EEG data post recording. EEG data were filtered using a bandpass filter (0.3–30 Hz, slope 24 dB per octave) with a notch-filter at 50 Hz for UK mains frequency. Eye-movement artefacts were detected algorithmically on the basis of channel maxima/minima (+/−100 µV) and/or slope (50 µV per time-step) and EEG segments 100 ms either side of such events were marked as bad. Data were separated into epochs of 350 ms duration, with each epoch commencing 50 ms prior to the onset of a trigger and extending for 300 ms thereafter. Epochs containing eye-movement artefacts were not included in averaging. Epoch data were baseline corrected by subtracting the mean of the pre-trigger period, and epochs associated with different conditions (Face and Object) were averaged to produce ERP waveforms.
Participants were excluded from further analysis if fewer than 90 trials were retained following artefact rejection for the Face condition of the WILD task, or fewer than 60 Non-Face trials in the WILD task. On the basis of these criteria, 22 participants were included in the subsequent analysis, having a sufficiently high number of good trials across all conditions in both tasks. On average, the number of trials contributing to the ERPs for each participant was as follows: WILD Faces 125.5 (SD 8.5), WILD Non-Faces 76.8 (SD 5.8), LAB Faces 146.3 (SD 17.8), LAB Objects 135.6 (SD 18.9) and LAB Scrambled 152.6 (SD 19.8).
Since there were more Face trials in the WILD task than Non-Face trials, and therefore a potential bias with respect to signal-to-noise ratios, we took the additional step of down-sampling the number of WILD Face trials for the purposes of making statistical comparisons to the WILD Non-Faces. We did this by splitting the WILD Face trials into odd and even numbered trials (post-artefact rejection) and generating separate ERPs to each of these. We designated these measures as WILD FacesA and WILD FacesB (in contrast to WILD Faces All, based on the complete set of WILD Face trials). In the statistical analysis within the WILD task, Non-Faces are compared to both FacesA and FacesB to give an indication of the stability of any effects. In correlation analyses examining whether the WILD and LAB N170 and VPP to Faces might be related phenomena, we compared WILD Faces All to LAB Faces to base analyses on ERPs involving comparable numbers of trials.
Following general conventions from the literature, the amplitudes of the ERP components N170 (the minima between 140 and 210 ms post-stimulus onset) and P1 (the maxima between 80 and 130 ms) were measured at lateral posterior electrode sites P7 and P8 (Bentin et al., 2007). VPP amplitudes (the maxima between 140 and 210 ms post-stimulus onset) were measured at electrode Cz (Joyce and Rossion, 2005).
RESULTS
Data inspection and data pooling
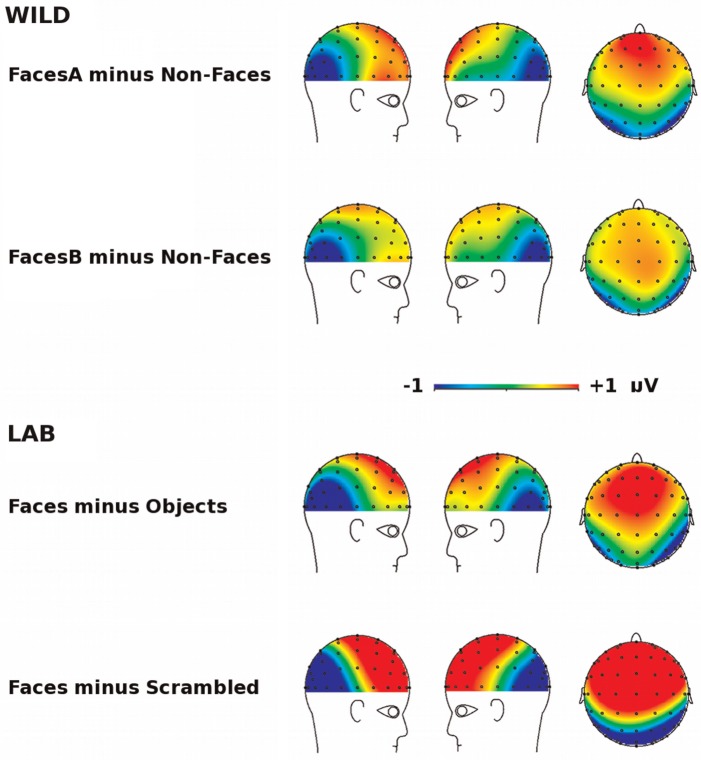
Scalp topographic maps of the difference between Face and Non-Face conditions for the N170 are shown for both the LAB and WILD tasks in Figure 2. Both the LAB and WILD tasks show a similar (and expected for the LAB task) pattern whereby the response difference is characterized by the presence of a posterior negativity that is observed maximally over lateral occipito-temporal electrode sites, and a positivity that is observed maximally over fronto-central electrode sites.
Fig. 2.
Scalp topographies of the difference in ERP response to Faces and Non-Faces for the WILD task and LAB task (averaged response between 140–210 ms).
We performed a two-factor repeated measures ANOVA comparing N170 amplitudes for the WILD task across stimulus type (FacesA, FacesB and Non-Faces) and lateral electrode sites (P8 and P7). The magnitude of the N170 was indistinguishable across electrode sites P7 and P8. For simplicity, ERPs triggered over these electrodes sites were collapsed across for all further analysis. This observation was supported statistically the absence of a main effect of electrode site (F(1,21) = 0.81, P = 0.378, ns, = 0.037) and the absence of an electrode site by stimulus type interaction (F(2,20) = 1.04, P = 0.363, ns, = 0.066), we pooled data across electrodes P8 and P7 for all subsequent analyses.
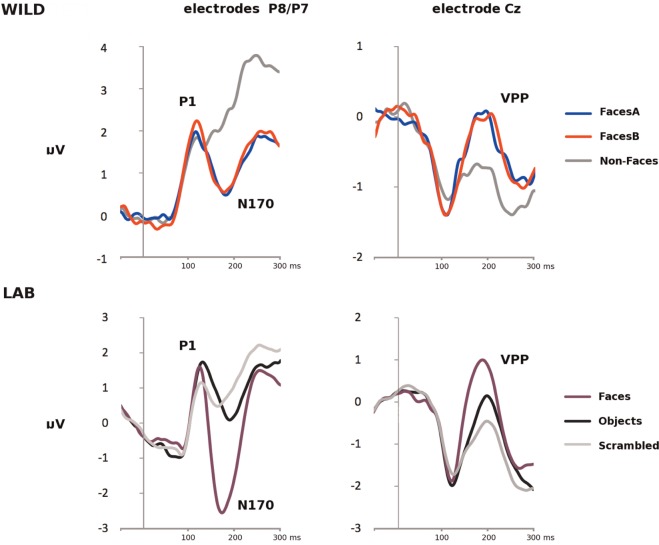
Grand-average ERP waveforms for all stimulus types for the WILD and LAB tasks are shown for electrodes Cz and pooled P8/P7 in Figure 3.
Fig. 3.
(Top) Grand-averaged ERP waveforms to FacesA, FacesB and Non-Faces at pooled electrodes P8/P7 site (Left) and electrode site Cz (Right) for the WILD task, and (Bottom) grand-averaged ERP waveforms to Faces, Objects and Scrambled images at pooled electrodes P8/P7 site (Left) and electrode site Cz (Right) for the LAB task.
P1 amplitudes
As can be seen (Figure 3—Left), there was a clearly observable P1 (i.e. a positivity peaking between 80 and 130 ms post-stimulus onset) at P8/P7, and a corresponding negative peak with a similar latency at Cz (Figure 3—Right). This pattern is typical for visual ERP experiments, and clearly establishes that transitions in our WILD experiment elicit a visual ERP.
For both the WILD and LAB tasks, P1 peak amplitudes for each condition were extracted (between 80 and 130 ms post-stimulus onset) for the electrode site P8/P7. These were entered into two repeated-measures ANOVAs comparing P1 amplitudes across stimulus type for each task separately. For the WILD task, there was no significant main effect of stimulus type (F(2,42) = 1.31, P = 0.281, ns, = 0.059). Similarly, for the LAB task, there was no main effect of stimulus type (F(2,42) = 1.99, P = 0.149, ns, = 0.087). Thus, for both the WILD and LAB tasks, P1 amplitudes did not differ as a function of stimulus type.
N170/VPP amplitudes
As can be seen (Figure 3—Left), there was a clearly observable N170 (i.e. a negative inflection of the ERP that is maximal 140–210 ms post-stimulus onset) at P8/P7, and a corresponding positivity (the VPP) with a similar latency at Cz (Figure 3—Right).
For both the WILD and LAB tasks, N170 peak amplitudes for each condition were extracted (between 140 and 210 ms post-stimulus onset) for the electrode site P8/P7, and VPP peak amplitudes were extracted for the same time-window for electrode Cz. We performed four repeated-measures ANOVAs: two comparing N170 amplitudes at electrode P8/P7 across stimulus type for each task separately and a further two comparing VPP amplitudes across stimulus type at electrode Cz for each task. N170 and VPP peak amplitudes for each for each task and stimulus type are shown in Table 1.
Table 1.
Mean ERP amplitudes, υV, (standard deviation in brackets) for the N170 and VPP components to Face and Non-Face stimuli for the WILD and LAB tasks
| N170 Mean Amplitude/(SD) | VPP Mean Amplitude/(SD) | |||||||
|---|---|---|---|---|---|---|---|---|
| WILD task | Faces |
Non-Faces | Faces |
Non-Faces | ||||
| FacesA | FacesB | FacesA | FacesB | |||||
| −0.46 (1.22) | −0.56 (1.13) | 1.03 (1.20) | 0.75 (0.81) | 0.82 (0.86) | −0.76 (0.90) | |||
| LAB task | Faces | Non-Faces |
Faces | Non-Faces |
||||
| Objects | Scrambled | Object | Scrambled | |||||
| −3.92 (1.79) | −1.41 (1.64) | −0.57 (1.29) | 1.85 (0.85) | 0.91 (1.14) | 0.11 (1.12) | |||
For the WILD task, there was a significant effect of stimulus type on N170 amplitudes at P8/P7 (F(2,42) = 15.25, P < 0.001, = 0.421). Follow-up Tukey HSD tests of all pair-wise comparisons revealed that FacesA differed significantly from Non-Faces (P < 0.01) and that FacesB differed significantly from Non-Faces (P < 0.01) but that FacesA and FacesB did not differ from each other. For the WILD task, there was also a significant effect of stimulus type on VPP amplitudes at electrode Cz (F(2,42) = 11.52, P < 0.001, = 0.354). Again, follow-up Tukey HSD tests revealed that FacesA and FacesB both differed from Non-Faces (both with P < 0.01) but not from each other. Thus N170 amplitudes and VPP amplitudes were greater to Faces than to Non-Faces, and these differences reflect stable and robust differences across conditions in the WILD task.
For the LAB task, there was a significant effect of stimulus type on N170 amplitudes at P8/P7 (F(2,42) = 49.84, P < 0.001, = 0.704). Follow-up Tukey HSD comparisons revealed that Faces differed from Objects (P < 0.01) and that Faces differed from Scrambled (P < 0.01), but that Objects and Scrambled did not differ from each other. For VPP measured at Cz, there was a significant effect of stimulus type (F(2,42) = 31.61, P < 0.001, = 0.601). Follow-up Tukey HSD tests showed that all pair-wise contrasts were significant (P < 0.01).
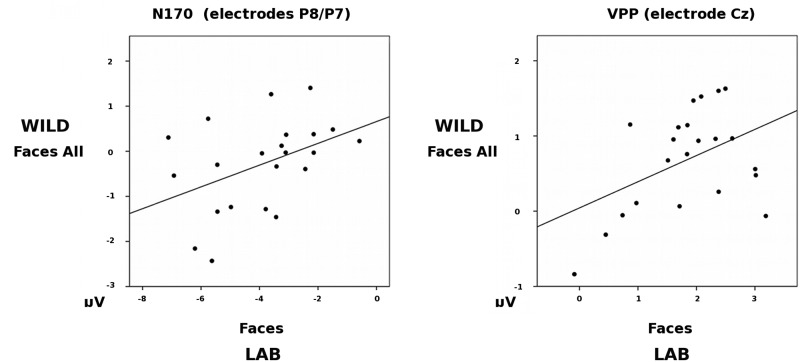
Are the WILD and LAB N170/VPP related?
We performed Pearson correlation analyses to examine, within participants, whether the amplitudes of the WILD Face N170 and the LAB Face N170 (at pooled electrodes P8/P7), and the WILD Face VPP and LAB Face VPP (at electrode Cz) were related. The amplitude of the WILD Face N170 was moderately correlated with the amplitude of the LAB Face N170 (r(22) = 0.43, P = 0.046), and the WILD Face VPP was moderately correlated with the LAB Face VPP (r(22) = 0.45, P = 0.038), (see Figure 4.)
Fig. 4.
Scatterplots showing the correlation between N170 amplitudes to Faces across the WILD and LAB tasks at pooled electrodes P8/P7, and VPP amplitudes across the WILD and LAB tasks at electrode site Cz.
These correlations are consistent with the inference that the WILD and LAB N170s are related phenomena that index, at least to some extent, the same underlying neural mechanisms.
DISCUSSION
We have addressed the question of whether there is evidence for a face N170 and a corresponding VPP occurring ‘in the wild'—that is, outside of the highly constrained and tightly controlled laboratory paradigms that have until now been the sole contexts in which these ERP components have been explored. To do so, we took a ‘found object' approach, using pop videos as stimuli on the basis that these are pre-exisiting artefacts produced with the primary intention that humans watch them for the purposes of entertainment. These videos, being visually rich, dynamic, rapidly changing but with contextual narrative, and accompanied by related auditory information, are much closer to the kind of stimulation that our perceptual systems deal with on an everyday basis than the materials usually adopted in laboratory studies.
We have shown that the onset of faces in such videos, following scene-cuts, can be used as trigger events from which it is possible to produce ERPs through the normal method of time-locked averaging of segments of the EEG. The resultant ‘wild' ERPs show the typical pattern observed in laboratory studies to visual stimuli in general, since there is a clear P1 component peaking at approximately 120 ms post-stimulus onset, which is maximally expressed at electrodes directly over the visual cortex, and which is accompanied by a temporally coincident negative component at frontal electrode sites (the N1). Moreover, these ERPs show the typical pattern observed in laboratory studies to faces, since the posterior P1 is followed by a negative inflection of the ERP with a latency of around 170 ms post-stimulus onset, which is maximally expressed in right lateral occipito-temporal electrodes. The N170 to Face onsets was greater than to Non-Faces, and the amplitude of this N170 was moderately correlated to that elicited to Faces in a typical laboratory paradigm. Thus, we have demonstrated a ‘wild-type N170' ERP to face onsets in pop videos which was highly similar in its topography and latency to its laboratory-based cousin. Furthermore, although the ‘wild-type N170' was smaller in amplitude than that observed in a more typical laboratory paradigm the moderate correlation between these two measures increases our conviction that both at least partially reflect the same underlying mechanisms. With respect to the ‘wild type' VPP, we have shown a highly comparable pattern of findings—that is to say, a clear VPP with the expected topography and latency, which was largest to Faces, and moderately correlated with that observed in the LAB task. In the LAB task, there was a greater VPP amplitude to Objects than to Scrambled images, which was not predicted, but which is not without precedent (i.e. see Rossion and Caharel, 2011).
We believe our findings to be important for a number of reasons. From a broad perspective, face perception is an essential and highly sophisticated human ability that is implicated in a range of neuropsychological, neurodevelopmental and psychiatric conditions (Kemp et al., 2003; Calder et al., 2011; Young, 2011; Bruce and Young, 2012). Since it forms the most frequently used electrophysiological index of face perception, understanding the N170/VPP is therefore of wide applicability. Indeed a number of existing studies report atypical N170/VPP components in a variety of clinical conditions including autistic spectrum disorders (Webb et al., 2102), schizophrenia (Johnston et al., 2005) and developmental prosopagnosia (Towler et al., 2012) and a deeper understanding of the N170 is likely to yield important insights into these conditions.
In terms of understanding the nature of the N170/VPP, as far as we are aware ours is the first direct evidence of a complex visual ERP (P1-N170) to ambient video stimuli. In this way, our study parallels and complements the landmark study by Hasson et al. (2004) which, using fMRI, demonstrated consistent patterns of activity in the sensory cortices across participants whilst they watched a portion of Sergio Leone’s classic western movie ‘The Good, the Bad and the Ugly'. In doing so, the Hassan study established a vital link between the spatial patterns of brain activity seen in tightly controlled laboratory studies and those elicited during the free-viewing of complex audio-visual narrative materials, confirming the ecological validity of previous laboratory findings. This study demonstrates a similar consistency between laboratory findings and measures derived from more ecologically valid stimuli with a high-temporal resolution brain imaging technology, thus validating the N170/VPP’s status as a real-time electrophysiological index of visual brain functioning that is elicited during the free-viewing of ambient stimuli.
Notably, in both the Hassan study and this study, the visual onset of faces was strongly related to identifiable indices of brain activity. In this study, replicating a common finding in the literature (Bentin et al., 2007; Rossion and Jacques, 2008; Eimer, 2011), we have shown a larger N170 to faces than to objects for both the ‘wild-type' and the lab-based N170. However, we note that our study may not support strong claims with respect to the putative face-specificity of the N170, since the non-Face stimuli were necessarily heterogeneous. Nonetheless, with respect to the claim made by Thierry et al. (2007) that the N170 is largely driven by low inter-stimulus perceptual variance, we can state with some confidence that this is not the case. In the pop videos, faces were highly variable with respect to their size, luminance, colour, and where they appeared in the visual field. They were dynamic, expressive, and attached to acting bodies, and they were accompanied by causally related (singing) and causally unrelated (music) auditory stimuli. Despite this substantial inter-stimulus perceptual variance between faces in the pop videos, the ‘wild-type' N170 to faces was clearly evident and correlated with that seen in the lab. From this, we conclude that the N170 is a robust, reliable index of real-world visual perceptual processes.
Conflict of Interest
None declared.
Acknowledgments
The authors thank Juliet Hodges and Catherine Kenyon for their contribution to participant recruitment and testing. The authors would also like to pay tribute to Shlomo Bentin’s influential role in shaping current understanding of the N170 between its discovery in 1996 and his untimely death in 2012.
REFERENCES
- Bentin S, Allison T, Perez E, Puce A, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–65. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Taylor M, Rousselet G, et al. Controlling interstimulus perceptual variance does not abolish N170 face sensitivity. Nature Neuroscience. 2007;10:801–2. doi: 10.1038/nn0707-801. [DOI] [PubMed] [Google Scholar]
- Blau V, Maurer U, Tottenham N, McCandliss B. The face-specific N170 component is modulated by emotional expression. Behavioral and Brain Functions. 2007;3(7) doi: 10.1186/1744-9081-3-7. ), doi:10.1186/1744-9081-3-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botzel K, Grusser OJ. Electric brain potentials evoked by pictures of faces and non-faces—a search for face-specific EEG-potentials. Experimental Brain Research. 1989;77:349–60. doi: 10.1007/BF00274992. [DOI] [PubMed] [Google Scholar]
- Bruce V, Young AW. Face Perception. Hove: East Sussex: Psychology Press; 2012. [Google Scholar]
- Calder AJ, Rhodes G, Johnson MH, Haxby JV, editors. The Oxford Handbook of Face Perception. Oxford: Oxford University Press; 2011. [Google Scholar]
- Cousineau D. Confidence intervals in within-subject designs: a simpler solution to Loftus and Masson’s method. Tutorials in Quantitative Methods for Psychology. 2005;1:42–5. [Google Scholar]
- Eimer M. The face-sensitive N170 component of the event-related brain potential. In: Calder AJ, Rhodes G, Johnson MH, Haxby JV, editors. The Oxford Handbook of Face Perception. Oxford: Oxford University Press; 2011. pp. 329–344. [Google Scholar]
- Focker J, Holig C, Best A, Roder B. Crossmodal interaction of facial and vocal person identity information: an event-related potential study. Brain Research. 2011;1385:229–45. doi: 10.1016/j.brainres.2011.02.021. [DOI] [PubMed] [Google Scholar]
- Ganis G, Smith D, Schendan HE. The N170, not the P1, indexes the earliest time for categorical perception of faces, regardless of interstimulus variance. Neuroimage. 2012;62:1563–74. doi: 10.1016/j.neuroimage.2012.05.043. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles M. Generalization and evaluation of eye-movement correction procedures. Journal of Psychophysiology. 1989;3:14–6. [Google Scholar]
- Hasson U, Nir Y, Levy I, Fuhrmann G, Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303:1634–40. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Itier R, Alain C, Kovacevic N, McIntosh A. Explicit versus implicit gaze processing assessed by ERPs. Brain Research. 2007;1177:79–89. doi: 10.1016/j.brainres.2007.07.094. [DOI] [PubMed] [Google Scholar]
- Jeffreys DA. A face-responsive potential recorded from the human scalp. Experimental Brain Research. 1989;78:193–202. doi: 10.1007/BF00230699. [DOI] [PubMed] [Google Scholar]
- Joassin F, Maurage P, Bruyer R, Crommelinck M, Campanella S. When audition alters vision: an event-related potential study of the cross-modal interactions between faces and voices. Neuroscience Letters. 2006;369(2):132–7. doi: 10.1016/j.neulet.2004.07.067. [DOI] [PubMed] [Google Scholar]
- Johnston P, Stojanov W, Devir H, Schall U. Functional MRI of facial emotion recognition deficits in schizophrenia and their electrophysiological correlates. European Journal of Neuroscience. 2005;22(5):1221–32. doi: 10.1111/j.1460-9568.2005.04294.x. [DOI] [PubMed] [Google Scholar]
- Joyce CA, Rossion B. The face-sensitive N170 and VPP components manifest the same brain processes: the effect of reference electrode site. Clinical Neurophysiology. 2005;116:2613–31. doi: 10.1016/j.clinph.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Kemp S, Young AW, Szulecka K, de Pauw KW. A case of paraprosopia and its treatment. Cognitive Neuropsychiatry. 2003;8:43–56. doi: 10.1080/713752239. [DOI] [PubMed] [Google Scholar]
- Levita L, Howsley P, Jordan J, Johnston P. Potentiation of the early visual response to learned danger signals in adults and adolescents. Social Cognitive and Affective Neuroscience. 2014;10(2):269–77. doi: 10.1093/scan/nsu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Woodman GE, Vogel EK. Event-related potential studies of attention. Trends in Cognitive Sciences. 2000;4:432–40. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- Mercure E, Cohen-Kadosh K, Johnson M. The N170 shows differential repetition effects for faces, objects, and orthographic stimuli. Frontiers in Human Neuroscience. 2011;5(6) doi: 10.3389/fnhum.2011.00006. doi: 10.3389/fnhum.2011.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morey RD. Confidence intervals from normalized data: a correction to Cousineau. Tutorials in Quantitative Methods for Psychology. 2008;4(2):61–4. [Google Scholar]
- Nummenmaa L, Calder A. Neural mechanisms of social attention. Trends in Cognitive Sciences. 2009;13(3):135–43. doi: 10.1016/j.tics.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Puce A, Smith A, Allison T. ERPs evoked by viewing facial movements. Cognitive Neuropsychology. 2000;17:221–39. doi: 10.1080/026432900380580. [DOI] [PubMed] [Google Scholar]
- Rossion B, Caharel S. ERP evidence for the speed of face categorization in the human brain: disentangling the contribution of low-level visual cues from face perception. Vision Research. 2011;51(12):1297–311. doi: 10.1016/j.visres.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Rossion B, Gauthier I, Tarr M, et al. The N170 occipito-temporal component is delayed and enhanced to inverted faces but not ojects: an electrophysiological account of face-specific processes in the human brain. Cognitive Neuroscience. 2000;11(1):69–72. doi: 10.1097/00001756-200001170-00014. [DOI] [PubMed] [Google Scholar]
- Rossion B, Jacques C. Does physical interstimulus variance account for early electrophysiological face sensitive responses in the human brain? Ten lessons on the N170. Neuroimage. 2008;39(4):1959–79. doi: 10.1016/j.neuroimage.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Spehlmann R. The average electrical responses to diffuse and to patterned light in the human. Electorencephology and Clinical Neuropsychology. 1965;19:560–9. doi: 10.1016/0013-4694(65)90241-5. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Curran T. A neural basis for expert object recognition. Psychological Science. 2001;12(1):43–7. doi: 10.1111/1467-9280.00308. [DOI] [PubMed] [Google Scholar]
- Thierry G, Martin C, Downing P, Pegna A. Controlling for interstimulus perceptual variance abolishes N170 face selectivity. Nature Neuroscience. 2007;10:505–11. doi: 10.1038/nn1864. [DOI] [PubMed] [Google Scholar]
- Tipples J, Johnston P, Mayes A. EEG correlates of congruent and incongruent gaze changes. Social Cognitive and Affective Neuroscience. 2013;8(5):509–14. doi: 10.1093/scan/nss024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler J, Gosling A, Duchaine B, Eimer M. The face-sensitive N170 component in developmental prosopagnosia. Neuropsychologia. 2012;50(14):3588–99. doi: 10.1016/j.neuropsychologia.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Webb SJ, Merkle K, Murias M, Richards T, Aylward E, Dawson G. ERP responses differentiate inverted but not upright face processing in adults with ASD. Social Cognitive and Affective Neuroscience. 2012;7:578–87. doi: 10.1093/scan/nsp002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young AW. Disorders of face perception. In: Calder AJ, Rhodes G, Johnson MH, Haxby JV, editors. The Oxford Handbook of Face Perception. Oxford: Oxford University Press; 2011. pp. 77–91. [Google Scholar]