Abstract
People evaluate members of their own social group more favorably and empathize more strongly with their ingroup members. Using electroencephalography (EEG), we explored whether resonant responses of sensorimotor cortex to the pain of others are modulated by the ethnicity of these others. White participants watched video clips of ethnic ingroup and outgroup hands, being either penetrated by a needle syringe or touched by a cotton swab, while EEG was recorded. Time-frequency analysis was applied to Laplacian-transformed signals from the sensors overlying sensorimotor cortex in order to assess event-related desynchronization and synchronization (ERD/ERS) of sensorimotor mu (7–12 Hz) and beta (13–30 Hz) rhythms. When watching needle injections, beta ERD was significantly stronger for ingroup compared with outgroup hands. This ethnicity bias was restricted to painful actions, as beta ERD for ingroup and outgroup hands neither differed when observing no-pain videos, nor during presentation of the hands without any treatment. Such vicarious sensorimotor activation could play a role in social interaction by enhancing the understanding of the feelings and reactions of others and hence facilitating behavioral coordination among group members.
Keywords: empathy, ingroup favoritism, racial bias, mu rhythm, mirror neurons
INTRODUCTION
One putative mechanism of how we bridge the divide between self and others is empathy, as it enables us to share and to experientially understand what others are feeling. Recent advances in social neuroscience have started to identify the neural mechanisms involved in empathy (Singer and Lamm, 2009; Decety, 2011, for review). Although earlier accounts predominantly stressed the role of affective representations (e.g. Singer et al., 2004), a large body of empirical evidence suggests that empathy can also be supported by sensorimotor resonance (for reviews, see Bastiaansen et al., 2009; Keysers et al., 2010; Bufalari and Ionta, 2013). While some of this evidence has been derived from functional magnetic resonance imaging (fMRI) studies (see Keysers et al., 2010, for review), specific signatures of sensorimotor mechanisms have been provided by electroencephalographic (EEG) studies. For instance, Bufalari et al. (2007) demonstrated that watching video clips which showed hands of others in painful situations resulted in modulation of early somatosensory evoked potential components.
Apart from event-related somatosensory potentials, EEG and magnetoencephalographic (MEG) investigations exploiting event-related changes in the central (Rolandic or sensorimotor) mu and beta rhythms provided another line of evidence. Mu (7–12 Hz) and beta (13–30 Hz) rhythms are spontaneous rhythmic oscillations that can be recorded over sensorimotor cortex using EEG/MEG (Niedermeyer, 2005). Both mu and beta rhythms are modulated in association with somatosensory and motor processing (for review, see Hari and Salmelin, 1997; Neuper and Pfurtscheller, 2001; Pfurtscheller and da Silva, 2005; Stančák, 2006), and the terms event-related desynchronization (ERD) and synchronization (ERS) have been introduced for event-related decreases and increases, respectively, in EEG/MEG oscillatory activity. Mu and beta ERD occur shortly after the onset of somatosensory stimulation, as well as before and during the execution and the imagination of movements. They are followed by a rebound (i.e. ERS) contingent upon the offset of somatosensory stimulation or movement execution/imagination. It has been repeatedly shown that mu/beta ERD and ERS, respectively, are associated with increases and decreases of regional cerebral blood flow and blood–oxygen-level dependent (BOLD) signal changes in sensorimotor cortex (Oishi et al., 2007; Formaggio et al., 2008, 2010; Ritter et al., 2009; Yuan et al., 2010; Arnstein et al., 2011; Stevenson et al., 2011).
Importantly, attenuation of sensorimotor mu and beta oscillations also occurs when individuals merely observe somatosensory stimulation or movements of other people (hitherto referred to as ‘targets’) (Babiloni et al., 2002; Cheyne et al., 2003; Avanzini et al., 2012). Furthermore, recent findings using both EEG (Perry et al., 2010) and MEG (Whitmarsh et al., 2011) suggest that observing pain inflicted in others results in stronger mu and beta ERD compared with observing non-painful interventions. There is also evidence that the vicarious experience of pain as reflected by ERD of the central rhythms can be modulated by processes such as the similarity between observer and target. More specifically, in line with previous fMRI findings (Lamm et al., 2010), mu ERD to the pain of others was modulated by the fact whether pain responses of a target are similar or dissimilar to the observer’s own pain responses (Perry et al., 2010).
Identification with a social group is one of the crucial determinants of personal identity (Ellemers et al., 2002). There is a substantial body of social psychology research demonstrating that group identity (e.g. ethnic, religious or political) has a powerful impact on social perceptions, emotions and behavior. This research revealed that people generally evaluate members of one’s own group more favorably than outgroup members, a phenomenon called ingroup favoritism (Allport, 1954; Hewstone et al., 2002). Several behavioral and neuroscientific studies revealed that people empathize more strongly with ingroup than outgroup members (Stürmer et al., 2006; Tarrant et al., 2009; Dovidio et al., 2010; Cikara et al., 2011; Forgiarini et al., 2011; Gutsell and Inzlicht, 2012; Montalan et al., 2012; Trawalter et al., 2012). Ethnicity1 is a dominant and an easily recognizable signal of group affiliation (Zebrowitz et al., 2007; Ito and Bartholow, 2009). A handful of studies have recently shown that observing ethnic ingroup members undergoing painful needle injections resulted in stronger neural responses as compared with observing ethnic outgroup members undergo the same procedures (Xu et al., 2009; Mathur et al., 2010; Sheng and Han, 2012; Azevedo et al., 2013; Contreras-Huerta et al., 2013; Sheng et al., 2013, 2014; Sessa et al., 2014). However, most of these findings have documented activation differences in areas associated with the affective components of empathy, such as the medial cingulate or the anterior insular cortex.
The fact that previous fMRI studies have not been able to demonstrate consistently that ethnic group affiliation modulates activity in sensorimotor cortex might be attributed to the lower sensitivity of fMRI (e.g. Brázdil et al., 2005; Yuan et al., 2010) and the use of less sensitive experimental paradigms to identify somatosensory modulation (e.g. Keysers et al., 2010; Lamm et al., 2011). On the other hand, in a transcranial magnetic stimulation (TMS) study, Avenanti et al. (2010) demonstrated that a target's ethnicity influenced the effect of pain observation on motor evoked potentials (MEPs). This finding of modulation of motor system excitability indicates that observing pain in others could engage sensorimotor processes, depending upon the target’s ethnic group. However, MEPs reflect excitability of selective muscle motor representations at a specific point in time and provide little insight into activity of extended sensorimotor cortical networks and their dynamic modulation over time.
In this study, we therefore employed EEG to specifically assess empathy-related sensorimotor responses to the pain of ethnic ingroup and outgroup targets. To this end, we presented white Caucasian volunteers with videos of needle injections into the hands of white and black targets. We took advantage of the high temporal resolution of EEG and used Laplacian current source density (CSD) spatial filtering to assess with enhanced spatial sensitivity modulations of oscillatory activity of the sensorimotor cortex (Srinivasan, 2005; Tenke and Kayser, 2012). In line with the hypothesis of the contribution of sensorimotor processes to empathy (see also Lamm and Majdandžić, 2015 for critical discussion), we expected to find desynchronization of mu and beta rhythms to be stronger when white participants observed pain inflicted on ethnic ingroup (white targets) as compared with outgroup members (black targets).
METHODS
In this section, we briefly describe the main methods used in this study. Additional details are provided in the Supplementary material.
Participants
Sixty-nine healthy white participants participated in this study. Thirty-seven individuals (20 females and 17 males, 19–36 years, mean = 23.7) participated in the main experiment in which naturalistic videos were used. Thirty-two other participants (17 females and 15 males, 19–30 years, mean = 22.5) participated in a control experiment in which violet colored versions of the stimuli of the main experiment were employed.
Stimuli
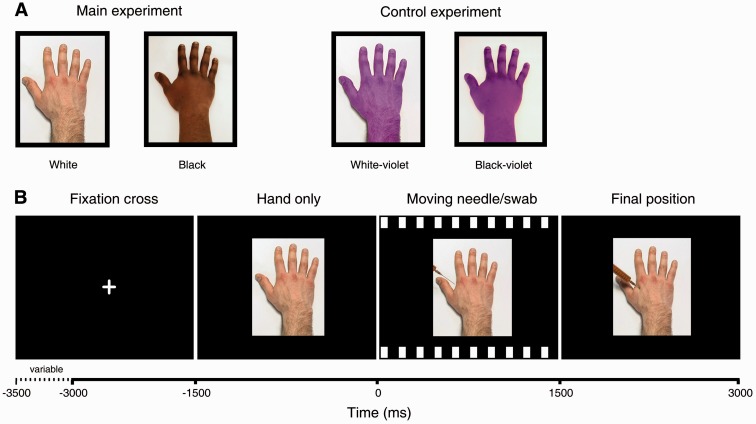
We used short videos developed by Avenanti et al. (2010) depicting the following situations: a needle syringe penetrating a white hand (White Pain condition) or a black hand (Black Pain); a cotton swab touching a white hand (White No-pain) or a black hand (Black No-pain). For the control experiment, we used the same stimuli, but the hands were digitally colored in violet (Figure 1A). In line with previous work (Avenanti et al., 2010; Azevedo et al., 2013), the aim of this experiment was to assess whether the expected ethnicity bias in the main experiment could be attributed to increased familiarity or similarity to ingroup white hands. Moreover, the control experiment allowed us to assess the influence of low-level visual features of the stimuli, such as brightness and shape, which might confound ethnicity effects. Figure 1B illustrates the sequence and the timing of stimuli within one trial. For each of the four experimental conditions, 60 trials were presented, resulting in a total of 240 trials. Stimuli were presented using E-Prime 2.0 (Psychology Software Tools, Inc., Sharpsburg, PA, USA).
Fig. 1.
Schematic display of the visual stimuli and their timing as used in the EEG experiment. A. Hands used as visual stimuli in the main and the control experiment. B. The trial sequence began with presenting a fixation cross (white on black background, duration varied between 1500 and 2000 ms), followed by a static display of a hand for a duration of 1500 ms. This static hand was followed by the video showing the action of hand treatment (i.e. motion of a needle syringe or a cotton swab, duration = 1500 ms). After the needle syringe or the cotton swab had reached their final position, a static display of this last frame of the video was shown for a duration of 1500 ms. This trial structure enabled us to assess unspecific responses to viewing pictures of hands of different ethnicity, as compared with the specific responses to painful or non-painful treatments of those hands.
Ratings of stimuli used in the EEG experiment
After finishing the EEG experiment participants were again presented with the videos to rate (1) how painful the situation was for the person whose hand was penetrated by a needle syringe or touched by the cotton swab (other-related painfulness) and (2) how unpleasant their own feelings were when watching the stimuli (self-related unpleasantness, i.e. negative affect). A 7-point Likert scale was used with values ranging from ‘not at all’ to ‘very much’.
Measures of ethnicity bias
Attitudes Towards Blacks (ATB) scale
To assess ethnic attitudes of study participants, we employed the ATB scale (Brigham, 1993). This questionnaire assesses ATB people in relation to various social issues such as urban crime, interracial marriage or racial integration in schools, businesses and residences.
Implicit Association Test (IAT)
We used the so-called race IAT to assess implicit ethnicity bias (Greenwald et al., 1998).2 Subjects categorized stimuli as belonging to the categories good or bad (words) and black or white (faces). Based on response latencies, the D-index was computed as a measure of implicit ethnicity bias according to the algorithm described by Greenwald et al. (2003).
Measures of dispositional empathy
Dispositional empathy was assessed by using the German version of the Interpersonal Reactivity Index (IRI; Davis, 1983; Paulus, 2009), a questionnaire measuring four aspects of empathy: Perspective Taking (PT), the ability to spontaneously take the perspective of others and to see things from their point of view; Fantasy Scale (FS) the tendency to identify with other persons; Empathic Concern (EC), addressing feelings of concern toward others; Personal Distress (PD), assessing feelings of distress when observing others in need.
EEG recording and processing
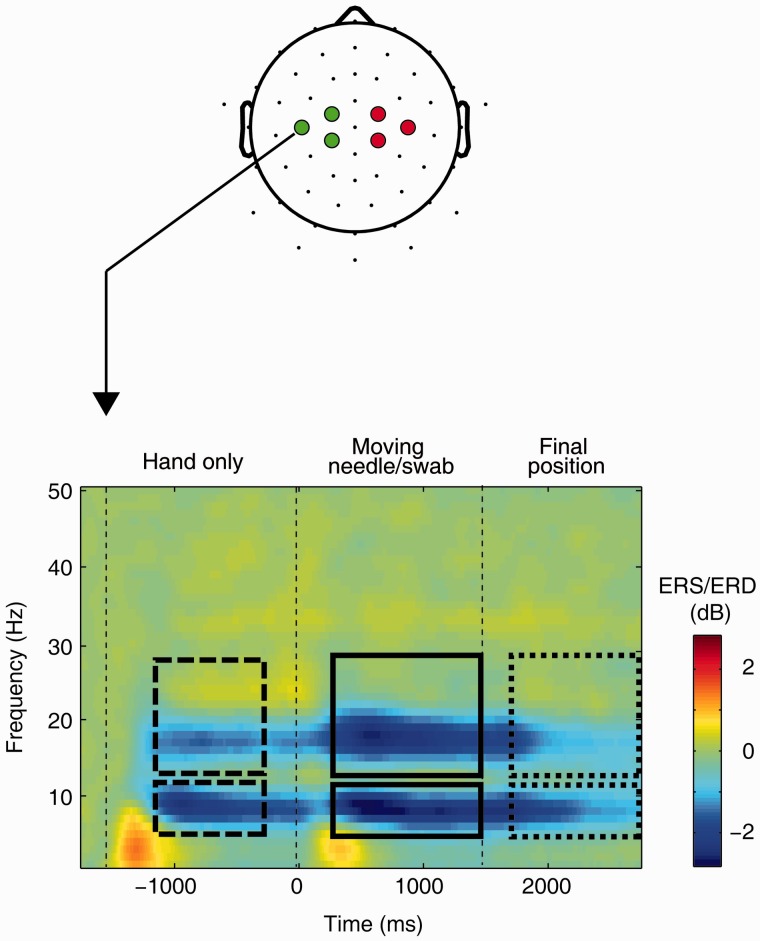
EEG was recorded from 59 equidistantly positioned electrodes. After initial signal processing and artifact removal using the EEGLAB toolbox (Delorme and Makeig, 2004), the EEG signals were transformed to reference-free scalp CSD in order to eliminate volume-conducted contributions from distant regions and hence signals likely not originating in sensorimotor cortex (Kayser, 2009). Sensors overlying left and right sensorimotor cortex were selected to represent regions of interest (ROIs, see Figure 2). In these channels, event-related spectral power modulation (ERD/ERS) was assessed for each subject and experimental condition and mean ERD/ERS was then calculated within the frequency bands 7–12 Hz (mu) and 13–30 Hz (beta) within each ROI.
Fig. 2.
Upper panel: Schematic drawing of a head depicting the EEG electrode positions (small black dots). Highlighted are those sensors selected for analysis, overlying the sensorimotor cortex (green circles: left ROI, red circles: right ROI). Lower panel: Grand mean CSD ERD/ERS from all subjects of the main experiment (n = 36), averaged across all four experimental conditions, at sensor C3. Rectangles depict mu and beta windows for analysis of experimental effects (dashed: perception of hand prior to treatment, solid: perception of dynamic treatment action, dotted: perception of static treatment endpoint). Note that since epoch finished at time = 3000 ms, the last window in which EEG spectrum was calculated was centered at time = 2746 ms (for more details see Supplementary material).
RESULTS
Behavioral data
We first assessed the effects of the videos on subjective ratings. Every participant rated needle penetration, compared with touching by a cotton swab, as more painful for the target and more unpleasant for themselves to observe, irrespective of hand skin color. Ratings of the no-pain videos showed a substantial floor effect. Ratings higher than zero occurred only sporadically and would have been classified as outliers by our outlier detection procedure (see Supplementary materials). Thus, the effects of target's ethnicity on the ratings were only assessed for the pain videos. This analysis revealed (Table 1) that, in line with our prediction, painfulness ratings were significantly higher for ethnic ingroup targets than outgroup targets (P = 0.039). Ratings of self-experienced unpleasantness showed a trend for an ethnicity ingroup bias (P = 0.064).
Table 1.
General linear model (GLM) analysis of the experimental effects on ratings of videos: naturalistic videos (main experiment)
| Ratings | White | Black | t(36) | P (one-tailed) |
|---|---|---|---|---|
| Painfulnessa | 4.48 ± 0.17 | 4.22 ± 0.23 | 1.815 | 0.039 |
| Unpleasantness | 4.18 ± 0.26 | 4.05 ± 0.27 | 1.556 | 0.064 |
Observers rated on a 7-point scale how painful the situation was for the person whose hand was penetrated by a needle syringe and how unpleasant their own feelings were when watching the stimuli.
aEffect size: r = 0.290.
In the control experiment, in which violet-colored hands were used, needle penetration was rated as more painful and unpleasant and a substantial floor effect in no-pain conditions was present again. In contrast to the main experiment, however, we found no statistically significant difference in the ratings of white–violet vs black–violet hands (P-values >0.05, Table 2).
Table 2.
GLM analysis of the experimental effects on ratings of videos: violet colored videos (control experiment)
| Ratings | White-violet | Black-violet | t(31) | P (two-tailed) |
|---|---|---|---|---|
| Painfulness | 4.19 ± 0.20 | 4.03 ± 0.20 | 1.429 | 0.163 |
| Unpleasantness | 2.49 ± 0.28 | 2.44 ± 0.26 | 0.473 | 0.639 |
Next, we analyzed the relationship between ratings of the pain videos and dispositional measures of empathy and ethnicity bias. For this purpose we calculated repeated measures analysis of covariance (RM ANCOVA) with the within-subject variable ethnicity (white vs black) and the test/questionnaire score as a covariate. The effects of IRI subscales on the painfulness ratings were not significant (see Supplementary Tables ST1a–d), but including IRI scores to the model decreased the effect of ethnicity on painfulness ratings. In contrast, IRI scores were significantly associated with the ratings of unpleasantness. More specifically, pain videos were rated as inducing more unpleasant feelings in participants with higher scores on the FS (r = 0.485, P = 0.002, Supplementary Table ST2a), PD (r = 0.469, P = 0.002, Supplementary Table ST2c), and EC (r = 0.320, P = 0.027, Supplementary Table ST2b) subscales of the IRI. Ethnicity had no significant influence on this relationship. In the IAT, there was a significant bias toward Whites and against Blacks (as indexed by a significant IAT D–index: mean ± SEM = 0.41 ± 0.08, t(35) = 5.153, P < 0.001), but no significant relationship between IAT score and ratings was observed (Supplementary Tables ST1e and ST2e). Similarly, there was no significant association of ratings and ATB score (Supplementary Tables ST1f and ST2f).
EEG data
Perception of the videos modulated sensorimotor mu as well as beta rhythm. In all experimental conditions, a prominent ERD of mu and beta oscillations occurred (Figure 2). More specifically, the time-course of EEG changes was as follows: After hand onset (time = −1500 ms), we observed a first ERD which then gradually decreased in magnitude. Upon the onset of the intervention with needle or cotton swab (time = 0 ms), ERD increased again. After the needle or swab had reached its final position (time = 1500 ms), the magnitude of ERD again gradually decreased, with the decrease being more evident in the beta than in the mu band. Given this ERD pattern, a time window from −1200 to −300 ms was selected to assess effects related to perception of the hands prior to the intervention, while a time windows of 300–1500 and 1800–3000 ms, respectively, were selected to assess the effects related to observing the dynamic action and the final treatment result (Figure 2). Within these time windows mean mu (7–12 Hz) and beta (13–30 Hz) ERD/ERS were separately calculated (see the rectangles in Figure 2) for the left and right ROIs (over sensorimotor cortex), and analyzed separately per frequency band using repeated measured analysis of variance (RM ANOVA) with within-subject variables Ethnicity (white vs black), Treatment (painful vs non-painful, not applicable for analyzing ERD prior to treatment onset) and Hemisphere (left vs right ROI). In the control experiment, the same approach was used, while the variable Ethnicity was replaced by Hand (white–violet vs black–violet). The rationale of the control experiment was to allow us to explore the nature and possible confounds of ethnicity bias in empathy in the main experiment. Hence, given the findings in the main experiment (see below), detailed analyses of mu ERD, the period prior to treatment onset and behavioral associations in the control experiment were deemed unnecessary and hence not carried out.
Perception of hand prior to treatment onset
The statistical analysis confirmed that perception of hands elicited a significant decrease in mu and beta power compared with pre-stimulus baseline period (grand mean mu ERD/ERS = −1.30 ± 0.19 dB, F(1,34) = 46.75, P < 0.001; grand mean beta ERD/ERS = −0.57 ± 0.09 dB, F(1,35) = 42.75, P < 0.001). However, the factors Ethnicity and Hemisphere had no significant effect on neither mu nor on beta ERD (all main and interaction effects: P-values > 0.05, Supplementary Table ST3).
Perception of hand treatment—dynamic action
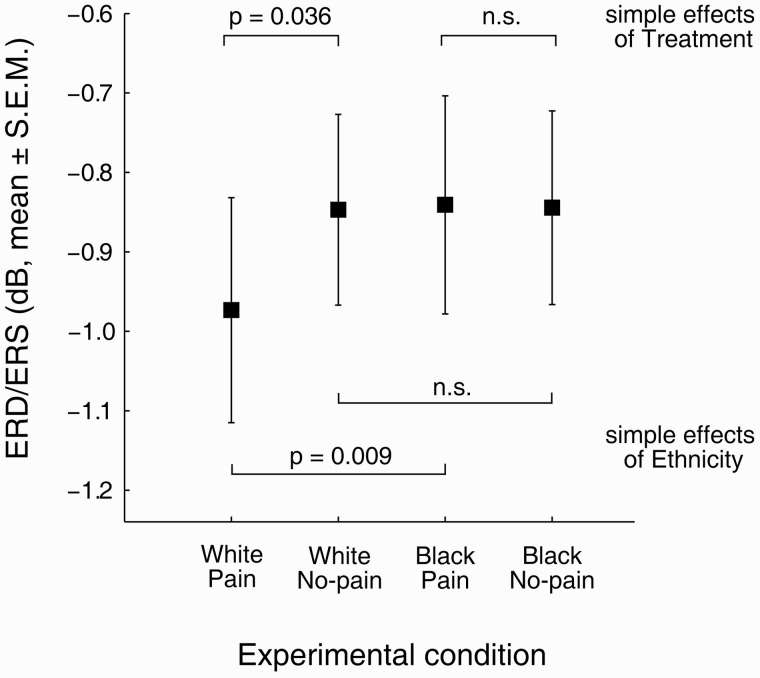
Perception of the hand treatment also elicited a significant suppression of mu and beta rhythms with respect to pre-stimulus baseline (grand mean mu ERD/ERS = −1.25 ± 0.33 dB, F(1,34) = 14.46, P = 0.001; grand mean beta ERD/ERS = −0.88 ± 0.13 dB, F(1,35) = 47.95, P < 0.001). The RM ANOVA (Table 3) showed that mu ERD was significantly stronger in response to videos of white compared with black hands (P < 0.001). However, this effect was observed irrespectively of whether pain or no pain was observed (Ethnicity × Treatment: P = 0.972). We also did not find a significant main effect of Treatment on mu ERD (P = 0.122). A different pattern of effects was observed for the beta rhythm (Table 4). Importantly, apart from a trend for a main effect of Ethnicity (P = 0.055) a significant interaction between Ethnicity and Treatment was obtained (P = 0.009, Figure 3). Simple effects analysis revealed that this interaction can be explained as follows: (i) for pain videos, white hands induced stronger beta ERD than black hands (effect of Ethnicity for pain videos: P = 0.009); in contrast, observation of non-painful treatments of black vs white hands did not differ (P = 0.940); (ii) for white hands, observing pain induced stronger ERD than observing no pain (effect of Treatment for white hands: P = 0.036) while no such effect of Treatment was present when observing videos of black hands (effect of Treatment for black hands: P = 0.942).
Table 3.
GLM analysis of the experimental effects on mu and beta ERD/ERS during perception of hand treatment action: mu ERD/ERS (7–12 Hz)
| Effect | F(1,34) | P |
|---|---|---|
| Ethnicitya | 13.936 | <0.001 |
| Treatment | 2.512 | 0.122 |
| Hemisphere | 0.019 | 0.890 |
| Ethnicity*Treatment | 0.001 | 0.972 |
| Ethnicity*Hemisphere | 2.050 | 0.161 |
| Treatment*Hemisphere | 0.115 | 0.736 |
| Ethnicity*Treatment*Hemisphere | 0.718 | 0.403 |
Model: 2 × 2 × 2 RM ANOVA; within-subject independent variables: Ethnicity, Treatment, Hemisphere; dependent variable: ERD/ERS (mean within a time window 300–1500 ms).
aEffect size: r = 0.539, White: −1.32 ± 0.33 dB, Black: −1.19 ± 0.33 dB
Table 4.
GLM analysis of the experimental effects on mu and beta ERD/ERS during perception of hand treatment action: beta ERD/ERS (13–30 Hz)
| Effect | F(1,35) | P |
|---|---|---|
| Ethnicity | 3.932 | 0.055 |
| Treatment | 1.600 | 0.214 |
| Hemisphere | 0.112 | 0.740 |
| Ethnicity*Treatmenta | 7.625 | 0.009 |
| Ethnicity*Hemisphere | 1.121 | 0.297 |
| Treatment*Hemisphere | 0.784 | 0.382 |
| Ethnicity*Treatment*Hemisphere | 0.049 | 0.827 |
Model: 2 × 2 × 2 RM ANOVA; within-subject independent variables: Ethnicity, Treatment, Hemisphere; dependent variable: ERD/ERS (mean within a time window 300–1500 ms).
aEffect size: r = 0.428, White Pain: −0.97 ± 0.14 dB, White No-pain: −0.85 ± 0.12, Black Pain: −0.84 ± 0.14 dB, Black No-pain: −0.84 ± 0.12 dB; simple effects of Treatment: (1) White Pain vs White No-pain, F(1,35) = 4.75, P = 0.036; (2) Black Pain vs Black No-pain, F(1,35) = 0.01, P = 0.942; simple effects of Ethnicity: (1) White Pain vs Black Pain, F(1,35) = 7.77, P = 0.009; (2) White No-pain vs Black No-pain, F(1,35) = 0.01, P = 0.940.
Fig. 3.
Mean beta (13–30 Hz) ERD/ERS for each experimental condition. Data are pooled from left and right ROIs. Negative values denote ERD, positive values denote ERS. Error bars represent the standard error of the mean.
In the control experiment, we did neither find a main effect of Treatment nor any interaction between Treatment and Hand on beta ERD (P-values >0.05, Supplementary Table ST4). The only significant effect was for the variable Hand (P = 0.010), which implied that white–violet hands generally resulted in more beta ERD, but irrespective of whether the intervention was painful or not.
In order to explore the nature of experimental effects on beta ERD in more detail, we analyzed the relationship between beta ERD and behavioral measures of ethnicity bias and empathy. Since no significant main and interaction effects of the factor Hemisphere were detected (Table 4) data from both hemispheres were pooled. In analogy to the analyses of the behavioral data, we added the score on the questionnaire/test as a continuous independent variable to the model and calculated RM ANCOVA. This revealed that beta ERD was significantly stronger in subjects with high scores on the IRI subscales of FS (r = −0.349, P = 0.010, Supplementary Table ST5a), EC (r = −0.374, P = 0.013, Supplementary Table ST5b), and PD (r = −0.360, P = 0.015, Supplementary Table ST5c). Two-way and three-way interactions of IRI with Ethnicity and Treatment were all not significant (P-values > 0.05), indicating that the relationship between IRI and beta ERD was similar in all experimental conditions. We found no significant main and interaction effects of IAT and ATB on beta ERD (P-values > 0.05, Supplementary Tables ST5e–f). For pain videos, we also tested the relationship between beta ERD and ratings of painfulness and unpleasantness, but found no significant associations.
Perception of hand treatment—static endpoint
Toward the end of the videos, when observing the motionless picture of a needle inserted into the hand or a swab contacting the hand, the magnitude of ERD decreased, especially in the beta band (Figure 2), but remained significant compared with the baseline period (grand mean mu ERD/ERS = −1.12 ± 0.20 dB, F(1,33) = 32.432, P < 0.001, grand mean beta ERD/ERS = −0.40 ± 0.10 dB, F(1,35) = 15.770, P < 0.001). The RM ANOVA (Supplementary Table ST6a) showed that mu ERD was significantly stronger in the pain than in the no-pain condition (main effect of Treatment: P = 0.046), but equally so for black and white hands (Treatment × Ethnicity: P = 0.177), and the main effect of Ethnicity was also not significant (P = 0.364). In contrast to the earlier, dynamic time period, we observed no significant main and interaction effects on beta ERD (Supplementary Table ST6b).
DISCUSSION
The aim of this study was to address the electrophysiological correlates of ethnicity bias during empathy for pain. Using spectral analysis of event-related EEG changes, we explored whether resonant responses of sensorimotor cortex elicited by observing painful treatment of target hands vary depending on the target’s ethnicity. We found that the sensorimotor beta rhythm was suppressed more strongly when observing painful compared with non-painful treatments of targets that were of the same (‘white’) ethnicity as the participants. In contrast, when observing targets of a different (‘black’) ethnicity, the suppression of beta oscillations was not different between painful and non-painful treatments. Similarly, in the control experiment using hands that had been colored in violet, beta changes also did not differ between painful and non-painful treatments. Notably, this evidence of an ethnicity bias was restricted to the observation of painful actions, as beta ERD for white and black hands neither differed when observing non-painful actions, nor during the initial presentation of static black and white hands at the onset of a trial. The control experiment also confirmed that the ingroup bias was not due to different brightness or shape of black and white hands since the difference in beta ERD between ‘white–violet’ and ‘black–violet’ stimuli was of equal magnitude when observing painful and non-painful treatments. Moreover, when debriefed at the completion of the control experiment, while participants had reported to notice differences in hand shape, none had reported suspicion that the hands varied in ethnicity (for additional commentary see Supplementary Discussion, paragraph 1).
In accordance with previous reports (Montalan et al., 2012; Trawalter et al., 2012; Contreras-Huerta et al., 2013), the estimates of how painful the observed needle injection might feel for the target were biased so that ethnic ingroup targets were considered to be in stronger pain than outgroup targets. Interestingly, the magnitude of this bias (and also of the IAT bias) was uncorrelated with the bias in beta ERD. Furthermore, in agreement with previous evidence (Cheng et al., 2008; Avenanti et al., 2009a; Martínez-Jauand et al., 2012; Schaefer et al., 2012), we found that reactive responses of sensorimotor cortex were positively associated with trait measures of interpersonal reactivity, being higher in individuals scoring higher in FS, EC as well as PD.
Our findings extend the knowledge on the involvement of sensorimotor processes in social cognition (see e.g. Keysers et al., 2010, for review). Previous studies had shown that the observation of bodily contacts activates sensorimotor cortex (Blakemore et al., 2005; Ebisch et al., 2008; Schaefer et al., 2009, 2012; Whitmarsh et al., 2011; Kuehn et al., 2013). We confirm these findings and, moreover, show that observing painful contacts activates sensorimotor cortex more strongly for ethnic ingroup than outgroup targets. We note, however, that conclusions must be drawn with caution since our findings are limited to a sample of white Caucasian subjects. In the remainder of the discussion, we will discuss the possible neurofunctional mechanisms underlying our findings and explore their implications for social cognition models of intergroup processes.
First, it is important to note that ethnicity bias in sensorimotor responses to pain in others was restricted to beta oscillatory activity. This may seem somewhat surprising given previous reports on changes in the mu range related to empathy (Cheng et al., 2008; Perry et al., 2010; Whitmarsh et al., 2011). We did find, however, stronger mu ERD in the pain compared with the no-pain conditions only within the last time window, when a static picture of the treatment endpoint was displayed, but this effect was similar for white and black hands. In contrast, beta ERD modulation related to ethnicity was only observed within the period in which the needle penetration was dynamically displayed. This suggests that ingroup bias in sensorimotor responses is only observable when using dynamic video stimuli. However, since the functional distinction between mu and beta ERD remains poorly understood, we have no straightforward explanation for the distinct reactivity of beta and mu oscillations.
Basically, our findings might be related to two specific functional processes, i.e. somatosensation and motor processing. Beta ERD is considered to index increased excitability of cortical neurons to incoming somatosensory signals (Steriade and Llinás, 1988; Pfurtscheller and da Silva, 2005; Stevenson et al., 2011) and numerous studies also implicate a specific role of beta oscillations in pain processing (see Hauck et al., 2008, for review). For instance, painful stimuli elicit stronger beta ERD than non-painful stimulation (Ploner et al., 2006; Stančák et al., 2007; Mancini et al., 2013; see also Fallon et al., 2013). Beta oscillations to painful stimuli also seem to be strongly influenced by contextual variables, such as attention, anticipation of stimuli and social signals, with accumulating evidence that such contextual effects are more readily observed for the beta compared with the mu/alpha band (Ohara et al., 2004; Senkowski et al., 2011; Voisin et al., 2011; Valentini et al., 2012; Yoshino et al., 2012; Mancini et al., 2013). Our finding may thus reflect biased vicarious somatosensory responding to observed pain, similar to that observed in touch perception (cf. Serino et al., 2009).
Apart from somatosensory/pain processing, a substantial body of research indicates that beta oscillations are also involved in motor processes (for review see Jenkinson and Brown, 2011; van Wijk et al., 2012; Kilavik et al., 2013). It is well established that beta oscillations decrease before and during movement execution (Pfurtscheller and da Silva, 2005; Kilavik et al., 2013) as well as during action observation (Babiloni et al., 2002; Avanzini et al., 2012). It has also been shown that the human motor system is activated in anticipation of a potential movement of the observed actor (Kilner et al., 2004; Avenanti et al., 2009b; Urgesi et al., 2010) and it is possible that such anticipatory motor activation, in this case related to an incoming defensive reaction to a painful bodily contact, is evoked more readily when observing people who are closer to us.
Engagement of motor processes in pain empathy is also indicated by a series of TMS experiments performed by Avenanti et al. They repeatedly reported that observing a needle penetrating the first dorsal interosseus (FDI) muscle attenuates MEPs of this muscle elicited by single TMS pulses delivered to contralateral primary motor cortex (e.g. Avenanti et al., 2005). Exploiting these findings in a study on ethnicity bias, MEP reduction of contralateral FDI was only obtained when participants witnessed painful injections into the FDI muscle of ethnic ingroup, but not of outgroup members (Avenanti et al., 2010). Using the same stimuli, we found an overall desynchronization of sensorimotor rhythms which, in the beta band, was strongest for pain of ingroup targets. Since mu and beta ERD is accompanied with MEP increase rather than decrease (Rossini et al., 1991; Chen et al., 1998; Lepage et al., 2008; Mäki and Ilmoniemi, 2010; Takemi et al., 2013) our findings seem at odds with those of Avenanti et al. There are several explanations that could reconcile these findings, though. First, in the paradigm we and Avenanti et al. had used, ERD might be related to somatosensory rather than motor responses. However, it would be unclear then why the recruitment of motor processes as indicated by the MEPs should not be accompanied by changes in EEG oscillations as well. Second, changes of MEPs could be caused by modulation of spinal, rather than cortical, excitability. This seems improbable either, since the findings of Avenanti et al. (2005) indicate that the modulation of MEPs by pain observation is of cortical origin. Third, because the MEP decrease is very selective, i.e. restricted to the muscle observed being penetrated, the spatially very restricted modulation of excitability may not affect scalp EEG. Moreover, it has been shown that while painful stimulation of fingers inhibits finger muscles it facilitates the effectors of the withdrawal reflex, including large muscles such as biceps brachii and deltoid (Kofler et al., 1998; Urban et al., 2004; for review see Bank et al., 2013), so that the net EEG oscillations detected on the scalp surface may be determined by this much more extensive excitability increase (cf. Avenanti et al., 2009b; for additional commentary, see also Supplementary Discussion, paragraph 2). This explanation seems the most probable as it is also consistent with fMRI findings, showing an increase in BOLD signal from motor cortex when observing painful compared with non-painful hand contacts (Lamm et al., 2007b). Further studies, however, are needed to draw more definite conclusions on the relationship between modulation of EEG and corticospinal excitability during perception of pain in others.
The increased readiness for reactive movements would be in line with the contention that pain acts as a particularly strong social signal which, apart from soliciting help, fulfills an important warning function in protection from imminent harm (e.g. Williams, 2002). It has been demonstrated that aversive visual primes, such as depictions of dangerous situations or painful injuries, increase excitability of motor cortex (Hajcak et al., 2007; Borgomaneri et al., 2014) and facilitate movements (Grecucci et al., 2011). The ingroup favoritism in action preparation is also indicated by a plethora of findings that people tend to behave in synchrony with ingroup rather than outgroup persons (Yabar et al., 2006; Bourgeois and Hess, 2008; Efferson et al., 2008; Gutsell and Inzlicht, 2010; Fu et al., 2012). Moreover, our own previous work has shown that increasing the self-relatedness of others by perspective taking manipulations results in increased activation in motor brain areas and increased motor mimetic reactions to others' pain (Lamm et al., 2007a, 2008; for additional commentary, see also Supplementary Discussion, paragraph 3).
While discussing our findings as either related to somatosensory or, respectively, to motor resonance, recent research has indicated that a principal conceptual distinction between somatosensory and motor processing may be arbitrary since these processes are tightly and inherently coupled (see Baker, 2007, for review). For instance, in macaques, several cortical areas located on both sides of the central sulcus contain multimodal sensory-motor neurons (for review see Fogassi et al., 2005; Graziano, 2006). Many multimodal cells, besides having motor fields, respond to tactile as well as visual stimuli, and such colocalization of neural responses was also demonstrated in human parietal and frontal cortices (Bremmer et al., 2001; Gentile et al., 2011). Typically, visual responses of the multimodal neurons are highest for objects moving toward their somatosensory receptive fields and approaching them (Graziano et al., 1997). Similarly, in our experiment beta ERD was maximal when the needle or the swab was moving toward the hand. Graziano et al. found that electric stimulations of regions of the precentral gyrus containing multimodal neurons elicit complex movements including defensive movements, such as fast retractions of the hand away from a potentially noxious object (Graziano et al., 2002). Recent findings indicate that these multimodal cortical areas map objects not only located near to one’s own body, but also to the body of another individual (Ishida et al., 2010; Brozzoli et al., 2013). Such a shared integrated representation of sensory events and actions is likely to support social interaction. This is confirmed by recent studies showing that negative implicit attitudes toward outgroup members are reduced after inducing illusions of membership of an ethnic outgroup body (Fini et al., 2013; Maister et al., 2013; Peck et al., 2013; Farmer et al., 2014; for additional commentary see Supplementary Discussion, paragraph 4).
In summary, considering our findings together with those of others on EEG changes related to sensorimotor processing, we would like to suggest that the ingroup bias revealed in our study could relate to activation of a sensorimotor network, which (i) detects with higher sensitivity when pain is inflicted in persons who are more similar to us, (ii) provides stronger predictive signals of others' immediate action and (iii) increases, in a mirror-like fashion, the readiness for reactive movements. However, based on the current experiment alone, this interpretation remains speculative and needs to be supported by additional data testing this claim.
CONCLUSIONS
Our study provides evidence that observing painful bodily contacts of ethnic ingroup targets evokes increased resonant activity of sensorimotor cortex, reflected by desynchronization of the beta rhythm. Such vicarious sensorimotor activation could play a role in social interaction by enhancing the understanding of the feelings and reactions of others and hence facilitating behavioral coordination among group members.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank Alessio Avenanti for providing the videos used in this study. C.L. acknowledges funding by the Viennese Science and Technology Fund (WWTF; projects CS11-005 and CS11-011). I.R. was supported by research grants by the Slovak Scientific Grant Agency (VEGA, projects 2/0080/13 and the 2/0093/14) and the Ministry of Health of the Slovak Republic (project 2012/52-SAV-2). N.P. and S.K. were supported by a master's thesis research grant of the University of Vienna.
Footnotes
1 Note that although the term racial has been mostly used in previous work, this term has some problematic connotations and implications in its public use (for instance motivating measures against certain racial groups based on their presumed biologically determined inferiority). We therefore prefer to use the term ethnicity as a more neutral description of what we are dealing with—i.e. differences between individuals in sociocultural and physical, but not in biological-genetic terms (AAPA, 1996).
2 There is an ongoing debate whether the race IAT (Greenwald et al., 1998) measures racism or rather basal ingroup/outgroup effects (see van Ravenzwaaij et al., 2011). Therefore, we decided to use the term ethnicity bias instead of racial bias also here.
REFERENCES
- AAPA. (1996). AAPA statement on biological aspects of race. American Journal of Physical Anthropology, 101(4), 569–70.
- Allport GW. The Nature of Prejudice. Reading, MA.: Addison-Wesley; 1954. [Google Scholar]
- Arnstein D, Cui F, Keysers C, Maurits NM, Gazzola V. μ-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. Journal of Neuroscience. 2011;31(40):14243–9. doi: 10.1523/JNEUROSCI.0963-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avanzini P, Fabbri-Destro M, Dalla Volta R, Daprati E, Rizzolatti G, Cantalupo G. The dynamics of sensorimotor cortical oscillations during the observation of hand movements: an EEG study. PLoS ONE. 2012;7(5):e37534. doi: 10.1371/journal.pone.0037534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nature Neuroscience. 2005;8(7):955–60. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Minio-Paluello I, Bufalari I, Aglioti SM. The pain of a model in the personality of an onlooker: influence of state-reactivity and personality traits on embodied empathy for pain. NeuroImage. 2009a;44(1):275–83. doi: 10.1016/j.neuroimage.2008.08.001. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Minio-Paluello I, Sforza A, Aglioti SM. Freezing or escaping? Opposite modulations of empathic reactivity to the pain of others. Cortex. 2009b;45(9):1072–7. doi: 10.1016/j.cortex.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Sirigu A, Aglioti SM. Racial bias reduces empathic sensorimotor resonance with other-race pain. Current Biology. 2010;20(11):1018–22. doi: 10.1016/j.cub.2010.03.071. [DOI] [PubMed] [Google Scholar]
- Azevedo RT, Macaluso E, Avenanti A, Santangelo V, Cazzato V, Aglioti SM. Their pain is not our pain: brain and autonomic correlates of empathic resonance with the pain of same and different race individuals. Human Brain Mapping. 2013;34(12):3168–81. doi: 10.1002/hbm.22133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babiloni C, Babiloni F, Carducci F, et al. Human cortical electroencephalography (EEG) rhythms during the observation of simple aimless movements: a high-resolution EEG study. NeuroImage. 2002;17(2):559–72. [PubMed] [Google Scholar]
- Baker SN. Oscillatory interactions between sensorimotor cortex and the periphery. Current Opinion in Neurobiology. 2007;17:649–55. doi: 10.1016/j.conb.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bank PJM, Peper CE, Marinus J, Beek PJ, van Hilten JJ. Motor consequences of experimentally induced limb pain: a systematic review. European Journal of Pain. 2013;17(2):145–57. doi: 10.1002/j.1532-2149.2012.00186.x. [DOI] [PubMed] [Google Scholar]
- Bastiaansen JA, Thioux M, Keysers C. Evidence for mirror systems in emotions. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2009;364(1528):2391–404. doi: 10.1098/rstb.2009.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J, Bristow D, Bird G, Frith C, Ward J. Somatosensory activations during the observation of touch and a case of vision-touch synaesthesia. Brain. 2005;128(Pt 7):1571–83. doi: 10.1093/brain/awh500. [DOI] [PubMed] [Google Scholar]
- Borgomaneri S, Gazzola V, Avenanti A. Temporal dynamics of motor cortex excitability during perception of natural emotional scenes. Social Cognitive and Affective Neuroscience. 2014;9(10):1451–7. doi: 10.1093/scan/nst139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois P, Hess U. The impact of social context on mimicry. Biological Psychology. 2008;77(3):343–52. doi: 10.1016/j.biopsycho.2007.11.008. [DOI] [PubMed] [Google Scholar]
- Brázdil M, Dobšík M, Mikl M, et al. Combined event-related fMRI and intracerebral ERP study of an auditory oddball task. NeuroImage. 2005;26(1):285–93. doi: 10.1016/j.neuroimage.2005.01.051. [DOI] [PubMed] [Google Scholar]
- Bremmer F, Schlack A, Shah NJ, et al. Polymodal motion processing in posterior parietal and premotor cortex: a human fMRI study strongly implies equivalencies between humans and monkeys. Neuron. 2001;29(1):287–96. doi: 10.1016/s0896-6273(01)00198-2. [DOI] [PubMed] [Google Scholar]
- Brigham JC. College students’ racial attitudes. Journal of Applied Social Psychology. 1993;23(23):1933–67. [Google Scholar]
- Brozzoli C, Gentile G, Bergouignan L, Ehrsson HH. A shared representation of the space near oneself and others in the human premotor cortex. Current Biology. 2013;23(18):1764–8. doi: 10.1016/j.cub.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Bufalari I, Aprile T, Avenanti A, Di Russo F, Aglioti SM. Empathy for pain and touch in the human somatosensory cortex. Cerebral Cortex. 2007;17(11):2553–61. doi: 10.1093/cercor/bhl161. [DOI] [PubMed] [Google Scholar]
- Bufalari I, Ionta S. The social and personality neuroscience of empathy for pain and touch. Frontiers in Human Neuroscience. 2013;7:393. doi: 10.3389/fnhum.2013.00393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Yaseen Z, Cohen LG, Hallett M. Time course of corticospinal excitability in reaction time and self-paced movements. Annals of Neurology. 1998;44(3):317–25. doi: 10.1002/ana.410440306. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Yang C-Y, Lin C-P, Lee P-L, Decety J. The perception of pain in others suppresses somatosensory oscillations: a magnetoencephalography study. NeuroImage. 2008;40(4):1833–40. doi: 10.1016/j.neuroimage.2008.01.064. [DOI] [PubMed] [Google Scholar]
- Cheyne D, Gaetz W, Garnero L, et al. Neuromagnetic imaging of cortical oscillations accompanying tactile stimulation. Brain Research. Cognitive Brain Research. 2003;17(3):599–611. doi: 10.1016/s0926-6410(03)00173-3. [DOI] [PubMed] [Google Scholar]
- Cikara M, Bruneau EG, Saxe RR. Us and them: intergroup failures of empathy. Current Directions in Psychological Science. 2011;20(3):149–53. [Google Scholar]
- Contreras-Huerta LS, Baker KS, Reynolds KJ, Batalha L, Cunnington R. Racial bias in neural empathic responses to pain. PLoS ONE. 2013;8(12):e84001. doi: 10.1371/journal.pone.0084001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44(1):113–26. [Google Scholar]
- Decety J. The neuroevolution of empathy. Annals of the New York Academy of Sciences. 2011;1231:35–45. doi: 10.1111/j.1749-6632.2011.06027.x. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods. 2004;134(1):9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Dovidio JF, Johnson JD, Gaertner SL, Pearson AR, Saguy T, Ashburn-Nardo L. Empathy and intergroup relations. In: Mikulincer M, Shaver PR, editors. Prosocial Motives, Emotions, and Behavior: The Better Angels of Our Nature (pp. 1–25) Washington, DC: American Psychological Association; 2010. [Google Scholar]
- Ebisch SJH, Perrucci MG, Ferretti A, Del Gratta C, Romani GL, Gallese V. The sense of touch: embodied simulation in a visuotactile mirroring mechanism for observed animate or inanimate touch. Journal of Cognitive Neuroscience. 2008;20(9):1611–23. doi: 10.1162/jocn.2008.20111. [DOI] [PubMed] [Google Scholar]
- Efferson C, Lalive R, Fehr E. The coevolution of cultural groups and ingroup favoritism. Science. 2008;321(5897):1844–9. doi: 10.1126/science.1155805. [DOI] [PubMed] [Google Scholar]
- Ellemers N, Spears R, Doosje B. Self and social identity. Annual Review of Psychology. 2002;53:161–86. doi: 10.1146/annurev.psych.53.100901.135228. [DOI] [PubMed] [Google Scholar]
- Fallon N, Chiu YH, Li X, Nurmikko TJ, Stancak A. Ipsilateral cortical activation in fibromyalgia patients during brushing correlates with symptom severity. Clinical Neurophysiology. 2013;124(1):154–63. doi: 10.1016/j.clinph.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Farmer H, Maister L, Tsakiris M. Change my body, change my mind: the effects of illusory ownership of an outgroup hand on implicit attitudes toward that outgroup. Frontiers in Psychology. 2014;4:1016. doi: 10.3389/fpsyg.2013.01016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fini C, Cardini F, Tajadura-Jiménez A, Serino A, Tsakiris M. Embodying an outgroup: the role of racial bias and the effect of multisensory processing in somatosensory remapping. Frontiers in Behavioral Neuroscience. 2013;7:165. doi: 10.3389/fnbeh.2013.00165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogassi L, Ferrari PF, Gesierich B, Rozzi S, Chersi F, Rizzolatti G. Parietal lobe: from action organization to intention understanding. Science. 2005;308(5722):662–7. doi: 10.1126/science.1106138. [DOI] [PubMed] [Google Scholar]
- Forgiarini M, Gallucci M, Maravita A. Racism and the empathy for pain on our skin. Frontiers in Psychology. 2011;2:108. doi: 10.3389/fpsyg.2011.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formaggio E, Storti SF, Avesani M, et al. EEG and FMRI coregistration to investigate the cortical oscillatory activities during finger movement. Brain Topography. 2008;21(2):100–11. doi: 10.1007/s10548-008-0058-1. [DOI] [PubMed] [Google Scholar]
- Formaggio E, Storti SF, Cerini R, Fiaschi A, Manganotti P. Brain oscillatory activity during motor imagery in EEG-fMRI coregistration. Magnetic Resonance Imaging. 2010;28(10):1403–12. doi: 10.1016/j.mri.2010.06.030. [DOI] [PubMed] [Google Scholar]
- Fu F, Tarnita CE, Christakis NA, et al. Evolution of in-group favoritism. Scientific Reports. 2012;2:460. doi: 10.1038/srep00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentile G, Petkova VI, Ehrsson HH. Integration of visual and tactile signals from the hand in the human brain: an fMRI study. Journal of Neurophysiology. 2011;105:910–22. doi: 10.1152/jn.00840.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano M. The organization of behavioral repertoire in motor cortex. Annual Review of Neuroscience. 2006;29:105–34. doi: 10.1146/annurev.neuro.29.051605.112924. [DOI] [PubMed] [Google Scholar]
- Graziano MSA, Taylor CSR, Moore T. Complex movements evoked by microstimulation of precentral cortex. Neuron. 2002;34:841–51. doi: 10.1016/s0896-6273(02)00698-0. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Hu XT, Gross CG. Visuospatial properties of ventral premotor cortex. Journal of Neurophysiology. 1997;77(5):2268–92. doi: 10.1152/jn.1997.77.5.2268. [DOI] [PubMed] [Google Scholar]
- Grecucci A, Koch I, Rumiati RI. The role of emotional context in facilitating imitative actions. Acta Psychologica. 2011;138(2):311–5. doi: 10.1016/j.actpsy.2011.07.005. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: the implicit association test. Journal of Personality and Social Psychology. 1998;74(6):1464–80. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and using the implicit association test: I. An improved scoring algorithm . Journal of Personality and Social Psychology. 2003;85(2):197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Gutsell JN, Inzlicht M. Empathy constrained: prejudice predicts reduced mental simulation of actions during observation of outgroups. Journal of Experimental Social Psychology. 2010;46(5):841–5. [Google Scholar]
- Gutsell JN, Inzlicht M. Intergroup differences in the sharing of emotive states: neural evidence of an empathy gap. Social Cognitive and Affective Neuroscience. 2012;7(5):596–603. doi: 10.1093/scan/nsr035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajcak G, Molnar C, George MS, Bolger K, Koola J, Nahas Z. Emotion facilitates action: a transcranial magnetic stimulation study of motor cortex excitability during picture viewing. Psychophysiology. 2007;44(1):91–7. doi: 10.1111/j.1469-8986.2006.00487.x. [DOI] [PubMed] [Google Scholar]
- Hari R, Salmelin R. Human cortical oscillations: a neuromagnetic view through the skull. Trends in Neurosciences. 1997;20(1):44–9. doi: 10.1016/S0166-2236(96)10065-5. [DOI] [PubMed] [Google Scholar]
- Hauck M, Lorenz J, Engel AK. Role of synchronized oscillatory brain activity for human pain perception. Reviews in the Neurosciences. 2008;19(6):441–50. doi: 10.1515/revneuro.2008.19.6.441. [DOI] [PubMed] [Google Scholar]
- Hewstone M, Rubin M, Willis H. Intergroup bias. Annual Review of Psychology. 2002;53:575–604. doi: 10.1146/annurev.psych.53.100901.135109. [DOI] [PubMed] [Google Scholar]
- Ishida H, Nakajima K, Inase M, Murata A. Shared mapping of own and others’ bodies in visuotactile bimodal area of monkey parietal cortex. Journal of Cognitive Neuroscience. 2010;22(1):83–96. doi: 10.1162/jocn.2009.21185. [DOI] [PubMed] [Google Scholar]
- Ito TA, Bartholow BD. The neural correlates of race. Trends in Cognitive Sciences. 2009;13(12):524–31. doi: 10.1016/j.tics.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson N, Brown P. New insights into the relationship between dopamine, beta oscillations and motor function. Trends in Neurosciences. 2011;34(12):611–8. doi: 10.1016/j.tins.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Kayser J. Current Source Density (CSD) Interpolation Using Spherical Splines—CSD Toolbox (Version 1.1) New York State Psychiatric Institute: Division of Cognitive Neuroscience; 2009. [Google Scholar]
- Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nature Reviews. Neuroscience. 2010;11(6):417–28. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- Kilavik BE, Zaepffel M, Brovelli A, Mackay W, Riehle A. The ups and downs of beta oscillations in sensorimotor cortex. Experimental Neurology. 2013;245:15–26. doi: 10.1016/j.expneurol.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Kilner JM, Vargas C, Duval S, Blakemore S-J, Sirigu A. Motor activation prior to observation of a predicted movement. Nature Neuroscience. 2004;7(12):1299–301. doi: 10.1038/nn1355. [DOI] [PubMed] [Google Scholar]
- Kofler M, Glocker FX, Leis AA, et al. Modulation of upper extremity motoneurone excitability following noxious finger tip stimulation in man: a study with transcranial magnetic stimulation. Neuroscience Letters. 1998;246(2):97–100. doi: 10.1016/s0304-3940(98)00243-2. [DOI] [PubMed] [Google Scholar]
- Kuehn E, Trampel R, Mueller K, Turner R, Schütz-Bosbach S. Judging roughness by sight—a 7-Tesla fMRI study on responsivity of the primary somatosensory cortex during observed touch of self and others. Human Brain Mapping. 2013;34(8):1882–95. doi: 10.1002/hbm.22031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. Journal of Cognitive Neuroscience. 2007a;19(1):42–58. doi: 10.1162/jocn.2007.19.1.42. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54(3):2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lamm C, Majdandžić J. The role of shared neural activations, mirror neurons, and morality for empathy—a critical comment. Neuroscience Research. 2015;90:15–24. doi: 10.1016/j.neures.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Lamm C, Meltzoff AN, Decety J. How do we empathize with someone who is not like us? A functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2010;22(2):362–76. doi: 10.1162/jocn.2009.21186. [DOI] [PubMed] [Google Scholar]
- Lamm C, Nusbaum HC, Meltzoff AN, Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS ONE. 2007b;2(12):e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Porges EC, Cacioppo JT, Decety J. Perspective taking is associated with specific facial responses during empathy for pain. Brain Research. 2008;1227:153–61. doi: 10.1016/j.brainres.2008.06.066. [DOI] [PubMed] [Google Scholar]
- Lepage J-F, Saint-Amour D, Théoret H. EEG and neuronavigated single-pulse TMS in the study of the observation/execution matching system: are both techniques measuring the same process? Journal of Neuroscience Methods. 2008;175(1):17–24. doi: 10.1016/j.jneumeth.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Maister L, Sebanz N, Knoblich G, Tsakiris M. Experiencing ownership over a dark-skinned body reduces implicit racial bias. Cognition. 2013;128(2):170–8. doi: 10.1016/j.cognition.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini F, Longo MR, Canzoneri E, Vallar G, Haggard P. Changes in cortical oscillations linked to multisensory modulation of nociception. European Journal of Neuroscience. 2013;37(5):768–76. doi: 10.1111/ejn.12080. [DOI] [PubMed] [Google Scholar]
- Martínez-Jauand M, González-Roldán AM, Muñoz MA, Sitges C, Cifre I, Montoya P. Somatosensory activity modulation during observation of other’s pain and touch. Brain Research. 2012;1467:48–55. doi: 10.1016/j.brainres.2012.05.055. [DOI] [PubMed] [Google Scholar]
- Mathur VA, Harada T, Lipke T, Chiao JY. Neural basis of extraordinary empathy and altruistic motivation. NeuroImage. 2010;51(4):1468–75. doi: 10.1016/j.neuroimage.2010.03.025. [DOI] [PubMed] [Google Scholar]
- Mäki H, Ilmoniemi RJ. EEG oscillations and magnetically evoked motor potentials reflect motor system excitability in overlapping neuronal populations. Clinical Neurophysiology. 2010;121(4):492–501. doi: 10.1016/j.clinph.2009.11.078. [DOI] [PubMed] [Google Scholar]
- Montalan B, Lelard T, Godefroy O, Mouras H. Behavioral investigation of the influence of social categorization on empathy for pain: a minimal group paradigm study. Frontiers in Psychology. 2012;3:389. doi: 10.3389/fpsyg.2012.00389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuper C, Pfurtscheller G. Event-related dynamics of cortical rhythms: frequency-specific features and functional correlates. International Journal of Psychophysiology. 2001;43(1):41–58. doi: 10.1016/s0167-8760(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Niedermeyer E. Normal EEG of the waking adult. In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. 5th edn. 2005. (pp. 167–192). Philadelphia: Lippincott, Williams and Wilkins. [Google Scholar]
- Ohara S, Crone NE, Weiss N, Lenz F. Attention to a painful cutaneous laser stimulus modulates electrocorticographic event-related desynchronization in humans. Clinical Neurophysiology. 2004;115(7):1641–52. doi: 10.1016/j.clinph.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Oishi N, Mima T, Ishii K, et al. Neural correlates of regional EEG power change. NeuroImage. 2007;36(4):1301–12. doi: 10.1016/j.neuroimage.2007.04.030. [DOI] [PubMed] [Google Scholar]
- Paulus C. The Saarbrueck Personality Questionnaire on Empathy: Psychometric evaluation of the German version of the Interpersonal Reactivity Index. Saarbrücken: Universität des Saarlandes; 2009. [Google Scholar]
- Peck TC, Seinfeld S, Aglioti SM, Slater M. Putting yourself in the skin of a black avatar reduces implicit racial bias. Consciousness and Cognition. 2013;22(3):779–87. doi: 10.1016/j.concog.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Perry A, Bentin S, Bartal IB-A, Lamm C, Decety J. ‘Feeling’ the pain of those who are different from us: modulation of EEG in the mu/alpha range. Cognitive, Affective & Behavioral Neuroscience. 2010;10(4):493–504. doi: 10.3758/CABN.10.4.493. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, da Silva FL. EEG event-related desynchronization (ERD) and event-related synchronization (ERS) In: Niedermeyer E, Lopes da Silva F, editors. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields, 2005. 5th edn. (pp. 1003–1016). Philadelphia: Lippincott, Williams and Wilkins. [Google Scholar]
- Ploner M, Gross J, Timmermann L, Pollok B, Schnitzler A. Pain suppresses spontaneous brain rhythms. Cerebral Cortex. 2006;16(4):537–40. doi: 10.1093/cercor/bhj001. [DOI] [PubMed] [Google Scholar]
- Ritter P, Moosmann M, Villringer A. Rolandic alpha and beta EEG rhythms’ strengths are inversely related to fMRI-BOLD signal in primary somatosensory and motor cortex. Human Brain Mapping. 2009;30(4):1168–87. doi: 10.1002/hbm.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Desiato MT, Lavaroni F, Caramia MD. Brain excitability and electroencephalographic activation: non-invasive evaluation in healthy humans via transcranial magnetic stimulation. Brain Research. 1991;567(1):111–9. doi: 10.1016/0006-8993(91)91442-4. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Heinze H-J, Rotte M. Embodied empathy for tactile events: interindividual differences and vicarious somatosensory responses during touch observation. NeuroImage. 2012;60(2):952–7. doi: 10.1016/j.neuroimage.2012.01.112. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Xu B, Flor H, Cohen LG. Effects of different viewing perspectives on somatosensory activations during observation of touch. Human Brain Mapping. 2009;30(9):2722–30. doi: 10.1002/hbm.20701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkowski D, Kautz J, Hauck M, Zimmermann R, Engel AK. Emotional facial expressions modulate pain-induced beta and gamma oscillations in sensorimotor cortex. Journal of Neuroscience. 2011;31(41):14542–50. doi: 10.1523/JNEUROSCI.6002-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serino A, Giovagnoli G, Làdavas E. I feel what you feel if you are similar to me. PLoS ONE. 2009;4(3):e4930. doi: 10.1371/journal.pone.0004930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa P, Meconi F, Castelli L, Dell’Acqua R. Taking one’s time in feeling other-race pain: an event-related potential investigation on the time-course of cross-racial empathy. Social Cognitive and Affective Neuroscience. 2014;9(4):454–63. doi: 10.1093/scan/nst003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng F, Han S. Manipulations of cognitive strategies and intergroup relationships reduce the racial bias in empathic neural responses. NeuroImage. 2012;61(4):786–97. doi: 10.1016/j.neuroimage.2012.04.028. [DOI] [PubMed] [Google Scholar]
- Sheng F, Liu Y, Zhou B, Zhou W, Han S. Oxytocin modulates the racial bias in neural responses to others’ suffering. Biological Psychology. 2013;92(2):380–6. doi: 10.1016/j.biopsycho.2012.11.018. [DOI] [PubMed] [Google Scholar]
- Sheng F, Liu Q, Li H, Fang F, Han S. Task modulations of racial bias in neural responses to others’ suffering. NeuroImage. 2014;88:263–70. doi: 10.1016/j.neuroimage.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Singer T, Lamm C. The social neuroscience of empathy. Annals of the New York Academy of Sciences. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Srinivasan R. High-resolution EEG: theory and practice. In: Handy TC, editor. Event-Related Potentials: A Methods Handbook. Cambrigde, MA.: MIT Press; 2005. [Google Scholar]
- Stančák A. Cortical oscillatory changes occurring during somatosensory and thermal stimulation. Progress in Brain Research. 2006;159:237–52. doi: 10.1016/S0079-6123(06)59016-8. [DOI] [PubMed] [Google Scholar]
- Stančák A, Poláček H, Vrána J, Mlynář J. Cortical oscillatory changes during warming and heating in humans. Neuroscience. 2007;147(3):842–52. doi: 10.1016/j.neuroscience.2007.04.055. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinás RR. The functional states of the thalamus and the associated neuronal interplay. Physiological Reviews. 1988;68(3):649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Stevenson CM, Brookes MJ, Morris PG. β-Band correlates of the fMRI BOLD response. Human Brain Mapping. 2011;32(2):182–97. doi: 10.1002/hbm.21016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stürmer S, Snyder M, Kropp A, Siem B. Empathy-motivated helping: the moderating role of group membership. Personality & Social Psychology Bulletin. 2006;32:943–56. doi: 10.1177/0146167206287363. [DOI] [PubMed] [Google Scholar]
- Takemi M, Masakado Y, Liu M, Ushiba J. Event-related desynchronization reflects downregulation of intracortical inhibition in human primary motor cortex. Journal of Neurophysiology. 2013;110(5):1158–66. doi: 10.1152/jn.01092.2012. [DOI] [PubMed] [Google Scholar]
- Tarrant M, Dazeley S, Cottom T. Social categorization and empathy for outgroup members. British Journal of Social Psychology. 2009;48(3):427–46. doi: 10.1348/014466608X373589. [DOI] [PubMed] [Google Scholar]
- Tenke CE, Kayser J. Generator localization by current source density (CSD): implications of volume conduction and field closure at intracranial and scalp resolutions. Clinical Neurophysiology. 2012;123(12):2328–45. doi: 10.1016/j.clinph.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trawalter S, Hoffman KM, Waytz A. Racial bias in perceptions of others’ pain. PLoS ONE. 2012;7(11):e48546. doi: 10.1371/journal.pone.0048546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban PP, Solinski M, Best C, Rolke R, Hopf HC, Dieterich M. Different short-term modulation of cortical motor output to distal and proximal upper-limb muscles during painful sensory nerve stimulation. Muscle and Nerve. 2004;29(5):663–9. doi: 10.1002/mus.20011. [DOI] [PubMed] [Google Scholar]
- Urgesi C, Maieron M, Avenanti A, Tidoni E, Fabbro F, Aglioti SM. Simulating the future of actions in the human corticospinal system. Cerebral Cortex. 2010;20(11):2511–21. doi: 10.1093/cercor/bhp292. [DOI] [PubMed] [Google Scholar]
- Valentini E, Liang M, Aglioti SM, Iannetti GD. Seeing touch and pain in a stranger modulates the cortical responses elicited by somatosensory but not auditory stimulation. Human Brain Mapping. 2012;33(12):2873–84. doi: 10.1002/hbm.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ravenzwaaij D, van der Maas HLJ, Wagenmakers E-J. Does the name-race Implicit Association Test measure racial prejudice? Experimental Psychology. 2011;58:271–7. doi: 10.1027/1618-3169/a000093. [DOI] [PubMed] [Google Scholar]
- Van Wijk BCM, Beek PJ, Daffertshofer A. Neural synchrony within the motor system: what have we learned so far? Frontiers in Human Neuroscience. 2012;6:252. doi: 10.3389/fnhum.2012.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voisin JIA, Marcoux L-A, Canizales DL, Mercier C, Jackson PL. I am touched by your pain: limb-specific modulation of the cortical response to a tactile stimulation during pain observation. Journal of Pain. 2011;12(11):1182–89. doi: 10.1016/j.jpain.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Whitmarsh S, Nieuwenhuis ILC, Barendregt HP, Jensen O. Sensorimotor alpha activity is modulated in response to the observation of pain in others. Frontiers in Human Neuroscience. 2011;5:91. doi: 10.3389/fnhum.2011.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AC. Facial expression of pain: an evolutionary account. The Behavioral and Brain Sciences. 2002;25(4):439–55. doi: 10.1017/s0140525x02000080. [DOI] [PubMed] [Google Scholar]
- Xu X, Zuo X, Wang X, Han S. Do you feel my pain? Racial group membership modulates empathic neural responses. Journal of Neuroscience. 2009;29(26):8525–9. doi: 10.1523/JNEUROSCI.2418-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabar Y, Johnston L, Miles L, Peace V. Implicit behavioral mimicry: investigating the impact of group membership. Journal of Nonverbal Behavior. 2006;30(3):97–113. [Google Scholar]
- Yoshino A, Okamoto Y, Onoda K, et al. Sadness enhances the experience of pain and affects pain-evoked cortical activities: an MEG study. Journal of Pain. 2012;13(7):628–35. doi: 10.1016/j.jpain.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Yuan H, Liu T, Szarkowski R, Rios C, Ashe J, He B. Negative covariation between task-related responses in alpha/beta-band activity and BOLD in human sensorimotor cortex: an EEG and fMRI study of motor imagery and movements. NeuroImage. 2010;49(3):2596–606. doi: 10.1016/j.neuroimage.2009.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zebrowitz LA, Bronstad PM, Leek HK. The contribution of face familiarity to ingroup favoritism and stereotyping. Social Cognition. 2007;25(2):306–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.