Abstract
In search of causative factors of social anxiety disorder (SAD), classical conditioning has been discussed as a potential trigger mechanism for many years. Recent findings suggest that the social relevance of the unconditioned stimulus (US) might play a major role in learning theories of SAD. Thus, this study applied a social conditioning paradigm with disorder-relevant US to examine the electrocortical correlates of affective learning. Twenty-four high socially anxious (HSA) and 23 age- and gender-matched low socially anxious (LSA) subjects were conditioned to 3 different faces flickering at a frequency of 15 Hz which were paired with auditory insults, compliments or neutral comments (US). The face-evoked electrocortical response was measured via steady-state visually evoked potentials and subjective measures of valence and arousal were obtained. Results revealed a significant interaction of social anxiety and conditioning, with LSA showing highest cortical activity to faces paired with insults and lowest activity to faces paired with compliments, while HSA did not differentiate between faces. No group differences were discovered in the affective ratings. The findings indicate a potentially impaired ability of HSA to discriminate between relevant and irrelevant social stimuli, which may constitute a perpetuating factor of SAD.
Keywords: fear conditioning, social anxiety, EEG, social stimuli, steady-state visually evoked potentials
INTRODUCTION
Learning mechanisms may play a major role in the etiology of anxiety disorders (Mineka and Zinbarg, 2006). Conditioning models discuss several factors as possible contributors to their onset, such as enhanced conditionability (Orr et al., 2000), difficulties to inhibit fear reactions in response to safety signals (Davis et al., 2000), a tendency to generalize conditioned fear to other stimuli (Lissek and Grillon, 2010) or delayed extinction (Hermann et al., 2002) (for a review, see Lissek et al., 2005).
Only a few studies examined the specific characteristics of learning mechanisms in patients with social anxiety disorder (SAD). An fMRI study found that SAD patients show enhanced activity in the amygdala and the hippocampus in response to neutral faces which had been paired with an aversive odor (Schneider et al., 1999). Another study using neutral faces [conditioned stimulus (CS)] and painful pressure [unconditioned stimulus (US)] reported insufficient differentiation among the reinforced danger (CS+) and the non-reinforced safety signal (CS−) in SAD patients (Veit et al., 2002). This effect might stem from a hyperactive fronto-limbic circuitry which had already been enhanced during habituation in comparison with healthy controls. A further study indicated delayed extinction and increased US expectancy for the CS− during acquisition in SAD (Hermann et al., 2002). The authors suggest that this bias refers to an overgeneralization of the conditioned response and adds to the development of pathologic anxiety. Similar effects were found in an investigation with colored lights serving as CS and air puffs serving as US. Although healthy controls kept their eyes open in response to the non-reinforced light, SAD patients did not only blink as a reaction to the danger, but also to the safety signal (Sachs et al., 2003).
Hence, SAD patients likely are not characterized by a generally enhanced conditionability, but differ in terms of resistance to extinction and discrimination of the CS+ and CS−. However, the aforementioned studies used stimuli of low ecological validity relating to the nature of SAD. As a consequence, it remains unclear whether socially anxious individuals react more sensitive to socially relevant stimuli.
So far, there is only little research investigating the neural correlates of social conditioning, which is the associative process whereby humans learn to identify individuals that have predicted threats or rewards in the past (Davis et al., 2010). Davis examined social learning with neutral faces and written verbal feedback and reported increased amygdala activation in response to faces paired with negative and positive comments. As fear of negative evaluation by others is a key criterion for SAD (American Psychiatric Association, 2000), Lissek et al. conducted a social conditioning study in SAD patients. Subjects were conditioned to three neutral faces with different types of audiovisual US: insults paired with angry faces, compliments paired with happy faces and neutral comments paired with neutral faces. The results indicated that only SAD showed a fear-potentiated startle in response to the CSneg compared with both CSneu and CSpos during conditioning, while HC did not (Lissek et al., 2008,b). However, it still has to be clarified whether the effect has to be attributed to the aversive verbal stimulus, the threatening facial expression or a mixture of both.
In terms of neurophysiology, functional anomalies in amygdala activity seem to be associated with social anxiety (Schneider et al., 1999; Veit et al., 2002). This is in line with numerous studies in animals and humans which provide evidence that the amygdala is involved in fear conditioning (Davis, 1992; LeDoux, 2003) and reacts with increased activity to the CS+ compared with the CS− (LaBar et al., 1998; Cheng et al., 2003). Because of its central position, the amygdala has multiple connections to brain areas which are essential for fear conditioning, such as sensory cortices, thalamus, hippocampus and temporal, orbito- and prefrontal cortices (LeDoux, 2000). Thus, it is provided with sensory information, possesses the ability to associate co-occurring stimuli and is endowed with fiber tracts which pass information on to structures responsible for long-term storage. Lesions of the amygdala, especially its lateral nucleus, impair fear conditioning (LeDoux et al., 1990; Campeau and Davis, 1995). In the same vein, it is reasonable to assume that chronically enhanced amygdala activity can impair conditioning processes as well. In this line, a meta-analysis found hyperactivation of the amygdala in SAD which might contribute to deviances in conditioning processes in this disorder (Etkin and Wager, 2007).
Although past research on fear conditioning often focused on differences in regional brain activations, recent investigations examined how acquired fears alter the temporal dynamics of cortical sensory processing (for a review, see Miskovic and Keil, 2012). A suitable method to measure cortical engagement in response to visual stimuli is the technique of steady-state visual evoked potentials (ssVEPs). SsVEPs are periodic signals with a constant frequency spectrum evoked by the presentation of oscillating visual stimuli (Vialatte et al., 2010). They are generated in the extended occipital cortex, especially V1, but also in higher order cortices (Di Russo et al., 2007), and reflect multiple excitations of the visual system as reaction to the same stimulus over a short period of time. Hence, changes in electrocortical activity can be caused by initial, bottom-up driven sensory processing as well as subsequent top-down regulation via higher order processing (Silberstein and Pipingas, 1995; Keil et al., 2001).
Besides their robustness to eye blink and movement artifacts (Perlstein et al., 2003), ssVEPs can be reliably separated from noise as their frequency equals that of the driving stimulus (Regan, 1989; Gray et al., 2003), and then be quantified in the frequency domain (Wang et al., 2007). In this regard, facilitated sensory processing is indicated by enhanced ssVEP amplitudes and can be interpreted as resource allocation to the flickering stimulus. As the frequency of the ssVEPs is known, they can not only be analyzed in the frequency domain but also in the time–frequency domain (Müller et al., 1998). This makes it possible to track the stimulus-evoked ssVEP response at the frequency of interest and get a continuous measure of visual cortical facilitation at near-optimum time resolution.
Numerous studies have shown that ssVEPs are sensitive to attentional and emotional processes with amplitude enhancement for attended compared with non-attended, and emotional compared with neutral stimuli (Müller et al., 1997, 1998; Kemp et al., 2002; Keil et al., 2003; Andersen and Müller, 2010; McTeague et al., 2011). With regard to conditioning, ssVEP amplitudes have been found to be reliably increased for CS+ compared with the CS− cues (Moratti and Keil, 2005; Moratti et al., 2006; Miskovic and Keil, 2012, 2013a,b; Wieser et al., 2014a,b). This effect has already been found with only 10 pairings of the CS+ with the US and is most prominent in a late temporal window of stimulus processing which precedes US onset. These adaptive changes in function of early visual cortices lead to augmented sensory gain and consequently enhanced perceptual processing of features predicting a temporally upcoming threatening event (Miskovic and Keil, 2012). It has been argued that this change in sensory processing during fear acquisition may reflect transient plasticity of sensory cortical networks (Keil et al., 2007).
In this study, ssVEPs were used to investigate the differences in visuocortical processing in response to differential conditioning with social stimuli in high and low socially anxious participants. Our aim was to test three hypotheses: first, we expected to find a general conditioning effect in all participants shown as enhanced ssVEP amplitude for faces paired with negative compared with neutral comments. Second, we assumed high socially anxious (HSA) to show higher ssVEP amplitudes to faces paired with socially threatening stimuli than low socially anxious (LSA). Third, we assumed HSA to be more resistant to extinction referring to socially threatening stimuli compared with LSA.
MATERIALS AND METHODS
Participants
Participants were undergraduate students at the University of Würzburg without any past or present psychiatric diagnosis (self-report), who were paid or received course credit for participation. 1112 students filled in a pre-screening questionnaire consisting of 5 items (Supplementary Table S1) based on the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria for social phobia (American Psychiatric Association, 2000), on a five-point Likert scale (0 = ‘strongly disagree’ to 4 = ‘strongly agree’), such that a maximum of 20 points could be achieved. Participants scoring from 2 to 4 points were classified as LSA and participants scoring from 12 to 14 points were classified as HSA. Twenty-four subjects per group took part in the study. One subject had to be excluded due to excessive artifacts in the electroencephalography (EEG) so that 47 subjects (HSA: n = 24; LSA: n = 23) were included in the statistical analysis.
Groups did not differ in terms of age [HSA: M = 20.1, s.d. = 2.0; LSA: M = 21.6, s.d. = 2.5; t(45) = 0.91, P = 0.37] and sex ratio [HSA: 15 women; LSA: 14 women; χ2 (1, N = 47) = 0.013, P = 0.91]. To ensure that the screening was successful, subjects again completed the pre-screening, the German version of the Social Phobia and Anxiety Inventory (SPAI; Turner et al., 1989; Fydrich, 2002) and the Social Phobia Inventory (SPIN; Connor et al., 2000). Because of missing data, the SPIN could not be analyzed for one person of each group. As expected, significant group differences were found in the total scores of the pre-screening [t(45) = 8.09, P < 0.001; HSA: M = 11.6, s.d. = 3.5; LSA: M = 3.7, s.d. = 3.1], SPIN [t(43) = 5.8, P < 0.001; HSA: M = 24.7, s.d. = 9.5; LSA: M = 10.2, s.d. = 6.9] and SPAI [t(45) = 8.8, P < 0.001; HSA: M = 91.6, s.d. = 14.5; LSA: M = 56.5, s.d. = 12.6]. Before the experimental task, subjects also completed a socio-demographic questionnaire, the State-Trait Anxiety Inventory (Spielberger et al., 1970), the Beck Depression Inventory (BDI; Beck et al., 1961; Hautzinger et al., 1994) and the Positive and Negative Affect Scale (Watson et al., 1988). Two of the HSA subjects scored above the cut-off of the BDI indicating sub-clinical depression. Because the exclusion of these subjects from analyses did not have a significant impact on the results, we decided to include them in our sample in order to enhance statistical power. Mean questionnaire scores are shown in Table 1.
Table 1.
Questionnaire characteristics of the participants
| Variable | HSA |
LSA |
t(45) | P value | ||
|---|---|---|---|---|---|---|
| M | s.d. | M | s.d. | |||
| Pre-screening | 11.58 | 3.54 | 3.70 | 3.13 | 8.09 | <0.001* |
| SPIN | 24.70 | 9.51 | 10.23 | 6.87 | 5.83 | <0.001* |
| SPAI | 91.55 | 14.45 | 56.48 | 12.62 | 8.84 | <0.001* |
| STAI state | 39.92 | 6.88 | 34.96 | 6.35 | 2.57 | 0.014* |
| STAI trait | 47.79 | 9.61 | 36.61 | 6.10 | 4.74 | <0.001* |
| BDI | 10.71 | 6.01 | 6.26 | 5.34 | 2.69 | 0.010* |
| PANAS_PA | 27.50 | 5.58 | 29.65 | 6.43 | 1.23 | 0.226 |
| PANAS_NA | 13.63 | 4.00 | 12.00 | 4.00 | 1.39 | 0.171 |
SPIN = Social Phobia Inventory; SPAI = Social Phobia and Anxiety Inventory; BDI = Beck Depression Inventory; STAI = State-Trait Anxiety Inventory; PANAS = Positive and Negative Affect Scale; PA = positive affect; NA = negative affect.
All participants gave written informed consent prior to participation. None of them had a family history of epilepsy and all reported normal or corrected-to-normal vision. The study was approved by the ethics committee of the medical department of the University of Würzburg.
Design and procedure
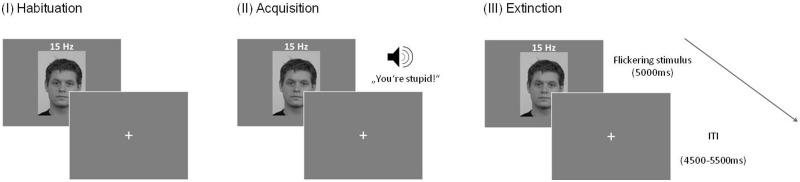
According to the standard of aversive conditioning paradigms, our experiment consisted of three phases: habituation, conditioning and extinction.
Three pictures of male actors with a neutral facial expression (CS) were selected from the Radboud Faces Database (Langner et al., 2010) and converted to grayscale to minimize differences in color and luminance (Supplementary Figure S1). All stimuli were displayed against a gray background on a 17-inch monitor using Presentation (Neurobehavioral Systems, Albany, CA). Faces were shown for 5000 msec, flickering at a driving frequency of 15 Hz to evoke ssVEPs. Each face was presented 20 times during habituation, conditioning and extinction (180 trials in total).
During conditioning, the faces were paired with acoustic stimuli (insults, neutral comments and compliments) serving as US. The US were created by recording 23 verbal comments of 1 sec length each with the audio-recorder Audacity (version 2.0), mostly adapted from Lissek et al. (2008a,b). They were rated in a pre-study relating to valence, arousal, naturalness and threat by 13 subjects. The five comments of each class which scored best (high valence for positive and low valence for negative comments, high arousal for negative and positive comments and low arousal for neutral comments, high instinctiveness for all classes, high threat for negative comments and low threat for neutral and positive comments) were chosen for the experiment (Supplementary Table S2). The US was replayed with a sound volume of 80 dB by Labtech speakers (Labtech International Ltd, East Sussex, UK) and a Kenwood KA-3010 amplifier (Kenwood Electronics, Heusenstamm, Germany). After each trial, a grey screen with a fixation cross was presented. These inter-trial intervals differed between 4500 and 5500 msec to prevent expectancy effects.
After completing the questionnaires, EEG electrodes were applied and participants were seated in a noise-reduced, darkened room 1 m distant to the screen. The experiment started with the habituation in which the three faces were presented to the participants in a randomized order. During acquisition, participants were conditioned to the faces by pairing each face with five US of the same category (negative, neutral or positive) in a pseudo-randomized order. Each of the five US was presented 4 times which resulted in 20 sentences per category and 60 trials in total. In the extinction phase, faces were presented again without reinforcement (Figure 1).
Fig. 1.
Schematic representation of the experimental procedure. In each phase, faces were presented for 5000 msec flickering at a frequency of 15 Hz, followed by an interstimulus interval (ISI) of 4500–5500 msec. During acquisition, the presentation of the faces was followed by negative, neutral or positive auditory comments. Each face was paired with comments of one of the three categories (negative, neutral or positive).
Subsequent to each experimental phase, participants rated the pictures on the dimensions of valence and arousal on the nine-point SAM scale (Self-Assessment Manikin; Bradley and Lang, 1994). After conditioning, they also conducted US expectancy ratings on a scale from 0% to 100% as a response to the question “What is the likelihood that the currently presented face is followed by a negative/neutral/positive comment?” as an index of the successful learning of the CS–US association.
EEG recording and data analysis
Electrocortical brain activity was measured via 129 electrodes using an Electrical Geodesics System (EGI, Eugene, OR) and referenced to the vertex electrode (Cz) (sampling rate 250 Hz, online bandpass 0.1–50 Hz). The threshold of impedances was kept below 50 kΩ as recommended for the Electrical Geodesics high-impedance amplifiers.
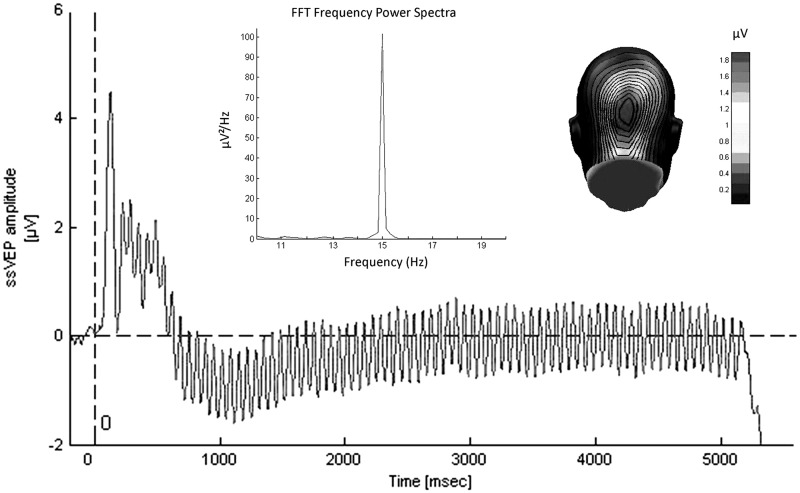
Off-line, the EEG signal was further processed using the software EMEGS (Electro Magnetic EncephaloGraphy) version 2.4 (Peyk et al., 2011) and MATLAB version 7.11.1 (Matrix Laboratory; MathWorks, Natick, MA). In a first step, a low-pass filter of 40 Hz was applied and the continuous signal was segmented into epochs of 600 msec before and 5600 msec after stimulus onset. In a second step, artifact rejection was conducted (according to Junghöfer et al., 2000). In a third step, the EEG signal was averaged for the nine different conditions. Figure 2 shows the raw ssVEP signal averaged across all subjects and conditions, its fast Fourier transformations and the spatial distribution of the driving frequency (15 Hz).
Fig. 2.
Grand mean ssVEPs averaged across all conditions and participants (N = 47), recorded from an occipital electrode. The upper panel shows the Fast Fourier Transformation of this signal with a peak at the driving frequency of the CS (15 Hz). The panel on the right depicts the topographic distribution of the 15 Hz signal on the back of a computer-modeled head. The strongest activity can be observed in the areas of the visual cortex.
The preprocessed data were further analyzed via time–frequency analysis to determine the strength of the driving frequency (15 Hz) during the time course of each trial. First, the ssVEP amplitude was extracted by means of Hilbert transformation using in-house written MATLAB scripts. A 20th-order Butterworth bandpass filter with a width of 0.5 Hz around the driving frequency of 15 Hz was applied to the data. Second, the time-varying amplitude was extracted by creating a phase-shifted version of the empirical signal with the help of the native Hilbert function executed in MATLAB and calculating the absolute value of the empirical and analytical signal. Thereupon, the mean ssVEP amplitude of each condition was assessed for the time interval between 200 and 5000 msec.
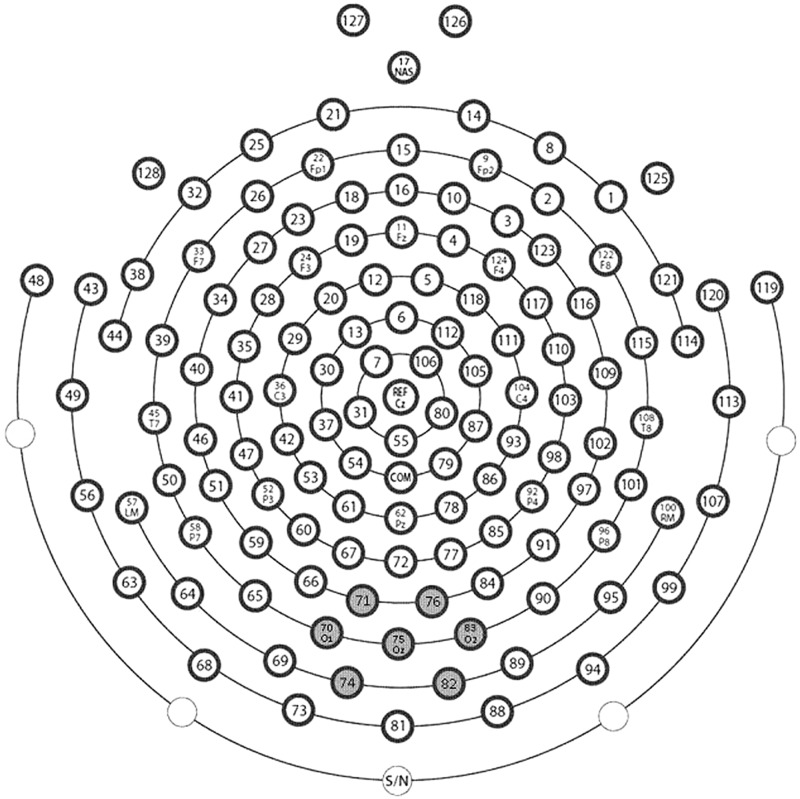
As the ssVEP signal was most pronounced over medial occipital sensors, it was spatially averaged across the occipital electrode and six surrounding electrodes (Figure 3).
Fig. 3.
Schematic representation of the electrode array. Gray background labels the electrodes used for the calculation of regional means in the statistical analysis. The inscriptions mark the approximated electrode names of the International 10–20 System.
Statistical analysis
Repeated measures analyses of variance (ANOVAs) with the within-subject factor CS type (negative vs neutral vs happy) and the between-subjects factor group (HSA vs LSA) were conducted for each phase of the experiment (habituation, conditioning, extinction) to analyze the mean ssVEP amplitudes. Significant differences including group were followed up by repeated measures ANOVAs for each group. In case of significant effects including valence of the CS, simple planned contrasts were calculated with the neutral condition as reference.
SAM and contingency ratings were analyzed by averaging the ratings across the three categories of the CS for valence, arousal and US expectancy, respectively, and calculating repeated measures ANOVAs with the within-subject factor CS type (negative vs neutral vs happy) and the between-subjects factor group (HSA vs LSA). For the US expectancy, we calculated hit and false alarm rates, indicating the probability by which subjects correctly identified which comments (negative, neutral or positive) followed a particular face, and wrongly assigned comments to a particular face, respectively. Follow-up tests were executed as described above.
A significance level of P < 0.05 (two tailed) was defined for all analyses. In case of violation of sphericity, Greenhouse–Geisser epsilon (GG-ε) and uncorrected degrees of freedom are reported (Picton et al., 2000). The partial eta-squared () is reported as a measure of effect size.
RESULTS
ssVEP amplitudes
No significant main effect of CS type, group or interaction was found during habituation, all P values > 0.10.
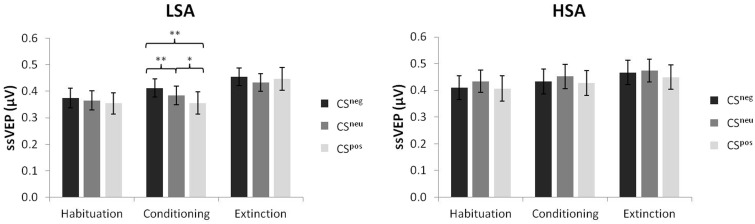
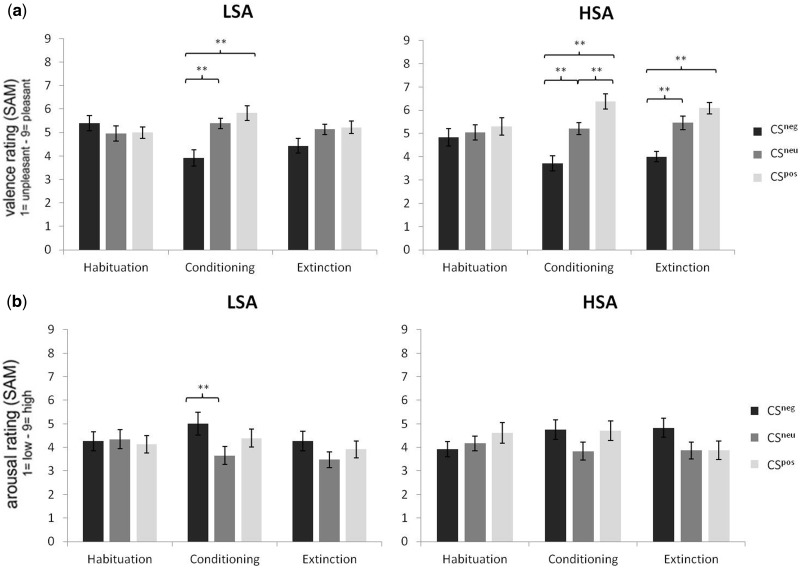
In the acquisition phase, a significant main effect of CS type [F(2,90) = 5.58, P = 0.005, = 0.11] and a significant CS type × group interaction [F(2,90) = 4.01, P = 0.021, = 0.08] were detected. Follow-up ANOVAs calculated for each group showed that this interaction was due to a significant main effect of CS type in the LSA group [F(2,44) = 9.13, P < 0.000, = 0.29], whereas in the HSA group the main effect of CS type did not reach significance [F(2,46) = 1.46, P = 0.243, = 0.06]. Planned contrasts indicated that LSA subjects reacted with significant larger ssVEP amplitudes to faces that had been paired with negative comments and marginally significant smaller ssVEP amplitudes to faces that had been paired with positive comments in comparison with neutral control comments [F(1,22) = 7.78, P = 0.011, = 0.26 and F(1,22) = 4.27, P = 0.051, = 0.16, respectively] (Figure 4). The main effect group was not significant [F(1,45) = 0.93, P = 0.339, = 0.02].
Fig. 4.
Mean ssVEP amplitudes and standard error across groups evoked by faces paired with negative, neutral or positive comments during habituation, conditioning and extinction (*P < 0.10; **P < 0.05).
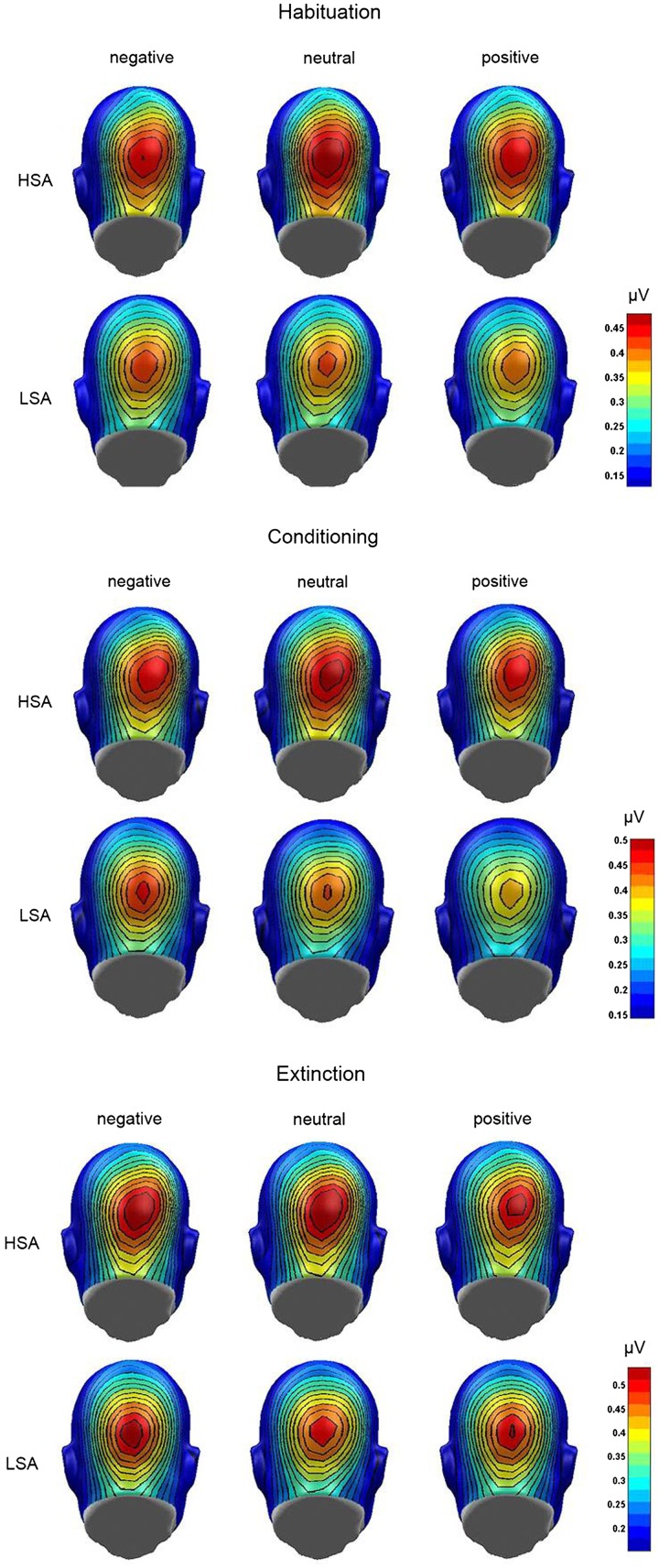
During extinction, neither significant main effects of CS type [F(2,90) = 0.63, P = 0.535, = 0.01] and group [F(1,45) = 0.09, P = 0.764, = 0.002] nor a significant interaction [F(2,90) = 1.46, P = 0.238, = 0.03] were found. Figure 5 displays the grand mean topographic distribution of the ssVEP signal in response to the CSpos, CSneu and CSneg for both groups during habituation, acquisition and extinction.
Fig. 5.
Grand mean topographic distribution of the ssVEP amplitudes across groups (LSA and HSA) in response to faces paired with negative, neutral or positive comments during habituation, conditioning and extinction.
In order to analyze the time course of electrocortical activity of affective learning, the ssVEP amplitudes were averaged in four consecutive segments (100–1300, 1300–2500, 2500–3700 and 3700–4900 msec), and an ANOVA including the factor Time was performed. As this analysis did not yield any new findings, only the results of the global analysis are reported (Table 2).
Table 2.
Mean ssVEP amplitude and standard deviations for the three phases of the experiment (habituation, acquisition and extinction) in LSA and HSA subjects
| LSA |
HSA |
|||
|---|---|---|---|---|
| M | s.d. | M | s.d. | |
| Habituation | ||||
| Negative | 0.37 | 0.18 | 0.41 | 0.22 |
| Neutral | 0.37 | 0.16 | 0.43 | 0.23 |
| Positive | 0.35 | 0.16 | 0.41 | 0.22 |
| Acquisition | ||||
| Negative | 0.41 | 0.17 | 0.43 | 0.20 |
| Neutral | 0.38 | 0.17 | 0.45 | 0.23 |
| Positive | 0.36 | 0.16 | 0.43 | 0.21 |
| Extinction | ||||
| Negative | 0.45 | 0.19 | 0.47 | 0.23 |
| Neutral | 0.43 | 0.20 | 0.47 | 0.23 |
| Positive | 0.45 | 0.21 | 0.45 | 0.23 |
SAM ratings
Valence
As expected, the ANOVA did not yield any significant effects during habituation. In the acquisition and extinction phase, the main effect CS type was significant, [F(2,90) = 24.27, GG-ε = 0.88, P < 0.001, = 0.35 and F(2,90) = 14.24, P < 0.001, = .24, respectively]. During extinction, this effect was further qualified by a marginally significant CS type × group interaction [F(2,90) = 2.73, P = 0.071, = 0.06].
Post hoc t-tests revealed that at acquisition, all subjects rated the CSpos (M = 6.11, s.d. = 1.54) as more pleasant and the CSneg (M = 3.81, s.d. = 1.64) as less pleasant in comparison with the CSneu (M = 5.30, s.d. = 1.18) [t(46) = 2.73, P = 0.009 and t(46) = 4.90, P < 0.001]. In the extinction phase, the CSneg (M = 4.21, s.d. = 1.32) was still rated as less pleasant in comparison with the CSneu (M = 5.30, s.d. = 1.25) and the CSpos (M = 5.66, s.d. = 1.31) [t(46) = 4.30, P < 0.001 and t(46) = 4.33, P < 0.001], but there was no longer a difference among the CSpos and the CSneu [t(46) = 1.39, P = 0.172].
Follow-up ANOVAs for each group during extinction indicated that although LSA did no longer discriminate between the three conditions referring to valence ratings [F(2,44) = 2.27, GG-ε = 0.75, P = 0.132, = 0.09], HSA still showed a significant main effect of CS type [F(2,46) = 15.18, P < 0.001, = 0.40]. This was due to the fact that HSA still rated the CSneg (M = 4.00, s.d. = 1.06) as less pleasant compared with both the CSneu (M = 5.46, s.d. = 1.44) and the CSpos (M = 6.08, s.d. = 1.21) [t(23) = 4.10, P < 0.001 and t(23) = 5.11, P < 0.001]. Means and standard deviations on the SAM scale are shown in Figure 6a.
Fig. 6.
Means and standard error on the SAM (Self-Assessment Manikin) scale for the self-report measures of valence (a) and arousal (b) during habituation, conditioning and extinction (*P < 0.10; **P < 0.05).
Arousal
As in the valence ratings, no differences were found during habituation, but a significant main effect of CS type was found during acquisition [F(2,90) = 5.39, P = 0.006, = 0.11] and extinction [F(2,90) = 4.13, P = 0.019, = 0.08]. The CS type × group interaction was neither significant during acquisition [F(2,90) = 0.35, P = 0.708, = 0.01] nor extinction [F(2,90) = 0.50, P = 0.610, = 0.01].
Further analyses with t-tests displayed higher arousal ratings for the CSneg (M = 4.87, s.d. = 2.17) and the CSpos (M = 4.55, s.d. = 1.92) compared with the CSneu (M = 3.74, s.d. = 1.82) [t(46) = 3.20, P = 0.003 and t(46) = 2.28, P = 0.027] in the acquisition phase. There was no significant difference between the CSneg and the CSpos [t(46) = 0.91, P = 0.368]. During extinction, the CSneg (M = 4.55, s.d. = 2.00) was still rated as more arousing in comparison with the CSneu (M = 3.68, s.d. = 1.67) [t(46) = 2.90, P = 0.006]. However, the difference among the CSpos (M = 3.89, s.d. = 1.80) and the CSneu was no longer significant [t(46) = 0.71, P = 0.483]. Comparison of CSneg and CSpos revealed that the CSpos was rated as marginally less arousing than the CSneg [t(46) = 1.95, P = 0.057] (Figure 6b).
US expectancy
Analysis of the contingency ratings revealed that there was no difference regarding the hit rate for faces paired with positive (M = 91.06, s.d. = 20.56), neutral (M = 91.49, s.d. = 18.06) or negative (M = 92.55, s.d. = 17.99) comments and there were no differences among groups. The calculated ANOVA yielded neither significant main effects of CS type or group [F(2,90) = 0.20, P = 0.819, = 0.004 and F(1,45) = 2.05, P = 0.159, = 0.044] nor interaction [F(2,90) = 1.92, P = 0.152, = 0.04].
Concerning the false alarm rate, we found a significant main effect of group [F(1,45) = 5.46, P = 0.024, < 0.11]. The main effect of CS type [F(2,90) = 0.09, GG-ε = 0.89, P = 0.891, = 0.002] and the CS type × group interaction [F(2,90) = 0.02, GG-ε = 0.89, P = 0.974, < 0.001] was not significant. Further analyses of the main effect group with t-tests indicated that HSA showed higher false alarm rates for faces paired with negative (M = 11.04, s.d. = 17.13) and neutral (M = 10.42, s.d. = 16.81) comments compared with LSA (M = 2.61, s.d. = 7.05 and M = 2.39, s.d. = 7.82, respectively) [t(45) = 2.22, P = 0.034 and t(45) = 2.11, P = 0.042]. The difference for faces paired with positive comments was marginally significant (HSA: M = 11.04, s.d. = 18.94; LSA: M = 3.26, s.d. = 8.20) [t(45) = 1.84, P = 0.075].
DISCUSSION
This study examined electrocortical activity in response to socially conditioned faces in HSA and LSA individuals. Results illustrate that although LSA showed differential visuocortical processing among the three different conditions, HSA only learned to discriminate them on a subjective level, but did not show any distinctions in the ssVEP amplitude. This indicates an impaired electrocortical differentiation between relevant and irrelevant social stimuli in HSA, and is consistent with data of studies in which reduced discrimination learning was observed in SAD (Hermann et al., 2002; Sachs et al., 2003). Interestingly, affective ratings did not reveal differences between groups, although a trend of diminished extinction was found in the valence ratings of HSA subjects. In line with this, former studies reported delayed extinction in social anxiety (Mineka and Zinbarg, 1996; Hermann et al., 2002; Sachs et al., 2003).
A primary aim of this study was to demonstrate that differential fear conditioning and concomitant changes in cortical activity cannot only be elicited by highly aversive non-social stimuli, such as loud tones (Morris and Dolan, 2004), aversive odors (Schneider et al., 1999; Hermann et al., 2002), or electric shocks (Straube et al., 2007; Alvarez et al., 2008), but also by less intensive social stimuli, such as verbal feedback. As association learning is facilitated when CS and US have a high (e.g. face/scream) compared with low belongingness (e.g. landscape/scream) (Hamm et al., 1989), we used faces as CS. So far, three studies reported that subjects can be conditioned to verbal comments in combination with simultaneously presented pictures of facial expressions (Lissek et al., 2008,b; Iidaka et al., 2010) or film clips as US (Pejic et al., 2013), but this is the first study showing successful conditioning using neutral faces as CS and isolated verbal comments as US.
In line with a recent study (Tinoco-González et al., 2015), our data do not support the hypothesis that SAD is characterized by enhanced conditionability relating to socially threatening stimuli: only LSA differed in their ssVEP amplitude in response to the three different categories of faces with highest amplitudes to the CSneg, indicating enhanced attention to events which predict something unpleasant or threatening (Öhman and Mineka, 2001). Interestingly, CSneu faces elicited slightly larger ssVEP amplitudes compared with CSpos faces, which might indicate that the faces paired with neutral comments gained ambiguous value, whereas the faces paired with positive comments clearly gained safety value. On the contrary, ssVEP amplitudes did not differ during conditioning in HSA no matter what verbal comment was paired with a face, which points to disturbed discrimination learning. Potentially, this impairment in HSA can be explained by hyperactivation of the amygdala during social situations. Support for this assumption stems from an fMRI study which found neutral social stimuli paired with aversive odor to activate the amygdala in social phobic individuals (Birbaumer et al., 1998). Moreover, a recent experiment found a positive correlation between social anxiety and amygdala activity in response to neutral faces paired with short film clips containing negative comments (Pejic et al., 2013). Furthermore, this view is strengthened by recent findings which suggest that enhanced sensory processing of the CS+ depends on the activation of the fear system (Moratti and Keil, 2005; Moratti et al., 2006; Miskovic and Keil, 2012).
Another plausible reason for our findings is that the experimental setting of ‘being tested’ itself, which might be experienced as potentially threatening in socially anxious, caused enough harassment to put them on the alert (Grillon, 2002). The therefore generally increased arousal might have demanded cognitive capacity which consequently blocked the acquisition of differences in danger and safety cues.
Overgeneralization may also underlie the pathology of SAD, which describes the transmission of a conditioned fear reaction from a particular stimulus to other stimuli which share its physical features. As reported above, patients with SAD have difficulties to discriminate danger and safety cues in differential conditioning (Hermann et al., 2002; Sachs et al., 2003). In line with this, our study found an US expectancy bias in HSA indicated by an increased false alarm rate which also points to poor discrimination learning. Decreased performance in stimulus differentiation might promote the tendency to generalize conditioned fear to stimuli which are similar to the CS+. In our case, it is possible that HSA transferred their fear of the CSneg to the CSneu and CSpos, as they expected the latter ones to be paired with negative comments as well. So far, overgeneralization has already been found in panic disorder and post-traumatic stress disorder (Lissek and Grillon, 2010; Lissek et al., 2010). Future studies might help to clarify whether overgeneralization is also a marker for SAD.
Although we found differences among groups in the electrocortical response, HSA and LSA did not differ in valence and arousal ratings of the CS. Discrepancies among implicit and explicit measures in social anxiety have been reported before with regard to electrocortical activity and ratings (Wieser et al., 2011), as well as behavioral and neural data (Bar-Haim et al., 2007; Moser et al., 2008). This may be due to different underlying learning mechanisms: electrocortical activity may represent expectancy as in signal learning, while valence ratings may rather represent cognitive aspects of learning as in evaluative conditioning (Blechert et al., 2008). Furthermore, in our study ssVEPs were collected online during the whole experiment, whereas ratings were obtained at the end of each phase which might have contributed to the observed findings. To rule out such an influence in the future, the experimental design could be improved by using online ratings, for instance for every second trial (Lissek et al., 2008a).
In contrast to acquisition, we did not find any differences among groups during extinction on the electrocortical level. A reason could be that the reinforcement rate of 100% during acquisition might have reduced the anxious apprehension related to the US which resulted in habituation during the last acquisition trials. Numerous investigations have shown the amygdala’s tendency to habituate toward a threatening stimulus over time (LaBar et al., 1998; Morris et al., 2001; Cheng et al., 2007). Future studies may address this issue by reducing the reinforcement rate, e.g. to 75%.
One limitation of this study is the fact that we examined a sub-clinical sample and no clinically diagnosed social phobics. Potentially, the observed effects would be more pronounced in a clinical sample or there might be additional effects, e.g. it has to be clarified whether diagnosed social phobics show differences during extinction in the ssVEP amplitude.
CONCLUSIONS
In summary, this study presents a paradigm to access social conditioning with disorder-specific stimuli as US in socially anxious subjects and controls. As our findings reveal, the distinctive feature of HSA might not be a selective enhanced conditionability to socially threatening stimuli but rather impaired discrimination learning, which deteriorates the ability to differentiate between friends and foes. Future studies need to explore to what extent the brain processes during social learning situations differ among social phobics and controls. The hereby gained insights might help to give new impulses for theories on learning mechanisms in SAD and their neural correlates.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This work was supported by the German Research Foundation (FOR 605, MU2299/1-3 and SFB/TRR-58 project B05).
REFERENCES
- Alvarez RP, Biggs A, Chen G, Pine DS, Grillon C. Contextual fear conditioning in humans: cortical-hippocampal and amygdala contributions. The Journal of Neuroscience. 2008;28(24):6211–9. doi: 10.1523/JNEUROSCI.1246-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. Washington, DC: American Psychiatric Publishing, Inc; 2000. [Google Scholar]
- Andersen SK, Müller MM. Behavioral performance follows the time course of neural facilitation and suppression during cued shifts of feature-selective attention. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(31):13878–82. doi: 10.1073/pnas.1002436107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4(6):561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Birbaumer N, Grodd W, Diedrich O, et al. fMRI reveals amygdala activation to human faces in social phobics. Neuroreport. 1998;9(6):1223–6. doi: 10.1097/00001756-199804200-00048. [DOI] [PubMed] [Google Scholar]
- Blechert J, Michael T, Williams SL, Purkis HM, Wilhelm FH. When two paradigms meet: does evaluative learning extinguish in differential fear conditioning? Learning and Motivation. 2008;39(1):58–70. [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Campeau S, Davis M. Involvement of subcortical and cortical afferents to the lateral nucleus of the amygdala in fear conditioning measured with fear-potentiated startle in rats trained concurrently with auditory and visual conditioned stimuli. The Journal of Neuroscience. 1995;15(3):2312–27. doi: 10.1523/JNEUROSCI.15-03-02312.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: stimulus processing versus response expression. Behavioral Neuroscience. 2003;117(1):3–10. doi: 10.1037//0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Richards J, Helmstetter FJ. Activity in the human amygdala corresponds to early, rather than late period autonomic responses to a signal for shock. Learning & Memory. 2007;14(7):485–90. doi: 10.1101/lm.632007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor KM, Davidson JRT, Churchill LE, Sherwood A, Weisler RH, Foa E. Psychometric properties of the Social Phobia Inventory (SPIN). New self-rating scale. The British Journal of Psychiatry. 2000;176(4):379–86. doi: 10.1192/bjp.176.4.379. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15(1):353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis FC, Johnstone T, Mazzulla EC, Oler JA, Whalen PJ. Regional response differences across the human amygdaloid complex during social conditioning. Cerebral Cortex. 2010;20(3):612–21. doi: 10.1093/cercor/bhp126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Falls WA, Gewirtz J. 2000. Neural systems involved in fear inhibition: extinction and conditioned inhibition. In: Myslobodsky, M., Weiner, I, editor. Contemporary Issues in Modeling Psychopathology. Boston: Kluwer Academic Publishers, 113–41. [Google Scholar]
- Di Russo F, Pitzalis S, Aprile T, et al. Spatiotemporal analysis of the cortical sources of the steady-state visual evoked potential. Human Brain Mapping. 2007;28(4):323–34. doi: 10.1002/hbm.20276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. The American Journal of Psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fydrich T. SPAI—Soziale Phobie und Angstinventar. In: Brähler E, Schumacher J, Strauß B, editors. Diagnostische Verfahren in der Psychotherapie. Göttingen: Hogrefe; 2002. 335–8. [Google Scholar]
- Gray M, Kemp AH, Silberstein RB, Nathan PJ. Cortical neurophysiology of anticipatory anxiety: an investigation utilizing steady state probe topography (SSPT) Neuroimage. 2003;20(2):975–86. doi: 10.1016/S1053-8119(03)00401-4. [DOI] [PubMed] [Google Scholar]
- Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biological Psychiatry. 2002;52(10):958–75. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Vaitl D, Lang PJ. Fear conditioning, meaning, and belongingness: a selective association analysis. Journal of Abnormal Psychology. 1989;98(4):395–406. doi: 10.1037//0021-843x.98.4.395. [DOI] [PubMed] [Google Scholar]
- Hautzinger M, Bailer M, Worall H, Keller F. Beck-depressions-inventar (BDI). Bearbeitung der deutschen Ausgabe. Testhandbuch. Bern: Huber; 1994. [Google Scholar]
- Hermann C, Ziegler S, Birbaumer N, Flor H. Psychophysiological and subjective indicators of aversive Pavlovian conditioning in generalized social phobia. Biological Psychiatry. 2002;52(4):328–37. doi: 10.1016/s0006-3223(02)01385-9. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Saito DN, Komeda H, et al. Transient neural activation in human amygdala involved in aversive conditioning of face and voice. Journal of Cognitive Neuroscience. 2010;22(9):2074–85. doi: 10.1162/jocn.2009.21347. [DOI] [PubMed] [Google Scholar]
- Junghöfer M, Elbert T, Tucker DM, Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37(4):523–32. [PubMed] [Google Scholar]
- Keil A, Gruber T, Müller MM. Functional correlates of macroscopic high-frequency brain activity in the human visual system. Neuroscience & Biobehavioral Reviews. 2001;25(6):527–34. doi: 10.1016/s0149-7634(01)00031-8. [DOI] [PubMed] [Google Scholar]
- Keil A, Gruber T, Müller M, et al. Early modulation of visual perception by emotional arousal: evidence from steady-state visual evoked brain potentials. Cognitive, Affective, & Behavioral Neuroscience. 2003;3(3):195–206. doi: 10.3758/cabn.3.3.195. [DOI] [PubMed] [Google Scholar]
- Keil A, Stolarova M, Moratti S, Ray WJ. Adaptation in human visual cortex as a mechanism for rapid discrimination of aversive stimuli. Neuroimage. 2007;36(2):472–9. doi: 10.1016/j.neuroimage.2007.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Gray MA, Eide P, Silberstein RB, Nathan PJ. Steady-state visually evoked potential topography during processing of emotional valence in healthy subjects. Neuroimage. 2002;17(4):1684–92. doi: 10.1006/nimg.2002.1298. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–45. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Langner O, Dotsch R, Bijlstra G, Wigboldus DHJ, Hawk ST, van Knippenberg A. Presentation and validation of the Radboud Faces Database. Cognition and Emotion. 2010;24(8):1377–88. [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23(1):155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The emotional brain, fear, and the amygdala. Cellular and Molecular Neurobiology. 2003;23(4–5):727–38. doi: 10.1023/A:1025048802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Cicchetti P, Xagoraris A, Romanski LM. The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. The Journal of Neuroscience. 1990;10(4):1062–9. doi: 10.1523/JNEUROSCI.10-04-01062.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Grillon C. Overgeneralization of conditioned fear in the anxiety disorders. Journal of Psychology. 2010;218(2):146–8. [Google Scholar]
- Lissek S, Biggs AL, Rabin SJ, et al. Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behaviour Research and Therapy. 2008a;46(5):678–87. doi: 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Levenson J, Biggs AL, et al. Elevated fear conditioning to socially relevant unconditioned stimuli in social anxiety disorder. The American Journal of Psychiatry. 2008b;165(1):124–32. doi: 10.1176/appi.ajp.2007.06091513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour Research and Therapy. 2005;43(11):1391–424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin S, Heller RE, et al. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. The American Journal of Psychiatry. 2010;167(1):47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Shumen JR, Wieser MJ, Lang PJ, Keil A. Social vision: sustained perceptual enhancement of affective facial cues in social anxiety. Neuroimage. 2011;54(2):1615–24. doi: 10.1016/j.neuroimage.2010.08.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. Conditioning and ethological models of anxiety disorders: stress-in-dynamic-context anxiety models. In: Hope DA, editor. Nebraska Symposium on Motivation, 1995: Perspectives on Anxiety, Panic, and Fear. Lincoln, Nebraska: University of Nebraska Press; 1996. 135–210. [PubMed] [Google Scholar]
- Mineka S, Zinbarg R. A contemporary learning theory perspective on the etiology of anxiety disorders: it's not what you thought it was. American Psychologist. 2006;61(1):10–26. doi: 10.1037/0003-066X.61.1.10. [DOI] [PubMed] [Google Scholar]
- Miskovic V, Keil A. Acquired fears reflected in cortical sensory processing: a review of electrophysiological studies of human classical conditioning. Psychophysiology. 2012;49(9):1230–41. doi: 10.1111/j.1469-8986.2012.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V, Keil A. Visuocortical changes during delay and trace aversive conditioning: evidence from steady-state visual evoked potentials. Emotion. 2013a;13(3):554–61. doi: 10.1037/a0031323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V, Keil A. Perceiving threat in the face of safety: excitation and inhibition of conditioned fear in human visual cortex. The Journal of Neuroscience. 2013b;33(1):72–8. doi: 10.1523/JNEUROSCI.3692-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moratti S, Keil A. Cortical activation during Pavlovian fear conditioning depends on heart rate response patterns: an MEG study. Cognitive Brain Research. 2005;25(2):459–71. doi: 10.1016/j.cogbrainres.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Moratti S, Keil A, Miller GA. Fear but not awareness predicts enhanced sensory processing in fear conditioning. Psychophysiology. 2006;43(2):216–226. doi: 10.1111/j.1464-8986.2006.00386.x. [DOI] [PubMed] [Google Scholar]
- Morris JS, Dolan RJ. Dissociable amygdala and orbitofrontal responses during reversal fear conditioning. Neuroimage. 2004;22(1):372–80. doi: 10.1016/j.neuroimage.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Morris JS, Buchel C, Dolan RJ. Parallel neural responses in amygdala subregions and sensory cortex during implicit fear conditioning. Neuroimage. 2001;13(6):1044–52. doi: 10.1006/nimg.2000.0721. [DOI] [PubMed] [Google Scholar]
- Moser JS, Huppert JD, Duval E, Simons RF. Face processing biases in social anxiety: an electrophysiological study. Biological Psychology. 2008;78(1):93–103. doi: 10.1016/j.biopsycho.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Müller MM, Teder-Sälejärvi W, Hillyard SA. Magnetoencephalographic recording of steadystate visual evoked cortical activity. Brain Topography. 1997;9(3):163–8. doi: 10.1007/BF01190385. [DOI] [PubMed] [Google Scholar]
- Müller MM, Teder-Sälejärvi W, Hillyard SA. The time course of cortical facilitation during cued shifts of spatial attention. Nature Neuroscience. 1998;1(7):631–4. doi: 10.1038/2865. [DOI] [PubMed] [Google Scholar]
- Öhman A, Mineka S. Fears, phobias, and preparedness: toward an evolved module of fear and fear learning. Psychological Review. 2001;108(3):483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109(2):290–8. [PubMed] [Google Scholar]
- Pejic T, Hermann A, Vaitl D, Stark R. Social anxiety modulates amygdala activation during social conditioning. Social Cognitive and Affective Neuroscience. 2013;8(3):267–76. doi: 10.1093/scan/nsr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlstein WM, Cole MA, Larson M, Kelly K, Seignourel P, Keil A. Steady-state visual evoked potentials reveal frontally-mediated working memory activity in humans. Neuroscience Letters. 2003;342(3):191–5. doi: 10.1016/s0304-3940(03)00226-x. [DOI] [PubMed] [Google Scholar]
- Peyk P, De Cesarei A, Junghöfer M. ElectroMagnetoEncephalography software: overview and integration with other EEG/MEG toolboxes. Computational Intelligence and Neuroscience, 2011. 2011 doi: 10.1155/2011/861705. doi:10.1155/2011/861705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, et al. Guidelines for using human event-related potentials to study cognition: recording standards and publication criteria. Psychophysiology. 2000;37(2):127–52. [PubMed] [Google Scholar]
- Regan D. Human Brain Electrophysiology: Evoked Potentials and Evoked Magnetic Fields in Science and Medicine. New York: Elsevier; 1989. [Google Scholar]
- Sachs G, Anderer P, Doby D, Saletu B, Dantendorfer K. Impaired conditional discrimination learning in social phobia. Neuropsychobiology. 2003;47(2):66–72. doi: 10.1159/000070011. [DOI] [PubMed] [Google Scholar]
- Schneider F, Weiss U, Kessler C, et al. Subcortical correlates of differential classical conditioning of aversive emotional reactions in social phobia. Biological Psychiatry. 1999;45(7):863–71. doi: 10.1016/s0006-3223(98)00269-8. [DOI] [PubMed] [Google Scholar]
- Silberstein RB, Pipingas A. Steady-state visually evoked potential topography during the Wisconsin card sorting test. Electroencephalography and Clinical Neurophysiology/Evoked Potentials Section. 1995;96(1):24–35. doi: 10.1016/0013-4694(94)00189-r. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. The State-Trait Anxiety Inventory. Palo Alto, California: Consulting Psychologists Press; 1970. [Google Scholar]
- Straube T, Weiss T, Mentzel HJ, Miltner WHR. Time course of amygdala activation during aversive conditioning depends on attention. Neuroimage. 2007;34(1):462–9. doi: 10.1016/j.neuroimage.2006.08.021. [DOI] [PubMed] [Google Scholar]
- Tinoco-González D, Fullana MA, Torrents-Rodas D, et al. Conditioned subjective responses to socially relevant stimuli in social anxiety disorder and subclinical social anxiety. Clinical Psychology & Psychotherapy. 2015;22(3):221–31. doi: 10.1002/cpp.1883. [DOI] [PubMed] [Google Scholar]
- Turner SM, Beidel DC, Dancu CV, Stanley MA. An empirically derived inventory to measure social fears and anxiety: the Social Phobia and Anxiety Inventory. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1989;1(1):35–40. [Google Scholar]
- Veit R, Flor H, Erb M, et al. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;328(3):233–6. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Vialatte F-B, Maurice M, Dauwels J, Cichocki A. Steady-state visually evoked potentials: focus on essential paradigms and future perspectives. Progress in Neurobiology. 2010;90(4):418–38. doi: 10.1016/j.pneurobio.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Wang J, Clementz BA, Keil A. The neural correlates of feature-based selective attention when viewing spatially and temporally overlapping images. Neuropsychologia. 2007;45(7):1393–9. doi: 10.1016/j.neuropsychologia.2006.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wieser MJ, Flaisch T, Pauli P. Raised middle-finger: electrocortical correlates of social conditioning with nonverbal affective gestures. PLoS One. 2014a;9(7):e102937. doi: 10.1371/journal.pone.0102937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser MJ, McTeague LM, Keil A. Sustained preferential processing of social threat cues: bias without competition? Journal of Cognitive Neuroscience. 2011;23(8):1973–86. doi: 10.1162/jocn.2010.21566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser MJ, Miskovic V, Rausch S, Keil A. Different time course of visuocortical signal changes to fear-conditioned faces with direct or averted gaze: A ssVEP study with single-trial analysis. Neuropsychologia. 2014b;62(9):101–10. doi: 10.1016/j.neuropsychologia.2014.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.