Abstract
Anxiety disorder patients were repeatedly found to overestimate the association between disorder-relevant stimuli and aversive outcomes despite random contingencies. Such an illusory correlation (IC) might play an important role in the return of fear after extinction learning; yet, little is known about how this cognitive bias emerges in the brain. In a functional magnetic resonance imaging study, 18 female patients with spider phobia and 18 healthy controls were exposed to pictures of spiders, mushrooms and puppies followed randomly by either a painful electrical shock or nothing. In advance, both patients and healthy controls expected more shocks after spider pictures. Importantly, only patients with spider phobia continued to overestimate this association after the experiment. The strength of this IC was predicted by increased outcome aversiveness ratings and primary sensory motor cortex activity in response to the shock after spider pictures. Moreover, increased activation of the left dorsolateral prefrontal cortex (dlPFC) to spider pictures predicted the IC. These results support the theory that phobia-relevant stimuli amplify unpleasantness and sensory motor representations of aversive stimuli, which in turn may promote their overestimation. Hyper-activity in dlPFC possibly reflects a pre-occupation of executive resources with phobia-relevant stimuli, thus complicating the accurate monitoring of objective contingencies and the unlearning of fear.
Keywords: illusory correlation, covariation bias, fMRI, spider phobia, pain
INTRODUCTION
Fear conditioning serves as a model for the development and maintenance of anxiety disorders, such as specific phobia (Mineka and Oehlberg, 2008). Indeed, several studies suggest abnormal conditioning processes in clinical and highly anxious populations (Lissek et al., 2009; Glotzbach-Schoon et al., 2013), but reported effect sizes differentiating patients from healthy controls are typically small (Lissek et al., 2005; Beckers et al., 2013). One reason may be that most conditioning studies realized a clear contingency between the conditioned stimulus (CS) and the aversive unconditioned stimulus (US). This may be problematic because differences between anxious and non-anxious individuals have often emerged particularly in ambiguous situations (Lissek et al., 2006), which is not the case if contingencies are clear. Alternatively, it might be assumed that individuals with anxiety disorders specifically suffer from a biased perception of the contingency between a fear-related CS (e.g. a spider) and an aversive US (e.g. an electric shock). In line with this approach, a so-called covariation bias or illusory correlation (IC; These terms may be used synonymously in this context. To avoid confusion, we focus on the term ICs in this article.) has been found in fearful and phobic individuals between phobia-relevant stimuli and aversive events (Tomarken et al., 1989; Mühlberger et al., 2006; De Jong and Peters, 2007; Davey, 2010). ICs have also been observed in panic-prone individuals and may be relevant for anxiety disorders in general (Pauli et al., 1996). In a classic IC experiment (Tomarken et al., 1989), participants are exposed to neutral and phobia-relevant pictures that are followed by different outcomes (i.e. aversive shocks, neutral tones or nothing). Although the relationship between pictures and outcomes is random, individuals suffering from phobia were found to specifically overestimate the association between phobia-relevant pictures and aversive outcomes. Interestingly, before such an experiment even non-fearful individuals are more likely to expect a shock following fear-relevant stimuli relative to neutral stimuli. Yet, only phobic patients associate spiders and shocks after confrontation with random contingencies (Davey and Dixon, 1996; Mühlberger et al., 2006).
We assume that abnormal neural processing of CS–US contingencies can in part explain why phobia patients have difficulties to learn that spiders are not specifically related to negative consequences. Therefore, we used functional magnetic resonance imaging (fMRI) to measure the brain activation of spider-phobic patients and healthy controls during an IC experiment.
Increased dorsolateral prefrontal cortex (dlPFC) activity has been associated with contingency awareness during fear conditioning (Carter et al., 2006). Moreover, the dlPFC was less active in a working memory task when participants were distracted by emotional stimuli (Dolcos and McCarthy, 2006). Finally, previous studies showed that phobic stimuli provoked enhanced (Paquette et al., 2003; Straube et al., 2004; Schienle et al., 2007; Aupperle et al., 2009) or reduced (Carlsson et al., 2004) activity in dlPFC. Its role in an IC experiment is yet unknown. Altered executive control and/or working memory performance (Miller and Cohen, 2001; Curtis and D’Esposito, 2003) may be reflected in altered dlPFC activity in the presence of phobia-relevant stimuli and account for impaired contingency monitoring in phobic individuals. Therefore, we expected that deviant dlPFC activity to spider images would prevent spider phobics from correcting biased contingency estimates, and should thus be correlated with the IC.
Furthermore, we expected increased responses to shocks following phobia-relevant stimuli within typical pain-processing areas, mainly comprising primary sensory motor cortex, secondary sensory cortex, anterior cingulate cortex (ACC) and insula (Peyron et al., 2000; Legrain et al., 2011). Despite several studies on the emotional modulation of pain (Kenntner-Mabiala and Pauli, 2005; Kenntner-Mabiala et al., 2008; Roy et al., 2009; Reicherts et al., 2013), the impact of phobic stimuli on the neural processing of painful stimuli has not yet been investigated. Since already the first IC study in animal phobia observed that the reported pain elicited by the shocks following spiders predicts ICs (Tomarken et al., 1989), we further expected that hyper-activity in these pain-processing areas should predict ICs.
Taken together, we expected amplified shock-related activity in response to spider-shock associations and aberrant dlPFC activity in response to spider images to be involved in phobia-relevant ICs. In addition, we examined the contribution of other brain areas typically involved in the processing of phobia-relevant stimuli to ICs (i.e. amygdala, ACC, insula). Importantly, further knowledge about the brain processes underlying ICs may help to improve treatment of anxiety disorders, since De Jong et al. (1995) found that the persistence of ICs immediately after exposure treatment was a significant predictor of relapse 2 years later.
MATERIALS AND METHODS
Participants
Thirty-eight female participants (20 patients with spider phobia and 18 non–spider-fearful individuals) were recruited via local advertisements. Specific phobia was confirmed by a trained psychologist using the structured clinical interview for DSM-IV (Wittchen et al., 1997). Screening criteria were high (≥20 of maximum 24) or low (≤8) scores in a short screening for fear of spiders (Rinck et al., 2002), being female and right handed, no history of other psychiatric or neurological disorders, no current psychoactive medication, and until now no treatment of specific phobia or other psychological disorders. Two of the 20 patients with spider phobia had to be excluded from data analysis (acute illness, respectively, stimulation electrode came off). One patient was excluded only from the analysis of ratings due to missing data, and as a consequence also from correlational analyses between fMRI and ratings.
Spider phobics and non–spider-fearful participants were matched for age and education (Table 1). The two groups significantly differed in the Spider Phobia Questionnaire (SPQ; Watts and Sharrock, 1984), the Fear of Spiders Questionnaire (FSQ; Szymanski and O’Donohue, 1995) and the state version of the State Trait Anxiety Inventory (STAI; Spielberger et al., 1970). In addition, the spider phobia group was slightly more trait anxious according to the STAI. On the Beck Depression Inventory, the groups were not significantly different (BDI-II; Beck et al., 1996). To ensure that differences in brain activation between groups were due to spider phobia and not trait anxiety, the fMRI analysis was run again with trait anxiety as a covariate. The core results remained unchanged in this analysis. That is, the identical regions of interest (ROIs) were still significantly activated and correlated with the IC.
Table 1.
Demographic and psychometric sample characteristics
| Spider phobia | N | Healthy control | N | P value | |
|---|---|---|---|---|---|
| Age | 21.4 ± 4.2 | 18 | 22.2 ± 2.2 | 18 | 0.461a |
| Education | 17 A/1 M | 18 | 16 A/2 M | 18 | 1.00b |
| SPQ | 23.2 ± 2.8 | 17 | 4.6 ± 2.4 | 16 | <0.001a |
| FSQ | 76.7 ± 4.8 | 17 | 4.8 ± 7.0 | 16 | <0.001a |
| STAI-state | 43.6 ± 7.3 | 18 | 36.2 ± 8.2 | 18 | 0.008a |
| STAI-trait | 42.4 ± 5.6 | 17 | 37.2 ± 8.4 | 16 | 0.044a |
| BDI-II | 9.2 ± 5.5 | 17 | 6.2 ± 4.0 | 16 | 0.082a |
Notes: Mean and standard deviation of demographic and psychometric sample characteristics. Age is specified in years and education is stated in the number of participants with higher education entrance qualification (A) and secondary education (M). N = number of participants (some participants had to be discarded due to missing data).
aTwo-sided t-tests.
bFisher’s exact test for cross tables.
Pictorial and electrical stimuli
Ninety different color pictures from a custom-made picture set were used in the IC experiment (30 spider pictures for phobia-relevant trials; 30 mushroom pictures for neutral trials; 30 puppy pictures for filler trials). The pictures were matched with regards to complexity, that is, each picture showed only one object centered in the foreground. After the IC experiment, the pictures were rated on Likert-like scales with verbal labels for the endpoints regarding valence (‘1 = very unpleasant’ to ‘9 = very pleasant’) and arousal (‘1 = not arousing at all’ to ‘9 = very arousing’).
Aversive electrical stimuli (400 V for 767 ms) were generated by a current stimulator (Digitimer DS7A; Digitimer Ltd, Welwyn Garden City, UK) and applied via two steel surface electrodes (9-mm diameter; GVB-geliMED, Bad Segeberg, Germany) to the inner side of the left calf. Next, a calibration procedure was executed in order to establish the necessary individual stimulation level to achieve comparably aversive pain intensities. Four individual pain thresholds were identified via painfulness ratings (0 = no sensation to 10 = maximal painful sensation) of two increasing and two decreasing series of electrical stimuli in steps of 0.5 mA. Participants were instructed that a rating of four indicated the beginning of painful sensation. To ensure that electrical stimuli were perceived as aversive, 20% were added to the mean of the four pain thresholds and the painfulness of this final stimulus was rated again. This procedure resulted in a mean intensity threshold of 2.02 mA ( ± s.d. = 0.94) for healthy controls and 1.27 mA ( ± 0.62) for spider phobics. This difference was statistically significant (P < 0.01, two sided). Importantly, the individual calibration of stimulus intensity succeeded to equalize the subjective evaluation of painfulness as indexed by the ratings of the final stimulus showing no group difference (healthy controls: M = 4.78 ± s.d. = 1.35; spider phobics: M = 4.44 ± s.d. = 0.71; P = 0.36, two sided).
Procedure
The study was approved by the ethics committee of the Medical Faculty of the University of Würzburg and carried out in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki). Signed informed consent was obtained from participants after the procedure was explained. Next, all participants filled out the STAI state. Then, individual pain thresholds were determined while participants were lying on the scanner bed. Before the IC experiment started, they were shown a random example picture of each category (spiders, mushrooms, puppies) and were asked to estimate the expected probability at which a specific category would be followed by a shock on a continuous visual scale (0–100%).
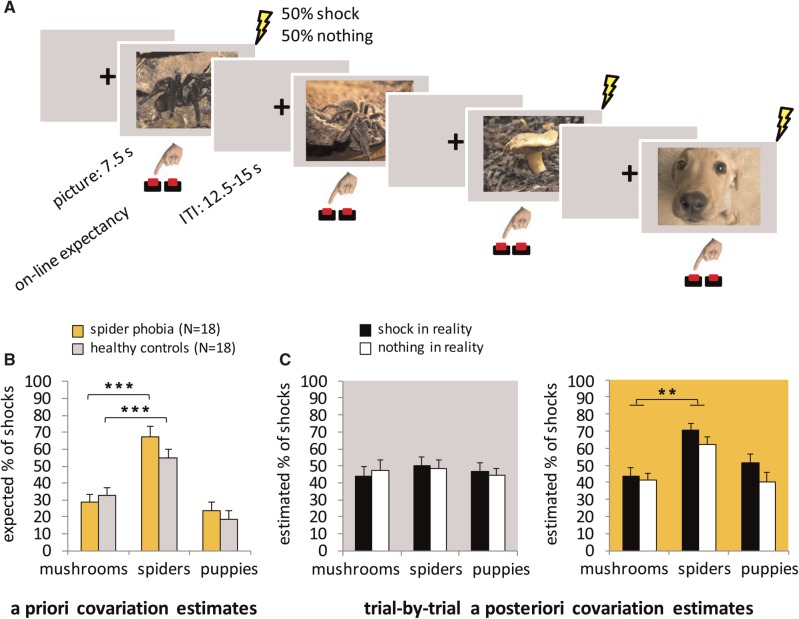
The IC experiment took about 35 min. Each of the 90 pictures was presented for 7.5 s. Fifteen of the 30 pictures of each category were followed by a shock. Pictures were separated by a varying inter-trial interval ranging from 12.5 to 15 s, showing a black fixation cross on a grey background (Figure 1A). The onset of every picture was jittered in 500 ms steps relative to the beginning of a scan interval (2.5 s). The five jitter intervals were equally distributed to the six conditions [i.e. 3 (categories) × 2 (outcomes)] and randomly distributed within each condition. Stimuli were presented in one of the six predefined pseudo-randomized orders. Across these orders, the condition of the starting stimulus was counter balanced. Furthermore, every picture was equally associated with a shock or nothing as an outcome across the six orders. Within every six consecutive trials, all picture outcome combinations occurred. To keep attention focused on the task, on-line expectancy was rated throughout the experiment by pressing one of the two buttons depending on which outcome (shock/nothing) was expected during picture presentation (see Supplementary material).
Fig. 1.
IC paradigm and covariation bias. (A) Three categories of pictures were presented (spiders, mushrooms and puppies). Exactly 50% of the pictures of each category were followed by a painful electrical shock. In order to keep attention focused on contingencies, participants were asked to press one of the two buttons depending on whether they expected a shock in a trial or not. The histograms show the expected proportion of shocks before (a priori, B) and the estimated proportion of shocks after the IC experiment (trial-by-trial a posteriori ICs, C). For trial-by-trial a posteriori ICs (C), each picture was presented again and the participants were asked to indicate whether a shock had followed that particular picture. Black and white bars display whether there had actually been presented a shock or nothing as an outcome. Both spider phobic and control participants expected more shocks following spiders before the experiment (B). Importantly, only spider-phobic participants (C, orange background) but not healthy controls (C, gray background) overestimated this association after the experiment. **P < 0.01; ***P < 0.001.
Outside the scanner, after the IC experiment, participants rated the aversiveness of the shock and a posteriori covariation estimates (see Supplementary material) for each picture category (in randomized order) on visual scales (0–100%, with 100% indicating perfect co-occurrence). To obtain a presumably more reliable measure of IC, we also assessed post-experimental covariation estimates on a trial-by-trial basis. Therefore, each picture was presented to the participants again and they were asked to decide whether a shock had been associated with this picture or not, using a scale ranging from −4 to + 4 (without 0). The participants were instructed to select a positive number if they thought that the picture had been associated with a shock and a negative number if they thought that there had not been a shock. Values from 1 to 4 (or −1 to − 4) served to express the certainty of their decision. Finally, the STAI trait, the BDI-II, the SPQ and the FSQ were completed.
Analysis of ratings
A priori covariation estimates were analyzed with a 3 × 2 repeated-measures ANOVA (analysis of variance) comprising the within-subjects factor ‘picture’ (spiders, mushrooms, puppies) and the between-subjects factor ‘group’ (phobics, controls). Trial-by-trial a posteriori covariation estimates were obtained by calculating the proportion of positive answers to the question whether a shock had been associated with a particular picture within each category depending on the actual outcome of the pictures. These proportions were analyzed with a 3 × 2 × 2 repeated-measures ANOVA comprising the within-subjects factor picture (spiders, mushrooms, puppies) and ‘outcome’ (shock, nothing) and the between-subjects factor group (phobics, controls). Trial-by-trial a posteriori covariation estimates were chosen as the index of IC because of three reasons: first, previous experiments showed dissociations in a posteriori estimates between phobics and controls (Davey and Dixon, 1996; Mühlberger et al., 2006). Second, in the present experiment, trial-by-trial covariation estimates differentiated the most between phobics and controls. Third, the trial-by-trial measurement of covariation estimates should subserve the reliability of the IC index.
fMRI acquisition and analysis
Scanning parameters
fMRI data were obtained using a 1.5 T Siemens Avanto MRI Scanner. Functional data included whole-brain T2*-weighted single-shot gradient echo-planar images (EPI) recorded with a repetition time of 2.5 s (echo time = 30 ms, flip angle = 90°, field-of-view = 200 mm, acquisition matrix = 64 × 64, voxel size = 3.1 × 3.1 × 5 mm). Each volume contained 25 axial slices parallel to the AC–PC line (from the anterior commissure [AC] to the posterior commissure [PC]) that were acquired in interleaved order. Slices were overlapping and 5 mm wide with a 1 mm gap. A total of 840 EPIs were recorded in every participant. The experimental procedure started only after the first eight EPIs to allow for a stabilization of the magnetic field. A high-resolution structural image of the brain was created via T1-weighted magnetization-prepared rapid gradient-echo imaging (repetition time = 2250 ms, echo time = 3.93 ms, flip angle = 8°, field-of-view = 256 mm, acquisition matrix = 256 × 256, voxel size = 1 × 1 × 1 mm). If the magnetic field is inhomogeneous, EPI images are often spatially distorted (Hutton et al., 2002). Therefore, a gradient echo (GRE) field mapping (TR [repetition time] = 1000 ms, TE [echo time] = 10 ms, slices = 25, slice thickness = 5 mm, FOV [field of view] = 240 mm, matrix size: 64 × 64) was performed prior to the acquisition of the functional MRI data to compensate for inhomogeneity of the magnetic field.
fMRI preprocessing
fMRI data were analyzed using Statistical Parametric Mapping software (SPM8; Wellcome Department of Imaging Neuroscience, London, UK). First, functional images were slice time corrected and realigned (second degree b-spline interpolation) using an individual voxel displacement map on the basis of GRE field mapping. The individual structural images were then coregistered to the mean individual functional image and segmented. Then functional images were spatially normalized into standard Montreal Neurological Institute space using a voxel size of 2 × 2 × 2 mm, and smoothed with an 8 mm full-width half maximum Gaussian kernel. Unless indicated otherwise, these steps were performed with SPM8 default settings.
First-level analysis
Twelve regressors of interest were defined including onsets of both pictures and outcomes of the six conditions: ‘spider-before-shock’, ‘spider-before-nothing’, ‘mushroom-before-shock’, ‘mushroom-before-nothing’, ‘puppy-before-shock’, ‘puppy-before-nothing’, ‘shock-after-spider’, ‘shock-after-mushroom’, ‘shock-after-puppy’, ‘nothing-after-spider’, ‘nothing-after-mushroom’ and ‘nothing-after-puppy’. In addition, realignment parameters were included as nuisance regressors accounting for movement artifacts during scanning. Event-related brain activation was modeled by convolving stick functions with the canonical hemodynamic response function. Parameter estimation was corrected for temporal autocorrelations using a first-order autoregressive model. The high-pass filter cutoff was set to 128 s.
Second-level analysis
We were primarily interested in two kinds of brain activities: responses to spider pictures and responses to shocks following spider pictures. To obtain fear-related modulation of shock processing, the interaction contrast [(shock-after-spider > nothing-after-spider) > (shock-after-mushroom > nothing-after-mushroom)] was computed. This approach controls for residual picture-related activity and reveals activity in response to only the electrical shock. To obtain activity related to picture onset, we defined the contrast [(spider-before-shock and spider-before-nothing) > (mushroom-before-shock and mushroom-before-nothing)], from now on referred to as (spider > mushroom). Since we were interested in the effects of fear-relevant stimuli in comparison with neutral stimuli, the pictures of puppies served as filler trials and were not included in this analysis. All analyses used a random-effects model for contrast maps of t-scores. Regions with significant activations are reported according to the automatic anatomic labeling (aal) in the Wake Forest University (WFU) PickAtlas (Maldjian et al., 2003).
Effects of experimental conditions
dlPFC, ACC, amygdala and insula were chosen a priori (see Introduction) as ROIs for the analysis of picture-related brain activation. The same regions plus the right paracentral lobule (PCL) as the primary sensory motor area contralateral to painful stimulation (Roy et al., 2009) were chosen as ROI for the analysis of shock-related activity. ROI masks were created using predefined regions in the aal WFU PickAtlas. The dlPFC mask was defined as the combination of the lateral parts of Brodmann areas (BA) 9 and 46, that is, the intersection of combined superior frontal gyrus and medial frontal gyrus on the one hand and combined BA 9 and 46 on the other hand. For ROI analyses, the alpha error level was set to P < 0.05, family-wise error (FWE) corrected, with a cluster threshold of k ≥ 5 voxels. ROI analyses were carried out bilaterally with the exception of the right PCL in response to contralateral electrical stimulation and the amygdala which is mostly lateralized to the left hemisphere in response to emotional stimuli (Baas et al., 2004). A supplementary finite impulse response analysis was performed to validate that picture-related effects were present before the onset of outcomes (see Supplementary material). For whole-brain analyses, the threshold was set to P < 0.001, uncorrected, with a minimal cluster extent of k ≥ 10 voxels. In addition to this common statistical threshold, we also marked those clusters in the tables exceeding an extent of k ≥ 43 voxels, which is equivalent to a whole-brain false discovery rate of P = 0.05, according to a Monte Carlo simulation with respect to imaging parameters (Slotnick et al., 2003). If several significant clusters were found inside one brain region in the whole-brain analysis, only the one with the lowest P value is reported.
Brain-behavior correlation analysis
We were interested in which brain activity predicted the tendency to overestimate the contingency between spider stimuli and shocks. We used MarsBaR (http://marsbar.sourceforge.net) to extract mean beta-values from all voxels in ROI with significant differences between conditions (see Effects of Experimental Conditions section). Next, we computed Pearson correlations (two-tailed test with alpha = 0.05) between these beta-values and the IC score obtained from trial-by-trial covariation estimates after the experiment. This IC score was calculated by subtracting the proportion of positive answers to mushroom pictures from the proportion of positive answers to spider pictures. In addition, for whole-brain analyses of brain-behavior correlations, the same IC score was entered as a covariate in the second-level analyses (P < 0.001, uncorrected; k ≥ 10 voxels).
RESULTS
Ratings
A priori covariation estimates
In the repeated-measures ANOVA with the factors picture (spiders, mushrooms, puppies) and group (phobics, controls), the main effect picture was significant (F2,33 = 14.55, P < 0.001, = 0.72), while there was no significant main effect of group (P = 0.37, = 0.02) and no significant interaction of Picture × Group (P = 0.13, = 0.06). Both patients and healthy controls expected more electrical shocks after spiders than after mushrooms or puppies (spider phobia patients: spiders M = 67.11 ± s.d. = 23.31 vs mushrooms 29.06 ± 19.26, t17 = 6.29, P < 0.001, d = 1.48, or puppies 23.94 ± 17.89, t17 = 6.72, P < 0.001, d = 1.59; healthy controls: spiders 55.11 ± 20.20 vs mushrooms 33.00 ± 18.66, t17 = 4.20, P < 0.001, d = 0.99, or puppies 18.78 ± 20.24, t17 = 6.02, P < 0.001, d = 1.42, Figure 1B).
Trial-by-trial a posteriori covariation estimates
In the repeated-measures ANOVA with the factors picture (spiders, mushrooms, puppies), outcome (shock, nothing) and group (phobics, controls), there was a significant main effect of picture (F2,32 = 5.44, P < 0.01, = 0.25) and a marginal significant interaction of Picture × Group (F2,32 = 2.69, P = 0.08, = 0.14) (If only the relevant categories spiders and mushrooms were included in this analysis, the interaction becomes significant at P < 0.05). Because of many previous findings of ICs in spider phobia, the probability of finding a false positive effect should be low, and therefore we further examined this marginal significant interaction. Patients with spider phobia overestimated the contingency between shocks and spiders (0.67 ± 0.16) relative to mushrooms (0.43 ± 0.17; t16 = 2.73, P < 0.01, d = 0.92) or puppies (0.46 ± 0.20; t16 = 3.87, P < 0.05, d = 0.68). In contrast, control participants did not overestimate the contingency between shocks and spiders compared with other pictures (both P > 0.43; Figure 1C). In addition, there was a significant effect of outcome, a significant interaction of Picture × Outcome and a significant interaction of Outcome × Group (see Supplementary material for a follow-up analysis).
fMRI data
Brain activity during picture processing
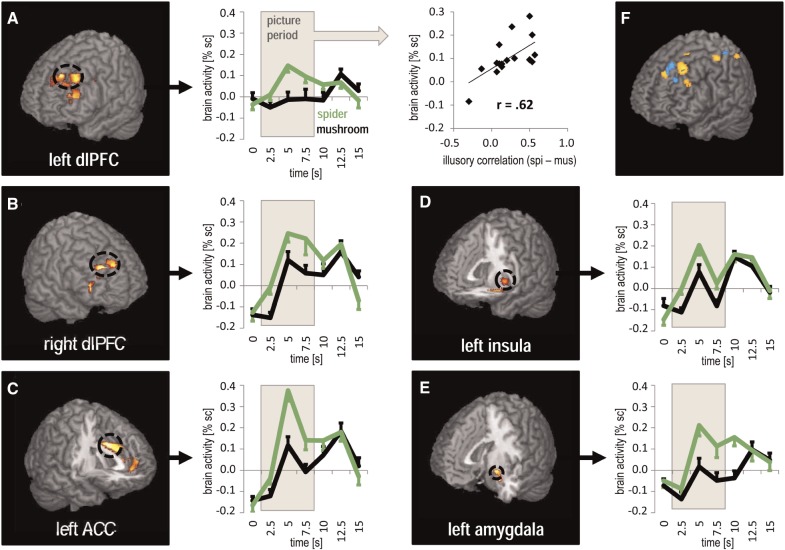
In the spider phobia group, spider images elicited activation (spider > mushroom) in areas typically involved in the processing of phobia-relevant stimuli, including bilateral dlPFC. Moreover, in the ROI analysis, we found activity within left amygdala, left ACC and left insula (Figure 2). Increased activation in left dlPFC was also observed in the control group, but was still higher in spider phobics. Likewise, the left amygdala, the left ACC and the left insula were significantly stronger activated in the spider phobia group than in the control group (Tables 2 for ROI and supplementary Table S1 for whole-brain analysis).
Fig. 2.
Spider-phobic patients’ responses to picture onsets. The five ROIs (A–E) with significant differences in the contrast (spider > mushroom) are depicted (ROI analysis: P < 0.05, few corrected, k ≥ 5 voxels; display threshold: P < 0.005, uncorrected, k ≥ 10 voxels). Line diagrams show the time course of percent signal change for spider (green) and mushroom (black) pictures during picture presentation (grey background from 0 to 7.5 s) in spider phobia. The dlPFC (A), the right dlPFC (B), the left ACC (C), the left insula (D) and the left amygdala (E) showed enhanced activity in response to spiders vs mushrooms within spider phobics. However, only the left dlPFC correlated with the IC. The scatter plot (A) visualizes this correlation between the difference in brain activity in the left dlPFC during picture presentation and the trial-by-trial IC after the experiment (see Supplementary material and supplementary Table S2 for time course analysis). In the upper right corner (F), clusters that correlated with the trial-by-trial IC can be seen mainly in the left fronto-parietal regions including the dlPFC, BA 8 and the superior parietal cortex (spider phobia = yellow; healthy controls = blue; whole-brain analysis: P < 0.001, uncorrected; k ≥ 10 voxels).
Table 2.
Significant activations at the contrast (spider > mushroom)
| Region | MNI co-ordinates |
k | t | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Spider phobics | ROI | ||||||
| dlPFC (BA 9) | L | −20 | 44 | 42 | 12 | 6.84 | |
| R | 32 | 44 | 30 | 22 | 6.57 | ||
| R | 18 | 56 | 32 | 8 | 5.56 | ||
| L | −36 | 32 | 36 | 8 | 5.41 | ||
| Amygdala | L | −18 | −4 | −12 | 22 | 4.84 | |
| L | −28 | −2 | 22 | 19 | 4.26 | ||
| ACC (BA 24) | L | −2 | 10 | 28 | 54 | 5.77 | |
| Insula (BA 13) | L | −44 | 12 | 2 | 23 | 6.81 | |
| Control group | ROI | ||||||
| dlPFC (BA 9) | L | −12 | 48 | 30 | 14 | 7.10 | |
| Phobics > Controls | ROI | ||||||
| dlPFC (BA 9) | L | −34 | 30 | 36 | 13 | 4.86 | |
| Amygdala | L | −28 | −4 | −20 | 6 | 3.62 | |
| ACC (BA 24) | L | −2 | −12 | 20 | 46 | 4.83 | |
| Insula (BA 13) | L | −42 | 12 | 2 | 67 | 5.90 | |
Notes: The table shows properties of peak voxels within a cluster. ROI threshold: P < 0.05 (FWE corrected), k ≥ 5. k = voxels in whole cluster; MNI = Montreal Neurological Institute.
Correlation between picture-related brain activity and IC
Across spider phobia participants, correlation coefficients were calculated between mean activity in the relevant significant ROIs (spider > mushroom) and the trial-by-trial IC. The correlation was significant for the left dlPFC (r = 0.56, P = 0.02), but not for the right dlPFC, the left amygdala, the left insula and the left ACC (supplementary Table S2 and Supplementary material for a time course analysis). In a whole-brain analysis, multiple additional brain regions were found to be correlated with the IC (Table 3 and Figure 2F). In patients with spider phobia, a cluster in the left middle frontal gyrus (BA 8) superior to our defined ROI of the dlPFC was the most prominent cluster correlating with the IC score regarding cluster size and t value.
Table 3.
Activations at the contrast (spider > mushroom) correlating with the IC
| Region | MNI co-ordinates |
k | t | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Spider phobics | Whole brain | ||||||
| Middle frontal (BA 8) | L | −26 | 28 | 46 | 120a | 6.99 | |
| Precentral (BA 4) | L | −32 | −28 | 54 | 24 | 5.55 | |
| Postcentral (BA 3) | L | −22 | −36 | 54 | 12 | 5.22 | |
| Superior frontal (BA 8) | L | −10 | 48 | 44 | 20 | 4.82 | |
| Lingual (BA 30) | R | 0 | −62 | −2 | 41 | 4.69 | |
| SMA (BA 6) | R + L | 2 | −8 | 56 | 10 | 4.37 | |
| Middle frontal (BA 9) | L | −40 | 34 | 34 | 11 | 4.25 | |
| Superior parietal (BA 7) | L | −38 | −60 | 50 | 10 | 4.09 | |
| Superior temporal (BA 12) | R | 56 | −44 | 12 | 12 | 4.04 | |
| Control group | Whole brain | ||||||
| Middle occipital | L | −34 | −78 | 6 | 63a | 6.44 | |
| Precentral (BA 6) | R | 34 | −4 | 44 | 42 | 6.38 | |
| Hippocampus | L | −32 | −32 | −10 | 35 | 5.69 | |
| Cerebellum | L | −6 | −36 | −22 | 41 | 4.85 | |
| Superior frontal (BA 8) | L | −10 | 32 | 50 | 35 | 4.83 | |
| Lingual (BA 30) | R | 8 | −62 | 4 | 11 | 4.66 | |
| Lingual (BA 19) | L | −10 | −56 | −8 | 44a | 4.58 | |
| Superior parietal (BA 7) | L | −22 | −70 | 46 | 10 | 4.31 | |
| Cuneus (BA 7) | L | −16 | −82 | 36 | 10 | 4.08 | |
Notes: The table shows properties of peak voxels within a cluster. Whole-brain threshold: P < 0.001 (uncorrected), k ≥ 10. k = voxels in whole cluster; MNI = Montreal Neurological Institute.
aCluster size exceeds voxel threshold (43) based on Monte Carlo simulation.
Modulation of shock processing by phobic stimuli
For spider phobia patients, ROI analysis of the contrast [(shock-after-spider > nothing-after-spider) > (shock-after-mushroom > nothing-after-mushroom)] representing activation related to shock only while controlling for effects of picture processing revealed significant activation in the right PCL. Whole-brain analysis for spider phobia patients returned an activation pattern mainly contralateral to electrical stimulation comprising right supplementary motor area (SMA), right postcentral gyrus, right fusiform gyrus, right cerebellum, right precentral gyrus and left middle temporal gyrus (Table 4). For control participants, ROI analysis of this contrast returned a significant cluster in the right insula, and whole-brain analysis indicated additional activity in right cerebellum and left cuneus. In a comparison between groups (phobia > control), ROI analyses did not confirm spider-specific shock processing when using FWE-corrected statistics. Whole-brain analysis indicated that right dlPFC, right SMA, left PCL and left parahippocampal gyrus were more active in the spider phobia group than in the control group (all P < .001). At a more lenient threshold of P < .005, k ≥ 10 (Lieberman and Cunningham, 2009), we also found a significant difference in the right PCL (t = 2.99; k = 14; x = 8, y = −40, z = 58). There was no significant activity in the reverse contrast (controls > phobics), even at this less conservative threshold.
Table 4.
Significant activations at the contrast [(shock-after-spider > nothing-after-spider) > (shock-after-mushroom > nothing-after-mushroom)]
| Region | MNI co-ordinates |
k | t | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Spider phobics | ROI | ||||||
| PCL (BA 6) | R | 8 | −26 | 60 | 13 | 4.59 | |
| Whole brain | |||||||
| SMA (BA 6) | R | −12 | −32 | 60 | 386a | 5.61 | |
| Postcentral (BA 40) | R | 50 | −30 | 52 | 72a | 5.19 | |
| Fusiform (BA 20) | R | 28 | −28 | −28 | 40 | 4.48 | |
| Cerebellum | R | 10 | −64 | −42 | 44a | 4.46 | |
| Precentral (BA 4) | R | 36 | −26 | 64 | 15 | 4.19 | |
| Middle temporal (BA 37/20) | L | −52 | −38 | −14 | 12 | 4.13 | |
| Control group | ROI | ||||||
| Insula (BA 13) | R | 44 | −10 | −4 | 7 | 6.00 | |
| Whole brain | |||||||
| Cerebellum | R | 8 | −50 | −36 | 29 | 5.63 | |
| Cuneus (BA 7) | L | −14 | −80 | 26 | 16 | 4.64 | |
| Phobics > Controls | Whole brain | ||||||
| Inferior frontal (BA 9/46) | R | 56 | 22 | 30 | 59a | 4.85 | |
| SMA (BA 6) | R | 10 | −18 | 56 | 67a | 4.39 | |
| PCL (BA 6) | L | −12 | −30 | 60 | 58a | 4.22 | |
| Parahippocampal (BA 28) | L | −20 | −20 | −26 | 25 | 4.17 | |
Notes: The table shows properties of peak voxels within a cluster. ROI threshold: P < 0.05 (FWE corrected), k ≥ 5; whole-brain threshold: P < 0.001 (uncorrected), k ≥ 10. k = voxels in whole cluster; MNI = Montreal Neurological Institute.
aCluster size exceeds voxel threshold (43) based on Monte Carlo simulation.
Correlation between shock-related brain activity and IC
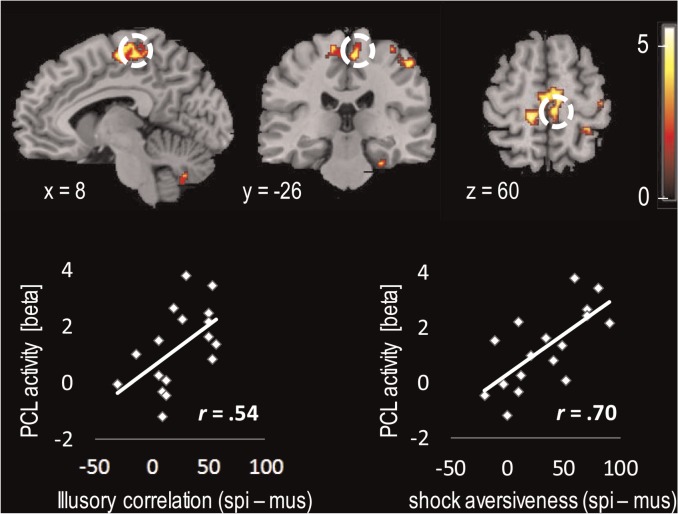
In the spider phobia group, mean right PCL activity in the contrast [(shock-after-spider > nothing-after-spider) > (shock-after-mushroom >nothing-after-mushroom)], that is, shock-associated activation, was significantly correlated with trial-by-trial IC (r = 0.54, P = 0.02; Figure 3). Right PCL response was also correlated with the difference between the aversiveness of the shock following spiders minus mushrooms (r = 0.70, P = 0.002). Moreover, IC and the difference in aversiveness were significantly correlated (r = 0.67, P = 0.003).
Fig. 3.
Activity in the PCL of spider-phobic patients and correlations with IC and ratings of shock aversiveness. The brain slices correspond with the peak voxel in right PCL (ROI-analysis: P < 0.05, k = 5 voxels) and show significant activation for the contrast [(shock-after-spider > nothing-after-spider) > (shock-after-mushroom > nothing-after-mushroom)] (P < 0.001, k = 10 voxels for display purpose, color scale represents t values). Scatterplots depict the association between right PCL activity (mean beta values of all voxels in the ROI) and trial-by-trial IC (left), respectively, the rated aversiveness of the electrical shock (right) depending on the picture type.
In a whole-brain analysis (Table 5), we found additional regions correlating with IC: left precentral gyrus, left PCL, right precentral gyrus, right cerebellum and right fusiform gyrus. Again, at the more lenient threshold of P < 0.005, k ≥ 10, a significant cluster in the right PCL emerged (t = 3.68; k = 26; x = 4, y = −24, z = 68).
Table 5.
Activations at the contrast [(shock-after-spider > nothing-after-spider) > (shock-after-mushroom > nothing-after-mushroom)] correlating with the IC
| Region | MNI co-ordinates |
k | t | ||||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Spider phobics | Whole brain | ||||||
| Precentral (BA 6) | L | −28 | −20 | 68 | 28 | 5.31 | |
| PCL (BA 6) | L | 0 | −18 | 68 | 47a | 4.66 | |
| Precentral (BA 4) | R | 16 | −26 | 68 | 10 | 4.33 | |
| Cerebellum (BA 37) | R | 46 | −64 | −24 | 14 | 4.13 | |
| Fusiform (BA 19) | R | 40 | −66 | −20 | 10 | 4.01 | |
| Control group | Whole brain | ||||||
| Inferior parietal (BA 40) | L | −52 | −48 | 34 | 75a | 6.65 | |
| Mid cingulum (BA 31) | L | −18 | −32 | 42 | 30 | 4.75 | |
| Pallidum | L | −20 | −8 | 2 | 14 | 4.37 | |
| Cerebellum | R | 12 | −54 | −16 | 18 | 4.27 | |
Notes: The table shows properties of peak voxels within a cluster. Whole-brain threshold: P < 0.001 (uncorrected), k ≥ 10. k = voxels in whole cluster; MNI = Montreal Neurological Institute.
aCluster size exceeds voxel threshold (43) based on Monte Carlo simulation.
DISCUSSION
This study confirms previous findings that both individuals with spider phobia and healthy controls expect spiders to be associated with aversive experiences (i.e. painful shocks). Importantly, only spider phobics still overestimated this contingency after being exposed to a series of stimuli with random contingency between image and shock suggesting that healthy individuals learn corrective information during this exposure. This study further suggests that biased processing of both pictures and outcomes may be a core issue of spider phobics not only preventing this learning effect but possibly even further increasing the bias existing before the experimental exposure. On the one hand, spider images led to increased recruitment of dlPFC, a region that has consistently been associated with executive control. On the other hand, shocks following spider images were perceived as more aversive and evoked enhanced responses in primary sensory motor area (PCL). Importantly, both PCL and dlPFC activity predicted the tendency to overestimate the contingency between spiders and shocks within spider phobics.
Increased left dlPFC activity to phobic stimuli and the correlation with IC suggests that altered executive control or working memory processes may play a role in the maintenance of fear-relevant ICs. Increased dlPFC activity to phobic symptom provocation is commonly reported and has been proposed to reflect attempts of emotional downregulation (Paquette et al., 2003). Considering the highly threatening impression of spiders on spider phobic patients, it seems reasonable that they mobilize executive control mechanisms to cope with the threatening situation. However, the increased working memory load may well occupy resources that are missing for processing of correcting information, that is, that the value of spider images for predicting shock is at chance level. Notably, dlPFC has been shown to predict contingency awareness in fear conditioning, suggesting that this brain region may be involved in monitoring CS–US relationships (Carter et al., 2006). Moreover, emotional distraction can reduce dlPFC activity which predicts performance during a working memory task (Dolcos and McCarthy, 2006). Finally, dlPFC is a key structure for overcoming habit (Knoch et al., 2005) and may therefore also be important to overcome an a priori IC. Whole-brain analyses suggest that—in addition to BA 9 and 46—BA 8 in the dlPFC may also be involved in an IC. Moreover, in both spider phobics and healthy controls, a more widespread fronto-parietal attention network (Ptak, 2012) might play an important role in the emergence of an IC. This finding further supports the idea that attentional engagement in fear-relevant pictures prevents accurate contingency monitoring. Taken together, several mechanisms may account for the observed dlPFC activity. However, in any case these processes use the same cognitive resources required for contingency monitoring (Carter et al., 2006). And this appears to complicate contingency monitoring in a state of fear.
The result that phobic images amplified the aversiveness of painful stimuli in phobic patients replicates earlier findings (Tomarken et al., 1989), and is in line with studies showing increased pain perception under negative affect (Kenntner-Mabiala and Pauli, 2005; Kenntner-Mabiala et al., 2008; Roy et al., 2009; Reicherts et al., 2013). To our knowledge, this is the first study revealing a modulation of brain responses to painful stimuli by phobic fear. Similar to the modulation by negative affective images (Roy et al., 2009), we found increased activity in primary sensory motor area (PCL). In this circumstance, it was to our knowledge a novel approach here to carefully disentangle picture and pain responses by subtracting the sole emotional experience (pictures without shocks) from the emotional modulation of pain (pictures with shocks).
Importantly, in phobic patients, the PCL activity was positively correlated with the aversiveness of shocks and the IC after the experiment. This demonstrates that the relationship between aversiveness of shocks and IC is more than just a demand effect at the moment of the rating. In fact, this relationship is manifest in biased sensory processing at the moment of shock application. Increased PCL activity may reflect increased attention to the shock. Previous studies showed that activity in primary sensory cortex is reduced when attention is directed away from a painful stimulus (Bushnell et al., 1999). If increased PCL activity reflects attentional engagement, increased attention to shocks following spiders may lead to deeper encoding which in turn causes the overestimation of shocks following spiders. In consistence with this idea, the primary sensory cortex is not only important for sensory on-line processing, but probably also serves as a storage site for tactile information (Harris et al., 2002; Pasternak and Greenlee, 2005). However, it should be kept in mind that these findings are correlational and the PCL might not necessarily be a causal factor of IC. In addition, alternative explanations about underlying potentially causal mechanisms are possible. For example, the enhanced PCL activity may also reflect a (suppressed) motor response. This notion is supported by accompanying activity in SMA and the cerebellum. Aversive stimuli following phobic images may trigger a flight or avoidance tendency that is correlated with IC. At least, spider images alone have the potential to accelerate avoidance reactions in spider-fearful individuals (Rinck and Becker, 2007). Again, this reaction could have initiated deeper encoding. In any case, the heightened aversiveness of the shock can be reason enough for the maintenance of ICs. After all, overestimating the occurrence of aversive outcomes could be a consequence of preparing for the worst case, and the necessity of being prepared should be higher when the potential outcome is more aversive. In a recent study, we showed that the experimental manipulation of the aversiveness of outcomes is sufficient to induce an IC between neutral cues and aversive outcomes (Wiemer et al., 2014).
In the control group, shocks following spider pictures were not rated as more unpleasant. However, shocks were also expected to follow spiders more often than following mushroom pictures. In response to the shocks, we found increased activity in the posterior insula among control participants. We suppose that this might reflect this increased cognitive but rather non-emotional or non-fearful anticipation of pain. This interpretation is supported by previous findings showing that the posterior insula is more activated in response to non-painful stimuli if participants were not sure whether a painful or a non-painful stimulus would occur (Sawamoto et al., 2000). Possibly, the posterior insula reflects increased processing of sensory stimuli due to increased expectancy/attention toward such stimuli during an anticipation phase.
Some limitations of this study should be considered. First, the a posteriori IC assessed as global covariation estimates did not differ between groups. This may be because we used 90 trials to enable reliable measurement of the BOLD response. Previous studies typically used 72 trials. Moreover, we asked participants to rate outcome expectancy throughout the experiment to ensure that they were attending the contingencies. This may have led to unbiased global covariation estimates. However, IC was significant on a trial-by-trial basis in individuals with spider phobia, although this effect was only marginally significantly more pronounced in phobics than in controls. This conceptualization of an IC should be more reliable due to repeated measurement than a global estimation based on two questions. Besides, it should be similarly relevant for fear maintenance, because it may reflect the tendency to expect an aversive stimulus when confronted with a spider again. As a second limitation, we have to consider that patients with spider phobia and healthy controls did not only differ in fear of spiders, but also showed increased state anxiety, trait anxiety and by trend higher depressiveness. Thus, the current results might have been influenced by one of these factors and not only specifically by fear of spiders. Moreover, trait anxiety is discussed to be a risk factor for the development of anxiety disorders (Mineka and Oehlberg, 2008). Hence, higher scores are expected in a typical sample of spider phobics. However, we found effects specifically related to spider images, the particular phobic stimuli for the experimental group. Notably, inclusion of trait anxiety as a covariate in these analyses did not change our main results, indicating that these effects were not related to fearfulness or anxiety in general. Therefore, we consider it unlikely that anxiety in general accounts as an alternative explanation of our findings. Third, the correlational findings between brain areas and IC do not allow us to conclude that these areas are a cause of IC. Since both dlPFC and PCL are situated at the surface of the human brain, future investigations might test for a causal impact on IC by manipulating their activity by transcranial magnetic stimulation.
In summary, we found that ICs in spider phobia are associated with biased neural processing of both fear-relevant cues and aversive outcomes. In particular, enhanced aversiveness and sensory motor processing of negative outcomes following spider images and enhanced activation of the dlPFC by phobic stimuli predicted IC in spider phobia. These results further contribute to a neurobiological explanation of fear maintenance in addition to classic fear conditioning. On a clinical level, our findings suggest that changing the evaluation of feared consequences and/or directing executive resources away from phobic stimuli should give rise to a less dangerous cognitive representation of phobic stimuli. Hence, supporting these processes during stimulus exposure may help to cure fear and anxiety.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We are grateful for the assistance of Dorothea Neueder, Marika Skowronski, Chalid Hasan, Jan Meier and Enne Floß. This work was supported by the Federal Ministry of Education and Research, project 01EO1004 (S.M.S., Comprehensive Heart Failure Center, University of Würzburg, Germany), and the German Research Foundation, SFB-TRR 58, project B01 (P.P., E.G., M.A., J.W.; Department of Psychology, University of Würzburg, Germany), project B05 (P.R.), and Research Group ‘Emotion and Behavior’, FOR 605.
REFERENCES
- Aupperle RL, Hale LR, Chambers RJ, et al. An fMRI study examining effects of acute D-cycloserine during symptom provocation in spider phobia. CNS Spectrums. 2009;14:556–71. doi: 10.1017/s1092852900024044. [DOI] [PubMed] [Google Scholar]
- Baas D, Aleman A, Kahn RS. Lateralization of amygdala activation: a systematic review of functional neuroimaging studies. Brain Research Brain Research Review. 2004;45:96–103. doi: 10.1016/j.brainresrev.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. Journal of Personality Assessment. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- Beckers T, Krypotos AM, Boddez Y, Effting M, Kindt M. What’s wrong with fear conditioning? Biological Psychology. 2013;92:90–6. doi: 10.1016/j.biopsycho.2011.12.015. [DOI] [PubMed] [Google Scholar]
- Bushnell MC, Duncan GH, Hofbauer RK, Ha B, Chen JI, Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proceedings of the National Acadamy of Sciences of the United States of America. 1999;96:7705–9. doi: 10.1073/pnas.96.14.7705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson K, Petersson KM, Lundqvist D, Karlsson A, Ingvar M, Öhman A. Fear and the amygdala: manipulation of awareness generates differential cerebral responses to phobic and fear-relevant (but nonfeared) stimuli. Emotion. 2004;4:340–53. doi: 10.1037/1528-3542.4.4.340. [DOI] [PubMed] [Google Scholar]
- Carter RM, O’Doherty JP, Seymour B, Koch C, Dolan RJ. Contingency awareness in human aversive conditioning involves the middle frontal gyrus. Neuroimage. 2006;29:1007–12. doi: 10.1016/j.neuroimage.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences. 2003;7:415–23. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- Davey GCL. Preparedness and phobias: specific evolved associations or a generalized expectancy bias? Behavioral and Brain Sciences. 2010;18:289–325. [Google Scholar]
- Davey GC, Dixon AL. The expectancy bias model of selective associations: the relationship of judgments of CS dangerousness, CS-UCS similarity and prior fear to a priori and a posteriori covariation assessments. Behaviour Research and Therapy. 1996;34:235–52. doi: 10.1016/0005-7967(96)88487-7. [DOI] [PubMed] [Google Scholar]
- De Jong PJ, Peters ML. Contamination vs. harm-relevant outcome expectancies and covariation bias in spider phobia. Behaviour Research and Therapy. 2007;45:1271–84. doi: 10.1016/j.brat.2006.09.007. [DOI] [PubMed] [Google Scholar]
- De Jong PJ, Van Den Hout MA, Merckelbach H. Covariation bias and the return of fear. Behaviour Research and Therapy. 1995;33:211–3. doi: 10.1016/0005-7967(94)e0024-d. [DOI] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. The Journal of Neuroscience. 2006;26:2072–9. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glotzbach-Schoon E, Tadda R, Andreatta M, et al. Enhanced discrimination between threatening and safe contexts in high-anxious individuals. Biological Psychology. 2013;93:159–66. doi: 10.1016/j.biopsycho.2013.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Miniussi C, Harris IM, Diamond ME. Transient storage of a tactile memory trace in primary somatosensory cortex. The Journal of Neuroscience. 2002;22:8720–5. doi: 10.1523/JNEUROSCI.22-19-08720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton C, Bork A, Josephs O, Deichmann R, Ashburner J, Turner R. Image distortion correction in fMRI: a quantitative evaluation. Neuroimage. 2002;16:217–40. doi: 10.1006/nimg.2001.1054. [DOI] [PubMed] [Google Scholar]
- Kenntner-Mabiala R, Andreatta M, Wieser MJ, Mühlberger A, Pauli P. Distinct effects of attention and affect on pain perception and somatosensory evoked potentials. Biological Psychology. 2008;78:114–22. doi: 10.1016/j.biopsycho.2008.01.007. [DOI] [PubMed] [Google Scholar]
- Kenntner-Mabiala R, Pauli P. Affective modulation of brain potentials to painful and nonpainful stimuli. Psychophysiology. 2005;42:559–67. doi: 10.1111/j.1469-8986.2005.00310.x. [DOI] [PubMed] [Google Scholar]
- Knoch D, Brugger P, Regard M. Suppressing versus releasing a habit: frequency-dependent effects of prefrontal transcranial magnetic stimulation. Cerebral Cortex. 2005;15:885–7. doi: 10.1093/cercor/bhh196. [DOI] [PubMed] [Google Scholar]
- Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Progress in Neurobiology. 2011;93:111–24. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, Pine DS, Grillon C. The strong situation: a potential impediment to studying the psychobiology and pharmacology of anxiety disorders. Biological Psychology. 2006;72:265–70. doi: 10.1016/j.biopsycho.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Lissek S, Powers AS, McClure EB, et al. Classical fear conditioning in the anxiety disorders: a meta-analysis. Behaviour Research and Therapy. 2005;43:1391–424. doi: 10.1016/j.brat.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin SJ, McDowell DJ, et al. Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behaviour Research and Therapy. 2009;47:111–8. doi: 10.1016/j.brat.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mineka S, Oehlberg K. The relevance of recent developments in classical conditioning to understanding the etiology and maintenance of anxiety disorders. Acta Psychologica. 2008;127:567–80. doi: 10.1016/j.actpsy.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Mühlberger A, Wiedemann G, Herrmann MJ, Pauli P. Phylo- and ontogenetic fears and the expectation of danger: differences between spider- and flight-phobic subjects in cognitive and physiological responses to disorder-specific stimuli. Journal of Abnormal Psychology. 2006;115:580–9. doi: 10.1037/0021-843X.115.3.580. [DOI] [PubMed] [Google Scholar]
- Paquette V, Lévesque J, Mensour B, et al. Change the mind and you change the brain: effects of cognitive-behavioral therapy on the neural correlates of spider phobia. Neuroimage. 2003;18:401–9. doi: 10.1016/s1053-8119(02)00030-7. [DOI] [PubMed] [Google Scholar]
- Pasternak T, Greenlee MW. Working memory in primate sensory systems. Nature Review Neuroscience. 2005;6:97–107. doi: 10.1038/nrn1603. [DOI] [PubMed] [Google Scholar]
- Pauli P, Montoya P, Martz G. Covariation bias in panic-prone individuals. Journal of Abnormal Psychology. 1996;105:658–62. doi: 10.1037//0021-843x.105.4.658. [DOI] [PubMed] [Google Scholar]
- Peyron R, Laurent B, Garcia-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis. Clinical Neurophysiology. 2000;30:263–88. doi: 10.1016/s0987-7053(00)00227-6. [DOI] [PubMed] [Google Scholar]
- Ptak R. The frontoparietal attention network of the human brain action, saliency, and a priority map of the environment. The Neuroscientist. 2012;18:502–15. doi: 10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- Reicherts P, Gerdes A, Pauli P, Wieser MJ. On the mutual effects of pain and emotion: facial pain expressions enhance pain perception and vice versa are perceived as more arousing when feeling pain. Pain. 2013;154:793–800. doi: 10.1016/j.pain.2013.02.012. [DOI] [PubMed] [Google Scholar]
- Rinck M, Becker ES. Approach and avoidance in fear of spiders. Journal of Behavior Therapy and Experimental Psychiatry. 2007;38:105–20. doi: 10.1016/j.jbtep.2006.10.001. [DOI] [PubMed] [Google Scholar]
- Rinck M, Bundschuh S, Engler S, et al. Reliabilität und Validität dreier Instrumente zur Messung von Angst vor Spinnen. Diagnostica. 2002;48:141–9. [Google Scholar]
- Roy M, Piché M, Chen JI, Peretz I, Rainville P. Cerebral and spinal modulation of pain by emotions. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20900–5. doi: 10.1073/pnas.0904706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto N, Honda M, Okada T, et al. Expectation of pain enhances responses to nonpainful somatosensory stimulation in the anterior cingulate cortex and parietal operculum/posterior insula: an event-related functional magnetic resonance imaging study. The Journal of Neuroscience. 2000;20(19):7438–45. doi: 10.1523/JNEUROSCI.20-19-07438.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schienle A, Schäfer A, Hermann A, Rohrmann S, Vaitl D. Symptom provocation and reduction in patients suffering from spider phobia. European Archives of Psychiatry and Clinical Neuroscience. 2007;257:486–93. doi: 10.1007/s00406-007-0754-y. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Cognitive Brain Research. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- Straube T, Mentzel HJ, Glauer M, Miltner WH. Brain activation to phobia-related words in phobic subjects. Neuroscience Letters. 2004;372:204–8. doi: 10.1016/j.neulet.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Szymanski J, O’Donohue W. Fear of spiders questionnaire. Journal of Behavior Therapy and Experimental Psychiatry. 1995;26:31–4. doi: 10.1016/0005-7916(94)00072-t. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Mineka S, Cook M. Fear-relevant selective associations and covariation bias. Journal Abnormal Psychology. 1989;98:381–94. doi: 10.1037//0021-843x.98.4.381. [DOI] [PubMed] [Google Scholar]
- Watts FN, Sharrock R. Questionnaire dimensions of spider phobia. Behaviour Research and Therapy. 1984;22:575–80. doi: 10.1016/0005-7967(84)90061-5. [DOI] [PubMed] [Google Scholar]
- Wiemer J, Mühlberger A, Pauli P. Illusory correlations between neutral and aversive events can be induced by outcome aversiveness. Cognition and Emotion. 2014;28:193–207. doi: 10.1080/02699931.2013.809699. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Zaudig M, Fydrich T. Strukturiertes Klinisches Interview für DSM-IV. Göttingen: Hogrefe; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.