Abstract
Although much attention has been directed towards life satisfaction that refers to an individual’s general cognitive evaluations of his or her life as a whole, little is known about the neural basis underlying global life satisfaction. In this study, we used voxel-based morphometry to investigate the structural neural correlates of life satisfaction in a large sample of young healthy adults (n = 299). We showed that individuals’ life satisfaction was positively correlated with the regional gray matter volume (rGMV) in the right parahippocampal gyrus (PHG), and negatively correlated with the rGMV in the left precuneus and left ventromedial prefrontal cortex. This pattern of results remained significant even after controlling for the effect of general positive and negative affect, suggesting a unique structural correlates of life satisfaction. Furthermore, we found that self-esteem partially mediated the association between the PHG volume and life satisfaction as well as that between the precuneus volume and global life satisfaction. Taken together, we provide the first evidence for the structural neural basis of life satisfaction, and highlight that self-esteem might play a crucial role in cultivating an individual’s life satisfaction.
Keywords: life satisfaction, precuneus, parahippocampal gyrus, self-esteem, voxel-based morphometry
INTRODUCTION
Over the decades, an increasing amount of attention has been devoted to positive psychology (Seligman and Csikszentmihalyi, 2000). A central construct within the positive psychology literature is global life satisfaction. Global life satisfaction is defined as an individual’s general cognitive evaluations of his or her life as a whole, which, along with positive and negative affect, is regarded as three primary components of subjective well-being that reflects the experience of pleasure (Diener et al., 2003). This type of well-being was termed as hedonia by Aristotle, which is differentiated from the notion of eudaimonia—the highest human good involving virtue and the realization of one’s potential (Aristotle, 1925). Contemporary psychological research has continued to distinguish between eudaimonic well-being and hedonic well-being (e.g. Ryan and Deci, 2001; Urry et al., 2004), with empirical studies demonstrating that these two types of well-being are related but independent constructs using confirmatory factor analysis techniques (Gallagher et al., 2009; Linley et al., 2009).
Life satisfaction has been increasingly recognized as an important domain in individual differences (Diener et al., 2003). For instance, life satisfaction tends to reflect relatively stable, long-term judgments of subjective well-being, whereas affect measures reflect more short-term reports of subjective well-being and show situational variability (Kim-Prieto et al., 2005). Life satisfaction is heritable (Stubbe et al., 2005) and a lack of satisfaction with life acts as a risk factor for depression (Green et al., 1992). Higher life satisfaction has also been linked to higher self-esteem, better physical health, better psychological health, higher eudaimonic well-being, more positive affect and stronger social relationships, and linked to lower neuroticism, fewer stressful life events and fewer negative affect (Ryff and Keyes, 1995; Zullig et al., 2005; Ring et al., 2007; Abdel-Khalek, 2010; Kong et al., 2012a, b, 2014a, b; Kong and You, 2013; Zhao et al., 2014). In this study, we used structural magnetic resonance imaging to investigate the brain structures underlying individual differences in life satisfaction.
Although life satisfaction has drawn continuous attention in the past few decades (e.g. Diener et al., 2003), little work to date has directly addressed the structural basis of life satisfaction. To our knowledge, the only study directly exploring the neural correlates of life satisfaction has found that higher left than right superior frontal activation under resting electroencephalography is associated with individuals’ global life satisfaction (Urry et al., 2004). Although no study has used MRI techniques to investigate the structural correlates of life satisfaction, numerous studies have explored the neural correlates of constructs related to life satisfaction such as depression, neuroticism, eudaimonic well-being, perceived stress, physical health and psychological health. Specifically, depression, which is moderately negatively associated with life satisfaction (Swami et al., 2007), is often found to be associated with structural changes or altered activation in the amygdala, hippocampus and insula in response to certain specific activation procedures (von Gunten et al., 2000; Campbell et al., 2004; Videbech and Ravnkilde, 2004; Hamilton et al., 2008; Hwang et al., 2010; Sprengelmeyer et al., 2011; Bechdolf et al., 2012). Neuroticism, which is moderately negatively associated with life satisfaction (Steel et al., 2008), is reported to be mostly associated with several brain regions including the amygdala, hippocampus/parahippocampal gyrus (PHG), posterior cingulate cortex/precuneus, anterior cingulate cortex (ACC), dorsomedial prefrontal cortex (DMPFC) and dorsal lateral prefrontal cortex (LPFC) (Omura et al., 2005; Wright et al., 2007; DeYoung et al., 2010; Kunisato et al., 2011; Lu et al., 2014a; Servaas et al., 2014). Recently, using voxel-based morphometry (VBM), anxiety symptoms is found to be correlated with regional gray matter volume (rGMV) in the PHG (Wei et al., 2014), and perceived stress is correlated with rGMV in the PHG and insular cortex (Li et al., 2014a). Among the constructs positively related to life satisfaction, eudaimonic well-being is reported to associated with rGMV in the insula (Lewis et al., 2014) and the activation of the ventromedial prefrontal cortex (VMPFC) in response to negative stimuli and dorsal LPFC in response to positive stimuli (van Reekum et al., 2007; Heller et al., 2013). Using VBM, better physical health, which is moderately associated with life satisfaction (Abdel-Khalek, 2010), is found to be associated with smaller rGMV in the rostral LPFC (Takeuchi et al., 2014a) and the amygdala (Song et al., 2014), whereas psychological health, which is moderately associated with life satisfaction (Abdel-Khalek, 2010), is associated with smaller rGMV in the ACC (Takeuchi et al., 2014a). On the basis of these studies, we speculated that structural differences in these regions may be associated with life satisfaction.
After reviewing the literature on life satisfaction, among the constructs, self-esteem has been demonstrated to be a strong correlate of life satisfaction in many studies. For example, Campbell (1981) found self-esteem was the strongest predictor of life satisfaction (with a correlation of 0.55) in a national sample of American adults. Neto (1993) further confirmed that self-esteem was the strongest predictor of life satisfaction in adolescents. Subsequently, Diener and Diener (1995) found a correlation of 0.47 between these two variables in college student samples from 31countries (see also Kwan et al. 1997; Kong et al., 2012a; Kong and You, 2013). Recently, a cross-lagged study further revealed that self-esteem predicted life satisfaction 8 months later, whereas the effect of life satisfaction on subsequent self-esteem was not found (Ye et al., 2012), suggesting that self-esteem plays a causal role in life satisfaction. This causal relationship can be explained by the bottom-up theories of subjective well-being. The theories propose that life satisfaction judgment is based on an assessment of satisfaction in a relatively small number of life domains such as family, friends and oneself (Brief et al., 1993; Schimmack, 2002; Heller et al., 2004) and thus the correlation between life satisfaction and domain satisfaction reflects a causal influence of domain satisfaction on life satisfaction. According to Diener and Diener (1995), self-esteem reflects one’s judgment and evaluation of oneself, while life satisfaction involves evaluations of one’s life as a whole. From the life satisfaction perspective, self-esteem is considered as a component of life satisfaction, which involves evaluations of different life domains such as family and oneself (Huebner et al., 1999). Taken together, self-esteem likely plays a causal role in life satisfaction. Thus, another important goal of this study was to test whether individual differences in self-esteem may account for the association between brain structure and life satisfaction.
To probe these questions, we used well-validated assessments of life satisfaction and self-esteem, and VBM methodology, which can be used to explore the structural neural correlates of interindividual differences in behavior (e.g. those related to stable personality characteristics) (Kanai and Rees, 2011; Takeuchi et al., 2011, 2014a; Kong, et al., 2014c; Lewis et al., 2014). Specifically, we measured participants’ levels of life satisfaction using the Satisfaction with Life Scale (SWLS, Diener et al., 1985), which has been widely used to assess individual differences in life satisfaction in previous studies (e.g. Neto, 1993; Ring et al., 2007; Kong et al., 2012a,b; Kong and You, 2013). On the basis of previous neuroscience findings on constructs related to life satisfaction (e.g. Urry et al., 2004; van Reekum et al., 2007; Heller et al., 2013; Lewis et al., 2014; Takeuchi et al., 2014a), we hypothesized that individual differences in life satisfaction would be associated with rGMV of the prefrontal (i.e. DMPFC, VMPFC and LPFC), ACC, the precuneus, PHG, amygdala, hippocampus and insula. Furthermore, given the important role of self-esteem in life satisfaction (Campbell, 1981; Neto, 1993; Diener and Diener, 1995; Kwan et al. 1997; Kong et al., 2012a; Ye et al., 2012; Kong and You, 2013), we further examined whether self-esteem would be able to mediate the relationship between brain structures and life satisfaction.
METHODS
Participants
Two hundred and ninety-nine college students [159 females; mean age = 21.55 years, standard deviation (s.d.) = 1.01] participated in this study as a part of an ongoing project investigating associations among brain imaging, cognitive functions and genetics (Wang et al., 2012; Huang et al., 2014; Kong et al., 2014c; Li et al., 2014b; Lu et al., 2014b; Song et al., 2014). Data that are irrelevant to the scope of this study were not reported here. All participants were college students from Beijing Normal University, Beijing, China. Participants were screened to confirm their healthy development by a self-report questionnaire before the study, and thus, those who had past or current psychiatric illness or a history of neurological illness were excluded. The majority of the participants were right-handed (n = 280) based on a single-item handedness questionnaire (‘Are you (a) right-handed, (b) left-handed, (c) mixed-handed?’). Both behavioral and MRI protocols were approved by the Institutional Review Board of Beijing Normal University. Written informed consent was obtained from all participants prior to study onset.
Assessment of global life satisfaction
The SWLS (Diener et al., 1985) was administered to assess global life satisfaction, which is the cognitive component of subjective well-being. The SWLS reflects global cognitive evaluations of one’s life, rather than summing across their satisfaction with specific domains (e.g. family, friends, school, self and living environment), for obtaining an index of overall life satisfaction. In their review of the SWLS, Pavot and Diener (1993) concluded that it is a one-dimensional, internally consistent measure. The SWLS has also high criterion-related validity with related constructs of optimism, loneliness, positive affect, negative affect, self-esteem, self-concept, anxiety and depression (Diener et al., 1985; Neto, 1993; Kong et al., 2012a, b; Kong and You, 2013; Kong and Zhao, 2013). The scale consists of five statements, such as, ‘I am satisfied with my life’ and ‘In most ways my life is close to my ideal’. Participants were instructed to indicate the extent to which they agree or disagree with each statement using a 5-point Likert scale. Higher scores reflect higher levels of global life satisfaction. The Chinese version of the SWLS has been demonstrated to be a reliable and valid measurement in assessing life satisfaction in Chinese adults (e.g. Kong et al., 2012a,b, 2014a; Kong and You, 2013; Kong and Zhao, 2013). In this study, the SWLS exhibited adequate reliability (α = 0.82).
Assessment of self-esteem
Self-esteem was measured by the Rosenberg Self-esteem Scale (RSES; Rosenberg, 1965), which is a 10-item self-report measure of global self-esteem. Each item is answered on a 6-point Likert type scale ranging from 1 = strongly disagree to 6 = strongly agree. It includes items such as, ‘I am able to do things as well as most other people’ and ‘I take a positive attitude toward myself’. Scale scores are the sum of items with reverse coding of relevant items. A meta-analysis of the scale by Schmitt and Allik (2005) found that the scale was a very popular test whose validity and reliability tests are used in 53 countries. The RSES has also high criterion-related validity with related constructs of extraversion, neuroticism, social support, positive affect, negative affect, life satisfaction, loneliness and depression (Rice et al., 1998; Schmitt and Allik, 2005; Çivitci and Çivitci, 2009; Kong et al., 2012b; Kong and You, 2013; Zhao et al., 2012, 2013). The Chinese version of the RSES has been found to be a reliable and valid measurement in assessing self-esteem in Chinese populations (Kong et al., 2012b; Zhao et al., 2012; Kong and You, 2013). In the study, the SWLS exhibited adequate reliability (α = 0.89).
Assessment of positive and negative affect
The affective component of subjective well-being was assessed by a general version of the Positive and Negative Affect Schedule (PANAS–Gen; Watson et al., 1988). The PANAS–Gen consists of a word list describing two different affect states (10 positive and 10 negative), for example, ‘excited’ and ‘upset’. Participants are instructed to indicate the extent to which they generally feel each affect using a 5-point Likert scale. Positive and negative affect scores are calculated separately, with higher scores indicating that participants feel more of that affect. The scale has high internal consistency, test–retest reliability and criterion-related validity with related constructs of extraversion, neuroticism, anxiety, depression, hope, stress and life satisfaction (Watson et al., 1988; Lucas et al., 1996; Crawford and Henry, 2004; Steel et al., 2008; Kong and Zhao, 2013). The Chinese version of the scale has been demonstrated to be a reliable and valid measurement in assessing positive and negative affect in Chinese population (e.g. Kang et al., 2003; Kong and Zhao, 2013; Sun and Kong, 2013). In this study, the positive affect scale (α = 0.81) and negative affect scale (α = 0.78) exhibited adequate reliability.
MRI acquisition
Participants were scanned using a Siemens 3T scanner (MAGENTOM Trio, a Tim system) with a 12-channel phased-array head coil at BNU Imaging Center for Brain Research, Beijing, China. MRI structural images were acquired using a 3D magnetization prepared rapid gradient echo T1-weighted sequence (TR/TE/TI = 2530/3.39/1100 ms, flip angle = 7°, FOV = 256 × 256 mm2). One hundred and twenty-eight contiguous sagittal slices were acquired with 1 × 1 mm2 in-plane resolution and 1.33-mm slab thickness for whole brain coverage.
Image processing for VBM
VBM was performed using SPM8 (Statistical Parametric Mapping, Wellcome Department of Imaging Neuroscience, London, UK), with an optimized VBM protocol on T1-weighted structural MRI images. First, image quality was assessed by manual visual inspection. Six participants whose images had excessive scanner artifacts or showed gross anatomical abnormalities were excluded. Second, the origin of the brain was manually set to the anterior commissure for each participant. Third, images were segmented into gray matter, white matter and cerebrospinal fluid using the unified segmentation approach (Ashburner and Friston, 2005). Fourth, gray matter images were rigidly aligned and resampled to 2 × 2 × 2 mm3 and normalized to a study-specific template in MNI152 space using the Diffeomorphic Anatomical Registration through Exponential Lie algebra registration method (Ashburner, 2007). Fifth, gray matter voxel values were modulated by multiplying the Jacobian determinants derived from the normalization to preserve the volume of tissue from each structure after warping. The modulated gray matter images were then smoothed with an 8-mm full-width-at-half-maximum isotropic Gaussian kernel.
Statistical analysis of VBM
Statistical analyses of the gray matter volume (GMV) data were performed using SPM8. In the whole-brain analyses, we used a multiple linear regression analysis to detect the neuroanatomical correlates of individual differences in life satisfaction. In the analysis, the self-reported life satisfaction score was used as the covariate of interest and age, sex and total GMV were as the confounding covariates. To exclude boundary effects between gray matter and white matter, an absolute threshold masking of 0.2 was used. For all analyses, the cluster-level statistical threshold was set at P < 0.05, and corrected at the non-stationary cluster correction (Hayasaka et al., 2004) according to the random field theory with an underlying voxel level of P < 0.0025. In this test, a relatively higher cluster-determining threshold combined with high smoothing values of more than six voxels has been found to lead to appropriate conservativeness in real data (Silver et al., 2011). With high smoothing values, an uncorrected threshold of P < 0.01 appears to bring about anticonservativeness, whereas that of P < 0.001 appears to result in slight conservativeness (Silver et al., 2011).
Furthermore, small-volume corrections (SVCs) were performed in regions with a priori hypothesis. The regions of interest (ROIs) were chosen from previous structural and functional imaging studies that might play an important role in life satisfaction. The Wake Forest University Pick Atlas (Maldjian et al., 2003) was used to define the regions of the ACC, VMPFC, DMPFC, dorsal and rostral LPFC, hippocampus, parahippocampus, amygdala and insula, based on the automated anatomical labeling template. The significance of correlation coefficients between life satisfaction and the rGMV of these ROIs were examined at a corrected threshold of P < 0.05, using the non-stationary cluster correction for multiple comparisons.
Mediation analysis
To test whether self-esteem can reliably explain the relationships between brain anatomy and life satisfaction, we conducted a mediation analysis, using the SPSS macro programmed by Preacher and Hayes (2008). It is based on a three-variable mediation model that investigates whether a predictor variable (X, brain anatomy) affects an outcome variable (Y, life satisfaction) through a mediator (M, self-esteem). Variable M is a mediator if X significantly predicts M (Path a), X significantly predicts in Y (Path c; representing the total effect), M significantly predicts in Y (Path b) when controlling for X, and the effect of X on Y reduces significantly when M as well as X simultaneously predicts Y (Path c′; representing the direct effect). In order to test statistical significance of the indirect effect through M, bootstrapping tests were used (Preacher and Hayes, 2008). We used 10 000 bootstrap samples to generate bootstrap confidence intervals (99%) for the indirect effects. An empirical 99% confidence interval do not include 0, signifying that the indirect effect is significant at the 0.01 level.
RESULTS
VBM of life satisfaction
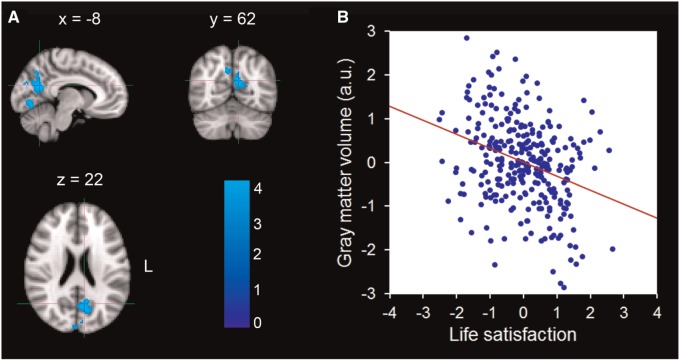
Table 1 lists the characteristics of demographics of the total sample. Behaviorally, we replicated the previous finding that life satisfaction is moderately positively correlated with positive affect (r = 0.28, P < 0.001, in our dataset), and negatively with negative affect (r = −0.26, P < 0.001, in our dataset), suggesting that these components of subjective well-being are related but distinct constructs. Neurally, in whole-brain analysis, multiple regression analysis revealed that life satisfaction was significantly and negatively correlated with GMV in an anatomical cluster that primarily included the left precuneus (MNI coordinate: −8, −62, 22; t = 4.66; Cluster size = 13 712; P < 0.05) (Figure 1; Table 2).
Table 1.
Demographic and psychometric measures (n = 293)
| Variables | Mean | s.d. | Range | r |
|---|---|---|---|---|
| Sex | – | – | – | 0.25** |
| Age | 21.56 | 1.01 | 18–25 | −0.06 |
| Total GMV | 0.49 | 0.04 | 0.39–0.60 | −0.16* |
| Positive affect | 34.15 | 4.78 | 20–48 | 0.28** |
| Negative affect | 24.40 | 4.69 | 12–40 | −0.26** |
| Life satisfaction | 20.03 | 5.34 | 6–35 | 1 |
Total GMV, Total gray matter volume. r, Pearson bivariate correlations with life satisfaction. *P < 0.01. **P < 0.001.
Fig. 1.
Brain regions that negatively correlated with life satisfaction. (A) The rGMV of the left precuneus was negatively correlated with life satisfaction. The coordinate is shown in the MNI stereotactic space. (B) Scatter plots depicting correlations between rGMV of the precuneus and individual variability in life satisfaction (r = −0.32, P < 0.001).
Table 2.
Brain structures correlating with life satisfaction
| Region | Side | MNI coordinate |
T | Cluster size (mm3) | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Positive correlation | ||||||
| PHG | R | 36 | −16 | −24 | 3.36 | 728* |
| Negative correlation | ||||||
| Precuneus | L | −8 | −62 | 22 | 4.66 | 13 712* |
| VMPFC | L | −4 | 54 | −14 | 3.58 | 952* |
MNI = Montreal Neurological Institute; L = left; R = right. All z-scores reflect a VBM threshold of P < 0.0025 (uncorrected). *P < 0.05 corrected at the non-stationary cluster level.
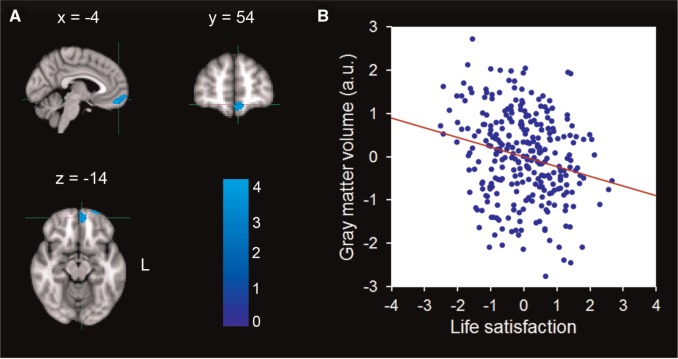
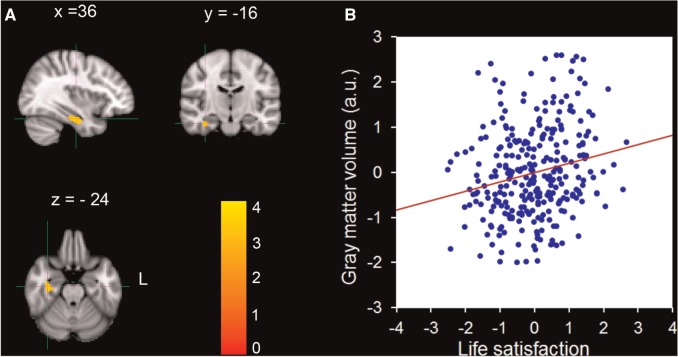
SVC analysis revealed a significant negative correlation between life satisfaction and rGMV in an anatomical cluster that mainly included the left VMPFC (MNI coordinate: −4, 50, −18; t = 3.86; Cluster size = 952; P < 0.05) (Figure 2; Table 2). A significant positive correlation between life satisfaction and rGMV was also identified in an anatomical cluster that included the right PHG (MNI coordinate: 36, −18, −24; t = 3.46; Cluster size = 728; P < 0.05) (Figure 3; Table 2). No significant relationships were observed in the ACC, DMPFC, dorsal and rostral LPFC, hippocampus, amygdala and insula. Because of the problem of multiple comparisons across nine ROIs, Bonferroni corrections were made to reduce the risk of Type I errors. We extracted the rGMV of the three aforementioned clusters from MRI scans of the participants and performed a correlation analysis between rGMV and life satisfaction. The results revealed that life satisfaction was negatively correlated with rGMV in the left precuneus (r = −0.32; P < 0.001, Bonferroni corrected) and left VMPFC (r = −0.22, P = 0.001, Bonferroni corrected), and positively with the rGMV in the right PHG (r = 0.21, P = 0.004, Bonferroni corrected).
Fig. 2.
Brain regions that negatively correlated with life satisfaction. (A) The rGMV of the left VMPFC was negatively correlated with life satisfaction. The coordinate is shown in the MNI stereotactic space. (B) Scatter plots depicting correlations between rGMV of the VMPFC and individual variability in life satisfaction (r = −0.22, P < 0.001).
Fig. 3.
Brain regions that positively correlated with life satisfaction. (A) The rGMV of the right PHG was positively correlated with life satisfaction. The coordinate is shown in the MNI stereotactic space. (B) Scatter plots depicting correlations between rGMV of the right PHG and individual variability in life satisfaction (r = 0.21, P < 0.001).
To examine whether these results are specific to life satisfaction, we also excluded a confounding factor of the affective component of subjective well-being (i.e. positive and negative affect). An additional model examining the association of life satisfaction with rGMV was tested with age, sex, global GMV and positive and negative affect as covariates. All correlations remained significant after age, sex, global GMV and positive and negative affect had been controlled (Left precuneus: MNI coordinate: −8, −62, 22; t = 5.22; Cluster size = 8699; P < 0.05; right PHG: MNI coordinate: 26, −28, −16; t = 3.51; Cluster size = 1304; P < 0.05; Left VMPFC: MNI coordinate: −2, 64, −8; t = 3.48; Cluster size = 920; P < 0.05). Although there were small variations in cluster size, significant regions were identical to those identified in initial analyses.
Brain structures mediated the relationship between self-esteem and life satisfaction
To test our hypothesis about the relationships between self-esteem, life satisfaction and brain structure, we collected the RSES measure from a subset (n = 274) of the participants studied in previous analysis and first performed a correlation analysis between self-esteem and life satisfaction. We replicated the previous finding that self-esteem has a strong correlation with life satisfaction (r = 0.45, P < 0.001, in our dataset). Next, we examined whether the rGMV of the regions related to life satisfaction could predict individual differences in self-esteem. The results showed that the rGMV in the right PHG (r = 0.23, P < 0.001, Bonferroni corrected) and left precuneus (r = −0.19, P = 0.003, Bonferroni corrected) was significantly correlated with self-esteem, even after adjusted for age, sex and total GMV. Furthermore, after age, sex, global GMV and positive and negative affect had been controlled, all correlations remained significant (right PHG: r = 0.24, P < 0.001, Bonferroni corrected; Left precuneus: r = −0.16, P = 0.021, Bonferroni corrected).
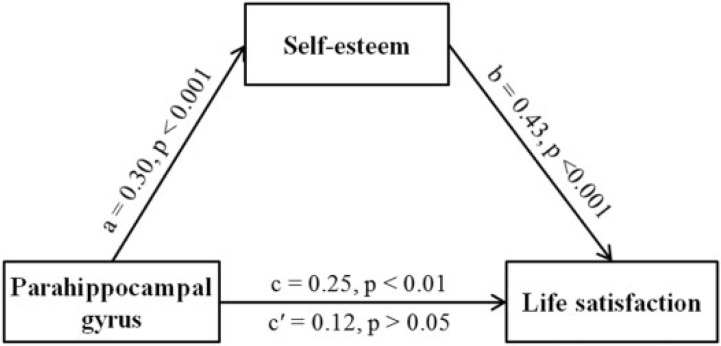
The results above indicated that self-esteem, life satisfaction and brain structure were linked closely to one another, but the exact relationships among these variables remain unknown. Here, we further performed two mediation analyses to examine whether self-esteem is able to mediate the relationship between brain structure and life satisfaction. Age, sex and total GMV were used as covariates in the mediational model. The results showed that the effect of the PHG volume on life satisfaction was not significant (β = 0.12, P > 0.05) after self-esteem was added as a mediator in the model. In contrast, the direct relationship was significant (β = 0.25, P = 0.001). Bootstrap simulation (n = 10 000) further confirmed that the indirect effect through self-esteem was significant (99% confidence interval = [0.26, 1.25], P < 0.01). Thus, self-esteem partially mediated the association between the PHG volume and life satisfaction (see Figure 4).
Fig. 4.
The PHG mediates the impact of self-esteem on life satisfaction. Depicted is the path diagram (including standard regression coefficients) of the mediation analysis demonstrating that the rGMV of the PHG affects individuals’ life satisfaction through self-esteem. All four requirements for a mediation effect are satisfied: Paths a–c are significant, and path c′ is significantly smaller than path c.
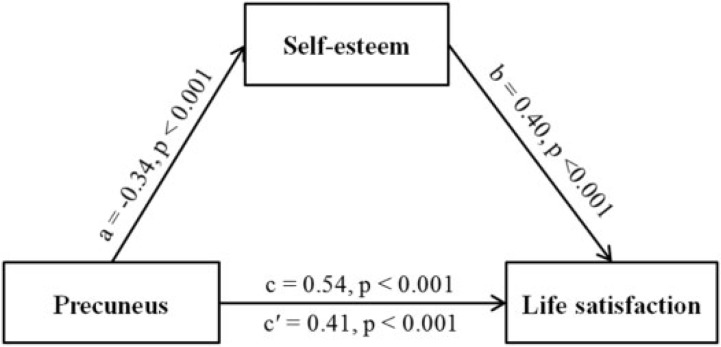
Same procedure was also implemented for the mediating effect of the precuneus. The results showed that the effect of the precuneus volume on life satisfaction reduced, though still significant (β = −0.41, P < 0.001) after self-esteem was added as a mediator in the model. In contrast, the direct relationship was significant (β = −0.54, P < 0.001). Bootstrap simulation (n = 10 000) further confirmed that the indirect effect through self-esteem was significant (99% confidence interval = [−1.47, −0.21], P < 0.01). Thus, self-esteem partially mediated the association between the precuneus volume and life satisfaction (see Figure 5).
Fig. 5.
The precuneus mediates the impact of self-esteem on life satisfaction. Depicted is the path diagram (including standard regression coefficients) of the mediation analysis demonstrating that the rGMV of the precuneus affects individuals’ life satisfaction through self-esteem. All four requirements for a mediation effect are satisfied: Paths a–c are significant, and path c′ is significantly smaller than path c.
DISCUSSION
The aim of this study was to investigate the structural neural correlates of global life satisfaction in a large sample of young healthy adults. Behavioral results showed that life satisfaction was positively correlated with self-esteem, which is consistent with previous studies (Campbell, 1981; Neto, 1993; Diener and Diener, 1995; Kwan et al., 1997; Kong et al., 2012a; Ye et al., 2012; Kong and You, 2013). The VBM analysis revealed that individuals’ life satisfaction was positively correlated with the rGMV in the right PHG, and negatively correlated with the rGMV in the left VMPFC and left precuneus. Furthermore, mediation analyses indicated that self-esteem mediated the association between the rGMV in the precuneus and PHG and life satisfaction. Taken together, our study provides the first evidence on the structural neural basis of life satisfaction, and highlight that self-esteem may play a crucial role in cultivating an individual’s life satisfaction.
In accordance with our expectation, our study revealed a significant negative correlation between life satisfaction and the rGMV in the left precuneus. The negative association between life satisfaction and regional structures is not surprising, because recent studies have often observed negative correlations between a range of cognitive functions (e.g. emotional intelligence) and the rGMV of brain regions (e.g. the medial prefrontal cortex) (Takeuchi et al., 2011, 2014a; for a review, see Kanai and Rees, 2011). The negative relationships are believed to be relevant to the intracortical myelination and synaptic pruning during development (Huttenlocher et al., 1982; Sowell et al., 2001; Paus, 2005). Previous studies have shown that the precuneus is involved in the controlling and switching of attention among objects and object features (Barber and Carter, 2005; Cavanna and Trimble, 2006). Furthermore, the region is also reported to play a central role in a range of highly integrated tasks, including visuo-spatial imagery (Knauff et al., 2002; Simon et al., 2002; Vanlierde et al., 2003; for a review, see Cavanna and Trimble, 2006), episodic memory retrieval (Wiggs et al., 1998; Lundstrom et al., 2003, 2005; Wagner et al., 2005) and self-processing operations such as self-consciousness, autobiographical memory, sense of agency and self-reflection (Kircher et al., 2002; Lou et al., 2004; Vogeley et al., 2004; Den Ouden et al., 2005; Freton et al., 2014; for a review, see Cavanna and Trimble, 2006), all of which seems consistent with the notion that the precuneus plays a central role in the modulation of conscious processes (Cavanna and Trimble, 2006; Cavanna, 2007). Precuneus dysfunction has been linked with sleep, vegetative state, drug-induced anesthesia and neuropsychiatric disorders including epilepsy, Alzheimer's disease and schizophrenia characterized by impaired consciousness (Vogt and Laureys, 2005; Cavanna, 2007). Thus, pruning of ineffective synapses during development may lead to less regional gray matter in the region, and increases in efficiency of conscious processes, both of which help individuals retrieve a positive self-image from positive episodic/autobiographical memories and lead to higher levels of life satisfaction.
We also revealed a significant negative correlation between life satisfaction and the rGMV in the left VMPFC. Previous studies have shown suggested that the VMPFC encodes the value of external rewards from various modalities including juice (Kim et al., 2011), faces (Smith et al., 2010; Lin et al., 2012) and non-monetary goods such as snack foods (Chib et al., 2009) and the affective value of emotional stimuli (Winecoff et al., 2013). Furthermore, the region is also shown to play an important role in emotional regulation, emotional perspective taking, sympathy, social decision making and collaborative attention and goals (Mitchell et al., 2005; Amodio and Frith, 2006; Saxe et al., 2006; Etkin et al., 2011; Rilling and Sanfey, 2011; Takeuchi et al., 2014b), which has immense value for social behavior and well-being. VMPFC dysfunction has been associated with psychopathy (Motzkin et al., 2011), depression (Brassen et al., 2008), schizophrenia (Park et al., 2008) and generalized social anxiety disorder (Evans et al., 2008). Thus, the highly developed VMPFC might help individuals to regulate emotional reactions jeopardizing valued relationships, sympathize with others’ experience and value long-term benefits associated with cooperative relationships, thus leading to higher levels of life satisfaction.
Furthermore, we revealed that the rGMV in the right PHG was positively associated with individuals’ life satisfaction, suggesting that the function of this structure contributes to life satisfaction. The PHG has been involved in perceptual processing, encoding and retrieval of scenes and places (Epstein et al., 1999; Hayes et al., 2007; Epstein, 2008; Rudy, 2009), emotional memory encoding and retrieval (Alkire et al., 1998; LaBar and Cabeza, 2006; Sterpenich et al., 2006; Murty et al., 2010) and emotional perceptual decision making (Pessoa and Padmala, 2005). This cortical region also plays an important role in stress regulation (Ulrich-Lai and Herman, 2009), and pain and stress perception (Cheng et al., 2007; Li et al., 2014a). PHG dysfunction has been linked with post-traumatic stress disorders (Etkin and Wager, 2007; Werner et al., 2009; Meng et al., 2014), anxiety disorders (Etkin and Wager, 2007; Goldin et al., 2009) and schizophrenia (Gradin et al., 2011). Therefore, higher levels of life satisfaction might be associated with larger rGMV in the right PHG through an array of capacities such as better emotional memory, more faithful pain/stress perception and improved stress regulation.
Interestingly, we found that self-esteem mediated the effect of the PHG volume on life satisfaction. The finding highlights that the PHG might play a crucial role in self-esteem, consistent with studies previously reporting the associations between measures related to self-esteem and hippocampal formation including the PHG and hippocampus (Pruessner et al., 2005; Onoda et al., 2010; Kubarych et al., 2012; Miyamoto and Kikuchi et al., 2012; Egenolf et al., 2013; Frewen et al., 2013). For example, individuals with low self-esteem showed greater activation in the PHG in response to social exclusion, relative to those with high self-esteem (Onoda et al., 2010). Low self-esteem has been found to be associated with greater amounts of perceived daily hassles and chronic stressors (Abouserie 1994; Lo, 2002), and associated with high cortisol responses to stress (Kirschbaum et al., 1995; Pruessner et al., 2005) and cardiovascular and inflammatory responses (O’Donnell et al., 2008). Furthermore, the hippocampus/PHG is also reported to be associated with cortisol responses to stress (Pruessner et al., 2005; Cunningham-Bussel et al., 2009; Root et al., 2009) and cardiovascular responses (Critchley et al., 2000). Therefore, given the role of the PHG in emotional memory (Alkire et al., 1998; LaBar and Cabeza, 2006; Sterpenich et al., 2006; Murty et al., 2010), stress regulation (Ulrich-Lai and Herman, 2009) and pain and stress perception (Cheng et al., 2007; Liet al., 2014a), it is not surprising that the PHG engagement can lead to higher levels of life satisfaction through striving for high self-esteem as a buffer for stressful life events.
We also found that self-esteem supported the association between the precuneus volume and life satisfaction. Numerous studies have demonstrated that the precuneus/posterior cingulate cortex is a central part of the default mode network (DMN; Fox et al., 2005; Fransson, 2005; Buckner et al. 2008; Fransson and Marrelec, 2008) and it serves as a functional core of the DMN (Utevsky et al., 2014). The DMN that consists of the precuneus/posterior cingulate cortex, medial prefrontal cortex, medial temporal lobe and posterior lateral cortices has been shown to be involved in self-referential processes such as conscious awareness of the internal and external environment (Buckner et al. 2008). These self-referential processes are believed to form the core of our self and are critical for elaborating experiential feelings of self (Northoff et al., 2006). Previous neuroimaging studies have demonstrated the importance of the precuneus in self-esteem (Onoda et al., 2010; Rameson et al., 2010; Eisenberger et al., 2011; Miyamoto and Kikuchi et al., 2012; Oikawa et al., 2012). In particular, self-esteem is found to be positively associated with the activity of the precuneus in the processing of positive self-face evaluation (Oikawa et al., 2012). Therefore, the highly developed precuneus seems to facilitate the cognition of self-referential processes, and thus help individuals get high positive self-evaluations (i.e. high self-esteem) from positive episodic/autobiographical memories, which in turn increases levels of life satisfaction.
In conclusion, we employed the VBM approach to investigate the structural neural correlates of individual differences in life satisfaction. We found that rGMV of the right PHG, left MPFC and left precuneus was related to life satisfaction. These findings were maintained even after controlling for individual differences on measures of positive and negative affect. As such, this research demonstrated a unique structural basis for individual differences in life satisfaction. Moreover, we also substantiated that self-esteem acted as a potential mechanism that accounted for the association between right PHG volume and life satisfaction as well as that between right precuneus volume and life satisfaction. The present findings seem to provide valuable guidance for how to implement psychological (self-esteem intervention) or neural-based interventions (e.g. neurofeedback training) aimed at enhancing an individual’ life satisfaction. Despite these strengths in our study, this sample was drawn from a college student population. The narrow age range may limit the generalizability of our findings, although it is common to choose college students as participants (Takeuchi, et al., 2011, 2014a; Kong et al., 2014c; Li et al., 2014a; Lewis et al., 2014; Song et al., 2014). In addition, we cannot determine the direction of causation between self-esteem, life satisfaction and brain structure. The implementation of longitudinal or experimental studies will help to elucidate these complex relationships in the future.
Conflict of Interest
None declared.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (31230031, 91132703, 31221003), the National Basic Research Program of China (2014CB846103), National Social Science Foundation of China (11&ZD187), and Changjiang Scholars Programme of China.
REFERENCES
- Aristotle. Nicomachean Ethics. 1925. (D. Ross, Trans.), New York, NY: Oxford University Press. [Google Scholar]
- Abdel-Khalek AM. Quality of life, subjective well-being, and religiosity in Muslim college students. Quality of Life Research. 2010;19:1133–43. doi: 10.1007/s11136-010-9676-7. [DOI] [PubMed] [Google Scholar]
- Abouserie R. Sources and levels of stress in relation to locus of control and self esteem in university students. Educational Psychology. 1994;14:323–30. [Google Scholar]
- Alkire MT, Haier RJ, Fallon JH, Cahill L. Hippocampal, but not amygdala, activity at encoding correlates with long-term, free recall of nonemotional information. Proceedings of the National Academy of Sciences. 1998;95:14506–10. doi: 10.1073/pnas.95.24.14506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Ashburner J. A fast diffeomorphic image registration algorithm. Neuroimage. 2007;38:95–113. doi: 10.1016/j.neuroimage.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–51. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Barber AD, Carter CS. Cognitive control involved in overcoming prepotent response tendencies and switching between tasks. Cerebral Cortex. 2005;15:899–912. doi: 10.1093/cercor/bhh189. [DOI] [PubMed] [Google Scholar]
- Bechdolf A, Wood SJ, Nelson B, et al. Amygdala and insula volumes prior to illness onset in bipolar disorder: a magnetic resonance imaging study. Psychiatry Research: Neuroimaging. 2012;201:34–9. doi: 10.1016/j.pscychresns.2011.06.010. [DOI] [PubMed] [Google Scholar]
- Brassen S, Kalisch R, Weber-Fahr W, Braus DF, Büchel C. Ventromedial prefrontal cortex processing during emotional evaluation in late-life depression: a longitudinal functional magnetic resonance imaging study. Biological Psychiatry. 2008;64:349–55. doi: 10.1016/j.biopsych.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Brief AP, Butcher AH, George JM, Link KE. Integrating bottom-up and top-down theories of subjective well-being: the case of health. Journal of Personality and Social Psychology. 1993;64:646–53. doi: 10.1037//0022-3514.64.4.646. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network. Annals of the New York Academy of Sciences. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Campbell A. The Sense of Well-Being in America: Recent Patterns and Trends. New York: McGraw-Hill; 1981. [Google Scholar]
- Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. American Journal of Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- Cavanna A. The precuneus and consciousness. CNS Spectrums. 2007;12:545–52. doi: 10.1017/s1092852900021295. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–83. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Lin C-P, Liu H-L, et al. Expertise modulates the perception of pain in others. Current Biology. 2007;17:1708–13. doi: 10.1016/j.cub.2007.09.020. [DOI] [PubMed] [Google Scholar]
- Chib VS, Rangel A, Shimojo S, O’Doherty JP. Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. The Journal of Neuroscience. 2009;29:12315–20. doi: 10.1523/JNEUROSCI.2575-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çivitci N, Çivitci A. Self-esteem as mediator and moderator of the relationship between loneliness and life satisfaction in adolescents. Personality and Individual Differences. 2009;47:954–8. [Google Scholar]
- Crawford JR, Henry JD. The Positive and Negative Affect Schedule (PANAS): construct validity, measurement properties and normative data in a large non-clinical sample. British Journal of Clinical Psychology. 2004;43:245–65. doi: 10.1348/0144665031752934. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Daly EM, Bullmore ET, et al. The functional neuroanatomy of social behaviour changes in cerebral blood flow when people with autistic disorder process facial expressions. Brain. 2000;123:2203–12. doi: 10.1093/brain/123.11.2203. [DOI] [PubMed] [Google Scholar]
- Cunningham-Bussel AC, Root JC, Butler T, et al. Diurnal cortisol amplitude and fronto-limbic activity in response to stressful stimuli. Psychoneuroendocrinology. 2009;34:694–704. doi: 10.1016/j.psyneuen.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Ouden HE, Frith U, Frith C, Blakemore S-J. Thinking about intentions. Neuroimage. 2005;28:787–96. doi: 10.1016/j.neuroimage.2005.05.001. [DOI] [PubMed] [Google Scholar]
- DeYoung CG, Hirsh JB, Shane MS, Papademetris X, Rajeevan N, Gray JR. Testing predictions from personality neuroscience brain structure and the big five. Psychological Science. 2010;21:820–8. doi: 10.1177/0956797610370159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener E, Diener M. Cross-cultural correlates of life satisfaction and self-esteem. Journal of Personality and Social Psychology. 1995;68:653–63. doi: 10.1037//0022-3514.68.4.653. [DOI] [PubMed] [Google Scholar]
- Diener E, Emmons RA, Larsen RJ, Griffin S. The satisfaction with life scale. Journal of Personality Assessment. 1985;49:71–5. doi: 10.1207/s15327752jpa4901_13. [DOI] [PubMed] [Google Scholar]
- Diener E, Oishi S, Lucas RE. Personality, culture, and subjective well-being: emotional and cognitive evaluations of life. Annual Review of Psychology. 2003;54:403–25. doi: 10.1146/annurev.psych.54.101601.145056. [DOI] [PubMed] [Google Scholar]
- Egenolf Y, Stein M, Koenig T, Holtforth MG, Dierks T, Caspar F. Tracking the implicit self using event-related potentials. Cognitive, Affective, & Behavioral Neuroscience. 2013;13:885–99. doi: 10.3758/s13415-013-0169-3. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Inagaki TK, Muscatell KA, Haltom KEB, Leary MR. The neural sociometer: brain mechanisms underlying state self-esteem. Journal of Cognitive Neuroscience. 2011;23:3448–55. doi: 10.1162/jocn_a_00027. [DOI] [PubMed] [Google Scholar]
- Epstein R, Harris A, Stanley D, Kanwisher N. The parahippocampal place area: recognition, navigation, or encoding? Neuron. 1999;23:115–25. doi: 10.1016/s0896-6273(00)80758-8. [DOI] [PubMed] [Google Scholar]
- Epstein RA. Parahippocampal and retrosplenial contributions to human spatial navigation. Trends in Cognitive Sciences. 2008;12:388–96. doi: 10.1016/j.tics.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in Cognitive Sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager T. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans KC, Simon NM, Dougherty DD, et al. A PET study of tiagabine treatment implicates ventral medial prefrontal cortex in generalized social anxiety disorder. Neuropsychopharmacology. 2008;34:390–8. doi: 10.1038/npp.2008.69. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:9673–78. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Human Brain Mapping. 2005;26:15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–84. doi: 10.1016/j.neuroimage.2008.05.059. [DOI] [PubMed] [Google Scholar]
- Freton M, Lemogne C, Bergouignan L, Delaveau P, Lehéricy S, Fossati P. The eye of the self: precuneus volume and visual perspective during autobiographical memory retrieval. Brain Structure and Function. 2014;219:959–68. doi: 10.1007/s00429-013-0546-2. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Lundberg E, Brimson-Théberge M, Théberge J. Neuroimaging self-esteem: a fMRI study of individual differences in women. Social Cognitive and Affective Neuroscience. 2013;8:546–55. doi: 10.1093/scan/nss032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher MW, Lopez SJ, Preacher KJ. The hierarchical structure of well-being. Journal of Personality. 2009;77:1025–50. doi: 10.1111/j.1467-6494.2009.00573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin PR, Manber T, Hakimi S, Canli T, Gross JJ. Neural bases of social anxiety disorder: emotional reactivity and cognitive regulation during social and physical threat. Archives of General Psychiatry. 2009;66:170–80. doi: 10.1001/archgenpsychiatry.2008.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M, Reid I, Hall J, Steele JD. Expected value and prediction error abnormalities in depression and schizophrenia. Brain. 2011;134:1751–64. doi: 10.1093/brain/awr059. [DOI] [PubMed] [Google Scholar]
- Green B, Copeland J, Dewey M, et al. Risk factors for depression in elderly people: a prospective study. Acta Psychiatrica Scandinavica. 1992;86:213–7. doi: 10.1111/j.1600-0447.1992.tb03254.x. [DOI] [PubMed] [Google Scholar]
- Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Molecular Psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka S, Phan KL, Liberzon I, Worsley KJ, Nichols TE. Nonstationary cluster-size inference with random field and permutation methods. Neuroimage. 2004;22:676–87. doi: 10.1016/j.neuroimage.2004.01.041. [DOI] [PubMed] [Google Scholar]
- Hayes SM, Nadel L, Ryan L. The effect of scene context on episodic object recognition: parahippocampal cortex mediates memory encoding and retrieval success. Hippocampus. 2007;17:873–89. doi: 10.1002/hipo.20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller AS, van Reekum CM, Schaefer SM, et al. Sustained striatal activity predicts eudaimonic well-being and cortisol output. Psychological Science. 2013;24:2191–200. doi: 10.1177/0956797613490744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller D, Watson D, Ilies R. The role of person versus situation in life satisfaction: a critical examination. Psychological Bulletin. 2004;130:574–600. doi: 10.1037/0033-2909.130.4.574. [DOI] [PubMed] [Google Scholar]
- Huang L, Song Y, Li J, Zhen Z, Yang Z, Liu J. Individual differences in cortical face selectivity predict behavioral performance in face recognition. Frontiers in Human Neuroscience. 2014;8:483. doi: 10.3389/fnhum.2014.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huebner ES, Gilman R, Laughlin J. A multimethod investigation of the multidimensionality of children's well-being reports: discriminant validity of life satisfaction and self-esteem. Social Indicators Research. 1999;46:1–22. [Google Scholar]
- Huttenlocher PR, de Courten C, Garey LJ, Van der Loos H. Synaptogenesis in human visual cortex—evidence for synapse elimination during normal development. Neuroscience Letters. 1982;33:247–52. doi: 10.1016/0304-3940(82)90379-2. [DOI] [PubMed] [Google Scholar]
- Hwang J-P, Lee T-W, Tsai S-J, et al. Cortical and subcortical abnormalities in late-onset depression with history of suicide attempts investigated with MRI and voxel-based morphometry. Journal of Geriatric Psychiatry and Neurology. 2010;23:171–84. doi: 10.1177/0891988710363713. [DOI] [PubMed] [Google Scholar]
- Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nature Reviews Neuroscience. 2011;12:231–42. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- Kang S-M, Shaver PR, Sue S, Min K-H, Jing H. Culture-specific patterns in the prediction of life satisfaction: roles of emotion, relationship quality, and self-esteem. Personality and Social Psychology Bulletin. 2003;29:1596–608. doi: 10.1177/0146167203255986. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O’Doherty JP. Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cerebral Cortex. 2011;21:769–76. doi: 10.1093/cercor/bhq145. [DOI] [PubMed] [Google Scholar]
- Kircher TT, Brammer M, Bullmore E, Simmons A, Bartels M, David AS. The neural correlates of intentional and incidental self processing. Neuropsychologia. 2002;40:683–92. doi: 10.1016/s0028-3932(01)00138-5. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Prussner JC, Stone AA, et al. Persistent high cortisol responses to repeated psychological stress in a subpopulation of healthy men. Psychosomatic Medicine. 1995;57:468–74. doi: 10.1097/00006842-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Knauff M, Mulack T, Kassubek J, Salih HR, Greenlee MW. Spatial imagery in deductive reasoning: a functional MRI study. Cognitive Brain Research. 2002;13:203–12. doi: 10.1016/s0926-6410(01)00116-1. [DOI] [PubMed] [Google Scholar]
- Kong F, Ding K, Zhao J. The relationships among gratitude, self-esteem, social support and life satisfaction among undergraduate students. Journal of Happiness Studies. 2014a , doi: 10.1007/s10902-014-9519-2. [Google Scholar]
- Kong F, Wang X, Zhao J. Dispositional mindfulness and life satisfaction: the role of core self-evaluations. Personality and Individual Differences. 2014b;56:165–9. [Google Scholar]
- Kong F, You X. Loneliness and self-esteem as mediators between social support and life satisfaction in late adolescence. Social Indicators Research. 2013;110:271–9. [Google Scholar]
- Kong F, Zhao J. Affective mediators of the relationship between trait emotional intelligence and life satisfaction in young adults. Personality and Individual Differences. 2013;54:197–201. [Google Scholar]
- Kong F, Zhao J, You X. Emotional intelligence and life satisfaction in Chinese university students: the mediating role of self-esteem and social support. Personality and Individual Differences. 2012a;53:1039–43. [Google Scholar]
- Kong F, Zhao J, You X. Social support mediates the impact of emotional intelligence on mental distress and life satisfaction in Chinese young adults. Personality and Individual Differences. 2012b;53:513–7. [Google Scholar]
- Kong F, Zhen Z, Li J, et al. Sex-related neuroanatomical basis of emotion regulation ability. PloS ONE. 2014c;9:e97071. doi: 10.1371/journal.pone.0097071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubarych TS, Prom-Wormley EC, Franz CE, et al. A multivariate twin study of hippocampal volume, self-esteem and well-being in middle-aged men. Genes, Brain and Behavior. 2012;11:539–44. doi: 10.1111/j.1601-183X.2012.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunisato Y, Okamoto Y, Okada G, et al. Personality traits and the amplitude of spontaneous low-frequency oscillations during resting state. Neuroscience Letters. 2011;492:109–13. doi: 10.1016/j.neulet.2011.01.067. [DOI] [PubMed] [Google Scholar]
- Kwan VSY, Bond MH, Singelis TM. Pancultural explanations for life satisfaction: adding relationship harmony to self-esteem. Journal of Personality and Social Psychology. 1997;73:1038–51. doi: 10.1037//0022-3514.73.5.1038. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Reviews Neuroscience. 2006;7:54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lewis GJ, Kanai R, Rees G, Bates TC. Neural correlates of the ‘good life’: eudaimonic well-being is associated with insular cortex volume. Social Cognitive and Affective Neuroscience. 2014;9:615–8. doi: 10.1093/scan/nst032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Li W, Wei D, et al. Examining brain structures associated with perceived stress in a large sample of young adults via voxel-based morphometry. Neuroimage. 2014a;92:1–7. doi: 10.1016/j.neuroimage.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Li X, De Beuckelaer A, Guo J, Ma F, Xu M, Liu J. The gray matter volume of the amygdala is correlated with the perception of melodic intervals: a voxel-based morphometry study. PloS ONE. 2014b;9:e99889. doi: 10.1371/journal.pone.0099889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A, Adolphs R, Rangel A. Social and monetary reward learning engage overlapping neural substrates. Social Cognitive and Affective Neuroscience. 2012;7:274–81. doi: 10.1093/scan/nsr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley PA, Maltby J, Wood AM, Osborne G, Hurling R. Measuring happiness: the higher order factor structure of subjective and psychological well-being measures. Personality and Individual Differences. 2009;47:878–84. [Google Scholar]
- Lo R. A longitudinal study of perceived level of stress, coping and self-esteem of undergraduate nursing students: an Australian case study. Journal of Advanced Nursing. 2002;39:119–26. doi: 10.1046/j.1365-2648.2000.02251.x. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, et al. Parietal cortex and representation of the mental self. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6827–32. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu F, Huo Y, Li M, et al. Relationship between personality and gray matter volume in healthy young adults: a voxel-based morphometric study. PloS ONE. 2014a;9:e88763. doi: 10.1371/journal.pone.0088763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Song Y, Xu M, Wang X, Li X, Liu J. The brain structure correlates of individual differences in trait mindfulness: a voxel-based morphometry study. Neuroscience. 2014b;272:21–8. doi: 10.1016/j.neuroscience.2014.04.051. [DOI] [PubMed] [Google Scholar]
- Lucas RE, Diener E, Suh E. Discriminant validity of well-being measures. Journal of Personality and Social Psychology. 1996;71:616–28. doi: 10.1037//0022-3514.71.3.616. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Ingvar M, Petersson KM. The role of precuneus and left inferior frontal cortex during source memory episodic retrieval. Neuroimage. 2005;27:824–34. doi: 10.1016/j.neuroimage.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Lundstrom BN, Petersson KM, Andersson J, Johansson M, Fransson P, Ingvar M. Isolating the retrieval of imagined pictures during episodic memory: activation of the left precuneus and left prefrontal cortex. Neuroimage. 2003;20:1934–1943. doi: 10.1016/j.neuroimage.2003.07.017. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Meng Y, Qiu C, Zhu H, et al. Anatomical deficits in adult posttraumatic stress disorder: a meta-analysis of voxel-based morphometry studies. Behavioural Brain Research. 2014 doi: 10.1016/j.bbr.2014.05.021. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17:1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Miyamoto R, Kikuchi Y. Gender differences of brain activity in the conflicts based on implicit self-esteem. PloS ONE. 2012;7:e37901. doi: 10.1371/journal.pone.0037901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M. Reduced prefrontal connectivity in psychopathy. The Journal of Neuroscience. 2011;31:17348–57. doi: 10.1523/JNEUROSCI.4215-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Ritchey M, Adcock RA, LaBar KS. fMRI studies of successful emotional memory encoding: a quantitative meta-analysis. Neuropsychologia. 2010;48:3459–69. doi: 10.1016/j.neuropsychologia.2010.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto F. The satisfaction with life scale: psychometrics properties in an adolescent sample. Journal of Youth and Adolescence. 1993;22:125–34. [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain—a meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- O’Donnell K, Brydon L, Wright CE, Steptoe A. Self-esteem levels and cardiovascular and inflammatory responses to acute stress. Brain, Behavior, and Immunity. 2008;22:1241–7. doi: 10.1016/j.bbi.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Oikawa H, Sugiura M, Sekiguchi A, et al. Self-face evaluation and self-esteem in young females: an fMRI study using contrast effect. Neuroimage. 2012;59:3668–76. doi: 10.1016/j.neuroimage.2011.10.098. [DOI] [PubMed] [Google Scholar]
- Omura K, Constable RT, Canli T. Amygdala gray matter concentration is associated with extraversion and neuroticism. Neuroreport. 2005;16:1905–8. doi: 10.1097/01.wnr.0000186596.64458.76. [DOI] [PubMed] [Google Scholar]
- Onoda K, Okamoto Y, Nakashima KI, et al. Does low self-esteem enhance social pain? The relationship between trait self-esteem and anterior cingulate cortex activation induced by ostracism. Social Cognitive and Affective Neuroscience. 2010;5:385–91. doi: 10.1093/scan/nsq002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IH, Park H-J, Chun J-W, Kim EY, Kim J-J. Dysfunctional modulation of emotional interference in the medial prefrontal cortex in patients with schizophrenia. Neuroscience Letters. 2008;440:119–24. doi: 10.1016/j.neulet.2008.05.094. [DOI] [PubMed] [Google Scholar]
- Paus T. Mapping brain maturation and cognitive development during adolescence. Trends in Cognitive Sciences. 2005;9:60–8. doi: 10.1016/j.tics.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Pavot W, Diener E. Review of the satisfaction with life scale. Psychological Assessment. 1993;5:164–72. [Google Scholar]
- Pessoa L, Padmala S. Quantitative prediction of perceptual decisions during near-threshold fear detection. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:5612–17. doi: 10.1073/pnas.0500566102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40:879–91. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Baldwin MW, Dedovic K, et al. Self-esteem, locus of control, hippocampal volume, and cortisol regulation in young and old adulthood. Neuroimage. 2005;28:815–26. doi: 10.1016/j.neuroimage.2005.06.014. [DOI] [PubMed] [Google Scholar]
- Rameson LT, Satpute AB, Lieberman MD. The neural correlates of implicit and explicit self-relevant processing. Neuroimage. 2010;50:701–8. doi: 10.1016/j.neuroimage.2009.12.098. [DOI] [PubMed] [Google Scholar]
- Rice KG, Ashby JS, Slaney RB. Self-esteem as a mediator between perfectionism and depression: a structural equations analysis. Journal of Counseling Psychology. 1998;45:304–14. [Google Scholar]
- Rilling JK, Sanfey AG. The neuroscience of social decision-making. Annual Review of Psychology. 2011;62:23–48. doi: 10.1146/annurev.psych.121208.131647. [DOI] [PubMed] [Google Scholar]
- Ring L, Höfer S, McGee H, Hickey A, O’Boyle C. Individual quality of life: can it be accounted for by psychological or subjective well-being? Social Indicators Research. 2007;82:443–61. [Google Scholar]
- Root JC, Tuescher O, Cunningham-Bussel A, et al. Frontolimbic function and cortisol reactivity in response to emotional stimuli. Neuroreport. 2009;20:429–34. doi: 10.1097/WNR.0b013e328326a031. [DOI] [PubMed] [Google Scholar]
- Rosenburg M. Society and the Adolescent Self-Image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- Rudy JW. Context representations, context functions, and the parahippocampal–hippocampal system. Learning & Memory. 2009;16:573–85. doi: 10.1101/lm.1494409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RM, Deci EL. On happiness and human potentials: a review of research on hedonic and eudaimonic well-being. Annual Review of Psychology. 2001;52:141–66. doi: 10.1146/annurev.psych.52.1.141. [DOI] [PubMed] [Google Scholar]
- Ryff CD, Keyes CLM. The structure of psychological well-being revisited. Journal of Personality and Social Psychology. 1995;69:719–27. doi: 10.1037//0022-3514.69.4.719. [DOI] [PubMed] [Google Scholar]
- Saxe R, Moran J, Scholz J, Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self reflection in individual subjects. Social Cognitive and Affective Neuroscience. 2006;1:229–34. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmack U. ETC: Frequency Processing and Cognition. 2002. Frequency judgments of emotions: the cognitive basis of personality assessment. (pp. 189–204). New York: Oxford University Press. [Google Scholar]
- Schmitt DP, Allik J. Simultaneous administration of the Rosenberg Self-Esteem Scale in 53 nations: exploring the universal and culture-specific features of global self-esteem. Journal of Personality and Social Psychology. 2005;89:623–42. doi: 10.1037/0022-3514.89.4.623. [DOI] [PubMed] [Google Scholar]
- Seligman M, Csikszentmihalyi M. Positive psychology. An introduction. American Psychologist. 2000;55:5–14. doi: 10.1037//0003-066x.55.1.5. [DOI] [PubMed] [Google Scholar]
- Servaas MN, Riese H, Ormel J, Aleman A. The neural correlates of worry in association with individual differences in neuroticism. Human Brain Mapping. 2014;35(9):4303–15. doi: 10.1002/hbm.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver M, Montana G, Nichols TE. False positives in neuroimaging genetics using voxel-based morphometry data. Neuroimage. 2011;54:992–1000. doi: 10.1016/j.neuroimage.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon O, Mangin J-F, Cohen L, Le Bihan D, Dehaene S. Topographical layout of hand, eye, calculation, and language-related areas in the human parietal lobe. Neuron. 2002;33:475–87. doi: 10.1016/s0896-6273(02)00575-5. [DOI] [PubMed] [Google Scholar]
- Smith DV, Hayden BY, Truong T-K, Song AW, Platt ML, Huettel SA. Distinct value signals in anterior and posterior ventromedial prefrontal cortex. The Journal of Neuroscience. 2010;30:2490–5. doi: 10.1523/JNEUROSCI.3319-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Y, Lu H, Hu S, Xu M, Li X, Liu J. Regulating emotion to improve physical health through the amygdala. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu083. doi: 10.1093/scan/nsu083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. Journal of Neuroscience. 2001;21:8819–29. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengelmeyer R, Steele JD, Mwangi B, et al. The insular cortex and the neuroanatomy of major depression. Journal of Affective Disorders. 2011;133:120–7. doi: 10.1016/j.jad.2011.04.004. [DOI] [PubMed] [Google Scholar]
- Steel P, Schmidt J, Shultz J. Refining the relationship between personality and subjective well-being. Psychological Bulletin. 2008;134:138–61. doi: 10.1037/0033-2909.134.1.138. [DOI] [PubMed] [Google Scholar]
- Sterpenich V, D’Argembeau A, Desseilles M, et al. The locus ceruleus is involved in the successful retrieval of emotional memories in humans. The Journal of Neuroscience. 2006;26:7416–23. doi: 10.1523/JNEUROSCI.1001-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbe JH, Posthuma D, Boomsma DI, De Geus EJ. Heritability of life satisfaction in adults: a twin-family study. Psychological Medicine. 2005;35:1581–8. doi: 10.1017/S0033291705005374. [DOI] [PubMed] [Google Scholar]
- Sun P, Kong F. Affective mediators of the influence of gratitude on life satisfaction in late adolescence. Social Indicators Research. 2013;114:1361–9. [Google Scholar]
- Swami V, Chamorro-Premuzic T, Sinniah D, et al. General health mediates the relationship between loneliness, life satisfaction and depression. Social Psychiatry and Psychiatric Epidemiology. 2007;42:161–6. doi: 10.1007/s00127-006-0140-5. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Nouchi R, et al. Anatomical correlates of quality of life: evidence from voxel-based morphometry. Human Brain Mapping. 2014a;35:1834–6. doi: 10.1002/hbm.22294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, et al. Regional gray matter density associated with emotional intelligence: evidence from voxel-based morphometry. Human Brain Mapping. 2011;32:1497–510. doi: 10.1002/hbm.21122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi H, Taki Y, Sassa Y, et al. Regional gray matter volume is associated with empathizing and systemizing in young adults. PLoS ONE. 2014b;9:e84782. doi: 10.1371/journal.pone.0084782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nature Reviews Neuroscience. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, Nitschke JB, Dolski I, et al. Making a life worth living neural correlates of well-being. Psychological Science. 2004;15:367–72. doi: 10.1111/j.0956-7976.2004.00686.x. [DOI] [PubMed] [Google Scholar]
- Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. The Journal of Neuroscience. 2014;34:932–40. doi: 10.1523/JNEUROSCI.4227-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Reekum CM, Urry HL, Johnstone T, et al. Individual differences in amygdala and ventromedial prefrontal cortex activity are associated with evaluation speed and psychological well-being. Journal of Cognitive Neuroscience. 2007;19:237–48. doi: 10.1162/jocn.2007.19.2.237. [DOI] [PubMed] [Google Scholar]
- Vanlierde A, De Volder AG, Wanet-Defalque M-C, Veraart C. Occipito-parietal cortex activation during visuo-spatial imagery in early blind humans. Neuroimage. 2003;19:698–709. doi: 10.1016/s1053-8119(03)00153-8. [DOI] [PubMed] [Google Scholar]
- Videbech P, Ravnkilde B. Hippocampal volume and depression: a meta-analysis of MRI studies. American Journal of Psychiatry. 2004;161:1957–66. doi: 10.1176/appi.ajp.161.11.1957. [DOI] [PubMed] [Google Scholar]
- Vogeley K, May M, Ritzl A, Falkai P, Zilles K, Fink G. Neural correlates of first-person perspective as one constituent of human self-consciousness. Journal of Cognitive Neuroscience. 2004;16:817–27. doi: 10.1162/089892904970799. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Progress in Brain Research. 2005;150:205–17. doi: 10.1016/S0079-6123(05)50015-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Gunten A, Fox NC, Cipolotti L, Ron MA. A volumetric study of hippocampus and amygdala in depressed patients with subjective memory problems. The Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:493–8. doi: 10.1176/jnp.12.4.493. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends in Cognitive Sciences. 2005;9:445–53. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Wang R, Li J, Fang H, Tian M, Liu J. Individual differences in holistic processing predict face recognition ability. Psychological Science. 2012;23:169–77. doi: 10.1177/0956797611420575. [DOI] [PubMed] [Google Scholar]
- Watson D, Clark L, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- Wei D, Du X, Li W, et al. Regional gray matter volume and anxiety-related traits interact to predict somatic complaints in a non-clinical sample. Social Cognitive and Affective Neuroscience. 2014 doi: 10.1093/scan/nsu033. doi: 10.1093/scan/nsu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner NS, Meindl T, Engel RR, et al. Hippocampal function during associative learning in patients with posttraumatic stress disorder. Journal of Psychiatric Research. 2009;43:309–18. doi: 10.1016/j.jpsychires.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Weisberg J, Martin A. Neural correlates of semantic and episodic memory retrieval. Neuropsychologia. 1998;37:103–18. doi: 10.1016/s0028-3932(98)00044-x. [DOI] [PubMed] [Google Scholar]
- Winecoff A, Clithero JA, Carter RM, Bergman SR, Wang L, Huettel SA. Ventromedial prefrontal cortex encodes emotional value. The Journal of Neuroscience. 2013;33:11032–9. doi: 10.1523/JNEUROSCI.4317-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright CI, Feczko E, Dickerson B, Williams D. Neuroanatomical correlates of personality in the elderly. Neuroimage. 2007;35:263–72. doi: 10.1016/j.neuroimage.2006.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye S, Yu L, Li K-K. A cross-lagged model of self-esteem and life satisfaction: gender differences among Chinese university students. Personality and Individual Differences. 2012;52:546–51. [Google Scholar]
- Zhao J, Kong F, Wang Y. Self-esteem and humor style as mediators of the effects of shyness on loneliness among Chinese college students. Personality and Individual Differences. 2012;52:686–90. [Google Scholar]
- Zhao J, Kong F, Wang Y. The role of social support and self-esteem in the relationship between shyness and loneliness. Personality and Individual Differences. 2013;54:577–81. [Google Scholar]
- Zhao J, Wang Y, Kong F. Exploring the mediation effect of social support and self-esteem on the relationship between humor style and life satisfaction in Chinese college students. Personality and Individual Differences. 2014;64:126–30. [Google Scholar]
- Zullig KJ, Valois RF, Huebner ES, Drane JW. Adolescent health-related quality of life and perceived satisfaction with life. Quality of Life Research. 2005;14:1573–84. doi: 10.1007/s11136-004-7707-y. [DOI] [PubMed] [Google Scholar]